Iproniazid: Difference between revisions
Tags: Mobile edit Mobile web edit |
Marijn1009 (talk | contribs) Added synthesis, structure&reactivity and properly divided the intro and History. Also added metabolism. |
||
| Line 42: | Line 42: | ||
|drug_name=|alt=|caption=|type=|MedlinePlus=|licence_EU=|pregnancy_AU=|pregnancy_US=|licence_US=|density=1.084|boiling_point=265.9}} |
|drug_name=|alt=|caption=|type=|MedlinePlus=|licence_EU=|pregnancy_AU=|pregnancy_US=|licence_US=|density=1.084|boiling_point=265.9}} |
||
'''Iproniazid''' ('''Marsilid''', '''Rivivol''', '''Euphozid''', '''Iprazid''', '''Ipronid''', '''Ipronin''') is a non-selective, [[irreversible inhibition|irreversible]] [[monoamine oxidase inhibitor]] (MAOI) of the [[hydrazine]] class.<ref name="bookDrug discovery">{{cite book |author1=Robert A. Maxwell |author2=Shohreh B. Eckhardt | title = Drug discovery | publisher = Humana Press | year = 1990 | pages = 143–154 | isbn = 0-89603-180-2 |id={{ISBN|9780896031807}} }}</ref><ref name="pmid2870717">{{cite journal |vauthors=Fagervall I, Ross SB |title=Inhibition of monoamine oxidase in monoaminergic neurones in the rat brain by irreversible inhibitors |journal=[[Biochemical Pharmacology (journal)|Biochemical pharmacology]] |volume=35 |issue=8 |pages=1381–7 |date=April 1986 |pmid=2870717 |doi= 10.1016/0006-2952(86)90285-6|url=http://linkinghub.elsevier.com/retrieve/pii/0006-2952(86)90285-6}}</ref> It was discontinued in most of the world in the 1960s, but remained in use in [[France]] until fairly recently.<ref name="pmid10592880">{{cite journal |vauthors=Maille F, Duvoux C, Cherqui D, Radier C, Zafrani ES, Dhumeaux D | title = [Auxiliary hepatic transplantation in iproniazid-induced subfulminant hepatitis. Should iproniazid still be sold in France?] | language = French | journal = Gastroenterol. Clin. Biol. | volume = 23 | issue = 10 | pages = 1083–5 |date=October 1999 | pmid = 10592880 | doi = | url = }}</ref> |
'''Iproniazid''' ('''Marsilid''', '''Rivivol''', '''Euphozid''', '''Iprazid''', '''Ipronid''', '''Ipronin''') is a non-selective, [[irreversible inhibition|irreversible]] [[monoamine oxidase inhibitor]] (MAOI) of the [[hydrazine]] class.<ref name="bookDrug discovery">{{cite book |author1=Robert A. Maxwell |author2=Shohreh B. Eckhardt | title = Drug discovery | publisher = Humana Press | year = 1990 | pages = 143–154 | isbn = 0-89603-180-2 |id={{ISBN|9780896031807}} }}</ref><ref name="pmid2870717">{{cite journal |vauthors=Fagervall I, Ross SB |title=Inhibition of monoamine oxidase in monoaminergic neurones in the rat brain by irreversible inhibitors |journal=[[Biochemical Pharmacology (journal)|Biochemical pharmacology]] |volume=35 |issue=8 |pages=1381–7 |date=April 1986 |pmid=2870717 |doi= 10.1016/0006-2952(86)90285-6|url=http://linkinghub.elsevier.com/retrieve/pii/0006-2952(86)90285-6}}</ref> It is a [[xenobiotic]] that was originally designed to treat [[tuberculosis]], but was later most prominently used as an [[antidepressant drug]]. However, it was withdrawn from the market because of its [[hepatotoxicity]]<ref name=":1">{{Cite book|url=https://doi.org/10.3109/9781420007084|title=Taylor & Francis Group|last=Timbrell|first=John|publisher=|year=|isbn=|location=|pages=324-326|language=en|doi=10.3109/9781420007084}}</ref><ref name=":0">{{Cite book|url=https://books.google.nl/books?id=Lur1CAAAQBAJ&pg=PA109&lpg=PA109&dq=iproniazid+synthesis&source=bl&ots=xUn5I9zzDe&sig=8aslgW6SUHHGL7UHNU93oqAcAqk&hl=nl&sa=X&ved=0ahUKEwjCwv2zh7rZAhXCasAKHVnzA_IQ6AEIcDAJ#v=onepage&q=iproniazid%20synthesis&f=false|title=Contemporary Psychiatry|last=Henn|first=Fritz|last2=Sartorius|first2=Norman|last3=Helmchen|first3=Hanfried|last4=Lauter|first4=Hans|date=2013-11-11|publisher=Springer Science & Business Media|year=|isbn=9783642595196|location=|pages=109|language=en}}</ref>. The medical use of iproniazid was discontinued in most of the world in the 1960s, but remained in use in [[France]] until fairly recently.<ref name="pmid10592880">{{cite journal |vauthors=Maille F, Duvoux C, Cherqui D, Radier C, Zafrani ES, Dhumeaux D | title = [Auxiliary hepatic transplantation in iproniazid-induced subfulminant hepatitis. Should iproniazid still be sold in France?] | language = French | journal = Gastroenterol. Clin. Biol. | volume = 23 | issue = 10 | pages = 1083–5 |date=October 1999 | pmid = 10592880 | doi = | url = }}</ref> |
||
== History == |
|||
Iproniazid was originally developed for the treatment of [[tuberculosis]],<ref name="bookDrug discovery"/> but in 1952, its antidepressant properties were discovered when researchers noted that patients given [[isoniazid]] |
Iproniazid was originally developed for the treatment of [[tuberculosis]],<ref name="bookDrug discovery" /> but in 1952, its antidepressant properties were discovered when researchers noted that patients became inappropriately happy when given [[isoniazid]], a [[structural analog]] of iproniazid.<ref name="bookDrug discovery" /><ref>{{Cite journal|last=Ramachandraih|first=Chaitra T.|last2=Subramanyam|first2=Narayana|last3=Bar|first3=Kral Jurgen|last4=Baker|first4=Glen|last5=Yeragani|first5=Vikram K.|date=2011|title=Antidepressants: From MAOIs to SSRIs and more|url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3136031/|journal=Indian Journal of Psychiatry|volume=53|issue=2|pages=180–182|doi=10.4103/0019-5545.82567|issn=0019-5545|pmc=3136031|pmid=21772661}}</ref> Subsequently N-isopropyl [[Addition reaction|addition]] led to development as an antidepressant and was approved for use in 1958.<ref name="bookDrug discovery" /> It was withdrawn a few years later in 1961 due to a high incidence of [[hepatitis]], and was replaced by less [[hepatotoxic]] drugs such as [[phenelzine]] and [[isocarboxazid]].<ref name="bookDrug discovery" /> Nevertheless, iproniazid has historic value as it helped establish the relationship between psychiatric disorders and the metabolism of neurotransmitters.<ref name=":0" /> |
||
Although iproniazid was one of the first [[antidepressant]]s ever marketed, [[amphetamine]] (marketed as [[Benzedrine]] from 1935, for "mild depression", amid other indications)<ref name="Amph Uses Dex">{{cite journal |vauthors=Heal DJ, Smith SL, Gosden J, Nutt DJ | title = Amphetamine, past and present – a pharmacological and clinical perspective | journal = J. Psychopharmacol. | volume = 27 | issue = 6 | pages = 479–96 |date=June 2013 | pmid = 23539642 | pmc = 3666194 | doi = 10.1177/0269881113482532}}</ref> predates it; and [[frankincense]] has been marketed traditionally for millennia for, among other things, altering mood, although it was not until 2012 that one of the components of its smoke was found to have antidepressant effects in mice.<ref>{{cite journal |
Although iproniazid was one of the first [[antidepressant]]s ever marketed, [[amphetamine]] (marketed as [[Benzedrine]] from 1935, for "mild depression", amid other indications)<ref name="Amph Uses Dex">{{cite journal |vauthors=Heal DJ, Smith SL, Gosden J, Nutt DJ | title = Amphetamine, past and present – a pharmacological and clinical perspective | journal = J. Psychopharmacol. | volume = 27 | issue = 6 | pages = 479–96 |date=June 2013 | pmid = 23539642 | pmc = 3666194 | doi = 10.1177/0269881113482532}}</ref> predates it; and [[frankincense]] has been marketed traditionally for millennia for, among other things, altering mood, although it was not until 2012 that one of the components of its smoke was found to have antidepressant effects in mice.<ref>{{cite journal |
||
| Line 86: | Line 87: | ||
| url=http://pubs.acs.org/cen/whatstuff/86/8651sci2.html |
| url=http://pubs.acs.org/cen/whatstuff/86/8651sci2.html |
||
| doi=10.1021/cen-v086n051.p038}}</ref> |
| doi=10.1021/cen-v086n051.p038}}</ref> |
||
== Structure and reactivity == |
|||
The structure of iproniazid is chemically, in both structure and reactivity, similar to isoniazid. Iproniazid is a substituted hydrazine of which the isopropyl hydrazine moiety is essential for the inhibition of monoamine oxidase activity.<ref name=":2">{{Cite journal|last=Smith|first=Thomas E.|last2=Weissbach|first2=Herbert|last3=Udenfriend|first3=Sidney|title=Studies on Monoamine Oxidase: The Mechanism of Inhibition of Monoamine Oxidase by Iproniazid|url=https://pubs.acs.org/doi/pdf/10.1021/bi00904a021|journal=Biochemistry|language=en|volume=2|issue=4|pages=746–751|doi=10.1021/bi00904a021}}</ref> |
|||
== Synthesis == |
|||
[[File:Synthesis of iproniazid.png|thumb|This figure shows the multiple synthesis pathways towards iproniazid.]] |
|||
There are multiple routes to synthesize iproniazid. The most common precursor is [[methyl isonicotinate]] which formes [[isonicotinohydrazide]] when it reacts with [[hydrazine]].<ref>{{Cite web|url=https://alchetron.com/Iproniazid|title=Iproniazid- Alchetron, The Free Social Encyclopedia|website=Alchetron.com|language=en-US|access-date=2018-03-26}}</ref> Isonicotinohydrazide can be converted into iproniazid via different pathways. |
|||
One synthesis pathway involves AcMe which results in the formation of N'-(propan-2-ylidene)isonicotinohydrazide. Subsequently, the C=N linkage is selectively [[hydrogenated]] in the presence of a platinum catalyst and with water, alcohol or acetic acid as solvent.<ref>{{Cite journal|last=Yale|first=Harry L.|last2=Losee|first2=Kathryn|last3=Martins|first3=Joseph|last4=Holsing|first4=Mary|last5=Perry|first5=Frances M.|last6=Bernstein|first6=Jack|title=Chemotherapy of Experimental Tuberculosis. VIII. The Synthesis of Acid Hydrazides, their Derivatives and Related Compounds1,2|url=https://pubs.acs.org/doi/pdf/10.1021/ja01104a046|journal=Journal of the American Chemical Society|language=en|volume=75|issue=8|pages=1933–1942|doi=10.1021/ja01104a046}}</ref><ref>Vigorita; Ottana; Maccari; Monforte; Bisignano; Pizzimenti Bollettino Chimico Farmaceutico, 1998 , vol. 137, # 7 p. 267 - 276</ref> |
|||
In another pathway isonicotinohydrazide reacts with either 2-bromopropane or 2-chloropropane in an N-isopropyl addition reaction to the hydrazine moiety. This directly results in the formation of iproniazid.<ref>''Journal of the American Pharmaceutical Association'' (1912-1977), '''42:''' 457,463</ref><ref>{{Cite journal|last=Società chimica italiana|first=|date=|title=Gazzetta chimica Italiana|url=https://catalog.hathitrust.org/Record/000679312|journal=European journal of organic chemistry|volume=88|pages=v.393, 398|issn=0016-5603|via=}}</ref> |
|||
== Reactions and Mechanism of action == |
|||
Iproniazid is a known [[monoamine oxidase inhibitor]], it inhibits the activity of [[Monoamine oxidase|monoamine oxidases]] (MAOs) by itself and through an active metabolite, [[isopropylhydrazine]]. The formation of isopropylhydrazine from iproniazid has been observed without MAOs present.<ref name=":2" /> Both iproniazid and isopropylhydrazine react near the [[active site]] of MAOs. The reaction is a progressive [[first-order reaction]] with a high [[activation energy]]. In the presence of oxygen it is an [[irreversible reaction]], as [[dehydrogenation]] of iproniazid at the active site of the enzyme takes place. This dehydrogenation resembles the first step of amine [[oxidation]]. After dehydrogenation iproniazid further reacts with the enzyme.<ref name=":3">{{Cite journal|last=Davinson|first=A.N.|date=21 November 1956|title=The Mechanism of the Irreversible Inhibition of Rat-Liver monoamine Oxidase by Iproniazid (Marsilid)|url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1200154/pdf/biochemj00840-0143.pdf|journal=Biochem J.|volume=67|pages=316-322|via=}}</ref> |
|||
Inhibition of MAOs by iproniazid is [[Competitive inhibition|competitive]] and sensitive to changes in pH and temperature, similar to oxidation of the monoamine substrate. Inhibition cannot be reversed by addition of the substrate.<ref name=":3" /> Iproniazid is able to displace non-hydrazine inhibitors, but not other hydrazine inhibitors from the active site of the enzyme.<ref name=":2" /> |
|||
To increase the inhibition of monoamine oxidase, [[cyanide]] can be used. The reaction however remains oxygen-dependent.<ref name=":3" /> MAO inhibition can be decreased by addition of [[glutathione]], suggesting non enzymatic conjugation of either iproniazid or isopropylhydrazine with glutathione.<ref name=":3" /> |
|||
== Metabolism and Toxicity == |
|||
[[File:Iproniazid Metabolism.png|thumb|This figure shows the metabolism of iproniazid. The most important (proposed) metabolite is the isopropyl radical which is thought to be responsible for the heptatoxicity of iproniazid.<ref name=":1" /><ref name=":4">{{Cite journal|last=Nelson|first=S. D.|last2=Mitchell|first2=J. R.|last3=Snodgrass|first3=W. R.|last4=Timbrell|first4=J. A.|date=1978-09-01|title=Hepatotoxicity and metabolism of iproniazid and isopropylhydrazine.|url=http://jpet.aspetjournals.org/content/206/3/574|journal=Journal of Pharmacology and Experimental Therapeutics|language=en|volume=206|issue=3|pages=574–585|issn=0022-3565|pmid=702322}}</ref>]] |
|||
Iproniazid is [[Metabolism|metabolized]] in the body. Iproniazid is converted to isopropyl hydrazine and isonicotinic acid in an initial [[Hydrolysis|hydrolysis reaction]]. Isopropyl hydrazine can either be released in the blood or it can be metabolically activated by microsomal [[Cytochrome P450|CYP450]] enzymes.<ref name=":4" /> This oxidation of isopropyl hydrazine is a [[Toxication|toxification]] reaction that eventually can lead to the formation of an [[Alkylation|alkylating]] agent: the isopropyl radical.<ref name=":1" /> |
|||
==== ''Isopropyl radical'' ==== |
|||
The presence of the isopropyl radical was indicated by another observed product of the metabolism of iproniazid: the gas propane.<ref name=":1" /> |
|||
[[Alkylation|Alkylating]] agents have the capability to bind to chemical groups such as [[Amine|amino]], [[Phosphate|phosphate hydroxyl]], [[imidazole]] and [[Thiol|sulfhydryl]] groups. The formed isopropyl radical is able to form S-isopropyl conjugates ''[[in vitro]]''. This diminishes covalent binding to other proteins, however it was only observed ''in vitro''. ''[[In vivo]]'', hepatotoxic doses of isopropyl hydrazine, the precursor of the isopropyl radical, did not deplete sulfhydryl-group containing compounds.<ref name=":1" /> |
|||
==== ''Liver necrosis'' ==== |
|||
The isopropyl radical formed as a result of the metabolism of iproniazid, is able to covalently bind to proteins and other [[Macromolecule|macromolecules]] in the liver. These interactions are the reason for the hepatotoxicity of iproniazid. Covalent binding results in liver [[necrosis]] by presumably changing protein function leading to [[organelle]] stress and acute toxicity.<ref>{{Cite journal|last=Iorga|first=Andrea|last2=Dara|first2=Lily|last3=Kaplowitz|first3=Neil|date=2017-05-09|title=Drug-Induced Liver Injury: Cascade of Events Leading to Cell Death, Apoptosis or Necrosis|url=http://www.mdpi.com/1422-0067/18/5/1018|journal=International Journal of Molecular Sciences|language=en|volume=18|issue=5|pages=1018|doi=10.3390/ijms18051018}}</ref><ref>{{Cite journal|last=Mitchell|first=J. R.|last2=Jollow|first2=D. J.|last3=Potter|first3=W. Z.|last4=Davis|first4=D. C.|last5=Gillette|first5=J. R.|last6=Brodie|first6=B. B.|date=October 1973|title=Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism|url=https://www.ncbi.nlm.nih.gov/pubmed/4746326|journal=The Journal of Pharmacology and Experimental Therapeutics|volume=187|issue=1|pages=185–194|issn=0022-3565|pmid=4746326}}</ref> However, the exact mechanism of how the binding of iproniazid derivatives to liver proteins would induce liver necrosis remains unclear.<ref name=":1" /> |
|||
Cytochrome P450 enzymes are present at the highest concentrations in the liver, causing most alkylating agents to be produced in the liver. This explains why the liver is mostly damaged by covalent binding of alkylating agents such as the isopropyl radical.<ref name=":4" /> Rat models and other animal models have shown that cytochrome P450 enzymes convert isopropyl hydrazine to alkylating compounds that induce liver necrosis. An inducer of a class of hepatic microsomal cytochrome P450 enzymes, [[phenobarbital]], highly increased the chance of necrosis. In contrast, the compounds [[Cobalt(II) chloride|cobalt chloride]], [[piperonyl butoxide]] and [[alpha-naphthylisothiocyanate]] inhibit microsomal enzymes which resulted in a decreased chance of necrosis due to isopropyl hydrazine.<ref name=":4" /> |
|||
==== ''Metabolism to other forms'' ==== |
|||
Iproniazid can also be metabolised by O-[[dealkylation]] from iproniazid to [[acetone]] and [[isoniazid]]. Isoniazid can undergo further metabolism via multiple metabolic pathways, of which one eventually results in alkylating agents as well. This toxifying metabolic pathway includes N-acetylation. Reactions involving acetylation are influenced by [[genetic variance]]: the [[N-acetyltransferase 2|acetylator phenotype]]. The toxicological response to isoniazid (and thus iproniazid) can therefore be subjected to interindividual differences. |
|||
Acetone can also be produced in alternative pathway as a metabolite of isopropyl hydrazine. It is eventually converted to CO<sub>2</sub> and exhaled.<ref name=":1" /> |
|||
==== ''Isonicotinic acid'' ==== |
|||
[[Isonicotinic acid]], formed during the hydrolysis of iproniazid, is described as a moderately toxic compound and [[allergen]] with [[wiktionary:cumulative_effect|cumulative]] effects.<ref>{{Cite journal|last=Tsarichenko|first=G. V.|last2=Bobrov|first2=V. I.|last3=Smarkov|first3=M. V.|date=1977-04-01|title=Toxicity of isonicotinic acid|url=https://link.springer.com/article/10.1007/BF01156485|journal=Pharmaceutical Chemistry Journal|language=en|volume=11|issue=4|pages=481–483|doi=10.1007/BF01156485|issn=0091-150X}}</ref> Isonicotinic acid is further metabolized by glycine-conjugation or glucuronic acid-conjugation.<ref name=":4" /><ref>{{Cite journal|last=Mahapatra|first=Sebabrata|last2=Woolhiser|first2=Lisa|last3=J Lenaerts|first3=Anne|last4=L Johnson|first4=John|last5=Eisenach|first5=Kathleen|last6=Joloba|first6=Moses|last7=Boom|first7=W|last8=T Belisle|first8=John|date=2012-01-01|title=A Novel Metabolite of Antituberculosis Therapy Demonstrates Host Activation of Isoniazid and Formation of the Isoniazid-NAD+ Adduct|url=https://www.researchgate.net/publication/51752557_A_Novel_Metabolite_of_Antituberculosis_Therapy_Demonstrates_Host_Activation_of_Isoniazid_and_Formation_of_the_Isoniazid-NAD_Adduct|journal=Antimicrobial agents and chemotherapy|volume=56|pages=28–35|doi=10.1128/AAC.05486-11}}</ref> |
|||
== See also == |
== See also == |
||
Revision as of 19:41, 26 March 2018
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.199 |
| Chemical and physical data | |
| Formula | C9H13N3O |
| Molar mass | 179.219 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.084 g/cm3 |
| Boiling point | 265.9 °C (510.6 °F) |
| |
| |
| (verify) | |
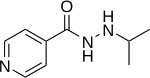
Iproniazid (Marsilid, Rivivol, Euphozid, Iprazid, Ipronid, Ipronin) is a non-selective, irreversible monoamine oxidase inhibitor (MAOI) of the hydrazine class.[1][2] It is a xenobiotic that was originally designed to treat tuberculosis, but was later most prominently used as an antidepressant drug. However, it was withdrawn from the market because of its hepatotoxicity[3][4]. The medical use of iproniazid was discontinued in most of the world in the 1960s, but remained in use in France until fairly recently.[5]
History
Iproniazid was originally developed for the treatment of tuberculosis,[1] but in 1952, its antidepressant properties were discovered when researchers noted that patients became inappropriately happy when given isoniazid, a structural analog of iproniazid.[1][6] Subsequently N-isopropyl addition led to development as an antidepressant and was approved for use in 1958.[1] It was withdrawn a few years later in 1961 due to a high incidence of hepatitis, and was replaced by less hepatotoxic drugs such as phenelzine and isocarboxazid.[1] Nevertheless, iproniazid has historic value as it helped establish the relationship between psychiatric disorders and the metabolism of neurotransmitters.[4]
Although iproniazid was one of the first antidepressants ever marketed, amphetamine (marketed as Benzedrine from 1935, for "mild depression", amid other indications)[7] predates it; and frankincense has been marketed traditionally for millennia for, among other things, altering mood, although it was not until 2012 that one of the components of its smoke was found to have antidepressant effects in mice.[8] [9] [10]
Structure and reactivity
The structure of iproniazid is chemically, in both structure and reactivity, similar to isoniazid. Iproniazid is a substituted hydrazine of which the isopropyl hydrazine moiety is essential for the inhibition of monoamine oxidase activity.[11]
Synthesis

There are multiple routes to synthesize iproniazid. The most common precursor is methyl isonicotinate which formes isonicotinohydrazide when it reacts with hydrazine.[12] Isonicotinohydrazide can be converted into iproniazid via different pathways.
One synthesis pathway involves AcMe which results in the formation of N'-(propan-2-ylidene)isonicotinohydrazide. Subsequently, the C=N linkage is selectively hydrogenated in the presence of a platinum catalyst and with water, alcohol or acetic acid as solvent.[13][14]
In another pathway isonicotinohydrazide reacts with either 2-bromopropane or 2-chloropropane in an N-isopropyl addition reaction to the hydrazine moiety. This directly results in the formation of iproniazid.[15][16]
Reactions and Mechanism of action
Iproniazid is a known monoamine oxidase inhibitor, it inhibits the activity of monoamine oxidases (MAOs) by itself and through an active metabolite, isopropylhydrazine. The formation of isopropylhydrazine from iproniazid has been observed without MAOs present.[11] Both iproniazid and isopropylhydrazine react near the active site of MAOs. The reaction is a progressive first-order reaction with a high activation energy. In the presence of oxygen it is an irreversible reaction, as dehydrogenation of iproniazid at the active site of the enzyme takes place. This dehydrogenation resembles the first step of amine oxidation. After dehydrogenation iproniazid further reacts with the enzyme.[17]
Inhibition of MAOs by iproniazid is competitive and sensitive to changes in pH and temperature, similar to oxidation of the monoamine substrate. Inhibition cannot be reversed by addition of the substrate.[17] Iproniazid is able to displace non-hydrazine inhibitors, but not other hydrazine inhibitors from the active site of the enzyme.[11]
To increase the inhibition of monoamine oxidase, cyanide can be used. The reaction however remains oxygen-dependent.[17] MAO inhibition can be decreased by addition of glutathione, suggesting non enzymatic conjugation of either iproniazid or isopropylhydrazine with glutathione.[17]
Metabolism and Toxicity

Iproniazid is metabolized in the body. Iproniazid is converted to isopropyl hydrazine and isonicotinic acid in an initial hydrolysis reaction. Isopropyl hydrazine can either be released in the blood or it can be metabolically activated by microsomal CYP450 enzymes.[18] This oxidation of isopropyl hydrazine is a toxification reaction that eventually can lead to the formation of an alkylating agent: the isopropyl radical.[3]
Isopropyl radical
The presence of the isopropyl radical was indicated by another observed product of the metabolism of iproniazid: the gas propane.[3]
Alkylating agents have the capability to bind to chemical groups such as amino, phosphate hydroxyl, imidazole and sulfhydryl groups. The formed isopropyl radical is able to form S-isopropyl conjugates in vitro. This diminishes covalent binding to other proteins, however it was only observed in vitro. In vivo, hepatotoxic doses of isopropyl hydrazine, the precursor of the isopropyl radical, did not deplete sulfhydryl-group containing compounds.[3]
Liver necrosis
The isopropyl radical formed as a result of the metabolism of iproniazid, is able to covalently bind to proteins and other macromolecules in the liver. These interactions are the reason for the hepatotoxicity of iproniazid. Covalent binding results in liver necrosis by presumably changing protein function leading to organelle stress and acute toxicity.[19][20] However, the exact mechanism of how the binding of iproniazid derivatives to liver proteins would induce liver necrosis remains unclear.[3]
Cytochrome P450 enzymes are present at the highest concentrations in the liver, causing most alkylating agents to be produced in the liver. This explains why the liver is mostly damaged by covalent binding of alkylating agents such as the isopropyl radical.[18] Rat models and other animal models have shown that cytochrome P450 enzymes convert isopropyl hydrazine to alkylating compounds that induce liver necrosis. An inducer of a class of hepatic microsomal cytochrome P450 enzymes, phenobarbital, highly increased the chance of necrosis. In contrast, the compounds cobalt chloride, piperonyl butoxide and alpha-naphthylisothiocyanate inhibit microsomal enzymes which resulted in a decreased chance of necrosis due to isopropyl hydrazine.[18]
Metabolism to other forms
Iproniazid can also be metabolised by O-dealkylation from iproniazid to acetone and isoniazid. Isoniazid can undergo further metabolism via multiple metabolic pathways, of which one eventually results in alkylating agents as well. This toxifying metabolic pathway includes N-acetylation. Reactions involving acetylation are influenced by genetic variance: the acetylator phenotype. The toxicological response to isoniazid (and thus iproniazid) can therefore be subjected to interindividual differences.
Acetone can also be produced in alternative pathway as a metabolite of isopropyl hydrazine. It is eventually converted to CO2 and exhaled.[3]
Isonicotinic acid
Isonicotinic acid, formed during the hydrolysis of iproniazid, is described as a moderately toxic compound and allergen with cumulative effects.[21] Isonicotinic acid is further metabolized by glycine-conjugation or glucuronic acid-conjugation.[18][22]
See also
References
- ^ a b c d e Robert A. Maxwell; Shohreh B. Eckhardt (1990). Drug discovery. Humana Press. pp. 143–154. ISBN 0-89603-180-2. ISBN 9780896031807.
- ^ Fagervall I, Ross SB (April 1986). "Inhibition of monoamine oxidase in monoaminergic neurones in the rat brain by irreversible inhibitors". Biochemical pharmacology. 35 (8): 1381–7. doi:10.1016/0006-2952(86)90285-6. PMID 2870717.
- ^ a b c d e f g Timbrell, John. Taylor & Francis Group. pp. 324–326. doi:10.3109/9781420007084.
- ^ a b Henn, Fritz; Sartorius, Norman; Helmchen, Hanfried; Lauter, Hans (2013-11-11). Contemporary Psychiatry. Springer Science & Business Media. p. 109. ISBN 9783642595196.
- ^ Maille F, Duvoux C, Cherqui D, Radier C, Zafrani ES, Dhumeaux D (October 1999). "[Auxiliary hepatic transplantation in iproniazid-induced subfulminant hepatitis. Should iproniazid still be sold in France?]". Gastroenterol. Clin. Biol. (in French). 23 (10): 1083–5. PMID 10592880.
- ^ Ramachandraih, Chaitra T.; Subramanyam, Narayana; Bar, Kral Jurgen; Baker, Glen; Yeragani, Vikram K. (2011). "Antidepressants: From MAOIs to SSRIs and more". Indian Journal of Psychiatry. 53 (2): 180–182. doi:10.4103/0019-5545.82567. ISSN 0019-5545. PMC 3136031. PMID 21772661.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Heal DJ, Smith SL, Gosden J, Nutt DJ (June 2013). "Amphetamine, past and present – a pharmacological and clinical perspective". J. Psychopharmacol. 27 (6): 479–96. doi:10.1177/0269881113482532. PMC 3666194. PMID 23539642.
- ^ Moussaieff, Arieh; Rimmerman, Neta; Bregman, Tatiana; Straiker, Alex; Felder, Christian C.; Shoham, Shai; Kashman, Yoel; Huang, Susan M.; Lee, Hyosang; Shohami, Esther; Mackie, Ken; Caterina, Michael J.; Walker, J. Michael; Fride, Ester; Mechoulam, Raphael (August 2008). "Incensole acetate, an incense component, elicits psychoactivity by activating TRPV3 channels in the brain". 22 (8). The FASEB Journal: 3024–3034. doi:10.1096/fj.07-101865. PMC 2493463. PMID 18492727.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: unflagged free DOI (link) - ^ Moussaieff, A; Gross, M; Nesher, E; Tikhonov, T; Yadid, G; Pinhasov, A (2012). "Incensole acetate reduces depressive-like behavior and modulates hippocampal BDNF and CRF expression of submissive animals". J Psychopharmacol. 26 (12). doi:10.1177/0269881112458729. PMID 23015543.
- ^ Drahl, Carmen (22 December 2008). "Frankincense And Myrrh". Chemical & Engineering News. 86 (51): 38. doi:10.1021/cen-v086n051.p038. ISSN 0009-2347.
- ^ a b c Smith, Thomas E.; Weissbach, Herbert; Udenfriend, Sidney. "Studies on Monoamine Oxidase: The Mechanism of Inhibition of Monoamine Oxidase by Iproniazid". Biochemistry. 2 (4): 746–751. doi:10.1021/bi00904a021.
- ^ "Iproniazid- Alchetron, The Free Social Encyclopedia". Alchetron.com. Retrieved 2018-03-26.
- ^ Yale, Harry L.; Losee, Kathryn; Martins, Joseph; Holsing, Mary; Perry, Frances M.; Bernstein, Jack. "Chemotherapy of Experimental Tuberculosis. VIII. The Synthesis of Acid Hydrazides, their Derivatives and Related Compounds1,2". Journal of the American Chemical Society. 75 (8): 1933–1942. doi:10.1021/ja01104a046.
- ^ Vigorita; Ottana; Maccari; Monforte; Bisignano; Pizzimenti Bollettino Chimico Farmaceutico, 1998 , vol. 137, # 7 p. 267 - 276
- ^ Journal of the American Pharmaceutical Association (1912-1977), 42: 457,463
- ^ Società chimica italiana. "Gazzetta chimica Italiana". European journal of organic chemistry. 88: v.393, 398. ISSN 0016-5603.
- ^ a b c d Davinson, A.N. (21 November 1956). "The Mechanism of the Irreversible Inhibition of Rat-Liver monoamine Oxidase by Iproniazid (Marsilid)" (PDF). Biochem J. 67: 316–322.
- ^ a b c d e Nelson, S. D.; Mitchell, J. R.; Snodgrass, W. R.; Timbrell, J. A. (1978-09-01). "Hepatotoxicity and metabolism of iproniazid and isopropylhydrazine". Journal of Pharmacology and Experimental Therapeutics. 206 (3): 574–585. ISSN 0022-3565. PMID 702322.
- ^ Iorga, Andrea; Dara, Lily; Kaplowitz, Neil (2017-05-09). "Drug-Induced Liver Injury: Cascade of Events Leading to Cell Death, Apoptosis or Necrosis". International Journal of Molecular Sciences. 18 (5): 1018. doi:10.3390/ijms18051018.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Mitchell, J. R.; Jollow, D. J.; Potter, W. Z.; Davis, D. C.; Gillette, J. R.; Brodie, B. B. (October 1973). "Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism". The Journal of Pharmacology and Experimental Therapeutics. 187 (1): 185–194. ISSN 0022-3565. PMID 4746326.
- ^ Tsarichenko, G. V.; Bobrov, V. I.; Smarkov, M. V. (1977-04-01). "Toxicity of isonicotinic acid". Pharmaceutical Chemistry Journal. 11 (4): 481–483. doi:10.1007/BF01156485. ISSN 0091-150X.
- ^ Mahapatra, Sebabrata; Woolhiser, Lisa; J Lenaerts, Anne; L Johnson, John; Eisenach, Kathleen; Joloba, Moses; Boom, W; T Belisle, John (2012-01-01). "A Novel Metabolite of Antituberculosis Therapy Demonstrates Host Activation of Isoniazid and Formation of the Isoniazid-NAD+ Adduct". Antimicrobial agents and chemotherapy. 56: 28–35. doi:10.1128/AAC.05486-11.
