Light-emitting diode
 Blue, green, and red LEDs in 5 mm diffused case | |
| Working principle | Electroluminescence |
|---|---|
| Invented | H. J. Round (1907)[1] Oleg Losev (1927)[2] James R. Biard (1961)[3] Nick Holonyak (1962)[4] |
| First production | October 1962 |
| Pin configuration | Anode and cathode |
| Electronic symbol | |



A light-emitting diode (LED) is a two-lead semiconductor light source. It is a p–n junction diode that emits light when activated.[5] When a suitable current is applied to the leads,[6][7] electrons are able to recombine with electron holes within the device, releasing energy in the form of photons. This effect is called electroluminescence, and the color of the light (corresponding to the energy of the photon) is determined by the energy band gap of the semiconductor. LEDs are typically small (less than 1 mm2) and integrated optical components may be used to shape the radiation pattern.[8]
Appearing as practical electronic components in 1962, the earliest LEDs emitted low-intensity infrared light.[9] Infrared LEDs are still frequently used as transmitting elements in remote-control circuits, such as those in remote controls for a wide variety of consumer electronics. The first visible-light LEDs were of low intensity and limited to red. Modern LEDs are available across the visible, ultraviolet, and infrared wavelengths, with very high brightness.
Early LEDs were often used as indicator lamps for electronic devices, replacing small incandescent bulbs. They were soon packaged into numeric readouts in the form of seven-segment displays and were commonly seen in digital clocks. Recent developments have produced LEDs suitable for environmental and task lighting. LEDs have led to new displays and sensors, while their high switching rates are useful in advanced communications technology.
LEDs have many advantages over incandescent light sources, including lower energy consumption, longer lifetime, improved physical robustness, smaller size, and faster switching. Light-emitting diodes are used in applications as diverse as aviation lighting, automotive headlamps, advertising, general lighting, traffic signals, camera flashes, lighted wallpaper and medical devices.[10] They are also significantly more energy efficient and, arguably, have fewer environmental concerns linked to their disposal.[11][12]
Unlike a laser, the color of light emitted from an LED is neither coherent nor monochromatic, but the spectrum is narrow with respect to human vision, and for most purposes the light from a simple diode element can be regarded as functionally monochromatic.[13][better source needed]
History
Discoveries and early devices

Electroluminescence as a phenomenon was discovered in 1907 by the British experimenter H. J. Round of Marconi Labs, using a crystal of silicon carbide and a cat's-whisker detector.[14][15] Russian inventor Oleg Losev reported creation of the first LED in 1927.[16] His research was distributed in Soviet, German and British scientific journals, but no practical use was made of the discovery for several decades.[17][18]
In 1936, Georges Destriau observed Electroluminescence could be produced when zinc sulphide (ZnS) powder is suspended in an insulator and an alternating electrical field is applied to it. In his publications, Destriau often referred to luminescence as Losev-Light. Destriau worked in the laboratories of Madame Marie Curie, also an early pioneer in the field of luminescence with research on radium.[19][20]
Kurt Lehovec, Carl Accardo, and Edward Jamgochian explained these first light-emitting diodes in 1951 using an apparatus employing SiC crystals with a current source of battery or pulse generator and with a comparison to a variant, pure, crystal in 1953.[21][22]
Rubin Braunstein[23] of the Radio Corporation of America reported on infrared emission from gallium arsenide (GaAs) and other semiconductor alloys in 1955.[24] Braunstein observed infrared emission generated by simple diode structures using gallium antimonide (GaSb), GaAs, indium phosphide (InP), and silicon-germanium (SiGe) alloys at room temperature and at 77 Kelvin.
In 1957, Braunstein further demonstrated that the rudimentary devices could be used for non-radio communication across a short distance. As noted by Kroemer[25] Braunstein "…had set up a simple optical communications link: Music emerging from a record player was used via suitable electronics to modulate the forward current of a GaAs diode. The emitted light was detected by a PbS diode some distance away. This signal was fed into an audio amplifier and played back by a loudspeaker. Intercepting the beam stopped the music. We had a great deal of fun playing with this setup." This setup presaged the use of LEDs for optical communication applications.

In September 1961, while working at Texas Instruments in Dallas, Texas, James R. Biard and Gary Pittman discovered near-infrared (900 nm) light emission from a tunnel diode they had constructed on a GaAs substrate.[9] By October 1961, they had demonstrated efficient light emission and signal coupling between a GaAs p-n junction light emitter and an electrically-isolated semiconductor photodetector.[26] On August 8, 1962, Biard and Pittman filed a patent titled "Semiconductor Radiant Diode" based on their findings, which described a zinc diffused p–n junction LED with a spaced cathode contact to allow for efficient emission of infrared light under forward bias. After establishing the priority of their work based on engineering notebooks predating submissions from G.E. Labs, RCA Research Labs, IBM Research Labs, Bell Labs, and Lincoln Lab at MIT, the U.S. patent office issued the two inventors the patent for the GaAs infrared (IR) light-emitting diode (U.S. Patent US3293513), the first practical LED.[9] Immediately after filing the patent, Texas Instruments (TI) began a project to manufacture infrared diodes. In October 1962, TI announced the first commercial LED product (the SNX-100), which employed a pure GaAs crystal to emit a 890 nm light output.[9] In October 1963, TI announced the first commercial hemispherical LED, the SNX-110.[27]
The first visible-spectrum (red) LED was developed in 1962 by Nick Holonyak, Jr. while working at General Electric. Holonyak first reported his LED in the journal Applied Physics Letters on December 1, 1962.[28][29] M. George Craford,[30] a former graduate student of Holonyak, invented the first yellow LED and improved the brightness of red and red-orange LEDs by a factor of ten in 1972.[31] In 1976, T. P. Pearsall created the first high-brightness, high-efficiency LEDs for optical fiber telecommunications by inventing new semiconductor materials specifically adapted to optical fiber transmission wavelengths.[32]
Initial commercial development
The first commercial LEDs were commonly used as replacements for incandescent and neon indicator lamps, and in seven-segment displays,[33] first in expensive equipment such as laboratory and electronics test equipment, then later in such appliances as TVs, radios, telephones, calculators, as well as watches (see list of signal uses). Until 1968, visible and infrared LEDs were extremely costly, in the order of US$200 per unit, and so had little practical use.[34] The Monsanto Company was the first organization to mass-produce visible LEDs, using gallium arsenide phosphide (GaAsP) in 1968 to produce red LEDs suitable for indicators.[34] Hewlett-Packard (HP) introduced LEDs in 1968, initially using GaAsP supplied by Monsanto. These red LEDs were bright enough only for use as indicators, as the light output was not enough to illuminate an area. Readouts in calculators were so small that plastic lenses were built over each digit to make them legible. Later, other colors became widely available and appeared in appliances and equipment. In the 1970s commercially successful LED devices at less than five cents each were produced by Fairchild Optoelectronics. These devices employed compound semiconductor chips fabricated with the planar process invented by Dr. Jean Hoerni at Fairchild Semiconductor.[35][36] The combination of planar processing for chip fabrication and innovative packaging methods enabled the team at Fairchild led by optoelectronics pioneer Thomas Brandt to achieve the needed cost reductions.[37] LED producers continue to use these methods.[38]

Most LEDs were made in the very common 5 mm T1¾ and 3 mm T1 packages, but with rising power output, it has grown increasingly necessary to shed excess heat to maintain reliability,[39] so more complex packages have been adapted for efficient heat dissipation. Packages for state-of-the-art high-power LEDs bear little resemblance to early LEDs.
Blue LED
Blue LEDs were first developed by Herbert Paul Maruska at RCA in 1972 using gallium nitride (GaN) on a sapphire substrate.[40] SiC-types were first commercially sold in the United States by Cree in 1989.[41] However, neither of these initial blue LEDs were very bright.
The first high-brightness blue LED was demonstrated by Shuji Nakamura of Nichia Corporation in 1994 and was based on InGaN.[42][43] In parallel, Isamu Akasaki and Hiroshi Amano in Nagoya were working on developing the important GaN nucleation on sapphire substrates and the demonstration of p-type doping of GaN. Nakamura, Akasaki, and Amano were awarded the 2014 Nobel prize in physics for their work.[44] In 1995, Alberto Barbieri at the Cardiff University Laboratory (GB) investigated the efficiency and reliability of high-brightness LEDs and demonstrated a "transparent contact" LED using indium tin oxide (ITO) on (AlGaInP/GaAs).
In 2001[45] and 2002,[46] processes for growing gallium nitride (GaN) LEDs on silicon were successfully demonstrated. In January 2012, Osram demonstrated high-power InGaN LEDs grown on silicon substrates commercially,[47] and GaN-on-silicon LEDs are in production at Plessey Semiconductors. As of 2017, some manufacturers are using SiC as the substrate for LED production, but sapphire is more common, as it has the most similar properties to that of gallium nitride, reducing the need for patterning the sapphire wafer. (Patterned wafers are known as epi wafers.) Samsung, the University of Cambridge, and Toshiba are performing research into GaN on Si(licon) LEDs. Toshiba has stopped research, possibly due to low yields.[48][49][50][51][52][53][54] Some opt towards epitaxy, which is difficult on silicon, while others, like the University of Cambridge, opt towards a multi layer structure, in order to reduce (crystal) lattice mismatch and different thermal expansion ratios, in order to avoid cracking of the led chip at high temperatures (e.g. during manufacturing), reduce heat generation and increase luminous efficiency. Epitaxy (or patterned sapphire) can be carried out with Nanoimprint lithography.[55][56][57][58][59][60][61]
White LEDs and the illumination breakthrough
Even though white light can be created using individual red, green and blue LEDs, perhaps using a single SMD (Surface Mount Device) or through-hole RGB LED, this setup results in poor color rendering or CRI, due to the fact that virtually only 3 wavelengths of light are being emitted, so the attainment of high efficiency in blue LEDs was quickly followed by the development of the first white LED. In this device a Y
3Al
5O
12:Ce (known as "YAG") cerium doped phosphor coating on the emitter absorbs some of the blue emission and produces yellow light through fluorescence. The combination of that yellow with remaining blue light appears white to the eye. However, using different phosphors (fluorescent materials) it also became possible to instead produce green and red light through fluorescence. The resulting mixture of red, green and blue is not only perceived by humans as white light but is superior for illumination in terms of color rendering, whereas one cannot appreciate the color of red or green objects illuminated only by the yellow (and remaining blue) wavelengths from the YAG phosphor.

The first white LEDs were expensive and inefficient. However, the light output of LEDs has increased exponentially, with a doubling occurring approximately every 36 months since the 1960s (similar to Moore's law). The latest research and development has been propagated by Japanese manufacturers such as Panasonic, Nichia, etc. and later by Korean and Chinese factories and investment such as: Samsung, Solstice, Kingsun, and countless others.[62] This trend is generally attributed to the parallel development of other semiconductor technologies and advances in optics[citation needed] and materials science and has been called Haitz's law after Dr. Roland Haitz.[63]
Light output and efficiency of blue and near-ultraviolet LEDs rose as the cost of reliable devices fell. This led to relatively high-power white-light LEDs for illumination, which are replacing incandescent and fluorescent lighting.[64][65]
Experimental white LEDs have been demonstrated to produce 303 lumens per watt of electricity (lm/w); some can last up to 100,000 hours.[66][67] However, commercially available LEDs have an efficiency of up to 223 lm/w.[68][69][70] Compared to incandescent bulbs, this is not only a huge increase in electrical efficiency but – over time – a similar or lower cost per bulb.[71] The LED chip is encapsulated inside a small, plastic, white mold. It can be encapsulated using resin, silicone, or epoxy containing (powdered) Cerium doped YAG phosphor. After allowing the solvents to evaporate, the LEDs are often tested, and placed on tapes for SMT placement equipment for use in LED light bulb production. Encapsulation is performed after probing, dicing, die transfer from wafer to package, and wire bonding or flip chip mounting, perhaps using Indium tin oxide, a transparent electrical conductor. In this case, the bond wire(s) are attached to the ITO film that has been deposited in the LEDs.
The YAG phosphor in the LED's encapsulation compound is why white LEDs look yellow when off. Some LED light bulbs use a single plastic cover with YAG phosphor (remote phosphor) for several blue LEDs instead of using a diffuser and single chip white LEDs. Remote phosphor LED light bulbs may have behind the plastic cover a white plastic reflector. Others shape the remote phosphor as a dome, or sphere, and place it atop a single PCB containing blue LEDs; this assembly may be behind a frosted glass or plastic cover. The PCB is often installed atop a pillar, which is lined with white plastic.
Working principle
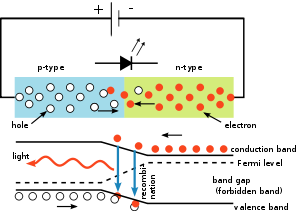
A P-N junction can convert absorbed light energy into a proportional electric current. The same process is reversed here (i.e. the P-N junction emits light when electrical energy is applied to it). This phenomenon is generally called electroluminescence, which can be defined as the emission of light from a semiconductor under the influence of an electric field. The charge carriers recombine in a forward-biased P-N junction as the electrons cross from the N-region and recombine with the holes existing in the P-region. Free electrons are in the conduction band of energy levels, while holes are in the valence energy band. Thus the energy level of the holes is less than the energy levels of the electrons. Some portion of the energy must be dissipated to recombine the electrons and the holes. This energy is emitted in the form of heat and light.
The electrons dissipate energy in the form of heat for silicon and germanium diodes but in gallium arsenide phosphide (GaAsP) and gallium phosphide (GaP) semiconductors, the electrons dissipate energy by emitting photons. If the semiconductor is translucent, the junction becomes the source of light as it is emitted, thus becoming a light-emitting diode. However, when the junction is reverse biased, the LED produces no light and—if the potential is great enough, the device is damaged.
Technology
Physics
The LED consists of a chip of semiconducting material doped with impurities to create a p-n junction. As in other diodes, current flows easily from the p-side, or anode, to the n-side, or cathode, but not in the reverse direction. Charge-carriers—electrons and holes—flow into the junction from electrodes with different voltages. When an electron meets a hole, it falls into a lower energy level and releases energy in the form of a photon.
The wavelength of the light emitted, and thus its color, depends on the band gap energy of the materials forming the p-n junction. In silicon or germanium diodes, the electrons and holes usually recombine by a non-radiative transition, which produces no optical emission, because these are indirect band gap materials. The materials used for the LED have a direct band gap with energies corresponding to near-infrared, visible, or near-ultraviolet light.
LED development began with infrared and red devices made with gallium arsenide. Advances in materials science have enabled making devices with ever-shorter wavelengths, emitting light in a variety of colors.
LEDs are usually built on an n-type substrate, with an electrode attached to the p-type layer deposited on its surface. P-type substrates, while less common, occur as well. Many commercial LEDs, especially GaN/InGaN, also use sapphire substrate.
Refractive index


Bare uncoated semiconductors such as silicon exhibit a very high refractive index relative to open air, which prevents passage of photons arriving at sharp angles relative to the air-contacting surface of the semiconductor due to total internal reflection. This property affects both the light-emission efficiency of LEDs as well as the light-absorption efficiency of photovoltaic cells. The refractive index of silicon is set at 3.96 (at 590 nm),[73] while air's refractive index is set at 1.0002926.[74]
In general, a flat-surface uncoated LED semiconductor chip emits light only perpendicular to the semiconductor's surface, and a few degrees to the side, in a cone shape referred to as the light cone, cone of light,[75] or the escape cone.[72] The maximum angle of incidence is referred to as the critical angle. When the critical angle is exceeded, photons no longer escape the semiconductor but are, instead, reflected internally inside the semiconductor crystal as if it were a mirror.[72]
Internal reflections can escape through other crystalline faces if the incidence angle is low enough and the crystal is sufficiently transparent to not re-absorb the photon emission. But for a simple square LED with 90-degree angled surfaces on all sides, the faces all act as equal angle mirrors. In this case, most of the light can not escape and is lost as waste heat in the crystal.[72]
A convoluted chip surface with angled facets similar to a jewel or fresnel lens can increase light output by distributing light perpendicular to the chip surface and far to the sides of the photon emission point.[76]
The ideal shape of a semiconductor with maximum light output would be a microsphere with the photon emission occurring at the exact center, with electrodes penetrating to the center to contact at the emission point. All light rays emanating from the center would be perpendicular to the entire surface of the sphere, resulting in no internal reflections. A hemispherical semiconductor would also work, with the flat back-surface serving as a mirror to back-scattered photons.[77]
Transition coatings
After the doping of the wafer, it is usually cut apart into individual dies. Each die is commonly called a chip.
Many LED semiconductor chips are encapsulated or potted in clear or colored molded solid plastic. The plastic encapsulation has three purposes:
- Mounting the semiconductor chip in devices is easier to accomplish.
- The tiny fragile electrical wiring is physically supported and protected from damage.
- The plastic acts as a refractive intermediary between the relatively high-index semiconductor and low-index open air.[78]
The third feature helps to boost the light emission from the semiconductor by acting as a diffusing lens, emitting light at a much higher angle of incidence from the light cone than the bare chip would alone.
Efficiency and operational parameters
Typical indicator LEDs are designed to operate with no more than 30–60 milliwatts (mW) of electrical power. Around 1999, Philips Lumileds introduced power LEDs capable of continuous use at one watt. These LEDs used much larger semiconductor die sizes to handle the large power inputs. Also, the semiconductor dies were mounted onto metal slugs to allow for greater heat dissipation from the LED die.
One of the key advantages of LED-based lighting sources is high luminous efficacy. White LEDs quickly matched and overtook the efficacy of standard incandescent lighting systems. In 2002, Lumileds made five-watt LEDs available with luminous efficacy of 18–22 lumens per watt (lm/W). For comparison, a conventional incandescent light bulb of 60–100 watts emits around 15 lm/W, and standard fluorescent lights emit up to 100 lm/W.
As of 2012[update], Philips had achieved the following efficacies for each color.[79] The efficiency values show the physics – light power out per electrical power in. The lumen-per-watt efficacy value includes characteristics of the human eye and is derived using the luminosity function.
| Color | Wavelength range (nm) | Typical efficiency coefficient | Typical efficacy (lm/W) | |
|---|---|---|---|---|
| Red | 620 < λ < 645 | 0.39 | 72 | |
| Red-orange | 610 < λ < 620 | 0.29 | 98 | |
| Green | 520 < λ < 550 | 0.15 | 93 | |
| Cyan | 490 < λ < 520 | 0.26 | 75 | |
| Blue | 460 < λ < 490 | 0.35 | 37 |
In September 2003, a new type of blue LED was demonstrated by Cree. This produced a commercially packaged white light giving 65 lm/W at 20 mA, becoming the brightest white LED commercially available at the time, and more than four times as efficient as standard incandescents. In 2006, they demonstrated a prototype with a record white LED luminous efficacy of 131 lm/W at 20 mA. Nichia Corporation has developed a white LED with luminous efficacy of 150 lm/W at a forward current of 20 mA.[80] Cree's XLamp XM-L LEDs, commercially available in 2011, produce 100 lm/W at their full power of 10 W, and up to 160 lm/W at around 2 W input power. In 2012, Cree announced a white LED giving 254 lm/W,[81] and 303 lm/W in March 2014.[82] Practical general lighting needs high-power LEDs, of one watt or more. Typical operating currents for such devices begin at 350 mA.
These efficiencies are for the light-emitting diode only, held at low temperature in a lab. Since LEDs installed in real fixtures operate at higher temperature and with driver losses, real-world efficiencies are much lower. United States Department of Energy (DOE) testing of commercial LED lamps designed to replace incandescent lamps or CFLs showed that average efficacy was still about 46 lm/W in 2009 (tested performance ranged from 17 lm/W to 79 lm/W).[83]
Efficiency droop
Efficiency droop is the decrease in luminous efficiency of LEDs as the electric current increases above tens of milliamperes.
This effect was initially thought related to elevated temperatures. Scientists proved the opposite is true: though the life of an LED is shortened, the efficiency droop is less severe at elevated temperatures.[84] The mechanism causing efficiency droop was identified in 2007 as Auger recombination, which was taken with mixed reaction.[85] In 2013, a study confirmed Auger recombination as the cause of efficiency droop.[86]
In addition to being less efficient, operating LEDs at higher electric currents creates higher heat levels, which can compromise LED lifetime. Because of this increased heat at higher currents, high-brightness LEDs have an industry standard of operating at only 350 mA, which is a compromise between light output, efficiency, and longevity.[85][87][88][89]
Possible solutions
Instead of increasing current levels, luminance is usually increased by combining multiple LEDs in one bulb. Solving the problem of efficiency droop would mean that household LED light bulbs would need fewer LEDs, which would significantly reduce costs.
Researchers at the U.S. Naval Research Laboratory have found a way to lessen the efficiency droop. They found that the droop arises from non-radiative Auger recombination of the injected carriers. They created quantum wells with a soft confinement potential to lessen the non-radiative Auger processes.[90]
Researchers at Taiwan National Central University and Epistar Corp are developing a way to lessen the efficiency droop by using ceramic aluminium nitride (AlN) substrates, which are more thermally conductive than the commercially used sapphire. The higher thermal conductivity reduces self-heating effects.[91]
Lifetime and failure
Solid-state devices such as LEDs are subject to very limited wear and tear if operated at low currents and at low temperatures. Typical lifetimes quoted are 25,000 to 100,000 hours, but heat and current settings can extend or shorten this time significantly.[92] One way to cool down the LED and use the energy efficiently, is to blink the LED in order to gain biomass for example in microalgae cultivation.[93] It is important to note that these projections are based on a standard test that may not accelerate all the potential mechanisms that can induce failures in LEDs.[94]
The most common symptom of LED (and diode laser) failure is the gradual lowering of light output and loss of efficiency. Sudden failures, although rare, can also occur. Early red LEDs were notable for their short service life. With the development of high-power LEDs, the devices are subjected to higher junction temperatures and higher current densities than traditional devices. This causes stress on the material and may cause early light-output degradation. To quantify useful lifetime in a standardized manner, some suggest using L70 or L50, which are runtimes (typically in thousands of hours) at which a given LED reaches 70% and 50% of initial light output, respectively.[95]
Whereas in most previous sources of light (incandescent lamps, discharge lamps, and those that burn combustible fuel, e.g. candles and oil lamps) the light results from heat, LEDs only operate if they are kept cool enough. The manufacturer commonly specifies a maximum junction temperature of 125 or 150 °C, and lower temperatures are advisable in the interests of long life. At these temperatures, relatively little heat is lost by radiation, which means that the light beam generated by an LED is cool.
The waste heat in a high-power LED (which as of 2015 can be less than half the power that it consumes) is conveyed by conduction through the substrate and package of the LED to a heat sink, which gives up the heat to the ambient air by convection. Careful thermal design is, therefore, essential, taking into account the thermal resistances of the LED’s package, the heat sink and the interface between the two. Medium-power LEDs are often designed to solder directly to a printed circuit board that contains a thermally conductive metal layer. (Often, it is a PCB with a white Solder mask (white pcb) and a ~1mm thick aluminum backing.) High-power LEDs are packaged in large-area ceramic packages that attach to a metal heat sink—the interface being a material with high thermal conductivity (thermal grease, phase-change material, thermally conductive pad, or thermal adhesive).
If an LED-based lamp is installed in an unventilated luminaire, or a luminaire is located in an environment that does not have free air circulation, the LED is likely to overheat, resulting in reduced life or early catastrophic failure. Thermal design is often based on an ambient temperature of 25 °C (77 °F). LEDs used in outdoor applications, such as traffic signals or in-pavement signal lights, and in climates where the temperature within the light fixture becomes very high, could experience reduced output or even failure.[96]
Since LED efficacy is higher at low temperatures, LED technology is well suited for supermarket freezer lighting.[97][98][99] Because LEDs produce less waste heat than incandescent lamps, freezer tube lighting[100] use can save on refrigeration costs as well. However, they may be more susceptible to frost and snow buildup than incandescent lamps,[96] so some LED lighting systems have been designed with an added heating circuit. Additionally, research has developed heat sink technologies that transfer heat produced within the junction to appropriate areas of the light fixture.[101]
Colors and materials
Conventional LEDs are made from a variety of inorganic semiconductor materials. The following table shows the available colors with wavelength range, voltage drop, and material:
| Color | Wavelength [nm] | Voltage drop [ΔV] | Semiconductor material | |
|---|---|---|---|---|
| Infrared | λ > 760 | ΔV < 1.63 | Gallium arsenide (GaAs) Aluminium gallium arsenide (AlGaAs) | |
| Red | 610 < λ < 760 | 1.63 < ΔV < 2.03 | Aluminium gallium arsenide (AlGaAs) Gallium arsenide phosphide (GaAsP) Aluminium gallium indium phosphide (AlGaInP) Gallium(III) phosphide (GaP) | |
| Orange | 590 < λ < 610 | 2.03 < ΔV < 2.10 | Gallium arsenide phosphide (GaAsP) Aluminium gallium indium phosphide (AlGaInP) Gallium(III) phosphide (GaP) | |
| Yellow | 570 < λ < 590 | 2.10 < ΔV < 2.18 | Gallium arsenide phosphide (GaAsP) Aluminium gallium indium phosphide (AlGaInP) Gallium(III) phosphide (GaP) | |
| Green | 500 < λ < 570 | 1.9[102] < ΔV < 4.0 | Traditional green: Gallium(III) phosphide (GaP) Aluminium gallium indium phosphide (AlGaInP) Aluminium gallium phosphide (AlGaP) Pure green: Indium gallium nitride (InGaN) / Gallium(III) nitride (GaN) | |
| Blue | 450 < λ < 500 | 2.48 < ΔV < 3.7 | Zinc selenide (ZnSe) Indium gallium nitride (InGaN) Synthetic sapphire, Silicon carbide (SiC) as substrate with or without epitaxy, Silicon (Si) as substrate—under development (epitaxy on silicon is hard to control) | |
| Violet | 400 < λ < 450 | 2.76 < ΔV < 4.0 | Indium gallium nitride (InGaN) | |
| Ultraviolet | λ < 400 | 3 < ΔV < 4.1 | Indium gallium nitride (InGaN) (385-400 nm)
Diamond (235 nm)[103] | |
| Pink | Multiple types | ΔV ≈3.3[108] | Blue with one or two phosphor layers, yellow with red, orange or pink phosphor added afterwards, white with pink plastic, | |
| Purple | Multiple types | 2.48 < ΔV < 3.7 | Dual blue/red LEDs, blue with red phosphor, or white with purple plastic | |
| White | Broad spectrum | 2.8 < ΔV < 4.2 | Cool / Pure White: Blue/UV diode with yellow phosphor Warm White: Blue diode with orange phosphor |
Blue and ultraviolet

| External videos | |
|---|---|
 | |
The first blue-violet LED using magnesium-doped gallium nitride was made at Stanford University in 1972 by Herb Maruska and Wally Rhines, doctoral students in materials science and engineering.[110][111] At the time Maruska was on leave from RCA Laboratories, where he collaborated with Jacques Pankove on related work. In 1971, the year after Maruska left for Stanford, his RCA colleagues Pankove and Ed Miller demonstrated the first blue electroluminescence from zinc-doped gallium nitride, though the subsequent device Pankove and Miller built, the first actual gallium nitride light-emitting diode, emitted green light.[112][113] In 1974 the U.S. Patent Office awarded Maruska, Rhines and Stanford professor David Stevenson a patent for their work in 1972 (U.S. Patent US3819974 A) and today, magnesium-doping of gallium nitride remains the basis for all commercial blue LEDs and laser diodes. In the early 1970s, these devices were too dim for practical use, and research into gallium nitride devices slowed. In August 1989, Cree introduced the first commercially available blue LED based on the indirect bandgap semiconductor, silicon carbide (SiC).[114] SiC LEDs had very low efficiency, no more than about 0.03%, but did emit in the blue portion of the visible light spectrum.[citation needed]
In the late 1980s, key breakthroughs in GaN epitaxial growth and p-type doping[115] ushered in the modern era of GaN-based optoelectronic devices. Building upon this foundation, Theodore Moustakas at Boston University patented a method for producing high-brightness blue LEDs using a new two-step process.[116] Two years later, in 1993, high-brightness blue LEDs were demonstrated again by Shuji Nakamura of Nichia Corporation using a gallium nitride growth process similar to Moustakas's.[117] Both Moustakas and Nakamura were issued separate patents, which confused the issue of who was the original inventor (partly because although Moustakas invented his first, Nakamura filed first).[citation needed] This new development revolutionized LED lighting, making high-power blue light sources practical, leading to the development of technologies like Blu-ray, as well as allowing the bright high-resolution screens of modern tablets and phones.[citation needed]
Nakamura was awarded the 2006 Millennium Technology Prize for his invention.[118] Nakamura, Hiroshi Amano and Isamu Akasaki were awarded the Nobel Prize in Physics in 2014 for the invention of the blue LED.[119][120][121] In 2015, a US court ruled that three companies (i.e. the litigants who had not previously settled out of court) that had licensed Nakamura's patents for production in the United States had infringed Moustakas's prior patent, and ordered them to pay licensing fees of not less than 13 million USD.[122]
By the late 1990s, blue LEDs became widely available. They have an active region consisting of one or more InGaN quantum wells sandwiched between thicker layers of GaN, called cladding layers. By varying the relative In/Ga fraction in the InGaN quantum wells, the light emission can in theory be varied from violet to amber. Aluminium gallium nitride (AlGaN) of varying Al/Ga fraction can be used to manufacture the cladding and quantum well layers for ultraviolet LEDs, but these devices have not yet reached the level of efficiency and technological maturity of InGaN/GaN blue/green devices. If un-alloyed GaN is used in this case to form the active quantum well layers, the device emits near-ultraviolet light with a peak wavelength centred around 365 nm. Green LEDs manufactured from the InGaN/GaN system are far more efficient and brighter than green LEDs produced with non-nitride material systems, but practical devices still exhibit efficiency too low for high-brightness applications.[citation needed]
With nitrides containing aluminium, most often AlGaN and AlGaInN, even shorter wavelengths are achievable. Ultraviolet LEDs in a range of wavelengths are becoming available on the market. Near-UV emitters at wavelengths around 375–395 nm are already cheap and often encountered, for example, as black light lamp replacements for inspection of anti-counterfeiting UV watermarks in some documents and paper currencies. Shorter-wavelength diodes, while substantially more expensive, are commercially available for wavelengths down to 240 nm.[123] As the photosensitivity of microorganisms approximately matches the absorption spectrum of DNA, with a peak at about 260 nm, UV LED emitting at 250–270 nm are expected in prospective disinfection and sterilization devices. Recent research has shown that commercially available UVA LEDs (365 nm) are already effective disinfection and sterilization devices.[124] UV-C wavelengths were obtained in laboratories using aluminium nitride (210 nm),[106] boron nitride (215 nm)[104][105] and diamond (235 nm).[103]
RGB

RGB LEDs consist of one red, one green, and one blue LED.[125] By independently adjusting each of the three, RGB LEDs are capable of producing a wide color gamut. Unlike dedicated-color LEDs, however, these obviously do not produce pure wavelengths. Moreover, such modules as commercially available are often not optimized for smooth color mixing.
White
There are two primary ways of producing white light-emitting diodes (WLEDs), LEDs that generate high-intensity white light. One is to use individual LEDs that emit three primary colors[126]—red, green, and blue—and then mix all the colors to form white light. The other is to use a phosphor material to convert monochromatic light from a blue or UV LED to broad-spectrum white light, much in the same way a fluorescent light bulb works. The 'whiteness' of the light produced is essentially engineered to suit the human eye. The yellow phosphor in LEDs is Cerium doped YAG crystal, in powder form and may be suspended in plastic, epoxy, synthetic rubber or silicone. Touching bare LEDs using synthetic rubber or silicone can damage the delicate wire bonds from LED to package or the LED itself, so they may be protected by a plastic or glass cover, such as in the LED flash in smartphones. It is this YAG phosphor which causes white LEDs without diffusers (such as those used in LED lamps and bulbs) to look yellow when off.
There are three main methods of mixing colors to produce white light from an LED:
- blue LED + green LED + red LED (color mixing; can be used as backlighting for displays, extremely poor for illumination due to gaps in spectrum)
- near-UV or UV LED + RGB phosphor (an LED producing light with a wavelength shorter than blue's is used to excite an RGB phosphor, a mixture of red, green, and blue phosphor)
- blue LED + yellow phosphor (two complementary colors combine to form white light; more efficient than the first two methods and more commonly used)[127]
Because of metamerism, it is possible to have quite different spectra that appear white. However, the appearance of objects illuminated by that light may vary as the spectrum varies, this is the issue of color rendition, quite separate from color temperature, where a really orange or cyan object could appear with the wrong color and much darker as the LED or phosphor does not emit the wavelength it reflects. The best color rendition CFL and LEDs use a mix of phosphors, resulting in less efficiency but better quality of light. Though incandescent halogen lamps have a more orange color temperature, they are still the best easily available artificial light sources in terms of color rendition.
RGB systems


White light can be formed by mixing differently colored lights; the most common method is to use red, green, and blue (RGB). Hence the method is called multicolor white LEDs (sometimes referred to as RGB LEDs). Because these need electronic circuits to control the blending and diffusion of different colors, and because the individual color LEDs typically have slightly different emission patterns (leading to variation of the color depending on direction) even if they are made as a single unit, these are seldom used to produce white lighting. Nonetheless, this method has many applications because of the flexibility of mixing different colors,[128] and in principle, this mechanism also has higher quantum efficiency in producing white light.[citation needed]
There are several types of multicolor white LEDs: di-, tri-, and tetrachromatic white LEDs. Several key factors that play among these different methods include color stability, color rendering capability, and luminous efficacy. Often, higher efficiency means lower color rendering, presenting a trade-off between the luminous efficacy and color rendering. For example, the dichromatic white LEDs have the best luminous efficacy (120 lm/W), but the lowest color rendering capability. However, although tetrachromatic white LEDs have excellent color rendering capability, they often have poor luminous efficacy. Trichromatic white LEDs are in between, having both good luminous efficacy (>70 lm/W) and fair color rendering capability.
One of the challenges is the development of more efficient green LEDs. The theoretical maximum for green LEDs is 683 lumens per watt but as of 2010 few green LEDs exceed even 100 lumens per watt. The blue and red LEDs approach their theoretical limits.
Multicolor LEDs offer not merely another means to form white light but a new means to form light of different colors. Most perceivable colors can be formed by mixing different amounts of three primary colors. This allows precise dynamic color control. As more effort is devoted to investigating this method, multicolor LEDs should have profound influence on the fundamental method that we use to produce and control light color. However, before this type of LED can play a role on the market, several technical problems must be solved. These include that this type of LED's emission power decays exponentially with rising temperature,[129] resulting in a substantial change in color stability. Such problems inhibit and may preclude industrial use. Thus, many new package designs aimed at solving this problem have been proposed and their results are now being reproduced by researchers and scientists. However multicolor LEDs without phosphors can never provide good quality lighting because each LED is a narrow band source (see graph). LEDs without phosphor while a poorer solution for general lighting are the best solution for displays, either backlight of LCD, or direct LED based pixels.
Correlated color temperature (CCT) dimming for LED technology is regarded as a difficult task since LED binning,[130] age and temperature drift effects of LEDs change the actual color value output. Feedback loop systems are used for example with color sensors, to actively monitor and control the color output of multiple color mixing LEDs.[131]
Phosphor-based LEDs

This method involves coating LEDs of one color (mostly blue LEDs made of InGaN) with phosphors of different colors to form white light; the resultant LEDs are called phosphor-based or phosphor-converted white LEDs (pcLEDs).[132] A fraction of the blue light undergoes the Stokes shift, which transforms it from shorter wavelengths to longer. Depending on the original LED's color, various color phosphors are used. Using several phosphor layers of distinct colors broadens the emitted spectrum, effectively raising the color rendering index (CRI).[133]
Phosphor-based LEDs have efficiency losses due to heat loss from the Stokes shift and also other phosphor-related issues. Their luminous efficacies compared to normal LEDs depend on the spectral distribution of the resultant light output and the original wavelength of the LED itself. For example, the luminous efficacy of a typical YAG yellow phosphor based white LED ranges from 3 to 5 times the luminous efficacy of the original blue LED because of the human eye's greater sensitivity to yellow than to blue (as modeled in the luminosity function). Due to the simplicity of manufacturing, the phosphor method is still the most popular method for making high-intensity white LEDs. The design and production of a light source or light fixture using a monochrome emitter with phosphor conversion is simpler and cheaper than a complex RGB system, and the majority of high-intensity white LEDs presently on the market are manufactured using phosphor light conversion.
Among the challenges being faced to improve the efficiency of LED-based white light sources is the development of more efficient phosphors. As of 2010, the most efficient yellow phosphor is still the YAG phosphor, with less than 10% Stokes shift loss. Losses attributable to internal optical losses due to re-absorption in the LED chip and in the LED packaging itself account typically for another 10% to 30% of efficiency loss. Currently, in the area of phosphor LED development, much effort is being spent on optimizing these devices to higher light output and higher operation temperatures. For instance, the efficiency can be raised by adapting better package design or by using a more suitable type of phosphor. Conformal coating process is frequently used to address the issue of varying phosphor thickness.
Some phosphor-based white LEDs encapsulate InGaN blue LEDs inside phosphor-coated epoxy. Alternatively, the LED might be paired with a remote phosphor, a preformed polycarbonate piece coated with the phosphor material. Remote phosphors provide more diffuse light, which is desirable for many applications. Remote phosphor designs are also more tolerant of variations in the LED emissions spectrum. A common yellow phosphor material is cerium-doped yttrium aluminium garnet (Ce3+:YAG).
White LEDs can also be made by coating near-ultraviolet (NUV) LEDs with a mixture of high-efficiency europium-based phosphors that emit red and blue, plus copper and aluminium-doped zinc sulfide (ZnS:Cu, Al) that emits green. This is a method analogous to the way fluorescent lamps work. This method is less efficient than blue LEDs with YAG:Ce phosphor, as the Stokes shift is larger, so more energy is converted to heat, but yields light with better spectral characteristics, which render color better. Due to the higher radiative output of the ultraviolet LEDs than of the blue ones, both methods offer comparable brightness. A concern is that UV light may leak from a malfunctioning light source and cause harm to human eyes or skin.
Other white LEDs
Another method used to produce experimental white light LEDs used no phosphors at all and was based on homoepitaxially grown zinc selenide (ZnSe) on a ZnSe substrate that simultaneously emitted blue light from its active region and yellow light from the substrate.[134]
A new style of wafers composed of gallium-nitride-on-silicon (GaN-on-Si) is being used to produce white LEDs using 200-mm silicon wafers. This avoids the typical costly sapphire substrate in relatively small 100- or 150-mm wafer sizes.[135] The sapphire apparatus must be coupled with a mirror-like collector to reflect light that would otherwise be wasted. It is predicted that by 2020, 40% of all GaN LEDs will be made with GaN-on-Si. Manufacturing large sapphire material is difficult, while large silicon material is cheaper and more abundant. LED companies shifting from using sapphire to silicon should be a minimal investment.[136]
Organic light-emitting diodes (OLEDs)
In an organic light-emitting diode (OLED), the electroluminescent material composing the emissive layer of the diode is an organic compound. The organic material is electrically conductive due to the delocalization of pi electrons caused by conjugation over all or part of the molecule, and the material therefore functions as an organic semiconductor.[137] The organic materials can be small organic molecules in a crystalline phase, or polymers.[138]
The potential advantages of OLEDs include thin, low-cost displays with a low driving voltage, wide viewing angle, and high contrast and color gamut.[139] Polymer LEDs have the added benefit of printable and flexible displays.[140][141][142] OLEDs have been used to make visual displays for portable electronic devices such as cellphones, digital cameras, and MP3 players while possible future uses include lighting and televisions.[138][139]
Quantum-dot LEDs
Quantum dots (QD) are semiconductor nanocrystals with optical properties that let their emission color be tuned from the visible into the infrared spectrum.[143][144] This allows quantum dot LEDs to create almost any color on the CIE diagram. This provides more color options and better color rendering than white LEDs since the emission spectrum is much narrower, characteristic of quantum confined states.
There are two types of schemes for QD excitation. One uses photo excitation with a primary light source LED (typically blue or UV LEDs are used). The other is direct electrical excitation first demonstrated by Alivisatos et al.[145]
One example of the photo-excitation scheme is a method developed by Michael Bowers, at Vanderbilt University in Nashville, involving coating a blue LED with quantum dots that glow white in response to the blue light from the LED. This method emits a warm, yellowish-white light similar to that made by incandescent light bulbs.[146] Quantum dots are also being considered for use in white light-emitting diodes in liquid crystal display (LCD) televisions.[147]
In February 2011 scientists at PlasmaChem GmbH were able to synthesize quantum dots for LED applications and build a light converter on their basis, which was able to efficiently convert light from blue to any other color for many hundred hours.[148] Such QDs can be used to emit visible or near infrared light of any wavelength being excited by light with a shorter wavelength.
The structure of QD-LEDs used for the electrical-excitation scheme is similar to basic design of OLEDs. A layer of quantum dots is sandwiched between layers of electron-transporting and hole-transporting materials. An applied electric field causes electrons and holes to move into the quantum dot layer and recombine forming an exciton that excites a QD. This scheme is commonly studied for quantum dot display. The tunability of emission wavelengths and narrow bandwidth is also beneficial as excitation sources for fluorescence imaging. Fluorescence near-field scanning optical microscopy (NSOM) utilizing an integrated QD-LED has been demonstrated.[149]
In February 2008, a luminous efficacy of 300 lumens of visible light per watt of radiation (not per electrical watt) and warm-light emission was achieved by using nanocrystals.[150]
Types
The main types of LEDs are miniature, high-power devices, and custom designs such as alphanumeric or multicolor.[151]
Miniature


These are mostly single-die LEDs used as indicators, and they come in various sizes from 2 mm to 8 mm, through-hole and surface mount packages. They usually do not use a separate heat sink.[152] Typical current ratings range from around 1 mA to above 20 mA. The small size sets a natural upper boundary on power consumption due to heat caused by the high current density and need for a heat sink. Often daisy chained as used in LED tapes.
Common package shapes include round, with a domed or flat top, rectangular with a flat top (as used in bar-graph displays), and triangular or square with a flat top. The encapsulation may also be clear or tinted to improve contrast and viewing angle.
Researchers at the University of Washington have invented the thinnest LED. It is made of two-dimensional (2-D) flexible materials. It is three atoms thick, which is 10 to 20 times thinner than three-dimensional (3-D) LEDs and is also 10,000 times smaller than the thickness of a human hair. These 2-D LEDs are going to make it possible to create smaller, more energy-efficient lighting, optical communication and nano lasers.[153][154]
There are three main categories of miniature single die LEDs:
- Low-current
- Typically rated for 2 mA at around 2 V (approximately 4 mW consumption)
- Standard
- 20 mA LEDs (ranging from approximately 40 mW to 90 mW) at around:
- 1.9 to 2.1 V for red, orange, yellow, and traditional green
- 3.0 to 3.4 V for pure green and blue
- 2.9 to 4.2 V for violet, pink, purple and white
- Ultra-high-output
- 20 mA at approximately 2 or 4–5 V, designed for viewing in direct sunlight
5 V and 12 V LEDs are ordinary miniature LEDs that incorporate a suitable series resistor for direct connection to a 5 V or 12 V supply.
High-power

High-power LEDs (HP-LEDs) or high-output LEDs (HO-LEDs) can be driven at currents from hundreds of mA to more than an ampere, compared with the tens of mA for other LEDs. Some can emit over a thousand lumens.[155][156] LED power densities up to 300 W/cm2 have been achieved. Since overheating is destructive, the HP-LEDs must be mounted on a heat sink to allow for heat dissipation. If the heat from an HP-LED is not removed, the device fails in seconds. One HP-LED can often replace an incandescent bulb in a flashlight, or be set in an array to form a powerful LED lamp.
Some well-known HP-LEDs in this category are the Nichia 19 series, Lumileds Rebel Led, Osram Opto Semiconductors Golden Dragon, and Cree X-lamp. As of September 2009, some HP-LEDs manufactured by Cree now exceed 105 lm/W.[157]
Examples for Haitz's law—which predicts an exponential rise in light output and efficacy of LEDs over time—are the CREE XP-G series LED, which achieved 105 lm/W in 2009[157] and the Nichia 19 series with a typical efficacy of 140 lm/W, released in 2010.[158]
AC-driven
LEDs developed by Seoul Semiconductor can operate on AC power without a DC converter. For each half-cycle, part of the LED emits light and part is dark, and this is reversed during the next half-cycle. The efficacy of this type of HP-LED is typically 40 lm/W.[159] A large number of LED elements in series may be able to operate directly from line voltage. In 2009, Seoul Semiconductor released a high DC voltage LED, named as 'Acrich MJT', capable of being driven from AC power with a simple controlling circuit. The low-power dissipation of these LEDs affords them more flexibility than the original AC LED design.[160]
Application-specific variations
Flashing
Flashing LEDs are used as attention seeking indicators without requiring external electronics. Flashing LEDs resemble standard LEDs but they contain an integrated multivibrator circuit that causes the LED to flash with a typical period of one second. In diffused lens LEDs, this circuit is visible as a small black dot. Most flashing LEDs emit light of one color, but more sophisticated devices can flash between multiple colors and even fade through a color sequence using RGB color mixing.
Bi-color
Bi-color LEDs contain two different LED emitters in one case. There are two types of these. One type consists of two dies connected to the same two leads antiparallel to each other. Current flow in one direction emits one color, and current in the opposite direction emits the other color. The other type consists of two dies with separate leads for both dies and another lead for common anode or cathode so that they can be controlled independently. The most common bi-color combination is red/traditional green, however, other available combinations include amber/traditional green, red/pure green, red/blue, and blue/pure green.
Tri-color
Tri-color LEDs contain three different LED emitters in one case. Each emitter is connected to a separate lead so they can be controlled independently. A four-lead arrangement is typical with one common lead (anode or cathode) and an additional lead for each color.
RGB
RGB LEDs are tri-color LEDs with red, green, and blue emitters, in general using a four-wire connection with one common lead (anode or cathode). Others, however, have only two leads (positive and negative) and have a built-in electronic control unit.
Decorative-multicolor
Decorative-multicolor LEDs incorporate several emitters of different colors supplied by only two lead-out wires. Colors are switched internally by varying the supply voltage.
Alphanumeric
Alphanumeric LEDs are available in seven-segment, starburst, and dot-matrix format. Seven-segment displays handle all numbers and a limited set of letters. Starburst displays can display all letters. Dot-matrix displays typically use 5x7 pixels per character. Seven-segment LED displays were in widespread use in the 1970s and 1980s, but rising use of liquid crystal displays, with their lower power needs and greater display flexibility, has reduced the popularity of numeric and alphanumeric LED displays.
Digital-RGB
Digital RGB Addressable LEDs are RGB LEDs that contain their own "smart" control electronics. In addition to power and ground, these provide connections for data-in, data-out, and sometimes a clock or strobe signal. These are connected in a daisy chain, with the data in of the first LED sourced by a microprocessor, which can control the brightness and color of each LED independently of the others. They are used where a combination of maximum control and minimum visible electronics are needed such as strings for Christmas and LED matrices. Some even have refresh rates in the kHz range, allowing for basic video applications. These devices are also known by their part number (WS2812 being the most common) or a brand name such as NeoPixel
Filament
An LED filament consists of multiple LED chips connected in series on a common longitudinal substrate that forms a thin rod reminiscent of a traditional incandescent filament.[161] These are being used as a low-cost decorative alternative for traditional light bulbs that are being phased out in many countries. The filaments require a rather high voltage to light to nominal brightness, allowing them to work efficiently and simply with mains voltages. Often a simple rectifier and capacitive current limiting are employed to create a low-cost replacement for a traditional light bulb without the complexity of the low voltage, high current converter that single die LEDs need.[162] Usually, they are packaged in a sealed enclosure with a shape similar to lamps they were designed to replace (e.g. a bulb) and filled with inert nitrogen or carbon dioxide gas to remove heat efficiently.
Considerations for use
Power sources

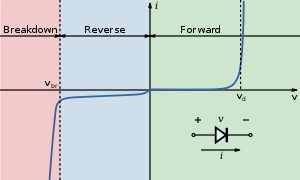
The current–voltage characteristic of an LED is similar to other diodes, in that the current is dependent exponentially on the voltage (see Shockley diode equation). This means that a small change in voltage can cause a large change in current.[163] If the applied voltage exceeds the LED's forward voltage drop by a small amount, the current rating may be exceeded by a large amount, potentially damaging or destroying the LED. The typical solution is to use constant-current power supplies to keep the current below the LED's maximum current rating. Since most common power sources (batteries, mains) are constant-voltage sources, most LED fixtures must include a power converter, at least a current-limiting resistor. However, the high resistance of three-volt coin cells combined with the high differential resistance of nitride-based LEDs makes it possible to power such an LED from such a coin cell without an external resistor.
Electrical polarity
As with all diodes, current flows easily from p-type to n-type material.[164] However, no current flows and no light is emitted if a small voltage is applied in the reverse direction. If the reverse voltage grows large enough to exceed the breakdown voltage, a large current flows and the LED may be damaged. If the reverse current is sufficiently limited to avoid damage, the reverse-conducting LED is a useful noise diode.
Safety and health
The vast majority of devices containing LEDs are "safe under all conditions of normal use", and so are classified as "Class 1 LED product"/"LED Klasse 1". At present, only a few LEDs—extremely bright LEDs that also have a tightly focused viewing angle of 8° or less—could, in theory, cause temporary blindness, and so are classified as "Class 2".[165] The opinion of the French Agency for Food, Environmental and Occupational Health & Safety (ANSES) of 2010, on the health issues concerning LEDs, suggested banning public use of lamps in the moderate Risk Group 2, especially those with a high blue component, in places frequented by children.[166][167]
In general, laser safety regulations—and the "Class 1", "Class 2", etc. system—also apply to LEDs.[168]
While LEDs have the advantage over fluorescent lamps that they do not contain mercury, they may contain other hazardous metals such as lead and arsenic. Regarding the toxicity of LEDs when treated as waste, a study published in 2011 stated: "According to federal standards, LEDs are not hazardous except for low-intensity red LEDs, which leached Pb [lead] at levels exceeding regulatory limits (186 mg/L; regulatory limit: 5). However, according to California regulations, excessive levels of copper (up to 3892 mg/kg; limit: 2500), lead (up to 8103 mg/kg; limit: 1000), nickel (up to 4797 mg/kg; limit: 2000), or silver (up to 721 mg/kg; limit: 500) render all except low-intensity yellow LEDs hazardous."[169]
In 2016 a statement of the American Medical Association (AMA) concerning the possible influence of blueish street lighting on the sleep-wake cycle of city-dwellers led to some controversy. So far high-pressure sodium lamps (HPS) with an orange light spectrum were the most efficient light sources commonly used in street-lighting. Now many modern street lamps are equipped with Indium gallium nitride LEDs (InGaN). These are even more efficient and mostly emit blue-rich light with a higher correlated color temperature (CCT). Since light with a high CCT resembles daylight it is thought that this might have an effect on the normal circadian physiology by suppressing melatonin production in the human body. There have been no relevant studies as yet and critics claim exposure levels are not high enough to have a noticeable effect.[170]
Advantages
- Efficiency: LEDs emit more lumens per watt than incandescent light bulbs.[171] The efficiency of LED lighting fixtures is not affected by shape and size, unlike fluorescent light bulbs or tubes.
- Color: LEDs can emit light of an intended color without using any color filters as traditional lighting methods need. This is more efficient and can lower initial costs.
- Size: LEDs can be very small (smaller than 2 mm2[172]) and are easily attached to printed circuit boards.
- Warmup time: LEDs light up very quickly. A typical red indicator LED achieves full brightness in under a microsecond.[173] LEDs used in communications devices can have even faster response times.
- Cycling: LEDs are ideal for uses subject to frequent on-off cycling, unlike incandescent and fluorescent lamps that fail faster when cycled often, or high-intensity discharge lamps (HID lamps) that require a long time before restarting.
- Dimming: LEDs can very easily be dimmed either by pulse-width modulation or lowering the forward current.[174] This pulse-width modulation is why LED lights, particularly headlights on cars, when viewed on camera or by some people, seem to flash or flicker. This is a type of stroboscopic effect.
- Cool light: In contrast to most light sources, LEDs radiate very little heat in the form of IR that can cause damage to sensitive objects or fabrics. Wasted energy is dispersed as heat through the base of the LED.
- Slow failure: LEDs mainly fail by dimming over time, rather than the abrupt failure of incandescent bulbs.[92]
- Lifetime: LEDs can have a relatively long useful life. One report estimates 35,000 to 50,000 hours of useful life, though time to complete failure may be shorter or longer.[175] Fluorescent tubes typically are rated at about 10,000 to 25,000 hours, depending partly on the conditions of use, and incandescent light bulbs at 1,000 to 2,000 hours. Several DOE demonstrations have shown that reduced maintenance costs from this extended lifetime, rather than energy savings, is the primary factor in determining the payback period for an LED product.[176]
- Shock resistance: LEDs, being solid-state components, are difficult to damage with external shock, unlike fluorescent and incandescent bulbs, which are fragile.[177]
- Focus: The solid package of the LED can be designed to focus its light. Incandescent and fluorescent sources often require an external reflector to collect light and direct it in a usable manner. For larger LED packages total internal reflection (TIR) lenses are often used to the same effect. However, when large quantities of light are needed many light sources are usually deployed, which are difficult to focus or collimate towards the same target.
Disadvantages
- Initial price: LEDs are currently slightly more expensive (price per lumen) on an initial capital cost basis, than other lighting technologies. As of March 2014[update], at least one manufacturer claims to have reached $1 per kilolumen.[178] The additional expense partially stems from the relatively low lumen output and the drive circuitry and power supplies needed.
- Temperature dependence: LED performance largely depends on the ambient temperature of the operating environment – or thermal management properties. Overdriving an LED in high ambient temperatures may result in overheating the LED package, eventually leading to device failure. An adequate heat sink is needed to maintain long life. This is especially important in automotive, medical, and military uses where devices must operate over a wide range of temperatures, which require low failure rates. Toshiba has produced LEDs with an operating temperature range of −40 to 100 °C, which suits the LEDs for both indoor and outdoor use in applications such as lamps, ceiling lighting, street lights, and floodlights.[135]
- Voltage sensitivity: LEDs must be supplied with a voltage above their threshold voltage and a current below their rating. Current and lifetime change greatly with a small change in applied voltage. They thus require a current-regulated supply (usually just a series resistor for indicator LEDs).[179]
- Color rendition: Most cool-white LEDs have spectra that differ significantly from a black body radiator like the sun or an incandescent light. The spike at 460 nm and dip at 500 nm can make the color of objects appear differently under cool-white LED illumination than sunlight or incandescent sources, due to metamerism,[180] red surfaces being rendered particularly poorly by typical phosphor-based cool-white LEDs. The same is true with green surfaces.
- Area light source: Single LEDs do not approximate a point source of light giving a spherical light distribution, but rather a lambertian distribution. So, LEDs are difficult to apply to uses needing a spherical light field; however, different fields of light can be manipulated by the application of different optics or "lenses". LEDs cannot provide divergence below a few degrees. In contrast, lasers can emit beams with divergences of 0.2 degrees or less.[181]
- Electrical polarity: Unlike incandescent light bulbs, which illuminate regardless of the electrical polarity, LEDs only light with correct electrical polarity. To automatically match source polarity to LED devices, rectifiers can be used.
- Blue hazard: There is a concern that blue LEDs and cool-white LEDs are now capable of exceeding safe limits of the so-called blue-light hazard as defined in eye safety specifications such as "ANSI/IESNA RP-27.1–05: Recommended Practice for Photobiological Safety for Lamp and Lamp Systems".[182][183] However, lighting physicist Sebastien Point and colleagues showed that there is no evidence of a risk in normal use at domestic illuminance for the general population,[184][185] and that caution is only needed for particular occupationnal situations or for specific populations.[186]
- Light pollution: Because white LEDs, especially those with high color temperature, emit much more short wavelength light than conventional outdoor light sources such as high-pressure sodium vapor lamps, the increased blue and green sensitivity of scotopic vision means that white LEDs used in outdoor lighting cause substantially more sky glow.[160][187][188][189][190] The American Medical Association warned on the use of high blue content white LEDs in street lighting, due to their higher impact on human health and environment, compared to low blue content light sources (e.g. High-Pressure Sodium, PC amber LEDs, and low CCT LEDs).[191]
- Efficiency droop: The efficiency of LEDs decreases as the electric current increases. Heating also increases with higher currents, which compromises LED lifetime. These effects put practical limits on the current through an LED in high power applications.[85][87][88][192]
- Impact on insects: LEDs are much more attractive to insects than sodium-vapor lights, so much so that there has been speculative concern about the possibility of disruption to food webs.[193][194]
- Use in winter conditions: Since they do not give off much heat in comparison to incandescent lights, LED lights used for traffic control can have snow obscuring them, leading to accidents.[195][196]
- Thermal runaway and negative resistance[citation needed] Some LEDs notably large area diodes have quite high reverse capacitance and it is possible to damage them if the dV/dT is too short due to destructive ringing oscillations with inadequate driver similar to a related effect in laser diodes. To some extent this can be mitigated by shunting the diode(s) with a resistor whose value can be calculated based on a very simple formula. Value can be quite high even up to 100K as the capacitance of a typical LED is in the 10-900pF range.Incidentally this effect is also used in some amateur radio optical receivers to help detect weak signals in the visible band eg Phlatlight(tm) paired with a bandpass filtered reverse biased silicon photovoltaic module using instrumentation amplifiers.Some newer GaN and InGaN LEDs show negative resistance at low to moderate drive current and this can be problematical as it can result in unpredictable behavior and RF emission under certain drive methodologies.
Applications
LED uses fall into four major categories:
- Visual signals where light goes more or less directly from the source to the human eye, to convey a message or meaning
- Illumination where light is reflected from objects to give visual response of these objects
- Measuring and interacting with processes involving no human vision[197]
- Narrow band light sensors where LEDs operate in a reverse-bias mode and respond to incident light, instead of emitting light[198][199][200][201]
Indicators and signs
The low energy consumption, low maintenance and small size of LEDs has led to uses as status indicators and displays on a variety of equipment and installations. Large-area LED displays are used as stadium displays, dynamic decorative displays, and dynamic message signs on freeways. Thin, lightweight message displays are used at airports and railway stations, and as destination displays for trains, buses, trams, and ferries.

One-color light is well suited for traffic lights and signals, exit signs, emergency vehicle lighting, ships' navigation lights or lanterns (chromacity and luminance standards being set under the Convention on the International Regulations for Preventing Collisions at Sea 1972, Annex I and the CIE) and LED-based Christmas lights. In cold climates, LED traffic lights may remain snow-covered.[202] Red or yellow LEDs are used in indicator and alphanumeric displays in environments where night vision must be retained: aircraft cockpits, submarine and ship bridges, astronomy observatories, and in the field, e.g. night time animal watching and military field use.

Because of their long life, fast switching times, and visibility in broad daylight due to their high output and focus, LEDs have been used in brake lights for cars' high-mounted brake lights, trucks, and buses, and in turn signals for some time. However, many vehicles now use LEDs for their rear light clusters. The use in brakes improves safety, due to a great reduction in the time needed to light fully, or faster rise time, up to 0.5 second faster[citation needed] than an incandescent bulb. This gives drivers behind more time to react. In a dual intensity circuit (rear markers and brakes) if the LEDs are not pulsed at a fast enough frequency, they can create a phantom array, where ghost images of the LED appear if the eyes quickly scan across the array. White LED headlamps are beginning to appear. Using LEDs has styling advantages because LEDs can form much thinner lights than incandescent lamps with parabolic reflectors.
Due to the relative cheapness of low output LEDs, they are also used in many temporary uses such as glowsticks, throwies, and the photonic textile Lumalive. Artists have also used LEDs for LED art.
Weather and all-hazards radio receivers with Specific Area Message Encoding (SAME) have three LEDs: red for warnings, orange for watches, and yellow for advisories and statements whenever issued.
Lighting
With the development of high-efficiency and high-power LEDs, it has become possible to use LEDs in lighting and illumination. To encourage the shift to LED lamps and other high-efficiency lighting, the US Department of Energy has created the L Prize competition. The Philips Lighting North America LED bulb won the first competition on August 3, 2011, after successfully completing 18 months of intensive field, lab, and product testing.[203]
LEDs are used as street lights and in other architectural lighting. The mechanical robustness and long lifetime are used in automotive lighting on cars, motorcycles, and bicycle lights. LED light emission may be efficiently controlled by using nonimaging optics principles.
LED street lights are employed on poles and in parking garages. In 2007, the Italian village of Torraca was the first place to convert its entire illumination system to LEDs.[204]
LEDs are used in aviation lighting. Airbus has used LED lighting in its Airbus A320 Enhanced since 2007, and Boeing uses LED lighting in the 787 and 737 Sky Interior. LEDs are also being used now in airport and heliport lighting. LED airport fixtures currently include medium-intensity runway lights, runway centerline lights, taxiway centerline and edge lights, guidance signs, and obstruction lighting.
LEDs are also used as a light source for DLP projectors, and to backlight LCD televisions (referred to as LED TVs) and laptop displays. RGB LEDs raise the color gamut by as much as 45%. Screens for TV and computer displays can be made thinner using LEDs for backlighting.[205]
The lack of IR or heat radiation makes LEDs ideal for stage lights using banks of RGB LEDs that can easily change color and decrease heating from traditional stage lighting, as well as medical lighting where IR-radiation can be harmful. In energy conservation, the lower heat output of LEDs also means air conditioning (cooling) systems have less heat in need of disposal.
LEDs are small, durable and need little power, so they are used in handheld devices such as flashlights. LED strobe lights or camera flashes operate at a safe, low voltage, instead of the 250+ volts commonly found in xenon flashlamp-based lighting. This is especially useful in cameras on mobile phones, where space is at a premium and bulky voltage-raising circuitry is undesirable.
LEDs are used for infrared illumination in night vision uses including security cameras. A ring of LEDs around a video camera, aimed forward into a retroreflective background, allows chroma keying in video productions.


LEDs are used in mining operations, as cap lamps to provide light for miners. Research has been done to improve LEDs for mining, to reduce glare and to increase illumination, reducing risk of injury to the miners.[206]
LEDs are now used commonly in all market areas from commercial to home use: standard lighting, AV, stage, theatrical, architectural, and public installations, and wherever artificial light is used.
LEDs are increasingly finding uses in medical and educational applications, for example as mood enhancement,[207] and new technologies such as AmBX, exploiting LED versatility. NASA has even sponsored research for the use of LEDs to promote health for astronauts.[208]
Data communication and other signalling
Light can be used to transmit data and analog signals. For example, lighting white LEDs can be used in systems assisting people to navigate in closed spaces while searching necessary rooms or objects.[209]
Assistive listening devices in many theaters and similar spaces use arrays of infrared LEDs to send sound to listeners' receivers. Light-emitting diodes (as well as semiconductor lasers) are used to send data over many types of fiber optic cable, from digital audio over TOSLINK cables to the very high bandwidth fiber links that form the Internet backbone. For some time, computers were commonly equipped with IrDA interfaces, which allowed them to send and receive data to nearby machines via infrared.
Because LEDs can cycle on and off millions of times per second, very high data bandwidth can be achieved.[210]
Sustainable lighting
Efficient lighting is needed for sustainable architecture. In 2009, US Department of Energy testing results on LED lamps showed an average efficacy of 35 lm/W, below that of typical CFLs, and as low as 9 lm/W, worse than standard incandescent bulbs. A typical 13-watt LED lamp emitted 450 to 650 lumens,[211] which is equivalent to a standard 40-watt incandescent bulb.
However, as of 2011, there are LED bulbs available as efficient as 150 lm/W and even inexpensive low-end models typically exceed 50 lm/W, so that a 6-watt LED could achieve the same results as a standard 40-watt incandescent bulb. The latter has an expected lifespan of 1,000 hours, whereas an LED can continue to operate with reduced efficiency for more than 50,000 hours.
See the chart below for a comparison of common light types:
| LED | CFL | Incandescent | |
|---|---|---|---|
| Lightbulb Projected Lifespan | 50,000 hours | 10,000 hours | 1,200 hours |
| Watts Per Bulb (equiv. 60 watts) | 10 | 14 | 60 |
| Cost Per Bulb | $7.00 | $2.00 | $1.25 |
| kWh of Electricity Used Over 50,000 Hours | 500 | 700 | 3000 |
| Cost of Electricity (@ 0.10 per kWh) | $50 | $70 | $300 |
| Bulbs Needed for 50,000 Hours of Use | 1 | 5 | 42 |
| Equivalent 50,000 Hours Bulb Expense | $7.00 | $10.00 | $52.50 |
| TOTAL Cost for 50,000 Hours | $57.00 | $80.00 | $352.50 |
Energy consumption
In the US, one kilowatt-hour (3.6 MJ) of electricity currently causes an average 1.34 pounds (610 g) of CO
2 emission.[212] Assuming the average light bulb is on for 10 hours a day, a 40-watt bulb causes 196 pounds (89 kg) of CO
2 emission per year. The 6-watt LED equivalent only causes 30 pounds (14 kg) of CO
2 over the same time span. A building’s carbon footprint from lighting can, therefore, be reduced by 85% by exchanging all incandescent bulbs for new LEDs—if a building previously used only incandescent bulbs.
In practice, most buildings that use a lot of lighting use fluorescent lighting, which has 22% luminous efficiency compared with 5% for filaments, so changing to LED lighting would still give a 34% reduction in electrical power use and carbon emissions.
The reduction in carbon emissions depends on the source of electricity. Nuclear power in the United States produced 19.2% of electricity in 2011, so reducing electricity consumption in the U.S. reduces carbon emissions more than in France (75% nuclear electricity) or Norway (almost entirely hydroelectric).
Replacing lights that spend the most time lit results in the most savings, so LED lights in infrequently used locations bring a smaller return on investment.
LED as a light source for machine vision systems
Machine vision systems, also known as Computer vision systems, often require bright and homogeneous illumination, so features of interest are easier to process. LEDs are often used to achieve this type of purpose, and this is likely to remain one of their major uses until their price drops low enough to make signaling and illumination uses more economically feasible.
Barcode scanners are the most common example of machine vision applications, and many of those scanners use low-cost products red LEDs instead of laser-based LEDs.[213] Optical computer mice are an example of LEDs in machine vision, as it is used to provide an even light source on the surface for the miniature camera within the mouse. LEDs constitute a nearly ideal light source for machine vision systems for several reasons:
- The size of the illuminated field is usually comparatively small and machine vision systems are often quite expensive, so the cost of the light source is usually a minor concern. However, it might not be easy to replace a broken light source placed within complex machinery, and here the long service life of LEDs is a benefit.
- LED elements tend to be small and can be placed with high density over flat or even-shaped substrates (PCBs etc.) so that bright and homogeneous sources that direct light from tightly controlled directions on inspected parts can be designed.
- LED lights can often be obtained with small, low-cost lenses and diffusers that help achieve high light densities with control over lighting levels and homogeneity.
- LED sources can also be shaped in several configurations (spot lights; ring lights; backlights; or linear assemblies ... etc).
- LEDs can be easily strobed (in the microsecond range and below) and synchronized with imaging. High-power LEDs are available allowing well-lit images even with very short light pulses. This is often used to obtain crisp and sharp still images of quickly moving parts.
- LEDs come in several different colors and wavelengths, allowing easy use of the best color for each need, where different color may provide better visibility of features of interest. Having a precisely known spectrum lets tightly matched filters be used to separate informative bandwidth or to reduce disturbing effects of ambient light. LEDs usually operate at comparatively low working temperatures, simplifying heat management, and dissipation. This allows using plastic lenses, filters, and diffusers. Waterproof units can also easily be designed, allowing use in harsh or wet environments (food, beverage, or oil industry).[213]
-
A large LED display behind a disc jockey
-
LED digital display that can display four digits and points
-
Traffic light using LED
-
daytime running light LEDs of an Audi A4
-
LED panel light source used in an experiment on plant growth. The findings of such experiments may be used to grow food in space on long duration missions.
-
LED lights reacting dynamically to video feed via AmBX
-
Different sized LEDs. 8 mm, 5 mm and 3 mm, with a wooden match-stick for scale.
Other applications


The light from LEDs can be modulated very quickly so they are used extensively in optical fiber and free space optics communications. This includes remote controls, such as for TVs, VCRs, and LED Computers, where infrared LEDs are often used. Opto-isolators use an LED combined with a photodiode or phototransistor to provide a signal path with electrical isolation between two circuits. This is especially useful in medical equipment where the signals from a low-voltage sensor circuit (usually battery-powered) in contact with a living organism must be electrically isolated from any possible electrical failure in a recording or monitoring device operating at potentially dangerous voltages. An optoisolator also lets information be transferred between circuits that don't share a common ground potential.
Many sensor systems rely on light as the signal source. LEDs are often ideal as a light source due to the requirements of the sensors. LEDs are used as motion sensors, for example in optical computer mice. The Nintendo Wii's sensor bar uses infrared LEDs. Pulse oximeters use them for measuring oxygen saturation. Some flatbed scanners use arrays of RGB LEDs rather than the typical cold-cathode fluorescent lamp as the light source. Having independent control of three illuminated colors allows the scanner to calibrate itself for more accurate color balance, and there is no need for warm-up. Further, its sensors only need be monochromatic, since at any one time the page being scanned is only lit by one color of light. Since LEDs can also be used as photodiodes, they can be used for both photo emission and detection. This could be used, for example, in a touchscreen that registers reflected light from a finger or stylus.[214] Many materials and biological systems are sensitive to, or dependent on, light. Grow lights use LEDs to increase photosynthesis in plants,[215] and bacteria and viruses can be removed from water and other substances using UV LEDs for sterilization.[124]
LEDs have also been used as a medium-quality voltage reference in electronic circuits. The forward voltage drop (e.g. about 1.7 V for a normal red LED) can be used instead of a Zener diode in low-voltage regulators. Red LEDs have the flattest I/V curve above the knee. Nitride-based LEDs have a fairly steep I/V curve and are useless for this purpose. Although LED forward voltage is far more current-dependent than a Zener diode, Zener diodes with breakdown voltages below 3 V are not widely available.
The progressive miniaturization of low-voltage lighting technology, such as LEDs and OLEDs, suitable to incorporate into low-thickness materials has fostered experimentation in combining light sources and wall covering surfaces for interior walls.[216] The new possibilities offered by these developments have prompted some designers and companies, such as Meystyle,[217] Ingo Maurer,[218] Lomox[219] and Samsung, to research and develop proprietary LED wallpaper technologies, some of which are currently available for commercial purchase. Other solutions mainly exist as prototypes or are in the process of being further refined.
See also
- History of display technology
- Laser diode
- LEAD (diode), a light-emitting and absorbing diode
- LED circuit
- LED lamp
- LED tattoo
- Li-Fi
- Light-emitting electrochemical cell
- List of LED failure modes
- Nixie tube
- OLED
- Photovoltaics
- Seven-segment display
- SMD LED Module
- Solar lamp
- Solid-state lighting
- Thermal management of high-power LEDs
- UV curing
References
- ^ "HJ Round Started It All By Discovering Electroluminescence". www.myledpassion.com.
- ^ "The life and times of the LED — a 100-year history" (PDF). The Optoelectronics Research Centre, University of Southampton. April 2007. Archived from the original (PDF) on September 15, 2012. Retrieved September 4, 2012.
{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ US Patent 3293513, "Semiconductor Radiant Diode", James R. Biard and Gary Pittman, Filed on Aug. 8th, 1962, Issued on Dec. 20th, 1966.
- ^ "Inventor of Long-Lasting, Low-Heat Light Source Awarded $500,000 Lemelson-MIT Prize for Invention". Washington, D.C. Massachusetts Institute of Technology. April 21, 2004. Archived from the original on October 9, 2011. Retrieved December 21, 2011.
{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "LED". The American heritage science dictionary. Houghton Mifflin Company. 2005. led and LED.
- ^ Gomez, Daniel. "TI LED Lighting Solution Overview" (PDF). Texas Microsystems. p. 4.
- ^ Steiger, S.; Veprek, R. G.; Witzigmann, B. (September 2009). tdkp/AQUA: Unified modelling of electroluminescence in nanostructures. 2009 9th International Conference on Numerical Simulation of Optoelectronic Devices. pp. 73–74. doi:10.1109/NUSOD.2009.5297218.
- ^ Moreno, Ivan; Sun, Ching-Cherng (February 4, 2008). "Modeling the radiation pattern of LEDs". Optics Express. 16 (3): 1808–1819. Bibcode:2008OExpr..16.1808M. doi:10.1364/oe.16.001808.
- ^ a b c d Okon, Thomas M.; Biard, James R. (2015). "The First Practical LED" (PDF). EdisonTechCenter.org. Edison Tech Center. Retrieved February 2, 2016.
- ^ Peláez, E. A; Villegas, E. R (2007). LED power reduction trade-offs for ambulatory pulse oximetry. Vol. 2007. pp. 2296–9. doi:10.1109/IEMBS.2007.4352784. ISBN 978-1-4244-0787-3. PMID 18002450.
{{cite book}}:|journal=ignored (help) - ^ "Cleaning Up a Broken CFL". www.epa.gov. United States Environmental Protection Agency. January 22, 2013.
- ^ Carlesi, F.; Oliveira, M.O.; Ando Junior, O.H.; Neto, J.M.; Spacek, A.D.; Coelho, V.L.; Schaeffer, L.; Bordon, H.; Perrone, O.E.; Bretas, A.S. (2013). "Evaluation of Alternative Disposal and Replacement of Fluorescent Lamps" (PDF). Renewable Energy and Power Quality Journal: 636–639. doi:10.24084/repqj11.399.
- ^ "LED Spectral Distribution". optiwave.com. July 25, 2013. Retrieved June 20, 2017.
- ^ Round, H. J. (1907). "A note on carborundum". Electrical World. 19: 309.
- ^ Margolin J. "The Road to the Transistor". jmargolin.com.
- ^ Losev, O. V. (1927). "Светящийся карборундовый детектор и детектирование с кристаллами" [Luminous carborundum detector and detection with crystals]. Телеграфия и Телефония без Проводов [Wireless Telegraphy and Telephony] (in Russian). 5 (44): 485–494.
{{cite journal}}: Italic or bold markup not allowed in:|journal=(help) English translation: Losev, O. V. (November 1928). "Luminous carborundum detector and detection effect and oscillations with crystals". Philosophical Magazine. 7th series. 5 (39): 1024–1044. doi:10.1080/14786441108564683. - ^ Zheludev, N. (2007). "The life and times of the LED: a 100-year history" (free-download PDF). Nature Photonics. 1 (4): 189–192. Bibcode:2007NaPho...1..189Z. doi:10.1038/nphoton.2007.34.
- ^ Lee, Thomas H. (2004). The design of CMOS radio-frequency integrated circuits. Cambridge University Press. p. 20. ISBN 978-0-521-83539-8.
- ^ Destriau, G. (1936). "Recherches sur les scintillations des sulfures de zinc aux rayons". Journal de Chemie Physique. 33: 587–625.
- ^ McGraw-Hill Concise Encyclopedia of Physics: electroluminescence. (n.d.) McGraw-Hill Concise Encyclopedia of Physics. (2002).
- ^ Lehovec, K; Accardo, C. A; Jamgochian, E (1951). "Injected Light Emission of Silicon Carbide Crystals". Physical Review. 83 (3): 603–607. Bibcode:1951PhRv...83..603L. doi:10.1103/PhysRev.83.603. Archived from the original (free-download HTML) on December 11, 2014.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Lehovec, K; Accardo, C. A; Jamgochian, E (1953). "Injected Light Emission of Silicon Carbide Crystals". Physical Review. 89 (1): 20–25. Bibcode:1953PhRv...89...20L. doi:10.1103/PhysRev.89.20.
- ^ Rubin Braunstein Archived February 4, 2012, at the Wayback Machine. physics.ucla.edu
- ^ Braunstein, Rubin (1955). "Radiative Transitions in Semiconductors". Physical Review. 99 (6): 1892–1893. Bibcode:1955PhRv...99.1892B. doi:10.1103/PhysRev.99.1892.
- ^ Kroemer, Herbert (September 16, 2013). "The Double-Heterostructure Concept: How It Got Started". Proceedings of the IEEE. 101 (10): 2183–2187. doi:10.1109/JPROC.2013.2274914.
- ^ Matzen, W. T. ed. (March 1963) "Semiconductor Single-Crystal Circuit Development," Texas Instruments Inc., Contract No. AF33(616)-6600, Rept. No ASD-TDR-63-281.
- ^ Carr, W. N.; G. E. Pittman (November 1963). "One-watt GaAs p-n junction infrared source". Applied Physics Letters. 3 (10): 173–175. Bibcode:1963ApPhL...3..173C. doi:10.1063/1.1753837.
- ^ Holonyak Nick; Bevacqua, S. F. (December 1962). "Coherent (Visible) Light Emission from Ga(As1−x Px) Junctions". Applied Physics Letters. 1 (4): 82. Bibcode:1962ApPhL...1...82H. doi:10.1063/1.1753706. Archived from the original on October 14, 2012.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Wolinsky, Howard (February 5, 2005). "U. of I.'s Holonyak out to take some of Edison's luster". Chicago Sun-Times. Archived from the original on March 28, 2006. Retrieved July 29, 2007.
{{cite news}}: Italic or bold markup not allowed in:|publisher=(help); Unknown parameter|dead-url=ignored (|url-status=suggested) (help) - ^ Perry, T.S. (1995). "M. George Craford [biography]". IEEE Spectrum. 32 (2): 52–55. doi:10.1109/6.343989.
- ^ "Brief Biography — Holonyak, Craford, Dupuis" (PDF). Technology Administration. Archived from the original (PDF) on August 9, 2007. Retrieved May 30, 2007.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Pearsall, T. P.; Miller, B. I.; Capik, R. J.; Bachmann, K. J. (1976). "Efficient, Lattice-matched, Double Heterostructure LEDs at 1.1 mm from GaxIn1-xAsyP1-y by Liquid-phase Epitaxy". Appl. Phys. Lett. 28 (9): 499. Bibcode:1976ApPhL..28..499P. doi:10.1063/1.88831.
- ^ Rostky, George (March 1997). "LEDs cast Monsanto in Unfamiliar Role". Electronic Engineering Times (EETimes) (944).
- ^ a b Schubert, E. Fred (2003). "1". Light-Emitting Diodes. Cambridge University Press. ISBN 978-0-8194-3956-7.
- ^ US 3025589, "Method of Manufacturing Semiconductor Devices", issued Mar 20, 1962
- ^ Patent number: 3025589 Retrieved May 17, 2013
- ^ Bausch, Jeffrey (December 2011). "The Long History of Light Emitting Diodes". Hearst Business Communications.
- ^ Park, S. -I.; Xiong, Y.; Kim, R. -H.; Elvikis, P.; Meitl, M.; Kim, D. -H.; Wu, J.; Yoon, J.; Yu, C. -J.; Liu, Z.; Huang, Y.; Hwang, K. -C.; Ferreira, P.; Li, X.; Choquette, K.; Rogers, J. A. (2009). "Printed Assemblies of Inorganic Light-Emitting Diodes for Deformable and Semitransparent Displays" (PDF). Science. 325 (5943): 977–981. Bibcode:2009Sci...325..977P. CiteSeerX 10.1.1.660.3338. doi:10.1126/science.1175690. PMID 19696346. Archived from the original (PDF) on October 24, 2015.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ LED Thermal Management. Lunaraccents.com. Retrieved on March 16, 2012.
- ^ Patel, Neel V. (October 9, 2014). "Nobel Shocker: RCA Had the First Blue LED in 1972". IEEE Spectrum. Retrieved June 21, 2016.
- ^ "History & Milestones". Cree.com. Cree. Retrieved September 14, 2015.
- ^ Nakamura, S.; Mukai, T.; Senoh, M. (1994). "Candela-Class High-Brightness InGaN/AlGaN Double-Heterostructure Blue-Light-Emitting-Diodes". Appl. Phys. Lett. 64 (13): 1687. Bibcode:1994ApPhL..64.1687N. doi:10.1063/1.111832.
- ^ Nakamura, Shuji. "Development of the Blue Light-Emitting Diode". SPIE Newsroom. Retrieved September 28, 2015.
- ^ "The Nobel Prize in Physics 2014 Isamu Akasaki, Hiroshi Amano, Shuji Nakamura". nobelprize.org. The Royal Swedish Academy of Sciences. Retrieved March 17, 2015.
- ^ Dadgar, A.; Alam, A.; Riemann, T.; Bläsing, J.; Diez, A.; Poschenrieder, M.; Strassburg, M.; Heuken, M.; Christen, J.; Krost, A. (2001). "Crack-Free InGaN/GaN Light Emitters on Si(111)". Physica Status Solidi A. 188: 155–158. doi:10.1002/1521-396X(200111)188:1<155::AID-PSSA155>3.0.CO;2-P.
- ^ Dadgar, A.; Poschenrieder, M.; BläSing, J.; Fehse, K.; Diez, A.; Krost, A. (2002). "Thick, crack-free blue light-emitting diodes on Si(111) using low-temperature AlN interlayers and in situ Si\sub x]N\sub y] masking". Applied Physics Letters. 80 (20): 3670. Bibcode:2002ApPhL..80.3670D. doi:10.1063/1.1479455.
- ^ "Success in research: First gallium-nitride LED chips on silicon in pilot stage" (PDF). Archived from the original (PDF) on September 15, 2012. Retrieved 2012-09-15.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help). www.osram.de, January 12, 2012 - ^ Lester, Steve (2014) Role of Substrate Choice on LED Packaging. Toshiba America Electronic Components
- ^ GaN on Silicon — Cambridge Centre for Gallium Nitride. Gan.msm.cam.ac.uk. Retrieved on 2018-07-31.
- ^ Bush, Steve. (2016-06-30) Toshiba gets out of GaN-on-Si leds. Electronicsweekly.com. Retrieved on 2018-07-31.
- ^ Nunoue, Shin-ya; Hikosaka, Toshiki; Yoshida, Hisashi; Tajima, Jumpei; Kimura, Shigeya; Sugiyama, Naoharu; Tachibana, Koichi; Shioda, Tomonari; Sato, Taisuke; Muramoto, Eiji; Onomura, Masaaki (2013). "LED manufacturing issues concerning gallium nitride-on-silicon (GaN-on-Si) technology and wafer scale up challenges". 2013 IEEE International Electron Devices Meeting. pp. 13.2.1–13.2.4. doi:10.1109/IEDM.2013.6724622. ISBN 978-1-4799-2306-9.
- ^ Wright, Maury (2 May 2016) Samsung's Tarn reports progress in CSP and GaN-on-Si LEDs. LEDs Magazine
- ^ Increasing The Competitiveness Of The GaN-on-silicon LED. compoundsemiconductor.net (30 March 2016)
- ^ Samsung To Focus on Silicon-based LED Chip Technology in 2015. LED Inside (17 March 2015)
- ^ Keeping, Steven. (2013-01-15) Material and Manufacturing Improvements. DigiKey. Retrieved on 2018-07-31.
- ^ Keeping, Steven. (2014-12-09) Manufacturers Shift Attention to Light Quality to Further LED Market Share Gains. DigiKey. Retrieved on 2018-07-31.
- ^ Keeping, Steven. (2013-09-24) Will Silicon Substrates Push LED Lighting. DigiKey. Retrieved on 2018-07-31.
- ^ Keeping, Steven. (2015-03-24) Improved Silicon-Substrate LEDs Address High Solid-State Lighting Costs. DigiKey. Retrieved on 2018-07-31.
- ^ Development of the Nano-Imprint Equipment ST50S-LED for High-Brightness LED. Toshiba Machine (2011-05-18). Retrieved on 2018-07-31.
- ^ The use of sapphire in mobile device and LED industries: Part 2 | Solid State Technology. Electroiq.com (2017-09-26). Retrieved on 2018-07-31.
- ^ Epitaxy. Applied Materials. Retrieved on 2018-07-31.
- ^ List of Top 10 LED light manufacturer in China. Ledcornbulbs.com (2013-06-07). Retrieved on 2018-07-31.
- ^ "Haitz's law". Nature Photonics. 1 (1): 23. 2007. Bibcode:2007NaPho...1...23.. doi:10.1038/nphoton.2006.78.
- ^ Morris, Nick (June 1, 2006). "LED there be light, Nick Morris predicts a bright future for LEDs". Electrooptics.com.
- ^ "The LED Illumination Revolution". Forbes. February 27, 2008.
- ^ Press Release, Official Nobel Prize website, 7 October 2014
- ^ Cree First to Break 300 Lumens-Per-Watt Barrier. Cree.com (2014-03-26). Retrieved on 2018-07-31.
- ^ LM301B | SAMSUNG LED | Samsung LED Global Website. Samsung.com. Retrieved on 2018-07-31.
- ^ Samsung Achieves 220 Lumens per Watt with New Mid-Power LED Package. Samsung.com (2017-06-16). Retrieved on 2018-07-31.
- ^ LED breakthrough promises ultra efficient luminaires | Lux Magazine. Luxreview.com (2018-01-19). Retrieved on 2018-07-31.
- ^ LED bulb efficiency expected to continue improving as cost declines. U.S. Energy Information Administration (March 19, 2014)
- ^ a b c d Mueller, Gerd (2000) Electroluminescence I, Academic Press, ISBN 0-12-752173-9, p. 67, "escape cone of light" from semiconductor, illustrations of light cones on p. 69
- ^ "Optical Properties of Silicon". PVCDROM.PVEducation.org. Archived from the original on June 5, 2009.
- ^ Refraction — Snell's Law. Interactagram.com. Retrieved on March 16, 2012.
- ^ Lipták, Bela G. (2005) Instrument Engineers' Handbook: Process control and optimization, CRC Press, ISBN 0-8493-1081-4 p. 537, "cone of light" in context of optical fibers
- ^ Capper, Peter; Mauk, Michael (2007). Liquid phase epitaxy of electronic, optical, and optoelectronic materials. Wiley. p. 389. ISBN 978-0-470-85290-3.
faceted structures are of interest for solar cells, LEDs, thermophotovoltaic devices, and detectors in that nonplanar surfaces and facets can enhance optical coupling and light-trapping effects, [with example microphotograph of a faceted crystal substrate].
- ^ Dakin, John and Brown, Robert G. W. (eds.) Handbook of optoelectronics, Volume 2, Taylor & Francis, 2006 ISBN 0-7503-0646-7 p. 356, "Die shaping is a step towards the ideal solution, that of a point light source at the center of a spherical semiconductor die."
- ^ Schubert, E. Fred (2006) Light-emitting diodes, Cambridge University Press, ISBN 0-521-86538-7 p. 97, "Epoxy Encapsulants", "The light extraction efficiency can be enhanced by using dome-shaped encapsulants with a large refractive index."
- ^ "All in 1 LED Lighting Solutions Guide". PhilipsLumileds.com. Philips. October 4, 2012. p. 15. Archived from the original (PDF) on March 14, 2013. Retrieved November 18, 2015.
- ^ "Nichia Unveils White LED with 150 lm/W Luminous Efficiency". Tech-On!. December 21, 2006. Retrieved August 13, 2007.
- ^ "Cree Sets New Record for White LED Efficiency", Tech-On, April 23, 2012.
- ^ "Cree First to Break 300 Lumens-Per-Watt Barrier", Cree news
- ^ DOE Solid-State Lighting CALiPER Program Summary of Results: Round 9 of Product Testing (PDF). U.S. Department of Energy. October 2009.
- ^ Identifying the Causes of LED Efficiency Droop Archived December 13, 2013, at the Wayback Machine, By Steven Keeping, Digi-Key Corporation Tech Zone
- ^ a b c Stevenson, Richard (August 2009) The LED’s Dark Secret: Solid-state lighting won't supplant the lightbulb until it can overcome the mysterious malady known as droop. IEEE Spectrum
- ^ Iveland, Justin; Martinelli, Lucio; Peretti, Jacques; Speck, James S.; Weisbuch, Claude. "Cause of LED Efficiency Droop Finally Revealed". Physical Review Letters, 2013. Science Daily. Retrieved April 23, 2013.
- ^ a b The LED's dark secret. EnergyDaily. Retrieved on March 16, 2012.
- ^ a b Smart Lighting: New LED Drops The 'Droop'. Sciencedaily.com (January 13, 2009). Retrieved on March 16, 2012.
- ^ Efremov, A. A.; Bochkareva, N. I.; Gorbunov, R. I.; Lavrinovich, D. A.; Rebane, Y. T.; Tarkhin, D. V.; Shreter, Y. G. (2006). "Effect of the joule heating on the quantum efficiency and choice of thermal conditions for high-power blue InGaN/GaN LEDs". Semiconductors. 40 (5): 605–610. Bibcode:2006Semic..40..605E. doi:10.1134/S1063782606050162.
- ^ McKinney, Donna (19 February 2014) A Roadmap to Efficient Green-Blue-Ultraviolet Light-Emitting Diodes, U.S. Naval Research Laboratory
- ^ Cooke, Mike (11 February 2014) Enabling high-voltage InGaN LED operation with ceramic substrate, Semiconductor Today
- ^ a b "Lifetime of White LEDs". Archived from the original on April 10, 2009. Retrieved 2009-04-10.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help), US Department of Energy - ^ Abu-Ghosh, Said; Fixler, Dror; Dubinsky, Zvy; Iluz, David (2016). "Flashing light in microalgae biotechnology". Bioresource Technology. 203: 357–363. doi:10.1016/j.biortech.2015.12.057. PMID 26747205.
- ^ Arnold, J. When the Lights Go Out: LED Failure Modes and Mechanisms. DfR Solutions
- ^ Narendran, N.; Y. Gu (2005). "Life of LED-based white light sources". IEEE/OSA Journal of Display Technology. 1 (1): 167–171. Bibcode:2005JDisT...1..167N. doi:10.1109/JDT.2005.852510.
- ^ a b Conway, K. M. and J. D. Bullough. 1999. Will LEDs transform traffic signals as they did exit signs? Proceedings of the Illuminating Engineering Society of North America Annual Conference (pp. 1–9), New Orleans, Louisiana, August 9–11. New York, NY: Illuminating Engineering Society of North America.
- ^ Narendran, N., J. Brons, and J. Taylor. 2006. Energy-efficient Alternative for Commercial Refrigeration. Project report prepared for the New York State Energy Research and Development Authority.
- ^ Dong, Tianming; et al. (2008). "Recommendations for Testing and Evaluating Luminaires for Refrigerated and Freezer Display Cases" (PDF). ASSIST Recommends. 5 (1).
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Narendran, N. (2006). Field Test DELTA Snapshots: LED Lighting In Freezer Cases. Troy, N.Y.: Lighting Research Center.
- ^ "4FT 18W T8 LED Freezer Tube Lights 120 Degree". LED Corporations. Retrieved November 14, 2017.
- ^ Gu, Y., A. Baker, and N. Narendran. 2007. Investigation of thermal management technique in blue LED airport taxiway fixtures. Seventh International Conference on Solid State Lighting, Proceedings of SPIE 6669: 66690U.
- ^ OSRAM: green LED. osram-os.com. Retrieved on March 16, 2012.
- ^ a b Koizumi, S.; Watanabe, K.; Hasegawa, M.; Kanda, H. (2001). "Ultraviolet Emission from a Diamond pn Junction". Science. 292 (5523): 1899–1901. Bibcode:2001Sci...292.1899K. doi:10.1126/science.1060258. PMID 11397942.
- ^ a b Kubota, Y.; Watanabe, K.; Tsuda, O.; Taniguchi, T. (2007). "Deep Ultraviolet Light-Emitting Hexagonal Boron Nitride Synthesized at Atmospheric Pressure". Science. 317 (5840): 932–934. Bibcode:2007Sci...317..932K. doi:10.1126/science.1144216. PMID 17702939.
- ^ a b Watanabe, K.; Taniguchi, T.; Kanda, H. (2004). "Direct-bandgap properties and evidence for ultraviolet lasing of hexagonal boron nitride single crystal". Nature Materials. 3 (6): 404–409. Bibcode:2004NatMa...3..404W. doi:10.1038/nmat1134. PMID 15156198.
- ^ a b Taniyasu, Y.; Kasu, M.; Makimoto, T. (2006). "An aluminium nitride light-emitting diode with a wavelength of 210 nanometres". Nature. 441 (7091): 325–328. Bibcode:2006Natur.441..325T. doi:10.1038/nature04760. PMID 16710416.
- ^ "LEDs move into the ultraviolet". physicsworld.com. May 17, 2006. Retrieved August 13, 2007.
- ^ How to Wire/Connect LEDs Archived March 2, 2012, at the Wayback Machine. Llamma.com. Retrieved on March 16, 2012.
- ^ LED types by Color, Brightness, and Chemistry. Donklipstein.com. Retrieved on March 16, 2012.
- ^ "Nobel Shocker: RCA Had the First Blue LED in 1972". IEEE Spectrum. October 9, 2014
- ^ "Oregon tech CEO says Nobel Prize in Physics overlooks the actual inventors". The Oregonian. October 16, 2014
- ^ Schubert, E. Fred (2006) Light-emitting diodes 2nd ed., Cambridge University Press. ISBN 0-521-86538-7 pp. 16–17
- ^ Maruska, H. (2005). "A Brief History of GaN Blue Light-Emitting Diodes". LIGHTimes Online – LED Industry News. Archived June 11, 2012, at the Wayback Machine
- ^ Major Business and Product Milestones. Cree.com. Retrieved on March 16, 2012. Archived April 13, 2011, at the Wayback Machine
- ^ "GaN-based blue light emitting device development by Akasaki and Amano" (PDF). Takeda Award 2002 Achievement Facts Sheet. The Takeda Foundation. April 5, 2002. Retrieved November 28, 2007.
- ^ Moustakas, Theodore D. U.S. patent 5686738A "Highly insulating monocrystalline gallium nitride thin films " Issue date: Mar 18, 1991
- ^ Iwasa, Naruhito; Mukai, Takashi and Nakamura, Shuji U.S. patent 5,578,839 "Light-emitting gallium nitride-based compound semiconductor device" Issue date: November 26, 1996
- ^ 2006 Millennium technology prize awarded to UCSB's Shuji Nakamura. Ia.ucsb.edu (June 15, 2006). Retrieved on March 16, 2012.
- ^ Overbye, Dennis (October 7, 2014). "Nobel Prize in Physics". The New York Times.
- ^ "The Nobel Prize in Physics 2014 – Press release". www.nobelprize.org. Retrieved October 7, 2014.
- ^ Jonathan Webb (October 7, 2014). "Invention of blue LEDs wins physics Nobel". BBC News.
- ^ Brown, Joel (December 7, 2015). "BU Wins $13 Million in Patent Infringement Suit". BU Today. Retrieved December 7, 2015.
- ^ Cooke, Mike (April–May 2010). "Going Deep for UV Sterilization LEDs" (PDF). Semiconductor Today. 5 (3): 82. Archived from the original (PDF) on May 15, 2013.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b Mori, M.; Hamamoto, A.; Takahashi, A.; Nakano, M.; Wakikawa, N.; Tachibana, S.; Ikehara, T.; Nakaya, Y.; Akutagawa, M.; Kinouchi, Y. (2007). "Development of a new water sterilization device with a 365 nm UV-LED". Medical & Biological Engineering & Computing. 45 (12): 1237–1241. doi:10.1007/s11517-007-0263-1. PMID 17978842.
- ^ Ting, Hua-Nong (June 17, 2011). 5th Kuala Lumpur International Conference on Biomedical Engineering 2011: BIOMED 2011, 20–23 June 2011, Kuala Lumpur, Malaysia. Springer Science & Business Media. ISBN 9783642217296.
- ^ Wold, J. H.; Valberg, A. (2000). "The derivation of XYZ tristimulus spaces: A comparison of two alternative methods". Color Research & Application. 26 (S1): S222. doi:10.1002/1520-6378(2001)26:1<::AID-COL47>3.0.CO;2-4 (inactive October 2, 2018).
{{cite journal}}: CS1 maint: DOI inactive as of October 2018 (link) - ^ Bessho, M; Shimizu, K (2012). "Latest trends in LED lighting". Electronics and Communications in Japan. 95 (1): 1–7. doi:10.1002/ecj.10394.
- ^ Moreno, I.; Contreras, U. (2007). "Color distribution from multicolor LED arrays". Optics Express. 15 (6): 3607–3618. Bibcode:2007OExpr..15.3607M. doi:10.1364/OE.15.003607. PMID 19532605.
- ^ Schubert, E. Fred; Kim, Jong Kyu (2005). "Solid-State Light Sources Getting Smart" (PDF). Science. 308 (5726): 1274–1278. Bibcode:2005Sci...308.1274S. doi:10.1126/science.1108712. PMID 15919985.
- ^ "What Exactly IS LED Binning?". LED Corporations. October 22, 2013. Retrieved October 23, 2017.
- ^ Nimz, Thomas; Hailer, Fredrik; Jensen, Kevin (November 2012). Sensors and Feedback Control of Multicolor LED Systems. LED Professional. pp. 2–5. ISSN 1993-890X. Archived from the original (PDF) on April 29, 2014.
{{cite book}}: Unknown parameter|dead-url=ignored (|url-status=suggested) (help) - ^ Tanabe, S.; Fujita, S.; Yoshihara, S.; Sakamoto, A.; Yamamoto, S. (2005). "YAG glass-ceramic phosphor for white LED (II): luminescence characteristics" (PDF). Proceedings of SPIE. Fifth International Conference on Solid State Lighting. 5941: 594112. doi:10.1117/12.614681. Archived from the original (PDF) on May 11, 2011.
- ^ Ohno, Y. (2004). "Color rendering and luminous efficacy of white LED spectra" (PDF). Proc. SPIE. Fourth International Conference on Solid State Lighting. 5530: 89. doi:10.1117/12.565757. Archived from the original (PDF) on May 11, 2011.
- ^ Whitaker, Tim (December 6, 2002). "Joint venture to make ZnSe white LEDs". Retrieved January 3, 2009.
- ^ a b Next-Generation GaN-on-Si White LEDs Suppress Costs, Electronic Design, 19 November 2013
- ^ GaN-on-Silicon LEDs Forecast to Increase Market Share to 40 Percent by 2020, iSuppli, 4 December 2013
- ^ Burroughes, J. H.; Bradley, D. D. C.; Brown, A. R.; Marks, R. N.; MacKay, K.; Friend, R. H.; Burns, P. L.; Holmes, A. B. (1990). "Light-emitting diodes based on conjugated polymers". Nature. 347 (6293): 539–541. Bibcode:1990Natur.347..539B. doi:10.1038/347539a0.
- ^ a b Kho, Mu-Jeong; Javed, T.; Mark, R.; Maier, E.; David, C (March 4, 2008). Final Report: OLED Solid State Lighting. Kodak European Research. Cambridge Science Park, Cambridge, UK.
- ^ a b Bardsley, J. N. (2004). "International OLED Technology Roadmap" (PDF). IEEE Journal of Selected Topics in Quantum Electronics. 10 (1): 3–4. Bibcode:2004IJSTQ..10....3B. doi:10.1109/JSTQE.2004.824077.
- ^ Hebner, T. R.; Wu, C. C.; Marcy, D.; Lu, M. H.; Sturm, J. C. (1998). "Ink-jet printing of doped polymers for organic light emitting devices". Applied Physics Letters. 72 (5): 519. Bibcode:1998ApPhL..72..519H. doi:10.1063/1.120807.
- ^ Bharathan, J.; Yang, Y. (1998). "Polymer electroluminescent devices processed by inkjet printing: I. Polymer light-emitting logo". Applied Physics Letters. 72 (21): 2660. Bibcode:1998ApPhL..72.2660B. doi:10.1063/1.121090.
- ^ Gustafsson, G.; Cao, Y.; Treacy, G. M.; Klavetter, F.; Colaneri, N.; Heeger, A. J. (1992). "Flexible light-emitting diodes made from soluble conducting polymers". Nature. 357 (6378): 477–479. Bibcode:1992Natur.357..477G. doi:10.1038/357477a0.
- ^ Quantum-dot LED may be screen of choice for future electronics Massachusetts Institute of Technology News Office, December 18, 2002
- ^ Neidhardt, H.; Wilhelm, L.; Zagrebnov, V. A. (February 2015). "A New Model for Quantum Dot Light Emitting-Absorbing Bevices: Proofs and Supplements". Nanosystems: Physics, Chemistry, Mathematics. 6 (1): 6–45. doi:10.17586/2220-8054-2015-6-1-6-45. Retrieved October 31, 2015.
- ^ Colvin, V. L.; Schlamp, M. C.; Alivisatos, A. P. (1994). "Light-emitting diodes made from cadmium selenide nanocrystals and a semiconducting polymer". Nature. 370 (6488): 354–357. Bibcode:1994Natur.370..354C. doi:10.1038/370354a0.
- ^ "Accidental Invention Points to End of Light Bulbs". LiveScience.com. October 21, 2005. Retrieved January 24, 2007.
- ^ Nanoco Signs Agreement with Major Japanese Electronics Company, nanocogroup.com (September 23, 2009)
- ^ Nanotechnologie Aktuell, pp. 98–99, v. 4, 2011, ISSN 1866-4997
- ^ Hoshino, K.; Gopal, A.; Glaz, M. S.; Vanden Bout, D. A.; Zhang, X. (2012). "Nanoscale fluorescence imaging with quantum dot near-field electroluminescence". Applied Physics Letters. 101 (4): 043118. Bibcode:2012ApPhL.101d3118H. doi:10.1063/1.4739235.
- ^ Inman, Mason (February 1, 2008). "Crystal Coat Warms up LED Light". newscientist.com. Retrieved January 30, 2012.
- ^ What is the difference between 3528 LEDs and 5050 LEDs |SMD 5050 SMD 3528. Flexfireleds.com. Retrieved on March 16, 2012.
- ^ LED-design. Elektor.com. Retrieved on March 16, 2012. Archived August 31, 2012, at the Wayback Machine
- ^ Ma, Michelle (10 March 2014) Researchers build thinnest-known LED, UW Today
- ^ Ross, J. S; Klement, P; Jones, A. M; Ghimire, N. J; Yan, J; Mandrus, D. G; Taniguchi, T; Watanabe, K; Kitamura, K; Yao, W; Cobden, D. H; Xu, X (2014). "Electrically tunable excitonic light-emitting diodes based on monolayer WSe2 p–n junctions". Nature Nanotechnology. 9 (4): 268–72. arXiv:1312.1435. doi:10.1038/nnano.2014.26. PMID 24608230.
- ^ "Luminus Products". Luminus Devices. Archived from the original on July 25, 2008. Retrieved October 21, 2009.
- ^ "Luminus Products CST-90 Series Datasheet" (PDF). Luminus Devices. Archived from the original (PDF) on March 31, 2010. Retrieved October 25, 2009.
- ^ a b "Xlamp Xp-G Led". Cree.com. Cree, Inc. Archived from the original on March 13, 2012. Retrieved March 16, 2012.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ High Power Point Source White Led NVSx219A. Nichia.co.jp, November 2, 2010.
- ^ "Seoul Semiconductor launches AC LED lighting source Acrich". LEDS Magazine. November 17, 2006. Retrieved February 17, 2008.
- ^ a b Visibility, Environmental, and Astronomical Issues Associated with Blue-Rich White Outdoor Lighting (PDF). International Dark-Sky Association. May 4, 2010. Archived from the original (PDF) on January 16, 2013.
{{cite book}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "The Next Generation of LED Filament Bulbs". LEDInside.com. Trendforce. Retrieved October 26, 2015.
- ^ "LED Filaments". Retrieved October 26, 2015.
- ^ Elektrotechnik Gesamtband Technische Mathematik Kommunikationselektronik (in German) (1st ed.). Westermann. 1997. p. 171. ISBN 978-3142212517.
- ^ Schubert, E. Fred (2005). "Chapter 4". Light-Emitting Diodes. Cambridge University Press. ISBN 978-0-8194-3956-7.
- ^ "Visible LED Device Classifications". Datasheetarchive.com. Retrieved on January 25, 2012.
- ^ Opinion of the French Agency for Food, Environmental and Occupational Health & Safety, ANSES Opinion, October 19, 2010. Archived April 29, 2014, at the Wayback Machine
- ^ Opinion of the French Agency for Food, Environmental and Occupational Health & Safety, ANSES Opinion update, September 21, 2016.
- ^
"Eye Safety and LED (Light Emitting Diode) diffusion" Archived January 8, 2010, at the Wayback Machine: "The relevant standard for LED lighting is EN 60825-1:2001 (Safety of laser products) ... The standard states that throughout the standard "light emitting diodes (LED) are included whenever the word "laser" is used."
"Archived copy". Archived from the original on January 8, 2010. Retrieved March 8, 2010.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help)CS1 maint: archived copy as title (link) - ^ Lim, S. R.; Kang, D.; Ogunseitan, O. A.; Schoenung, J. M. (2011). "Potential Environmental Impacts of Light-Emitting Diodes (LEDs): Metallic Resources, Toxicity, and Hazardous Waste Classification". Environmental Science & Technology. 45 (1): 320–327. Bibcode:2011EnST...45..320L. doi:10.1021/es101052q. PMID 21138290.
- ^ The reactions to the recommendations of the American Medical Association are cited in an article on LightNowBlog.com. The comments note that the AMA slogan "cool it and dim it" leads to some confusion, since this refers to lighting with cooler color temperatures, which is commonly perceived as having a warmer color.
- ^ "Solid-State Lighting: Comparing LEDs to Traditional Light Sources". eere.energy.gov. Archived from the original on May 5, 2009.
- ^ "Dialight Micro LED SMD LED "598 SERIES" Datasheet" (PDF). Dialight.com. Archived from the original (PDF) on February 5, 2009.
- ^ "Data Sheet — HLMP-1301, T-1 (3 mm) Diffused LED Lamps". Avago Technologies. Retrieved May 30, 2010.
- ^ Narra, Prathyusha; Zinger, D.S. (2004). An effective LED dimming approach. Vol. 3. pp. 1671–1676. doi:10.1109/IAS.2004.1348695. ISBN 978-0-7803-8486-6.
{{cite book}}:|journal=ignored (help) - ^ Lifetime of White LEDs. US Department of Energy. (PDF) . Retrieved on March 16, 2012.
- ^ "In depth: Advantages of LED Lighting". energy.ltgovernors.com.
- ^ "LED Light Bars For Off Road Illumination". Larson Electronics.
{{cite web}}: Cite has empty unknown parameter:|dead-url=(help) - ^ "Philips Lumileds" (PDF). Philipslumileds.com. March 25, 2014.
- ^ The Led Museum. The Led Museum. Retrieved on March 16, 2012.
- ^ Worthey, James A. "How White Light Works". LRO Lighting Research Symposium, Light and Color. Retrieved October 6, 2007.
- ^ Hecht, E. (2002). Optics (4 ed.). Addison Wesley. p. 591. ISBN 978-0-19-510818-7.
- ^ "Blue LEDs: A health hazard?". texyt.com. January 15, 2007. Retrieved September 3, 2007.
- ^ Raloff, Janet (May 27, 2006). "Light Impacts: Science News". Sciencenews.org. Archived from the original on May 1, 2007.
- ^ Some evidences that white LEDs are toxic for human at domestic radiance?. Radioprotection (2017-09-12). Retrieved on 2018-07-31.
- ^ Point, Sébastien (1 February 2018) Why you shouldn’t be afraid of LEDs. Europeanscientist.com.
- ^ Point, S. and Barlier-Salsi, A. (2018) LEDs lighting and retinal damage, technical information sheets, SFRP
- ^ Luginbuhl, C. (2014). "The impact of light source spectral power distribution on sky glow". Journal of Quantitative Spectroscopy and Radiative Transfer. 139: 21–26. Bibcode:2014JQSRT.139...21L. doi:10.1016/j.jqsrt.2013.12.004.
- ^ Aubé, M.; Roby, J.; Kocifaj, M. (2013). "Evaluating Potential Spectral Impacts of Various Artificial Lights on Melatonin Suppression, Photosynthesis, and Star Visibility". PLOS ONE. 8 (7): e67798. Bibcode:2013PLoSO...867798A. doi:10.1371/journal.pone.0067798. PMC 3702543. PMID 23861808.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Crawford, Mark. "LED light pollution: Can we save energy and save the night?". SPIE Newsroom. Retrieved October 5, 2015.
- ^ Flagstaff Dark Skies Coalition. "Lamp Spectrum and Light Pollution". Lamp Spectrum and Light Pollution. Retrieved April 10, 2016.
- ^ "AMA Adopts Community Guidance to Reduce the Harmful Human and Environmental Effects of High Intensity Street Lighting". www.ama-assn.org. Retrieved August 1, 2016.
- ^ Efremov, A. A.; Bochkareva, N. I.; Gorbunov, R. I.; Lavrinovich, D. A.; Rebane, Y. T.; Tarkhin, D. V.; Shreter, Y. G. (2006). "Effect of the joule heating on the quantum efficiency and choice of thermal conditions for high-power blue InGaN/GaN LEDs". Semiconductors. 40 (5): 605–610. Bibcode:2006Semic..40..605E. doi:10.1134/S1063782606050162.
- ^ "LEDs: Good for prizes, bad for insects". news.sciencemag.org. October 7, 2014. Retrieved October 7, 2014.
- ^ Pawson, S. M.; Bader, M. K.-F. (2014). "LED Lighting Increases the Ecological Impact of Light Pollution Irrespective of Color Temperature". Ecological Applications. 24 (7): 1561–1568. doi:10.1890/14-0468.1.
- ^ "Stoplights' Potentially Deadly Winter Problem". ABC News. January 8, 2010.
- ^ "LED Traffic Lights Can't Melt Snow, Ice".
- ^ European Photonics Industry Consortium (EPIC). This includes use in data communications over fiber optics as well as "broadcast" data or signaling.
- ^ Mims, Forrest M. III. "An Inexpensive and Accurate Student Sun Photometer with Light-Emitting Diodes as Spectrally Selective Detectors".
- ^ "Water Vapor Measurements with LED Detectors". cs.drexel.edu (2002).
- ^ Dziekan, Mike (February 6, 2009) "Using Light-Emitting Diodes as Sensors". soamsci.or. Archived May 31, 2013, at the Wayback Machine
- ^ Ben-Ezra, Moshe; Wang, Jiaping; Wilburn, Bennett; Xiaoyang Li; Le Ma (2008). "An LED-only BRDF measurement device". 2008 IEEE Conference on Computer Vision and Pattern Recognition. pp. 1–8. CiteSeerX 10.1.1.165.484. doi:10.1109/CVPR.2008.4587766. ISBN 978-1-4244-2242-5.
- ^ LED advantages outweigh potential snow hazards in traffic signals, LEDs magazine January 7, 2010
- ^ "L-Prize U.S. Department of Energy", L-Prize Website, August 3, 2011
- ^ LED There Be Light, Scientific American, March 18, 2009
- ^ Eisenberg, Anne (June 24, 2007). "In Pursuit of Perfect TV Color, With L.E.D.'s and Lasers". New York Times. Retrieved April 4, 2010.
- ^ "CDC – NIOSH Publications and Products – Impact: NIOSH Light-Emitting Diode (LED) Cap Lamp Improves Illumination and Decreases Injury Risk for Underground Miners". cdc.gov. 2011. doi:10.26616/NIOSHPUB2011192. Retrieved May 3, 2013.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Janeway, Kimberly (December 12, 2014). "LED lightbulbs that promise to help you sleep". Consumer Reports. Retrieved May 10, 2018.
- ^ "LED Device Illuminates New Path to Healing" (Press release). nasa.gov. Retrieved January 30, 2012.
- ^ Fudin, M. S.; Mynbaev, K. D.; Aifantis, K. E.; Lipsanen H.; Bougrov, V. E.; Romanov, A. E. (2014). "Frequency characteristics of modern LED phosphor materials". Scientific and Technical Journal of Information Technologies, Mechanics and Optics. 14 (6).
- ^ Green, Hank (October 9, 2008). "Transmitting Data Through LED Light Bulbs". EcoGeek. Archived from the original on December 12, 2008. Retrieved February 15, 2009.
{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ DOE Solid-State Lighting CALiPER Program Summary of Results: Round 7 of Product Testing (PDF). U.S. Department of Energy. February 2009.
- ^ US DOE EIA: Electricity Emission Factors Archived June 14, 2009, at the Wayback Machine. Eia.doe.gov. Retrieved on March 16, 2012.
- ^ a b Administrator. "Application of LED Lighting". www.abengroup.com. Retrieved February 12, 2016.
- ^ Dietz, P. H.; Yerazunis, W. S.; Leigh, D. L. (2004). "Very Low-Cost Sensing and Communication Using Bidirectional LEDs".
{{cite journal}}: Cite journal requires|journal=(help) - ^ Goins, G. D.; Yorio, N. C.; Sanwo, M. M.; Brown, C. S. (1997). "Photomorphogenesis, photosynthesis, and seed yield of wheat plants grown under red light-emitting diodes (LEDs) with and without supplemental blue lighting". Journal of Experimental Botany. 48 (7): 1407–1413. doi:10.1093/jxb/48.7.1407.
- ^ Schubert, E. Fred (2003). Light-emitting Diodes. Cambridge: Cambridge University Press. ISBN 978-0521823302.
- ^ "Winner of Maison & Objet Projects award 2014". Meystyle.com. Meystyle. Archived from the original on March 29, 2016. Retrieved March 31, 2016.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "LED Wallpaper". Ingo-maurer.com. Ingo Maurer. Retrieved March 31, 2016.
- ^ "LOMOX OLED Innovation". Lomox.co.uk. Lomox. Retrieved March 31, 2016.
Further reading
- Shuji Nakamura; Gerhard Fasol; Stephen J Pearton (2000). The Blue Laser Diode: The Complete Story. Springer Verlag. ISBN 978-3-540-66505-2.







