Rhodium(III) chloride
| |

| |

| |
| Names | |
|---|---|
| Other names
Rhodium trichloride
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.138 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| RhCl3 | |
| Molar mass | 209.26 g/mol |
| Appearance | dark red solid deliquescent |
| Density | 5.38 g/cm3, solid |
| Melting point | ca. 450 °C (842 °F; 723 K) |
| Boiling point | 717 °C (1,323 °F; 990 K) |
| insoluble | |
| Solubility | soluble in hydroxide and cyanide solutions, also soluble in aqua regia |
| Acidity (pKa) | acidic in solution |
| −-7.5·10−6 cm3/mol | |
| Structure | |
| Monoclinic, mS16 | |
| C12/m1, No. 12 | |
| octahedral | |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
−234 kJ/mol |
| Hazards | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
>500 mg/kg (rat, oral) 1302 mg/kg (rat, oral)[1] |
| Safety data sheet (SDS) | ICSC 0746 |
| Related compounds | |
Other anions
|
Rhodium(III) fluoride Rhodium(III) bromide Rhodium(III) iodide |
Other cations
|
Cobalt(II) chloride Iridium(III) chloride |
Related compounds
|
Ruthenium(III) chloride Palladium(II) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Rhodium(III) chloride refers to inorganic compounds with the formula RhCl3(H2O)n, where n varies from 0 to 3. These are diamagnetic solids featuring octahedral Rh(III) centres. Depending on the value of n, the material is either a dense brown solid or a soluble reddish salt. The soluble trihydrated (n = 3) salt is widely used to prepare compounds used in homogeneous catalysis, notably for the industrial production of acetic acid and hydroformylation.[2]
Structures
Aqueous solutions of RhCl3(H2O)3 have been characterized by 103Rh NMR spectroscopy, which shows the presence of several species. The proportions of which change with time and depend on the concentration of chloride. The relative distribution of these species determines the colour of the solutions, which can range from yellow (the hexaaquo ion) to "raspberry-red". Some of these species are [Rh(H2O)6]3+, [RhCl(H2O)5]2+, cis- and trans-[RhCl2(H2O)4]+, and [RhCl3(H2O)3].[3] Individual ions have been separated by ion exchange chromatography.[4]
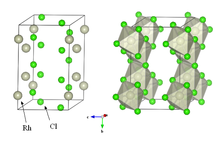
Anhydrous rhodium chloride crystallises in the YCl3 and AlCl3 motif (see image in upper right). The metal centres are octahedral, and the halides are doubly bridging.[5] It is a dense brown solid that is insoluble in common solvents and of little value in the laboratory.
Preparation
RhCl3(H2O)3 is produced from salts such as Na3RhCl6, the latter being obtained in the purification of rhodium from the other platinum group metals such as platinum and iridium. The sodium salt is converted to H3RhCl6 by ion exchange chromatography. Recrystallization of this acidic salt from water affords the hydrated trichloride, sometimes called "soluble rhodium trichloride."[6] Anhydrous RhCl3 is prepared by reaction of chlorine with rhodium sponge metal at 200–300 °C.[7] Above 800 °C, the anhydrous chloride reverts to Rh metal and chlorine.[6]
Various rhodium chloride complexes are intermediates in the purification of rhodium from its ores.[8]
Coordination complexes
RhCl3(H2O)3 is the precursor to a wide variety of complexes, some of which are commercially useful. It reacts with acetylacetone to give rhodium acetylacetonate.
Amines and pyridine
Solutions of RhCl3(H2O)3 react with ammonia in the presence of alcohol to give the salt pentamminerhodium chloride, [RhCl(NH3)5]Cl2. Zinc reduction of this cation followed by the addition of sulfate gives the colourless hydride complex [HRh(NH3)5]SO4.[9]
Upon boiling in a mixture of ethanol and pyridine (py), hydrated rhodium trichloride converts to trans-[RhCl2(py)4)]Cl. In the absence of a reductant, the reaction affords fac-[RhCl3(py)3], analogous to the thioether derivatives.[5] Oxidation of aqueous ethanolic solution of pyridine and RhCl3(H2O)3 by air affords a blue paramagnetic oxygen-bridged compound, [Cl(py)4Rh-O2-Rh(py)4Cl]5+.[10]
Thioethers and tertiary phosphines
Ethanolic solutions of hydrated rhodium trichloride react with dialkyl sulfides.
- RhCl3(H2O)3 + 3 SR2 RhCl3(SR2)3 + 3 H2O
Both fac and mer stereoisomers of such compounds have been isolated.[5]
Reaction of RhCl3(H2O)3 under mild conditions with tertiary phosphines affords adducts akin to the aforementioned thioether complexes. When these reactions are conducted in boiling ethanol solution, reduction of rhodium(III) occurs, leading to rhodium(I) derivatives such as [RhCl(PPh3)3], Wilkinson's catalyst, with oxidation of the solvent or more commonly with an excess of the phosphine:[11][12]
- RhCl3(H2O)3 + 3 PPh3 + CH3CH2OH RhCl(PPh3)3 + CH3CHO + 2 HCl + 3 H2O
- RhCl3(H2O)3 + 4 PPh3 RhCl(PPh3)3 + OPPh3 + 2 HCl + 2 H2O
Alkenes and carbon monoxide
Reaction of RhCl3(H2O)3 with olefins affords compounds of the type Rh2Cl2(alkene)4. With 1,5-cyclooctadiene, RhCl3(H2O)3 react in ethanol to give cyclooctadiene rhodium chloride dimer.[13]

RhCl3(H2O)3 in methanol reacts with carbon monoxide (1 bar) to produce H[RhCl2(CO)2], which contains the dicarbonyldichloridorhodate(I) anion; further carbonylation in the presence of sodium citrate leads to the formation of tetrarhodium dodecacarbonyl, Rh4(CO)12, a rhodium(0) cluster compound.[14] Treatment of solid RhCl3(H2O)3 with flowing CO gives the dimeric rhodium(I) compoundrhodium carbonyl chloride, [(CO)2Rh(μ-Cl)]2.[15]
Numerous Rh-CO-PR3 (R = organic group) compounds have been prepared in the course of extensive investigations on hydroformylation catalysis. RhCl(PPh3)3 reacts with CO to give trans-RhCl(CO)(PPh3)2, stoichiometrically analogous to but less reactive than Vaska's complex. Trans-RhCl(CO)(PPh3)2 reacts with a mixture of NaBH4 and PPh3 to give HRh(CO)(PPh3)3, a highly active catalyst for hydroformylation of alkenes.[16]
When treated with cyclopentadienes or its derivatives, organometallic half sandwich compounds can be produced. For example, reacting the trihydrate with pentamethylcyclopentadiene (Cp*H) in hot methanol leads to the precipitation of the pentamethylcyclopentadienyl rhodium dichloride dimer, [Cp*RhCl2]2:[17]
- 2 Cp*H + 2 RhCl3(H2O)3 [Cp*RhCl2]2 + 2 HCl + 6 H2O
This compound was first prepared from hexamethyl Dewar benzene and RhCl3(H2O)3.[18][19][20] The hydrohalic acid necessary for the ring-contraction rearrangement is generated in situ in methanolic solutions of the rhodium salt, and the second step has been carried out separately, confirming this mechanistic description.[21] The reaction occurs with the formation of 1,1-dimethoxyethane, CH3CH(OCH3)2, and hexamethylbenzene is produce by a side reaction.[20][21] This rhodium(III) dimer can be reduced with zinc in the presence of CO to produce the rhodium(I) complex [Cp*Rh(CO)2].[22]
![Synthesis of the rhodium(III) dimer [Cp*RhCl2]2 from hexamethyl Dewar benzene](http://upload.wikimedia.org/wikipedia/commons/thumb/d/db/Hexamethyl_Dewar_benzene_reacting_with_rhodium_chloride_under_acidic_conditions.PNG/700px-Hexamethyl_Dewar_benzene_reacting_with_rhodium_chloride_under_acidic_conditions.PNG)
Catalysis
Beginning especially in the 1960s, RhCl3(H2O)3 was demonstrated to be catalytically active for a variety of reactions involving CO, H2, and alkenes.[23] For example, RhCl3(H2O)3 was shown to dimerise ethene to a mixture of cis and trans 2-butene:
Unfortunately this reaction fails for higher alkenes.
Ethylene dimerization was shown to involve catalysis by the dimeric rhodium(I) compound [(η2-C2H4)2Rh(μ-Cl)2Rh(η2-C2H4)2]. This and many related discoveries nurtured the then young field of homogeneous catalysis, wherein the catalysts are dissolved in the medium with the substrate. Previous to this era, most metal catalysts were "heterogeneous", i.e. the catalysts were solids and the substrates were either liquid or gases. Another advance in homogeneous catalysis was the finding that PPh3-derived complexes were active catalytically as well as soluble in organic solvents,[16] the best known such catalyst being Wilkinson's catalyst that catalyzes the hydrogenation and isomerization of alkenes.[23] The hydroformylation of alkenes is catalyzed by the related RhH(CO)(PPh3)3. Catalysis by rhodium is so efficient that it has significantly displaced the previous technology based on less expensive cobalt catalysts.
Safety
Rhodium(III) chloride is not listed under Annex I of Directive 67/548/EEC, but is usually classified as harmful, R22: Harmful if swallowed. Some Rh compounds have been investigated as anti-cancer drugs. It is listed in the inventory of the Toxic Substances Control Act (TSCA).
References
- ^ "Rhodium (metal fume and insoluble compounds, as Rh)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Greenwood, N. N. & Earnshaw, A. (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. ISBN 0-7506-3365-4.
- ^ Carr, Christopher; Glaser, Julius; Sandström, Magnus (1987). "103Rh NMR chemical shifts of all ten [RhCln(OH2)6−n]3−n complexes in aqueous solution". Inorg. Chim. Acta. 131 (2): 153–156. doi:10.1016/S0020-1693(00)96016-X.
- ^ Wolsey, Wayne C.; Reynolds, Charles A.; Kleinberg, Jacob (1963). "Complexes in the Rhodium(III)-Chloride System in Acid Solution". Inorg. Chem. 2 (3): 463–468. doi:10.1021/ic50007a009.
- ^ a b c Cotton, Simon A. (1997). Chemistry of the Precious Metals. Chapman & Hall. ISBN 0-7514-0413-6.
- ^ a b Brauer, Georg, ed. (1965). "Rhodium(III) Chloride". Handbook of Preparative Inorganic Chemistry. Vol. 2 (2nd ed.). New York: Academic Press. pp. 1587–1588. ISBN 9780323161299.
- ^ Renner, Hermann; Schlamp, Günther; Kleinwächter, Ingo; Drost, Ernst; Lüschow, Hans M.; Tews, Peter; Panster, Peter; Diehl, Manfred; Lang, Jutta; Kreuzer, Thomas; Knödler, Alfons; Starz, Karl A.; Dermann, Klaus; Rothaut, Josef; Drieselmann, Ralf; Peter, Catrin; Schiele, Rainer (2005). "Platinum Group Metals and Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a21_075. ISBN 3527306730.
- ^ Benguerel, E.; Demopoulos, G. P.; Harris, G. B. (1996). "Speciation and separation of rhodium(III) from chloride solutions: A critical review". Hydrometallurgy. 40 (1–2): 135–152. doi:10.1016/0304-386X(94)00086-I.
- ^ Osborn, J. A.; Thomas, K.; Wilkinson, G. (1972). Pentaamminechlororhodium(III) Dichloride and Pentaamminehydridorhodium(III) Sulfate. Inorganic Syntheses. Vol. 13. pp. 213–215. doi:10.1002/9780470132449.ch43. ISBN 9780470132449.
{{cite book}}:|journal=ignored (help) - ^ Gillard, R. D.; Wilkinson, G. (1967). trans-Dichlorotetra(pyridine)rhodium(III) Salts. Inorganic Syntheses. Vol. 10. pp. 64–67. doi:10.1002/9780470132418.ch11. ISBN 9780470132418.
{{cite book}}:|journal=ignored (help) - ^ Osborn, J. A.; Jardine, F. H.; Young, J. F.; Wilkinson, G. (1966). "The Preparation and Properties of Tris(triphenylphosphine)halogenorhodium(I) and Some Reactions Thereof Including Catalytic Homogeneous Hydrogenation of Olefins and Acetylenes and Their Derivatives". J. Chem. Soc. A. 1966: 1711–1732. doi:10.1039/J19660001711.
- ^ Osborn, J. A.; Wilkinson, G. (1967). Tris(Triphenylphosphine)Halorhodium(I). Inorganic Syntheses. Vol. 10. pp. 67–71. doi:10.1002/9780470132418.ch12. ISBN 9780470132418.
{{cite book}}:|journal=ignored (help) - ^ Giordano, G.; Crabtree, R. H. (1990). Di-μ-chloro-bis(η4-1,5-cyclooctadiene)dirhodium(I). Inorganic Syntheses. Vol. 28. pp. 88–90. doi:10.1002/9780470132500.ch50. ISBN 9780470132500.
{{cite book}}:|journal=ignored (help) - ^ Serp, P. H.; Kalck, P. H.; Feurer, R.; Morancho, R. (1998). Tri(μ-carbonyl)Nonacarbonyltetrarhodium, Rh4(μ-CO)3(CO)4. Inorganic Syntheses. Vol. 32. pp. 284–287. doi:10.1002/9780470132630.ch45. ISBN 9780470132630.
{{cite book}}:|journal=ignored (help) - ^ McCleverty, J. A.; Wilkinson, G. (1966). Dichlorotetracarbonyldirhodium: (Rhodium Carbonyl Chloride). Inorganic Syntheses. Vol. 8. pp. 211–214. doi:10.1002/9780470132395.ch56. ISBN 9780470132395.
{{cite book}}:|journal=ignored (help) - ^ a b Hartwig, John F. (2010). Organotransition Metal Chemistry: From Bonding to Catalysis. New York: University Science Books. ISBN 978-1-891389-53-5.
- ^ White, C.; Yates, A.; Maitlis, Peter M. (1992). (η5-Pentamethylcyclopentadienyl)Rhodium and -Iridium Compounds. Inorganic Syntheses. Vol. 29. pp. 228–234. doi:10.1002/9780470132609.ch53. ISBN 9780470132609.
{{cite book}}:|journal=ignored (help) - ^ Paquette, Leo A.; Krow, Grant R. (1968). "Electrophilic Additions to Hexamethyldewarbenzene". Tetrahedron Lett. 9 (17): 2139–2142. doi:10.1016/S0040-4039(00)89761-0.
- ^ Criegee, Rudolf; Grüner, H. (1968). "Acid-catalyzed Rearrangements of Hexamethyl-prismane and Hexamethyl-Dewar-benzene". Angew. Chem. Int. Ed. 7 (6): 467–468. doi:10.1002/anie.196804672.
- ^ a b Herrmann, Wolfgang A.; Zybill, Christian (1996). "Bis{(μ-chloro)[chloro(η-pentamethylcyclopentadienyl)rhodium]} — {Rh(μ-Cl)Cl[η-C5(CH3)5]}2". In Herrmann, Wolfgang A.; Salzer, Albrecht (eds.). Synthetic Methods of Organometallic and Inorganic Chemistry – Volume 1: Literature, Laboratory Techniques, and Common Starting Materials. Georg Thieme Verlag. pp. 148–149. ISBN 9783131791610.
- ^ a b Heck, Richard F. (1974). "Reactions of Dienes Trienes and Tetraenes with Transition Metal Compounds". Organotransition Metal Chemistry: A Mechanistic Approach. Academic Press. pp. 116–117. ISBN 9780323154703.
- ^ Herrmann, Wolfgang A.; Zybill, Christian (1996). "Dicarbonyl(η-pentamethylcyclopentadienyl)rhodium — Rh[η-C5(CH3)5](CO)2". In Herrmann, Wolfgang A.; Salzer, Albrecht (eds.). Synthetic Methods of Organometallic and Inorganic Chemistry – Volume 1: Literature, Laboratory Techniques, and Common Starting Materials. Georg Thieme Verlag. pp. 147–148. ISBN 9783131791610.
- ^ a b Bennett, Martin A.; Longstaff, P. A. (1965). "Complexes of Rhodium(I) with Triphenylphosphine". Chem. Ind. (London): 846.

![{\displaystyle {\ce {->[{\ce {CH3CH2OH}}]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/7a6552044024d3bcd2e07972f7ae82ca8ca646a1)
![{\displaystyle {\ce {->[{\ce {CH3CH2OH}}{\text{ / Δ}}]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/12a08389489aa7ab9fbd60f946daba3c1a06e582)
![{\displaystyle {\ce {->[{\ce {CH3OH}}{\text{ / Δ}}]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/8b38287ac05102ae2efa8432ce004a22c2dc924e)
![{\displaystyle {\ce {->[{\ce {RhCl3(H2O)3}}]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/bb2c131af4d6b9deaacb2b34406a3bdff68a87cb)