Uranium: Difference between revisions
m clean up, typos fixed: THe → The, stablity → stability (4) using AWB |
No edit summary |
||
| Line 2: | Line 2: | ||
<!-- moved otheruses below to align infobox; eases flipping through atomic numbers --> |
<!-- moved otheruses below to align infobox; eases flipping through atomic numbers --> |
||
{{Infobox uranium}} |
{{Infobox uranium}} |
||
'''Uranium''' ({{pron-en|jʊˈreɪniəm}} {{respell|yoo|RAY|nee-əm}}) is a silvery-white [[metal]]lic [[chemical element]] in the [[actinide]] series of the [[periodic table]] that has the [[chemical symbol|symbol]] '''U''' and [[atomic number]] 92. Besides its 92 [[proton]]s, a uranium nucleus can |
'''Uranium''' ({{pron-en|jʊˈreɪniəm}} {{respell|yoo|RAY|nee-əm}}) is a silvery-white [[metal]]lic [[chemical element]] in the [[actinide]] series of the [[periodic table]] that has the [[chemical symbol|symbol]] '''U''' and [[atomic number]] 92. Besides its 92 [[proton]]s, a uranium nucleus can haand then i will goa to the bathroom and take a wicked between 141 and 146 [[neutron]]s. The most common uranium isotopes are U-238 (146 neutrons) and U-235 (143 neutrons). A uranium atom has 92 [[electron]]s, 6 of them [[valence electron]]s. Uranium has the highest atomic weight of the naturally occurring elements. It is about 70% [[density|denser]] than [[lead]], but not as dense as [[gold]] or [[tungsten]]. Uranium is weakly [[radioactive decay|radioactive]]. It occurs naturally in low concentrations (a few [[Parts-per notation#Parts-per expressions|parts per million]]) in soil, rock and water, and is commercially extracted from uranium-bearing [[mineral]]s such as [[uraninite]] (see [[uranium mining]]). |
||
In nature, uranium atoms exist as [[uranium-238]] (99.284%), [[uranium-235]] (0.711%),<ref>{{cite web|url=http://www.afrri.usuhs.mil/www/outreach/pdf/mcclain_NATO_2005.pdf|title= Health Concerns about Military Use of Depleted Uranium|format=PDF}}</ref> and a very small amount of [[uranium-234]] (0.0058%). Uranium decays slowly by emitting an [[alpha particle]]. The [[half-life]] of uranium-238 is about 4.47 [[1000000000 (number)|billion]] years and that of uranium-235 is 704 [[million]] years,<ref>{{cite web|url=http://ie.lbl.gov/toi/nucSearch.asp|title=WWW Table of Radioactive Isotopes}}</ref> making them useful in dating the [[age of the Earth]] (see [[uranium-thorium dating]], [[uranium-lead dating]] and [[uranium-uranium dating]]). |
In nature, uranium atoms exist as [[uranium-238]] (99.284%), [[uranium-235]] (0.711%),<ref>{{cite web|url=http://www.afrri.usuhs.mil/www/outreach/pdf/mcclain_NATO_2005.pdf|title= Health Concerns about Military Use of Depleted Uranium|format=PDF}}</ref> and a very small amount of [[uranium-234]] (0.0058%). Uranium decays slowly by emitting an [[alpha particle]]. The [[half-life]] of uranium-238 is about 4.47 [[1000000000 (number)|billion]] years and that of uranium-235 is 704 [[million]] years,<ref>{{cite web|url=http://ie.lbl.gov/toi/nucSearch.asp|title=WWW Table of Radioactive Isotopes}}</ref> making them useful in dating the [[age of the Earth]] (see [[uranium-thorium dating]], [[uranium-lead dating]] and [[uranium-uranium dating]]). |
||
Revision as of 16:19, 5 October 2009
 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uranium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /jʊˈreɪniəm/ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery gray metallic; corrodes to a spalling black oxide coat in air | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(U) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Uranium in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 92 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | f-block groups (no number) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | f-block | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Rn] 5f3 6d1 7s2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 21, 9, 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 1405.3 K (1132.2 °C, 2070 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 4404 K (4131 °C, 7468 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (at 20° C) | 19.050 g/cm3 [3] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 17.3 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 9.14 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 417.1 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 27.665 J/(mol·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | −1,[4] +1, +2, +3,[5] +4, +5, +6 (an amphoteric oxide) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.38 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 156 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 196±7 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 186 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | orthorhombic (oS4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lattice constants | a = 285.35 pm b = 586.97 pm c = 495.52 pm (at 20 °C)[3] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 15.46×10−6/K (at 20 °C)[a] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 27.5 W/(m⋅K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 0.280 µΩ⋅m (at 0 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | paramagnetic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 208 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 111 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 100 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 3155 m/s (at 20 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.23 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vickers hardness | 1960–2500 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 2350–3850 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7440-61-1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Naming | after planet Uranus, itself named after Greek god of the sky Uranus | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | Martin Heinrich Klaproth (1789) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| First isolation | Eugène-Melchior Péligot (1841) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes of uranium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Uranium (Template:Pron-en yoo-RAY-nee-əm) is a silvery-white metallic chemical element in the actinide series of the periodic table that has the symbol U and atomic number 92. Besides its 92 protons, a uranium nucleus can haand then i will goa to the bathroom and take a wicked between 141 and 146 neutrons. The most common uranium isotopes are U-238 (146 neutrons) and U-235 (143 neutrons). A uranium atom has 92 electrons, 6 of them valence electrons. Uranium has the highest atomic weight of the naturally occurring elements. It is about 70% denser than lead, but not as dense as gold or tungsten. Uranium is weakly radioactive. It occurs naturally in low concentrations (a few parts per million) in soil, rock and water, and is commercially extracted from uranium-bearing minerals such as uraninite (see uranium mining).
In nature, uranium atoms exist as uranium-238 (99.284%), uranium-235 (0.711%),[8] and a very small amount of uranium-234 (0.0058%). Uranium decays slowly by emitting an alpha particle. The half-life of uranium-238 is about 4.47 billion years and that of uranium-235 is 704 million years,[9] making them useful in dating the age of the Earth (see uranium-thorium dating, uranium-lead dating and uranium-uranium dating).
Many contemporary uses of uranium exploit its unique nuclear properties. Uranium-235 has the distinction of being the only naturally occurring fissile isotope. Uranium-238 is both fissionable by fast neutrons, and fertile (capable of being transmuted to fissile plutonium-239 in a nuclear reactor). An artificial fissile isotope, uranium-233, can be produced from natural thorium and is also important in nuclear technology. While uranium-238 has a small probability to fission spontaneously or when bombarded with fast neutrons, the much higher probability of uranium-235 and to a lesser degree uranium-233 to fission when bombarded with slow neutrons generates the heat in nuclear reactors used as a source of power, and provides the fissile material for nuclear weapons. Both uses rely on the ability of uranium to produce a sustained nuclear chain reaction. Depleted uranium (uranium-238) is used in kinetic energy penetrators and armor plating.[10]
Uranium is used as a colorant in uranium glass, producing orange-red to lemon yellow hues. It was also used for tinting and shading in early photography. The 1789 discovery of uranium in the mineral pitchblende is credited to Martin Heinrich Klaproth, who named the new element after the planet Uranus. Eugène-Melchior Péligot was the first person to isolate the metal, and its radioactive properties were uncovered in 1896 by Antoine Becquerel. Research by Enrico Fermi and others starting in 1934 led to its use as a fuel in the nuclear power industry and in Little Boy, the first nuclear weapon used in war. An ensuing arms race during the Cold War between the United States and the Soviet Union produced tens of thousands of nuclear weapons that used enriched uranium and uranium-derived plutonium. The security of those weapons and their fissile material following the breakup of the Soviet Union in 1991 is an ongoing concern for public health and safety.
Characteristics

When refined, uranium is a silvery white, weakly radioactive metal, which is slightly softer than steel,[11] strongly electropositive and a poor electrical conductor.[12] It is malleable, ductile, and slightly paramagnetic.[11] Uranium metal has very high density, being approximately 70% denser than lead, but slightly less dense than gold.
Uranium metal reacts with almost all nonmetallic elements and their compounds, with reactivity increasing with temperature.[13] Hydrochloric and nitric acids dissolve uranium, but nonoxidizing acids attack the element very slowly.[12] When finely divided, it can react with cold water; in air, uranium metal becomes coated with a dark layer of uranium oxide.[11] Uranium in ores is extracted chemically and converted into uranium dioxide or other chemical forms usable in industry.
Uranium was the first element that was found to be fissile. Upon bombardment with slow neutrons, its uranium-235 isotope will most of the time divide into two smaller nuclei, releasing nuclear binding energy and more neutrons. If these neutrons are absorbed by other uranium-235 nuclei, a nuclear chain reaction occurs and, if there is nothing to absorb some neutrons and slow the reaction, the reaction is explosive. As little as 15 lb (7 kg) of uranium-235 can be used to make an atomic bomb.[14] The first nuclear bomb used in war, Little Boy, relied on uranium fission, while the very first nuclear explosive (The gadget) and the bomb that destroyed Nagasaki (Fat Man) were plutonium bombs.
Uranium metal has three allotropic forms:[15]
- α (orthorhombic) stable up to 660 °C
- β (tetragonal) stable from 660 °C to 760 °C
- γ (body-centered cubic) from 760 °C to melting point—this is the most malleable and ductile state.
Applications
Military

The major application of uranium in the military sector is in high-density penetrators. This ammunition consists of depleted uranium (DU) alloyed with 1–2% other elements. At high impact speed, the density, hardness, and flammability of the projectile enable destruction of heavily armored targets. Tank armor and the removable armor on combat vehicles are also hardened with depleted uranium plates. The use of DU became a contentious political-environmental issue after the use of DU munitions by the US, UK and other countries during wars in the Persian Gulf and the Balkans raised questions of uranium compounds left in the soil (see Gulf War Syndrome).[14]
Depleted uranium is also used as a shielding material in some containers used to store and transport radioactive materials.[12] Other uses of DU include counterweights for aircraft control surfaces, as ballast for missile re-entry vehicles and as a shielding material.[11] Due to its high density, this material is found in inertial guidance devices and in gyroscopic compasses.[11] DU is preferred over similarly dense metals due to its ability to be easily machined and cast as well as its relatively low cost.[16] Counter to popular belief, the main risk of exposure to DU is chemical poisoning by uranium oxide rather than radioactivity (uranium being only a weak alpha emitter).
During the later stages of World War II, the entire Cold War, and to a lesser extent afterwards, uranium has been used as the fissile explosive material to produce nuclear weapons. Two major types of fission bombs were built: a relatively simple device that uses uranium-235 and a more complicated mechanism that uses uranium-238-derived plutonium-239. Later, a much more complicated and far more powerful fusion bomb that uses a plutonium-based device in a uranium casing to cause a mixture of tritium and deuterium to undergo nuclear fusion was built.[17]
Civilian


The main use of uranium in the civilian sector is to fuel commercial nuclear power plants; by the time it is completely fissioned, one kilogram of uranium-235 can theoretically produce about 80 trillion joules of energy (8×1013 joules); as much energy as 1500 tonnes of coal.[10]
Commercial nuclear power plants use fuel that is typically enriched to around 3% uranium-235.[10] The CANDU reactor is the only commercial reactor capable of using unenriched uranium fuel. Fuel used for United States Navy reactors is typically highly enriched in uranium-235 (the exact values are classified). In a breeder reactor, uranium-238 can also be converted into plutonium through the following reaction:[11] 238U (n, gamma) → 239U -(beta) → 239Np -(beta) → 239Pu.
One of the major yet-unresolved issues with uranium nuclear fuel is the creation of large amount of nuclear waste. Traditional nuclear reactors burn only 1-2% of uranium fuel. However, it is worth noting that other designs of nuclear reactors using alternative, liquid thorium fuel in molten salt reactors produce virtually no long-lasting nuclear waste.
Prior to the discovery of radiation, uranium was primarily used in small amounts for yellow glass and pottery glazes (such as uranium glass and in Fiestaware).
After Marie Curie discovered radium in uranium ore, a huge industry developed to mine uranium so as to extract the radium, which was used to make glow-in-the-dark paints for clock and aircraft dials.[18] This left a prodigious quantity of uranium as a 'waste product', since it takes three metric tons of uranium to extract one gram of radium. This 'waste product' was diverted to the glazing industry, making uranium glazes very inexpensive and abundant. In addition to the pottery glazes, uranium tile glazes accounted for the bulk of the use, including common bathroom and kitchen tiles which can be produced in green, yellow, mauve, black, blue, red and other colors.
Uranium was also used in photographic chemicals (esp. uranium nitrate as a toner),[11] in lamp filaments, to improve the appearance of dentures, and in the leather and wood industries for stains and dyes. Uranium salts are mordants of silk or wool. Uranyl acetate and uranyl formate are used as electron-dense "stains" in transmission electron microscopy, to increase the contrast of biological specimens in ultrathin sections and in negative staining of viruses, isolated cell organelles and macromolecules.
The discovery of the radioactivity of uranium ushered in additional scientific and practical uses of the element. The long half-life of the isotope uranium-238 (4.51×109 years) makes it well-suited for use in estimating the age of the earliest igneous rocks and for other types of radiometric dating (including uranium-thorium dating and uranium-lead dating). Uranium metal is used for X-ray targets in the making of high-energy X-rays.[11]
History
Pre-discovery use
The use of uranium in its natural oxide form dates back to at least the year 79 CE, when it was used to add a yellow color to ceramic glazes.[11] Yellow glass with 1% uranium oxide was found in a Roman villa on Cape Posillipo in the Bay of Naples, Italy by R. T. Gunther of the University of Oxford in 1912.[19] Starting in the late Middle Ages, pitchblende was extracted from the Habsburg silver mines in Joachimsthal, Bohemia (now Jáchymov in the Czech Republic) and was used as a coloring agent in the local glassmaking industry.[20] In the early 19th century, the world's only known sources of uranium ores were these mines.
Discovery

The discovery of the element is credited to the German chemist Martin Heinrich Klaproth. While he was working in his experimental laboratory in Berlin in 1789, Klaproth was able to precipitate a yellow compound (likely sodium diuranate) by dissolving pitchblende in nitric acid and neutralizing the solution with sodium hydroxide.[20] Klaproth mistakenly assumed the yellow substance was the oxide of a yet-undiscovered element and heated it with charcoal to obtain a black powder, which he thought was the newly discovered metal itself (in fact, that powder was an oxide of uranium).[20][21] He named the newly discovered element after the planet Uranus, which had been discovered eight years earlier by William Herschel.[22]
In 1841, Eugène-Melchior Péligot, who was Professor of Analytical Chemistry at the Conservatoire National des Arts et Métiers (Central School of Arts and Manufactures) in Paris, isolated the first sample of uranium metal by heating uranium tetrachloride with potassium.[20][23] Uranium was not seen as being particularly dangerous during much of the 19th century, leading to the development of various uses for the element. One such use for the oxide was the aforementioned but no longer secret coloring of pottery and glass.
Antoine Henri Becquerel discovered radioactivity by using uranium in 1896.[13] Becquerel made the discovery in Paris by leaving a sample of a uranium salt on top of an unexposed photographic plate in a drawer and noting that the plate had become 'fogged'.[24] He determined that a form of invisible light or rays emitted by uranium had exposed the plate.
Fission research

A team led by Enrico Fermi in 1934 observed that bombarding uranium with neutrons produces the emission of beta rays (electrons or positrons; see beta particle).[25] The fission products were at first mistaken for new elements of atomic numbers 93 and 94, which the Dean of the Faculty of Rome, Orso Mario Corbino, christened ausonium and hesperium, respectively.[26][27][28][29] The experiments leading to the discovery of uranium's ability to fission (break apart) into lighter elements and release binding energy were conducted by Otto Hahn and Fritz Strassmann[25] in Hahn's laboratory in Berlin. Lise Meitner and her nephew, physicist Otto Robert Frisch, published the physical explanation in February 1939 and named the process 'nuclear fission'.[30] Soon after, Fermi hypothesized that the fission of uranium might release enough neutrons to sustain a fission reaction. Confirmation of this hypothesis came in 1939, and later work found that on average about 2.5 neutrons are released by each fission of the rare uranium isotope uranium-235.[25] Further work found that the far more common uranium-238 isotope can be transmuted into plutonium, which, like uranium-235, is also fissionable by thermal neutrons. These discoveries led numerous countries to begin working on the development of nuclear weapons and nuclear power.
On 2 December 1942, as part of the Manhattan Project, another team led by Enrico Fermi was able to initiate the first artificial self-sustained nuclear chain reaction, Chicago Pile-1. Working in a lab below the stands of Stagg Field at the University of Chicago, the team created the conditions needed for such a reaction by piling together 400 tons (360 tonnes) of graphite, 58 tons (53 tonnes) of uranium oxide, and six tons (five and a half tonnes) of uranium metal.[25]
Bombs

Two major types of atomic bomb were developed by the United States during World War II: a uranium-based device (codenamed "Little Boy") whose fissile material was highly enriched uranium, and a plutonium-based device (see Trinity test and "Fat Man") whose plutonium was derived from uranium-238. The uranium-based Little Boy device became the first nuclear weapon used in war when it was detonated over the Japanese city of Hiroshima on 6 August 1945. Exploding with a yield equivalent to 12,500 tonnes of TNT, the blast and thermal wave of the bomb destroyed nearly 50,000 buildings and killed approximately 75,000 people (see Atomic bombings of Hiroshima and Nagasaki).[24] Initially it was believed that uranium was relatively rare, and that nuclear proliferation could be avoided by simply buying up all known uranium stocks, but within a decade large deposits of it were discovered in many places around the world.[31]
Reactors

The X-10 Graphite Reactor at Oak Ridge National Laboratory (ORNL) in Oak Ridge, Tennessee, formerly known as the Clinton Pile and X-10 Pile, was the world's second artificial nuclear reactor (after Enrico Fermi's Chicago Pile) and was the first reactor designed and built for continuous operation. The Experimental Breeder Reactor I at the Idaho National Laboratory (INL) near Arco, Idaho became the first nuclear reactor to create electricity on 20 December 1951. Initially, four 150-watt light bulbs were lit by the reactor, but improvements eventually enabled it to power the whole facility (later, the town of Arco became the first in the world to have all its electricity come from nuclear power).[32] The world's first commercial scale nuclear power station, Obninsk in the Soviet Union, began generation with its reactor AM-1 on 27 June 1954. Other early nuclear power plants were Calder Hall in England which began generation on 17 October 1956[33] and the Shippingport Atomic Power Station in Pennsylvania which began on 26 May 1958. Nuclear power was used for the first time for propulsion by a submarine, the USS Nautilus, in 1954.[25]
Naturally occurring nuclear fission
Fifteen ancient and no longer active natural nuclear fission reactors were found in three separate ore deposits at the Oklo mine in Gabon, West Africa in 1972. Discovered by French physicist Francis Perrin, they are collectively known as the Oklo Fossil Reactors. The ore they exist in is 1.7 billion years old; at that time, uranium-235 constituted about three percent of the total uranium on Earth.[34] This is high enough to permit a sustained nuclear fission chain reaction to occur, providing other conditions are right. The ability of the surrounding sediment to contain the nuclear waste products in less than ideal conditions has been cited by the U.S. federal government as evidence of their claim that the Yucca Mountain nuclear waste repository could safely be a repository of waste for the nuclear power industry.[34]
Cold War legacy and waste
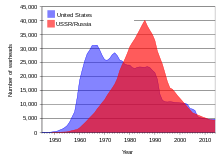
During the Cold War between the Soviet Union and the United States, huge stockpiles of uranium were amassed and tens of thousands of nuclear weapons were created using enriched uranium and plutonium made from uranium.
Since the break-up of the Soviet Union in 1991, an estimated 600 tons (540 tonnes) of highly enriched weapons grade uranium (enough to make 40,000 nuclear warheads) have been stored in often inadequately guarded facilities in the Russian Federation and several other former Soviet states.[14] Police in Asia, Europe, and South America on at least 16 occasions from 1993 to 2005 have intercepted shipments of smuggled bomb-grade uranium or plutonium, most of which was from ex-Soviet sources.[14] From 1993 to 2005 the Material Protection, Control, and Accounting Program, operated by the federal government of the United States, spent approximately US $550 million to help safeguard uranium and plutonium stockpiles in Russia.[14] This money was used for improvements and security enhancements at research and storage facilities. Scientific American reported in February 2006 that some of the facilities security consisted of chain link fences which were in severe states of disrepair. According to an interview from the article, one facility had been storing samples of enriched (weapons grade) uranium in a broom closet prior to the improvement project; another had been keeping track of its stock of nuclear warheads using index cards kept in a shoe box.[35]
Above-ground nuclear tests by the Soviet Union and the United States in the 1950s and early 1960s and by France into the 1970s and 1980s[16] spread a significant amount of fallout from uranium daughter isotopes around the world.[36] Additional fallout and pollution occurred from several nuclear accidents.
The Windscale fire at the Sellafield nuclear plant in 1957 spread iodine-131, a short lived radioactive isotope, over much of Northern England.
In 1979, the Three Mile Island accident released a small amount of iodine-131. The amounts released by the partial meltdown of the Three Mile Island power plant were minimal, and an environmental survey found only trace amounts in a few field mice dwelling nearby. As I-131 has a half life of slightly more than eight days, any danger posed by the radioactive material has long since passed for both of these incidents.
However, the Chernobyl disaster in 1986 was a complete core breach meltdown and partial detonation of the reactor, which ejected iodine-131 and strontium-90 over a large area of Europe. The 28 year half-life of strontium-90 has only recently allowed some of the surrounding countryside around the reactor to be habitable.[16] Since this is less than one half life after the accident, more than half of the original release of strontium-90 will still be present.
Occurrence
Biotic and abiotic

Uranium is a naturally occurring element that can be found in low levels within all rock, soil, and water. Uranium is also the highest-numbered element to be found naturally in significant quantities on earth and is always found combined with other elements.[11] Along with all elements having atomic weights higher than that of iron, it is only naturally formed in supernovas.[37] The decay of uranium, thorium, and potassium-40 in the Earth's mantle is thought to be the main source of heat[38][39] that keeps the outer core liquid and drives mantle convection, which in turn drives plate tectonics.
Uranium's average concentration in the Earth's crust is (depending on the reference) 2 to 4 parts per million,[12][16] or about 40 times as abundant as silver.[13] The Earth's crust from the surface to 25 km (15 mi) down is calculated to contain 1017 kg (2×1017 lb) of uranium while the oceans may contain 1013 kg (2×1013 lb).[12] The concentration of uranium in soil ranges from 0.7 to 11 parts per million (up to 15 parts per million in farmland soil due to use of phosphate fertilizers), and its concentration in sea water is 3 parts per billion.[16]
Uranium is more plentiful than antimony, tin, cadmium, mercury, or silver, and it is about as abundant as arsenic or molybdenum.[11][16] Uranium is found in hundreds of minerals including uraninite (the most common uranium ore), carnotite, autunite, uranophane, torbernite, and coffinite.[11] Significant concentrations of uranium occur in some substances such as phosphate rock deposits, and minerals such as lignite, and monazite sands in uranium-rich ores[11] (it is recovered commercially from sources with as little as 0.1% uranium[13]).

Some organisms, such as the lichen Trapelia involuta or microorganisms such as the bacterium Citrobacter, can absorb concentrations of uranium that are up to 300 times higher than in their environment.[40] Citrobacter species absorb uranyl ions when given glycerol phosphate (or other similar organic phosphates). After one day, one gram of bacteria can encrust themselves with nine grams of uranyl phosphate crystals; this creates the possibility that these organisms could be used in bioremediation to decontaminate uranium-polluted water.[20][41]
In nature, uranium (VI) forms highly soluble carbonate complexes at alkaline pH. This leads to an increase in mobility and availability of uranium to groundwater and soil from nuclear wastes which leads to health hazards. However, it is difficult to precipitate uranium as phosphate in the presence of excess carbonate at alkaline pH. A Sphingomonas sp. strain BSAR-1 has been found to express a high activity alkaline phosphatase (PhoK) that has been applied for bioprecipitation of uranium as uranyl phosphate species from alkaline solutions. The precipitation ability was enhanced by overexpressing PhoK protein in E. coli.[42]
Plants absorb some uranium from soil. Dry weight concentrations of uranium in plants range from 5 to 60 parts per billion, and ash from burnt wood can have concentrations up to 4 parts per million.[20] Dry weight concentrations of uranium in food plants are typically lower with one to two micrograms per day ingested through the food people eat.[20]
Production and mining

The worldwide production of uranium in 2006 amounted to 39 655 tonnes, of which 25% was mined in Canada. Other important uranium mining countries are Australia (19.1%), Kazakhstan (13.3%), Niger (8.7%), Russia (8.6%), and Namibia (7.8%).
Uranium ore is mined in several ways: by open pit, underground, in-situ leaching, and borehole mining (see uranium mining).[10] Low-grade uranium ore mined in 2006 typically contains 0.01 to 0.25% uranium oxides. Extensive measures must be employed to extract the metal from its ore.[43] High-grade ores found in Athabasca Basin deposits in Saskatchewan, Canada can contain up to 23% uranium oxides on average.[44] Uranium ore is crushed and rendered into a fine powder and then leached with either an acid or alkali. The leachate is subjected to one of several sequences of precipitation, solvent extraction, and ion exchange. The resulting mixture, called yellowcake, contains at least 75% uranium oxides. Yellowcake is then calcined to remove impurities from the milling process prior to refining and conversion.[45]
Commercial-grade uranium can be produced through the reduction of uranium halides with alkali or alkaline earth metals.[11] Uranium metal can also be made through electrolysis of KU
5 or
UF
4, dissolved in molten calcium chloride (CaCl
2) and sodium chloride (NaCl) solution.[11] Very pure uranium can be produced through the thermal decomposition of uranium halides on a hot filament.[11]
Miners who worked in uranium filled mines have a very high incidence of cancer relative to the rest of the United States population. Though the Navajo workers and families noticed this in the 1950s, bureaucrats dragged their feet, and companies disregarded warnings. The miners, especially the Navajo miners, were kept from receiving compensation for the suffering they went through. In 1990 a law was passed known as the Radiation Exposure Compensation Act of 1990 (RECA) (Eichstaedt, 1994). The law required $100,000 in "compassion payments" to uranium miners diagnosed with cancer or other respiratory ailments (Eichstaedt, 1994; Benally Sr., 1995). To qualify for compensation, a miner had to prove that s/he had worked in the mines and was now suffering from one of the diseases on the compensation list (Eichstaedt, 1994; Benally Sr. 1995).[46]
Resources and reserves
Current economic uranium resources will last for over 100 years at 2006 consumption rates, while it is expected there is twice that amount awaiting discovery. With reprocessing and recycling, the reserves are good for thousands of years.[47] It is estimated that 5.5 million tonnes of uranium ore reserves are economically viable at US$59/lb,[47] while 35 million tonnes are classed as mineral resources (reasonable prospects for eventual economic extraction).[48] An additional 4.6 billion tonnes of uranium are estimated to be in sea water (Japanese scientists in the 1980s showed that extraction of uranium from sea water using ion exchangers was technically feasible).[49][50]
Exploration for uranium is increasing with US$200 million being spent world wide in 2005, a 54% increase on the previous year.[48] This trend continued through 2006, when expenditure on exploration rocketed to over $774 million, an increase of over 250% compared to 2004. The OECD Nuclear Energy Agency said exploration figures for 2007 would likely match those for 2006.[47]
Australia has 23% of the world's uranium ore reserves[51] and the world's largest single uranium deposit, located at the Olympic Dam Mine in South Australia.[52] Almost all Australia's mined uranium is exported, under strict International Atomic Energy Agency safeguards against use in nuclear weapons.
Some nuclear fuel comes from nuclear weapons being dismantled.[53]
Supply
In 2005, seventeen countries produced concentrated uranium oxides, with Canada (27.9% of world production) and Australia (22.8%) being the largest producers and Kazakhstan (10.5%), Russia (8.0%), Namibia (7.5%), Niger (7.4%), Uzbekistan (5.5%), the United States (2.5%), Argentina (2.1%), Ukraine (1.9%) and China (1.7%) also producing significant amounts.[54] Kazakhstan continues to increase production and may become the world's largest producer of uranium by this year (2009) with an expected production of 12,826 tonnes, compared to Canada with 11,100 tonnes and Australia with 9,430 tonnes.[55][56] The ultimate available uranium is believed to be sufficient for at least the next 85 years[48] although some studies indicate underinvestment in the late twentieth century may produce supply problems in the 21st century.[57]
Some claim that production of uranium will peak similar to peak oil. Kenneth S. Deffeyes and Ian D. MacGregor point out that uranium deposits seem to be log-normal distributed. There is a 300-fold increase in the amount of uranium recoverable for each tenfold decrease in ore grade."[58] In other words, there is little high grade ore and proportionately much more low grade ore available.
Compounds
Oxidation states and oxides
Oxides

Calcined uranium yellowcake as produced in many large mills contains a distribution of uranium oxidation species in various forms ranging from most oxidized to least oxidized. Particles with short residence times in a calciner will generally be less oxidized than those with long retention times or particles recovered in the stack scrubber. Uranium content is usually referenced to U
3O
8, which dates to the days of the Manhattan project when U
3O
8 was used as an analytical chemistry reporting standard.
Phase relationships in the uranium-oxygen system are complex. The most important oxidation states of uranium are uranium(IV) and uranium(VI), and their two corresponding oxides are, respectively, uranium dioxide (UO
2) and uranium trioxide (UO
3).[59] Other uranium oxides such as uranium monoxide (UO), diuranium pentoxide (U
2O
5), and uranium peroxide (UO
4•2H
2O) also exist.
The most common forms of uranium oxide are triuranium octaoxide (U
3O
8) and UO
2.[60] Both oxide forms are solids that have low solubility in water and are relatively stable over a wide range of environmental conditions. Triuranium octaoxide is (depending on conditions) the most stable compound of uranium and is the form most commonly found in nature. Uranium dioxide is the form in which uranium is most commonly used as a nuclear reactor fuel.[60] At ambient temperatures, UO
2 will gradually convert to U
3O
8. Because of their stability, uranium oxides are generally considered the preferred chemical form for storage or disposal.[60]
Aqueous chemistry
The four different oxidation states of uranium are soluble and therefore can be studied in aqueous solutions. They are: U3+ (red), U4+ (green), UO+
2 (unstable), and UO
22+ (yellow).[61] A few solid and semi-metallic compounds such as UO and US exist for the formal oxidation state uranium(II), but no simple ions are known to exist in solution for that state. Ions of U3+ liberate hydrogen from water and are therefore considered to be highly unstable. The UO2+
2 ion represents the uranium(VI) state and is known to form compounds such as carbonate, chloride and sulfate. UO2+
2 also forms complexes with various organic chelating agents, the most commonly encountered of which is uranyl acetate.[61]
Carbonates
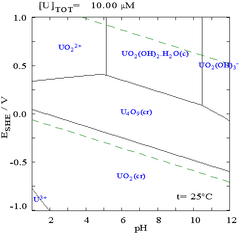

The interactions of carbonate anions with uranium(VI) cause the Pourbaix diagram to change greatly when the medium is changed from water to a carbonate containing solution. It is interesting to note that while the vast majority of carbonates are insoluble in water (students are often taught that all carbonates other than those of alkali metals are insoluble in water), uranium carbonates are often soluble in water. This is due to the fact that a U(VI) cation is able to bind two terminal oxides and three or more carbonates to form anionic complexes.
The effect of pH


The uranium fraction diagrams in the presence of carbonate illustrate this further: when the pH of a uranium(VI) solution increases, the uranium is converted to a hydrated uranium oxide hydroxide and at high pHs it becomes an anionic hydroxide complex.
When carbonate is added, uranium is converted to a series of carbonate complexes if the pH is increased. One effect of these reactions is increased solubility of uranium in the pH range 6 to 8, a fact which has a direct bearing on the long term stability of spent uranium dioxide nuclear fuels.
Hydrides, carbides and nitrides
Uranium metal heated to 250 to 300 °C (482 to 572 °F) reacts with hydrogen to form uranium hydride. Even higher temperatures will reversibly remove the hydrogen. This property makes uranium hydrides convenient starting materials to create reactive uranium powder along with various uranium carbide, nitride, and halide compounds.[63] Two crystal modifications of uranium hydride exist: an α form that is obtained at low temperatures and a β form that is created when the formation temperature is above 250 °C.[63]
Uranium carbides and uranium nitrides are both relatively inert semimetallic compounds that are minimally soluble in acids, react with water, and can ignite in air to form U
3O
8.[63] Carbides of uranium include uranium monocarbide (UC), uranium dicarbide (UC
2), and diuranium tricarbide (U
2C
3). Both UC and UC
2 are formed by adding carbon to molten uranium or by exposing the metal to carbon monoxide at high temperatures. Stable below 1800 °C, U
2C
3 is prepared by subjecting a heated mixture of UC and UC
2 to mechanical stress.[64] Uranium nitrides obtained by direct exposure of the metal to nitrogen include uranium mononitride (UN), uranium dinitride (UN
2), and diuranium trinitride (U
2N
3).[64]
Halides
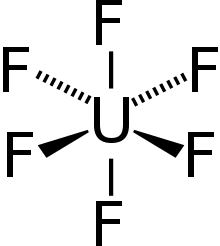
All uranium fluorides are created using uranium tetrafluoride (UF
4); UF
4 itself is prepared by hydrofluorination of uranium dioxide.[63] Reduction of UF
4 with hydrogen at 1000 °C produces uranium trifluoride (UF
3). Under the right conditions of temperature and pressure, the reaction of solid UF
4 with gaseous uranium hexafluoride (UF
6) can form the intermediate fluorides of U
2F
9, U
4F
17, and UF
5.[63]
At room temperatures, UF
6 has a high vapor pressure, making it useful in the gaseous diffusion process to separate uranium-235 from the common uranium-238 isotope. This compound can be prepared from uranium dioxide and uranium hydride by the following process:[63]
- UO
2 + 4 HF → UF
4 + 2 H
2O (500 °C, endothermic) - UF
4 + F
2 → UF
6 (350 °C, endothermic)
The resulting UF
6, a white solid, is highly reactive (by fluorination), easily sublimes (emitting a nearly perfect gas vapor), and is the most volatile compound of uranium known to exist.[63]
One method of preparing uranium tetrachloride (UCl
4) is to directly combine chlorine with either uranium metal or uranium hydride. The reduction of UCl
4 by hydrogen produces uranium trichloride (UCl
3) while the higher chlorides of uranium are prepared by reaction with additional chlorine.[63] All uranium chlorides react with water and air.
Bromides and iodides of uranium are formed by direct reaction of, respectively, bromine and iodine with uranium or by adding UH
3 to those element's acids.[63] Known examples include: UBr
3, UBr
4, UI
3, and UI
4. Uranium oxyhalides are water-soluble and include UO
2F
2, UOCl
2, UO
2Cl
2, and UO
2Br
2. Stability of the oxyhalides decrease as the atomic weight of the component halide increases.[63]
Isotopes
Natural concentrations
Natural uranium consists of three major isotopes: uranium-238 (99.28% natural abundance), uranium-235 (0.71%), and uranium-234 (0.0054%). All three are radioactive. Uranium-238 is the most stable isotope, with a half-life of 4.51×109 years (close to the age of the Earth). Uranium-235 has a half-life of 7.13×108 years, and uranium-234 has a half-life of 2.48×105 years.[65]
Uranium-238 is an α emitter, decaying through the 18-member uranium natural decay series into lead-206.[13] The decay series of uranium-235 (also called actino-uranium) has 15 members that ends in lead-207.[13] The constant rates of decay in these series makes comparison of the ratios of parent to daughter elements useful in radiometric dating. Uranium-234 decays to lead-206 through a series of short-lived intermediaries. Uranium-233 is made from thorium-232 by neutron bombardment;[11] its decay series ends with thallium-205.
The isotope uranium-235 is important for both nuclear reactors and nuclear weapons because it is the only isotope existing in nature to any appreciable extent that is fissile, that is, can be broken apart by thermal neutrons.[13] The isotope uranium-238 is also important because it absorbs neutrons to produce a radioactive isotope that subsequently decays to the isotope plutonium-239, which is also fissile.[25]
Enrichment

Isotope separation concentrates (enriches) the fissionable uranium-235 for nuclear weapons and most nuclear power plants, with the exception of gas cooled reactors and pressurised heavy water reactors. Most neutrons released by a fissioning atom of uranium-235 must impact other uranium-235 atoms to sustain the nuclear chain reaction. The concentration and amount of uranium-235 needed to achieve this is called a 'critical mass'.
To be considered 'enriched', the uranium-235 fraction should be between 3% and 5%.[66] This process produces huge quantities of uranium that is depleted of uranium-235 and with a correspondingly increased fraction of uranium-238, called depleted uranium or 'DU'. To be considered 'depleted', the uranium-235 isotope concentration should be no more than 0.2% to 0.3%.[67] The price of uranium has risen since 2001, so enrichment tailings containing more than 0.35% uranium-235 are being considered for re-enrichment, driving the price of depleted uranium hexafluoride above $130 per kilogram in July, 2007 from $5 in 2001.[67]
The gas centrifuge process, where gaseous uranium hexafluoride (UF
6) is separated by the difference in molecular weight between 235UF6 and 238UF6 using high-speed centrifuges, is the cheapest and leading enrichment process.[24] The gaseous diffusion process had been the leading method for enrichment and was used in the Manhattan Project. In this process, uranium hexafluoride is repeatedly diffused through a silver-zinc membrane, and the different isotopes of uranium are separated by diffusion rate (since uranium 238 is heavier it diffuses slightly slower than uranium-235).[24] The molecular laser isotope separation method employs a laser beam of precise energy to sever the bond between uranium-235 and fluorine. This leaves uranium-238 bonded to fluorine and allows uranium-235 metal to precipitate from the solution.[10] Another method used is liquid thermal diffusion.[12]
Precautions
Exposure
A person can be exposed to uranium (or its radioactive daughters such as radon) by inhaling dust in air or by ingesting contaminated water and food. The amount of uranium in air is usually very small; however, people who work in factories that process phosphate fertilizers, live near government facilities that made or tested nuclear weapons, live or work near a modern battlefield where depleted uranium weapons have been used, or live or work near a coal-fired power plant, facilities that mine or process uranium ore, or enrich uranium for reactor fuel, may have increased exposure to uranium.[68][69] Houses or structures that are over uranium deposits (either natural or man-made slag deposits) may have an increased incidence of exposure to radon gas.
Most ingested uranium is excreted during digestion. Only 0.5% is absorbed when insoluble forms of uranium, such as its oxide, are ingested, whereas absorption of the more soluble uranyl ion can be up to 5%.[20] However, soluble uranium compounds tend to quickly pass through the body whereas insoluble uranium compounds, especially when ingested via dust into the lungs, pose a more serious exposure hazard. After entering the bloodstream, the absorbed uranium tends to bioaccumulate and stay for many years in bone tissue because of uranium's affinity for phosphates.[20] Uranium is not absorbed through the skin, and alpha particles released by uranium cannot penetrate the skin.
Effects
Normal functioning of the kidney, brain, liver, heart, and other systems can be affected by uranium exposure, because, in addition to being weakly radioactive, uranium is a toxic metal.[20][70][71] Uranium is also a reproductive toxicant.[72][73] Radiological effects are generally local because alpha radiation, the primary form of U-238 decay, has a very short range, and will not penetrate skin. Uranyl (UO+
2) ions, such as from uranium trioxide or uranyl nitrate and other hexavalent uranium compounds, have been shown to cause birth defects and immune system damage in laboratory animals.[74] While the CDC has published one study that no human cancer has been seen as a result of exposure to natural or depleted uranium,[75] exposure to uranium and its decay products, especially radon, are widely known and significant health threats.[16] Exposure to strontium-90, iodine-131, and other fission products is unrelated to uranium exposure, but may result from medical procedures or exposure to spent reactor fuel or fallout from nuclear weapons.[76]
Although accidental inhalation exposure to a high concentration of uranium hexafluoride has
resulted in human fatalities, those deaths were associated with generation of highly toxic hydrofluoric acid and uranyl fluoride rather than with uranium itself.[77] Finely divided uranium metal presents a fire hazard because uranium is pyrophoric; small grains will ignite spontaneously in air at room temperature.[11]
| Compilation of 2004 review on uranium toxicity[70] | |||
|---|---|---|---|
| Body system | Human studies | Animal studies | In vitro |
| Renal | Elevated levels of protein excretion, urinary catalase and diuresis | Damage to Proximal convoluted tubules, necrotic cells cast from tubular epithelium, glomerular changes | No studies |
| Brain/CNS | Decreased performance on neurocognitive tests | Acute cholinergic toxicity; Dose-dependent accumulation in cortex, midbrain, and vermis; Electrophysiological changes in hippocampus | No studies |
| DNA | Increased reports of cancers | Increased urine mutagenicity and induction of tumors | Binucleated cells with micronuclei, Inhibition of cell cycle kinetics and proliferation; Sister chromatid induction, tumorigenic phenotype |
| Bone/muscle | No studies | Inhibition of periodontal bone formation; and alveolar wound healing | No studies |
| Reproductive | Uranium miners have more first born female children | Moderate to severe focal tubular atrophy; vacuolization of Leydig cells | No studies |
| Lungs/respiratory | No adverse health effects reported | Severe nasal congestion and hemorrage, lung lesions and fibrosis, edema and swelling, lung cancer | No studies |
| Gastrointestinal | Vomiting, diarrhea, albuminuria | n/a | n/a |
| Liver | No effects seen at exposure dose | Fatty livers, focal necrosis | No studies |
| Skin | No exposure assessment data available | Swollen vacuolated epidermal cells, damage to hair follicles and sebaceous glands | No studies |
| Tissues surrounding embedded DU fragments | Elevated uranium urine concentrations | Elevated uranium urine concentrations, perturbations in biochemical and neuropsychological testing | No studies |
| Immune system | Chronic fatigue, rash, ear and eye infections, hair and weight loss, cough. May be due to combined chemical exposure rather than DU alone | No studies | No studies |
| Eyes | No studies | Conjunctivitis, irritation inflammation, edema, ulceration of conjunctival sacs | No studies |
| Blood | No studies | Decrease in RBC count and hemoglobin concentration | No studies |
| Cardiovascular | Myocarditis resulting from the uranium ingestion, which ended 6 months after ingestion | No effects | No studies |
See also
Notes
- ^ "Standard Atomic Weights: Uranium". CIAAW. 1999.
- ^ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (2022-05-04). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- ^ a b Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- ^ Th(-I) and U(-I) have been detected in the gas phase as octacarbonyl anions; see Chaoxian, Chi; Sudip, Pan; Jiaye, Jin; Luyan, Meng; Mingbiao, Luo; Lili, Zhao; Mingfei, Zhou; Gernot, Frenking (2019). "Octacarbonyl Ion Complexes of Actinides [An(CO)8]+/− (An=Th, U) and the Role of f Orbitals in Metal–Ligand Bonding". Chemistry (Weinheim an der Bergstrasse, Germany). 25 (50): 11772–11784. 25 (50): 11772–11784. doi:10.1002/chem.201902625. ISSN 0947-6539. PMC 6772027. PMID 31276242.
- ^ Morss, L.R.; Edelstein, N.M.; Fuger, J., eds. (2006). The Chemistry of the Actinide and Transactinide Elements (3rd ed.). Netherlands: Springer. ISBN 978-9048131464.
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ Magurno, B.A.; Pearlstein, S, eds. (1981). Proceedings of the conference on nuclear data evaluation methods and procedures. BNL-NCS 51363, vol. II (PDF). Upton, NY (USA): Brookhaven National Lab. pp. 835 ff. Retrieved 2014-08-06.
- ^ "Health Concerns about Military Use of Depleted Uranium" (PDF).
- ^ "WWW Table of Radioactive Isotopes".
- ^ a b c d e Emsley, page 479
- ^ a b c d e f g h i j k l m n o p q r C. R. Hammond (2000). The Elements, in Handbook of Chemistry and Physics 81th edition (PDF). CRC press. ISBN 0849304814.
- ^ a b c d e f "Uranium". The McGraw-Hill Science and Technology Encyclopedia (5th edition ed.). The McGraw-Hill Companies, Inc.
{{cite encyclopedia}}:|edition=has extra text (help) - ^ a b c d e f g "uranium". Columbia Electronic Encyclopedia (6th Edition ed.). Columbia University Press.
{{cite encyclopedia}}:|edition=has extra text (help) - ^ a b c d e "uranium". Encyclopedia of Espionage, Intelligence, and Security. The Gale Group, Inc.
- ^ A. D. Rollett (2008). Applications of Texture Analysis. John Wiley and Sons. p. 108. ISBN 0470408359.
- ^ a b c d e f g Emsley, page 480
- ^ "Nuclear Weapon Design". Federation of American Scientists. 1998. Retrieved 2007-02-19.
- ^ "Dial R for radioactive - 12 July 1997 - New Scientist". Newscientist.com. Retrieved 2008-09-12.
- ^ Emsley, page 482
- ^ a b c d e f g h i j Emsley, page 477
- ^ M. H. Klaproth (1789). "Chemische Untersuchung des Uranits, einer neuentdeckten metallischen Substanz". Chemische Annalen. 2: 387–403.
- ^ "Uranium". The American Heritage Dictionary of the English Language (4th edition ed.). Houghton Mifflin Company.
{{cite encyclopedia}}:|edition=has extra text (help) - ^ E.-M. Péligot (1842). "Recherches Sur L'Uranium". Annales de chimie et de physique. 5 (5): 5–47.
- ^ a b c d Emsley, page 478
- ^ a b c d e f Seaborg, page 773
- ^ Fermi, Enrico (December 12, 1938). "Artificial radioactivity produced by neutron bombardment: Nobel Lecture" (PDF). Royal Swedish Academy of Sciences.
- ^ De Gregorio, A. (2003). "A Historical Note About How the Property was Discovered that Hydrogenated Substances Increase the Radioactivity Induced by Neutrons". arXiv:physics/0309046.
{{cite arXiv}}: Unknown parameter|accessdate=ignored (help) - ^ Nigro, M, (2004). "Hahn, Meitner e la teoria della fissione" (PDF). Retrieved 2009-05-05.
{{cite web}}: CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ Peter van der Krogt. "Elementymology & Elements Multidict". Retrieved 2009-05-05.
- ^ L. Meitner, O. Frisch (1939). "Disintegration of Uranium by Neutrons: a New Type of Nuclear Reaction". Nature. 143: 239–240. doi:10.1038/224466a0.
- ^ J.E. Helmreich, Gathering Rare Ores: The Diplomacy of Uranium Acquisition, 1943-1954, Princeton UP, 1986: ch. 10
- ^ "History and Success of Argonne National Laboratory: Part 1". U.S. Department of Energy, Argonne National Laboratory. 1998. Retrieved 2007-01-28.
- ^ "1956:Queen switches on nuclear power". BBC news. Retrieved June 28.
{{cite web}}: Check date values in:|accessdate=(help); Unknown parameter|accessyear=ignored (|access-date=suggested) (help) - ^ a b "Oklo: Natural Nuclear Reactors". Office of Civilian Radioactive Waste Management. Retrieved June 28.
{{cite web}}: Check date values in:|accessdate=(help); Unknown parameter|accessyear=ignored (|access-date=suggested) (help) - ^ Glaser, Alexander and von Hippel, Frank N. "Thwarting Nuclear Terrorism" Scientific American Magazine, February 2006
- ^ T. Warneke, I. W. Croudace, P. E. Warwick, R. N. Taylor (2002). "A new ground-level fallout record of uranium and plutonium isotopes for northern temperate latitudes". Earth and Planetary Science Letters. 203 (3–4): 1047–1057. doi:10.1016/S0012-821X(02)00930-5.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "WorldBook@NASA: Supernova". NASA. Retrieved 2007-02-19.
- ^ Biever, Celeste (27 July 2005). "First measurements of Earth's core radioactivity". New Scientist.
{{cite journal}}: Cite journal requires|journal=(help) - ^ "Potassium-40 heats up Earth's core". physicsweb. 7 May 2003. Retrieved 2007-01-14.
- ^ Emsley, pages 476 and 482
- ^ L. E. Macaskie, R. M. Empson, A. K. Cheetham, C. P. Grey, A. J. Skarnulis (1992). "Uranium bioaccumulation by a Citrobacter sp. as a result of enzymically mediated growth of polycrystalline HUO
2PO
4". Science. 257: 782–784. doi:10.1126/science.1496397. PMID 1496397.{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^
K.S. Nilgiriwala, A. Alahari, A. S. Rao & S.K. Apte (2008). "Cloning and overexpression of an alkaline phosphatase PhoK from Sphingomonas sp. BSAR-1 for uranium bioprecipitation from alkaline solutions". Applied and Environmental Microbiology. 74 (17): 5516–5523. doi:10.1128/AEM.00107-08. ISSN 1098-5336. PMID 18641147.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Seaborg, page 774
- ^ "Athabasca Basin, Saskatchewan". Retrieved 2009-09-04.
- ^ C. K. Gupta, T. K. Mukherjee (1990). Hydrometallurgy in extraction processes, Volume 1. CRC Press. pp. 74–75. ISBN 0849368049.
- ^ "Environmental Justice for the Navajo : Uranium Mining in the Southwest". University of Michigan. Retrieved 2009-08-08.
- ^ a b c "Exploration drives uranium resources up 17%<!- Bot generated title ->". World-nuclear-news.org. Retrieved 2008-09-12.
- ^ a b c "Global Uranium Resources to Meet Projected Demand". International Atomic Energy Agency. 2006. Retrieved 2007-03-29.
- ^ "Uranium recovery from Seawater". Japan Atomic Energy Research Institute. 1999-08-23. Retrieved 2008-09-03.
- ^ "How long will nuclear energy last?". 1996-02-12. Retrieved 2007-03-29.
- ^ "Supply of Uranium".
- ^ "Uranium Mining and Processing in South Australia". South Australian Chamber of Mines and Energy. 2002. Retrieved 2007-01-14.
- ^ Military Warheads as a Source of Nuclear Fuel
- ^ "World Uranium Production". UxC Consulting Company, LLC. Retrieved 2007-02-11.
- ^ Posted by Mithridates (July 24, 2008). "Page F30: Kazakhstan to surpass Canada as the world's largest producer of uranium by next year (2009)<!- Bot generated title ->". Mithridates.blogspot.com. Retrieved 2008-09-12.
- ^ "ZAMAN GAZETESİ [İnternetin İlk Türk Gazetesi] - Kazakistan uranyum üretimini artıracak<!- Bot generated title ->" (in Turkish). Zaman.com.tr. Retrieved 2008-09-12.
- ^ "Lack of fuel may limit U.S. nuclear power expansion". Massachusetts Institute of Technology. 2007-03-21. Retrieved 2007-03-29.
- ^ Kenneth S. Deffeyes and Ian D. MacGregor (1980-01). "World Uranium Resources". Scientific American. p. p 66. Retrieved 2008-04-21.
{{cite web}}:|page=has extra text (help); Check date values in:|date=(help) - ^ Seaborg, page 779
- ^ a b c "Chemical Forms of Uranium". Argonne National Laboratory. Retrieved 2007-02-18.
- ^ a b Seaborg, page 778
- ^ a b c d Ignasi Puigdomenech, Hydra/Medusa Chemical Equilibrium Database and Plotting Software (2004) KTH Royal Institute of Technology, freely downloadable software at [1]
- ^ a b c d e f g h i j Seaborg, page 782
- ^ a b Seaborg, page 780
- ^ Seaborg, page 777
- ^ "Uranium Enrichment". Argonne National Laboratory. Retrieved 2007-02-11.
- ^ a b Peter Diehl. "Depleted Uranium: a by-product of the Nuclear Chain". Laka Foundation. Retrieved 2009-07-31. Cite error: The named reference "paducah" was defined multiple times with different content (see the help page).
- ^ "Radiation Information for Uranium". U.S. Environmental Protection Agency. Retrieved 2009-07-31.
- ^ "ToxFAQ for Uranium". Agency for Toxic Substances and Disease Registry. 1999. Retrieved 2007-02-18.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ a b E. S. Craft, A. W. Abu-Qare, M. M. Flaherty, M. C. Garofolo, H. L. Rincavage, M. B. Abou-Donia (2004). "Depleted and natural uranium: chemistry and toxicological effects". Journal of Toxicology and Environmental Health Part B: Critical Reviews. 7 (4): 297–317. doi:10.1080/10937400490452714.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Toxicological Profile for Uranium" (PDF). Atlanta, GA: Agency for Toxic Substances and Disease Registry (ATSDR). 1999. CAS# 7440-61-1 month=September.
- ^ Hindin, et al. (2005) "Teratogenicity of depleted uranium aerosols: A review from an epidemiological perspective," Environ Health, vol. 4, pp. 17
- ^ Arfsten, D.P.; K.R. Still; G.D. Ritchie (2001). "A review of the effects of uranium and depleted uranium exposure on reproduction and fetal development". Toxicology and Industrial Health. 17 (5–10): 180–91. doi:10.1191/0748233701th111oa. PMID 12539863.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Domingo, J. (2001) "Reproductive and developmental toxicity of natural and depleted uranium: a review," Reproductive Toxicology, vol. 15, pp. 603–609, doi: 10.1016/S0890-6238(01)00181-2 PMID 2711400
- ^ "Public Health Statement for Uranium". CDC. Retrieved 2007-02-15.
- ^ Chart of the Nuclides, US Atomic Energy Commission 1968
- ^ Richard C. Dart (2004). Medical Toxicology. Lippincott Williams & Wilkins. p. 1468. ISBN 0781728452.
References
Full reference information for multi-page works cited
- John Emsley (2001). "Uranium". Nature's Building Blocks: An A to Z Guide to the Elements. Oxford: Oxford University Press. pp. 476–82. ISBN 0-19-850340-7.
- Glenn T. Seaborg (1968). "Uranium". The Encyclopedia of the Chemical Elements. Skokie, Illinois: Reinhold Book Corporation. pp. 773–86. LCCCN 68-29938.
External links
- ATSDR Case Studies in Environmental Medicine: Uranium Toxicity U.S. Department of Health and Human Services
- "Public Health Statement for Uranium". CDC.
- Uranium Resources and Nuclear Energy
- U.S. EPA: Radiation Information for Uranium
- "What is Uranium?" from World Nuclear Association
- Nuclear fuel data and analysis from the U.S. Energy Information Administration
- Current market price of uranium
- World Uranium deposit maps
- Annotated bibliography for uranium from the Alsos Digital Library
- NLM Hazardous Substances Databank—Uranium, Radioactive
- 'Pac-Man' molecule chews up uranium contamination - earth - 17 January 2008 - New Scientist Environment
- Mining Uranium at Namibia's Langer Heinrich Mine
- Uranium futures market
- World Nuclear News
Cite error: There are <ref group=lower-alpha> tags or {{efn}} templates on this page, but the references will not show without a {{reflist|group=lower-alpha}} template or {{notelist}} template (see the help page).


