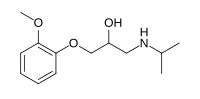
Moprolol
| |
| Names | |
|---|---|
| IUPAC name
1-(2-Methoxyphenoxy)-3-(propan-2-ylamino)propan-2-ol
| |
| Other names
(±)-Moprolol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.024.777 |
| EC Number |
|
| MeSH | C009976 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C13H21NO3 | |
| Molar mass | 239.315 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Moprolol is a beta-adrenergic antagonist,[1] or beta blocker. Typically moprolol is prescribed to treat hypertension, high blood pressure, anxiety, and glaucoma[2]. Moprolol was first introduced by SIMS, or S.p.A Societa Italiana Medicinali e Sintetici, in 1982. SIMS is a private Italian company that has been present in the pharmaceutical industry since 1937. In 1972 SIMS first submitted a Drug Master file to the US FDA and has continued to do so over the years.[3] In addition to formulating moprolol SIMES has also manufactured Omeral and Levotensin, derivatives of moprolol.
Regulation History
Moprolol was prominent in both literature and clinical trials up until the 1990s. At this point moprolol was pulled from the market. This could have been for a number of reasons, including FDA recalls and ineffective formulations. Through some clinical trials it was seen that moprolol was not as effective for treating blood pressure as originally thought, and this will be covered in the clinical section.
Even though there were no specific FDA recalls issued for moprolol, the FDA has issued warnings to SIMS. In 2000 the FDA and the United States Department of Health and Sciences issued a warning letter to the SIMS facility.[4] In this letter it stated that during a routine inspection of the SIMS facility it was found that there were significant deviations from the US good manufacturing practices when manufacturing active pharmaceutical ingredients. SIMS was then notified that until these deviations were corrected and another FDA inspection was completed the FDA will disapprove any applications for active pharmaceutical ingredients from the SIMS facility.
Then in June 2015 the FDA issued an Import alert including SIMS.[5] This alert stated that some active pharmaceutical ingredients appeared to be misbranded. Therefore, any active pharmaceutical ingredients can be refused admission if the facility they are from is listed in this alert.
Before being pulled from the market moprolol was a prescription drug, meaning it must be prescribed from a doctor, and could not be given over the counter.
Clinical Studies
Moprolol has been the subject for many clinical trials to study the effect moprolol has on both blood pressure and glaucoma.
In many hypertension and blood pressure studies the moprolol is prescribed as a tablet given once a day, typically on a schedule. For glaucoma studies moprolol is typically prescribed as an ophthalmic solution where ½ a drop of 1% moprolol solution was given 2-3 times a day.[6] The specific dosages and concentrations can vary depending on the severity of the condition.
One significant clinical study in hypertension ran in 2013 using non-selective beta-blocker to study the effects on lowering blood pressure.[7] In this study 30 participants were given 75 mg of moprolol twice a day. Through this study it was found that moprolol had no significant effect on lowering the heart rate.
Another important clinical trial for the treatment of glaucoma with moprolol was conducted in 1994.[1] This was a double-blind prospective trial to test the efficacy of lowering intraocular pressure when using l-moprolol alone and in combination with dipivefrin. In this study 27 patients that had glaucoma or ocular hypertension were given l-moprolol eye drops twice a day for 4 weeks. After this a combination of l-moprolol and dipivefrin eye drops were given for another 4 weeks. Through this study it was found that l-moprolol was a good treatment for glaucoma but that a treatment consisting of a combination of hypertension medications can have a greater effect at lowering ocular pressure.
Both studies show that moprolol, or its derivatives, has some effect at lowering blood pressure but may not have an effect on the heart rate. This opens a way for future studies, especially in the treatment for glaucoma.
Commercialization
Moprolol was first patented by SIMS in 1987 under US patent number 4647590.[8] In this patent SIMS states that moprolol can be used to treat glaucoma when administered locally as well as arterial hypertension. Then it goes into the studies conducted that proved the effectiveness of moprolol to treat these conditions without having the side effects of treatments on the market. This led to many other patents over the years, a few of which are listed in the table below:
| Filing Date | Publication Date | Applicant | Title | |
|---|---|---|---|---|
| US 4683245[9] | Oct 14, 1982 | Jul 28, 1987 | SIMS | Laevorotatory antipode of moprolol as an antihypertensive |
| EP 0118940[10] | Feb 9, 1984 | Jul 29, 1988 | SIMS | Use of l-moprolol for the manufacture of a fluid ophthalmic composition for treating glaucoma |
| US 20030060477[11] | Mar 27, 2001 | Mar 27, 2003 | Goran Fondjers, Olov Wiklund, John Wikstrand | Combination of a betablocker and a cholesterol-lowering agent |
As previously mentioned moprolol is currently off the market, most likely due to the SIMS facility being in violation of US good manufacturing practices. This means that currently there are no sales being brought in by moprolol.
That being said there is still a huge market for moprolol, both for hypertension and glaucoma treatment. A similar beta-blocker, metoprolol, that is very popular both overseas and in the US brought in an estimated €2.2 million in 2010 in Europe.[12] Metoprolol is also sold for $2.93-$0.76 per pill here in the US.[13] Since moprolol and metoprolol both treat hypertension the market and estimated sales should be the same, all of which is reasonable for this type of non-invasive treatment.
See also
- Levomoprolol, the (S)-enantiomer of moprolol
- Metoprolol
References
- ^ a b Rossetti, L; Barbieri, P; Velati, P; Bujtar, E; Orzalesi, N (1994). "The efficacy of the combination of l-moprolol and dipivefrin in reducing the intraocular pressure in primary open-angle glaucoma or in ocular hypertension". Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv für klinische und experimentelle Ophthalmologie. 232 (11): 670–4. PMID 7843592.
- ^ "Human Metabolome Database: Showing metabocard for Moprolol (HMDB41935)". www.hmdb.ca. Retrieved 2015-11-30.
- ^ "Storia SIMS Italy". www.simsitaly.it. Retrieved 2015-12-05.
- ^ Research, Center for Drug Evaluation and. "Warning Letters and Notice of Violation Letters to Pharmaceutical Companies - Warning Letters 2000". www.fda.gov. Retrieved 2015-12-14.
- ^ "Import Alert 66-66". www.accessdata.fda.gov. Retrieved 2015-12-14.
- ^ "0118940 USE OF L-MOPROLOL FOR THE MANUFACTURE OF A FLUID OPHTHALMIC COMPOSITION FOR TREATING GLAUCOMA". patentscope.wipo.int. Retrieved 2015-12-14.
- ^ Wong, Gavin WK; Wright, James M. Blood pressure lowering efficacy of nonselective beta-blockers for primary hypertension. doi:10.1002/14651858.cd007452.pub2.
- ^ Pharmaceutical compositions and their use in the treatment of glaucoma, retrieved 2015-12-14
- ^ Laevorotatory antipode of moprolol as an antihypertensive, retrieved 2015-12-14
- ^ Use of l-moprolol for the manufacture of a fluid ophthalmic composition for treating glaucoma, retrieved 2015-12-14
- ^ Combination of a betablocker and a cholesterol-lowering agent, retrieved 2015-12-14
- ^ "Annual Report 2010" (PDF). Recordati. Recordati S.p.A.
- ^ "Buy The Cheapest Offers". plusvision.eu. Retrieved 2015-12-14.
