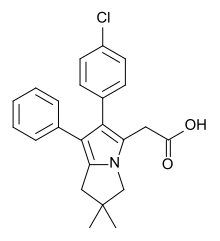
Licofelone
Appearance
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.222.821 |
| Chemical and physical data | |
| Formula | C23H22ClNO2 |
| Molar mass | 379.88 g·mol−1 |
| |
| | |
Licofelone is a dual COX/LOX inhibitor[1][2] that was studied in clinical trials as a treatment for osteoarthritis[3] and which was under development by Merckle GmbH with partners Alfa Wassermann and Lacer.
Licofelone is both an analgesic and an anti-inflammatory. Inhibition of 5-lipoxygenase (5-LOX) may reduce the gastrointestinal toxicity associated with other nonsteroidal anti-inflammatory drugs (NSAID), which only inhibit cyclooxygenase (COX). Licofelone is the first drug to inhibit both.
Phase III trials for osteoarthritis were conducted in the early 2000s,[4][5] but results were mixed and the drug has never been submitted for regulatory approval.
References
- ^ Fischer L, Hornig M, Pergola C, Meindl N, Franke L, Tanrikulu Y, et al. (October 2007). "The molecular mechanism of the inhibition by licofelone of the biosynthesis of 5-lipoxygenase products". British Journal of Pharmacology. 152 (4): 471–80. doi:10.1038/sj.bjp.0707416. PMC 2050828. PMID 17704828.
- ^ Vidal C, Gómez-Hernández A, Sánchez-Galán E, González A, Ortega L, Gómez-Gerique JA, et al. (January 2007). "Licofelone, a balanced inhibitor of cyclooxygenase and 5-lipoxygenase, reduces inflammation in a rabbit model of atherosclerosis". The Journal of Pharmacology and Experimental Therapeutics. 320 (1): 108–16. doi:10.1124/jpet.106.110361. PMID 17015640. S2CID 73105840.
- ^ Alvaro-Gracia JM (February 2004). "Licofelone--clinical update on a novel LOX/COX inhibitor for the treatment of osteoarthritis". Rheumatology. 43 Suppl 1 (90001): i21-5. doi:10.1093/rheumatology/keh105. PMID 14752172.
- ^ "Licofelone – Novel Analgesic and Anti-Inflammatory Agent for Osteoarthritis". Retrieved January 12, 2018.
- ^ Wildi LM, Raynauld JP, Martel-Pelletier J, Abram F, Dorais M, Pelletier JP (December 2010). "Relationship between bone marrow lesions, cartilage loss and pain in knee osteoarthritis: results from a randomised controlled clinical trial using MRI". Annals of the Rheumatic Diseases. 69 (12): 2118–24. doi:10.1136/ard.2009.127993. PMID 20610445. S2CID 206864399.
