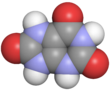
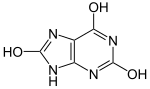
Uric acid
| |||
 Crystals of urate in polarized light
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
7,9-Dihydro-1H-purine-2,6,8(3H)-trione | |||
| Other names
2,6,8-Trioxypurine; 2,6,8-Trihydroxypurine; 2,6,8-Trioxopurine; 1H-Purine-2,6,8-trione
| |||
| Identifiers | |||
3D model (JSmol)
|
| ||
| 3DMet | |||
| 156158 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.655 | ||
| EC Number |
| ||
| KEGG | |||
| MeSH | Uric+Acid | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| Properties | |||
| C5H4N4O3 | |||
| Molar mass | 168.112 g·mol−1 | ||
| Appearance | White crystals | ||
| Melting point | 300 °C (572 °F; 573 K) | ||
| 6 mg/100 mL (at 20 °C) | |||
| log P | −1.107 | ||
| Acidity (pKa) | 5.6 | ||
| Basicity (pKb) | 8.4 | ||
| −6.62×10−5 cm3 mol−1 | |||
| Thermochemistry | |||
Heat capacity (C)
|
166.15 J K−1 mol−1 (at 24.0 °C) | ||
Std molar
entropy (S⦵298) |
173.2 J K−1 mol−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
−619.69 to −617.93 kJ mol−1 | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−1921.2 to −1919.56 kJ mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Uric acid is a heterocyclic compound of carbon, nitrogen, oxygen, and hydrogen with the formula C5H4N4O3. It forms ions and salts known as urates and acid urates, such as ammonium acid urate. Uric acid is a product of the metabolic breakdown of purine nucleotides, and it is a normal component of urine. High blood concentrations of uric acid can lead to gout and are associated with other medical conditions, including diabetes and the formation of ammonium acid urate kidney stones.
Chemistry
Uric acid was first isolated from kidney stones in 1776 by Swedish chemist Carl Wilhelm Scheele.[1] In 1882, the Ukrainian chemist Ivan Horbaczewski first synthesized uric acid by melting urea with glycine.[2]
Uric acid displays lactam–lactim tautomerism (also often described as keto–enol tautomerism[3]). Although the lactim form is expected to possess some degree of aromaticity, uric acid crystallizes in the lactam form,[4] with computational chemistry also indicating that tautomer to be the most stable.[5] Uric acid is a diprotic acid with pKa1 = 5.4 and pKa2 = 10.3,[6] thus at physiological pH, it predominately exists as the monoionic urate ion.
Water solubility
In general, the water solubility of uric acid and its alkali metal and alkaline earth salts is rather low. All these salts exhibit greater solubility in hot water than cold, allowing for easy recrystallization. This low solubility is significant for the etiology of gout. The solubility of the acid and its salts in ethanol is very low or negligible. In ethanol/water mixtures, the solubilities are somewhere between the end values for pure ethanol and pure water.
Solubility of urate salts (grams of water per gram of compound) Compound Cold water Boiling water Uric acid 15,000 2,000 Ammonium hydrogen urate — 1,600 Lithium hydrogen urate 370 39 Sodium hydrogen urate 1,175 124 Potassium hydrogen urate 790 75 Magnesium dihydrogen diurate 3,750 160 Calcium dihydrogen diurate 603 276 Disodium urate 77 — Dipotassium urate 44 35 Calcium urate 1,500 1,440 Strontium urate 4,300 1,790 Barium urate 7,900 2,700
The figures given indicate what mass of water is required to dissolve a unit mass of compound indicated. The lower the number, the more soluble the substance in the said solvent.[7][8][9]
Biochemistry
Xanthine oxidase (found in mammals primarily as xanthine dehydrogenase and rarely as an oxidase[10]) is an enzyme which catalyzes the formation of uric acid from xanthine and hypoxanthine, which in turn are produced from other purines. Xanthine oxidase is a large enzyme whose active site consists of the metal molybdenum bound to sulfur and oxygen.[11] Within cells, xanthine oxidase can exist as xanthine dehydrogenase and xanthine oxireductase, which has also been purified from bovine milk and spleen extracts.[12] Uric acid is released in hypoxic conditions (low oxygen saturation).[13]
Genetic and physiological diversity
Primates
In humans and other great apes, uric acid (actually hydrogen urate ion) is the final oxidation (breakdown) product of purine metabolism and is excreted in urine, whereas in most other mammals, the enzyme uricase further oxidizes uric acid to allantoin.[14] The loss of uricase in higher primates parallels the similar loss of the ability to synthesize ascorbic acid, leading to the suggestion that urate may partially substitute for ascorbate in such species.[15] Both uric acid and ascorbic acid are strong reducing agents (electron donors) and potent antioxidants. In humans, over half the antioxidant capacity of blood plasma comes from hydrogen urate ion.[16]
Humans
The normal concentration range of uric acid (or hydrogen urate ion) in human blood is 25 to 80 mg/L for men and 15 to 60 mg/L for women[17] (but see below for slightly different values). An individual can have serum values as high as 96 mg/L and not have gout.[18] In humans, about 70% of daily uric acid disposal occurs via the kidneys, and in 5–25% of humans, impaired renal (kidney) excretion leads to hyperuricemia.[19] Normal excretion of uric acid in the urine is 270 to 360 mg per day (concentration of 270 to 360 mg/L if one litre of urine is produced per day – higher than the solubility of uric acid because it is in the form of dissolved acid urates), roughly 1% as much as the daily excretion of urea.[20]
Dogs
The Dalmatian has a genetic defect in uric acid uptake by the liver and kidneys, resulting in decreased conversion to allantoin, so this breed excretes uric acid, and not allantoin, in the urine.[21]
Birds and reptiles
In birds and reptiles, and in some desert-dwelling mammals (such as the kangaroo rat), uric acid also is the end product of purine metabolism, but it is excreted in feces as a dry mass. This involves a complex metabolic pathway that is energetically costly in comparison to processing of other nitrogenous wastes such as urea (from the urea cycle) or ammonia, but has the advantages of reducing water loss and preventing dehydration.[22]
Invertebrates
Platynereis dumerilii, a marine polychaete worm, uses uric acid as a sexual pheromone. The female of the species releases uric acid into the water during mating, to induce males to release sperm.[23]
Genetics
Although foods such as meat and seafood can elevate serum urate levels, genetic variation is a much greater contributor to high serum urate.[24][25] A proportion of people have mutations in the urate transport proteins responsible for the excretion of uric acid by the kidneys. Variants of a number of genes, linked to serum urate, have so far been identified: SLC2A9; ABCG2; SLC17A1; SLC22A11; SLC22A12; SLC16A9; GCKR; LRRC16A; and PDZK1.[26][27][28] GLUT9, encoded by the SLC2A9 gene, is known to transport both uric acid and fructose.[19][29][30]
Clinical significance and research
In human blood plasma, the reference range of uric acid is typically 3.4–7.2 mg per 100 mL(200–430 μmol/L) for men, and 2.4–6.1 mg per 100 mL for women (140–360 μmol/L).[31] Uric acid concentrations in blood plasma above and below the normal range are known as, respectively, hyperuricemia and hypouricemia. Likewise, uric acid concentrations in urine above and below normal are known as hyperuricosuria and hypouricosuria. Uric acid levels in saliva may be associated with blood uric acid levels.[32]
High uric acid
Hyperuricemia (high levels of uric acid), which induces gout, has various potential origins:
- Diet may be a factor. High intake of dietary purine, high-fructose corn syrup, and sucrose can increase levels of uric acid.[33][34]
- Serum uric acid can be elevated by reduced excretion via the kidneys.[35]
- Fasting or rapid weight loss can temporarily elevate uric acid levels.[36]
- Certain drugs, such as thiazide diuretics, can increase blood uric acid levels by interfering with renal clearance.[37]
- Tumor lysis syndrome, a metabolic complication of certain cancers or chemotherapy, due to nucleobase and potassium release into the plasma.[38]
Gout
A 2011 survey in the United States indicated that 3.9% of the population had gout, whereas 21.4% had hyperuricemia without having symptoms.[39]
Excess blood uric acid can induce gout,[40] a painful condition resulting from needle-like crystals of uric acid precipitating in joints, capillaries, skin, and other tissues.[41] Gout can occur where serum uric acid levels are as low as 6 mg per 100 mL (357 μmol/L), but an individual can have serum values as high as 9.6 mg per 100 mL (565 μmol/L) and not have gout.[18]
In humans, purines are metabolized into uric acid, which is then excreted in the urine. Consumption of large amounts of some types of purine-rich foods, particularly meat and seafood, increases gout risk.[42] Purine-rich foods include liver, kidney, and sweetbreads, and certain types of seafood, including anchovies, herring, sardines, mussels, scallops, trout, haddock, mackerel, and tuna.[43] Moderate intake of purine-rich vegetables, however, is not associated with an increased risk of gout.[42]
One treatment for gout in the 19th century was administration of lithium salts;[44] lithium urate is more soluble. Today, inflammation during attacks is more commonly treated with NSAIDs, colchicine, or corticosteroids, and urate levels are managed with allopurinol.[45] Allopurinol, which weakly inhibits xanthine oxidase, is an analog of hypoxanthine that is hydroxylated by xanthine oxidoreductase at the 2-position to give oxipurinol.[46]
Tumor lysis syndrome
Tumor lysis syndrome, an emergency condition that may result from blood cancers, produces high uric acid levels in blood when tumor cells release their contents into the blood, either spontaneously or following chemotherapy.[38] Tumor lysis syndrome may lead to acute kidney injury when uric acid crystals are deposited in the kidneys.[38] Treatment includes hyperhydration to dilute and excrete uric acid via urine, rasburicase to reduce levels of poorly soluble uric acid in blood, or allopurinol to inhibit purine catabolism from adding to uric acid levels.[38]
Lesch–Nyhan syndrome
Lesch–Nyhan syndrome, a rare inherited disorder, is also associated with high serum uric acid levels.[47] Spasticity, involuntary movement, and cognitive retardation as well as manifestations of gout are seen in this syndrome.[48]
Cardiovascular disease
Hyperuricemia is associated with an increase in risk factors for cardiovascular disease.[49] It is also possible that high levels of uric acid may have a causal role in the development of atherosclerotic cardiovascular disease, but this is controversial and the data are conflicting.[50]
Type 2 diabetes
Hyperuricemia may be a consequence of insulin resistance in diabetes rather than its precursor.[51] One study showed high serum uric acid was associated with higher risk of type 2 diabetes, independent of obesity, dyslipidemia, and hypertension.[52] Hyperuricemia is associated with components of metabolic syndrome, including in children.[53][54]
Uric acid stone formation
Kidney stones can form through deposits of sodium urate microcrystals.[55][56]
Saturation levels of uric acid in blood may result in one form of kidney stones when the urate crystallizes in the kidney. These uric acid stones are radiolucent, so do not appear on an abdominal plain X-ray.[57] Uric acid crystals can also promote the formation of calcium oxalate stones, acting as "seed crystals".[58]
Low uric acid
Low uric acid (hypouricemia) can have numerous causes. Low dietary zinc intakes cause lower uric acid levels. This effect can be even more pronounced in women taking oral contraceptive medication.[59] Sevelamer, a drug indicated for prevention of hyperphosphataemia in people with chronic kidney failure, can significantly reduce serum uric acid.[60]
Multiple sclerosis
Meta-analysis of 10 case-control studies found that the serum uric acid levels of patients with multiple sclerosis were significantly lower compared to those of healthy controls, possibly indicating a diagnostic biomarker for multiple sclerosis.[61]
Normalizing low uric acid
Correcting low or deficient zinc levels can help elevate serum uric acid.[62]
See also
- Theacrine or 1,3,7,9-tetramethyluric acid, a purine alkaloid found in some teas
- Uracil – purine nucleobase named by Robert Behrend who was attempting to synthesize derivatives of uric acid
References
- ^ Scheele, C. W. (1776). "Examen Chemicum Calculi Urinari" [A chemical examiniation of kidney stones]. Opuscula. 2: 73.
- ^ Horbaczewski, J. (1882). "Synthese der Harnsäure" [Synthesis of uric acid]. Monatshefte für Chemie und Verwandte Teile Anderer Wissenschaften. 3: 796–797. doi:10.1007/BF01516847. S2CID 92323943.
- ^ Lieberman, M.; Marks, A. D.; Smith, C. M.; Marks, D. B. (2007). Marks' Essential Medical Biochemistry. Philadelphia: Lippincott Williams & Wilkins. pp. 47–. ISBN 978-0-7817-9340-7.
- ^ Ringertz, H. (1 March 1966). "The molecular and crystal structure of uric acid". Acta Crystallographica. 20 (3): 397–403. doi:10.1107/S0365110X66000914.
- ^ Jiménez, V.; Alderete, J. B. (November 2005). "Theoretical calculations on the tautomerism of uric acid in gas phase and aqueous solution". Journal of Molecular Structure: THEOCHEM. 755 (1–3): 209–214. doi:10.1016/j.theochem.2005.08.001.
- ^ McCrudden, F. H. (2008) [1905]. Uric Acid: The Chemistry, Physiology and Pathology of Uric Acid and the Physiologically Important Purin Bodies, with a Discussion of the Metabolism in Gout. Charleston, SC: BiblioBazaar. ISBN 978-0-554-61991-0.
- ^ Weast, R. C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. OCLC 7842683.
- ^ Windholz, M., ed. (1976). Merck Index (9th ed.). Merck. ISBN 978-0-911910-26-1.
- ^ McCrudden, Francis H. Uric acid. p. 58.
- ^ Ichida, K.; Amaya, Y.; Noda, K.; Minoshima, S.; Hosoya, T.; Sakai, O.; Shimizu, N.; Nishino, T. (November 1993). "Cloning of the cDNA encoding human xanthine dehydrogenase (oxidase): Structural analysis of the protein and chromosomal location of the gene". Gene. 133 (2): 279–284. doi:10.1016/0378-1119(93)90652-J. PMID 8224915.
- ^ Hille, R. (2005). "Molybdenum-containing hydroxylases". Archives of Biochemistry and Biophysics. 433 (1): 107–116. doi:10.1016/j.abb.2004.08.012. PMID 15581570.
- ^ Hori, N.; Uehara, K.; Mikami, Y. (1992). "Enzymatic Synthesis of 5-Methyluridine from Adenosine and Thymine with High Efficiency". Bioscience, Biotechnology, and Biochemistry. 56 (4): 580–582. doi:10.1271/bbb.56.580. PMID 27280651.
- ^ Baillie, J. K.; Bates, M. G.; Thompson, A. A.; Waring, W. S.; Partridge, R. W.; Schnopp, M. F.; Simpson, A.; Gulliver-Sloan, F.; Maxwell, S. R.; Webb, D. J. (May 2007). "Endogenous urate production augments plasma antioxidant capacity in healthy lowland subjects exposed to high altitude". Chest. 131 (5): 1473–1478. doi:10.1378/chest.06-2235. PMID 17494796.
- ^ Angstadt, C. N. (4 December 1997). "Purine and Pyrimidine Metabolism: Purine Catabolism". NetBiochem.
- ^ Proctor, P. (November 1970). "Similar functions of uric acid and ascorbate in man?". Nature. 228 (5274): 868. Bibcode:1970Natur.228..868P. doi:10.1038/228868a0. PMID 5477017. S2CID 4146946.
- ^ Maxwell, S. R. J.; Thomason, H.; Sandler, D.; Leguen, C.; Baxter, M. A.; Thorpe, G. H. G.; Jones, A. F.; Barnett, A. H. (1997). "Antioxidant status in patients with uncomplicated insulin-dependent and non-insulin-dependent diabetes mellitus". European Journal of Clinical Investigation. 27 (6): 484–490. doi:10.1046/j.1365-2362.1997.1390687.x. PMID 9229228. S2CID 11773699.
- ^ Braunwald, E., ed. (1987). Harrison's Principles of Internal Medicine (11th ed.). New York: McGraw-Hill. p. A-3. ISBN 978-0-07-079454-2.
- ^ a b Tausche, A. K.; Unger, S.; Richter, K.; et al. (May 2006). "Hyperurikämie und Gicht" [Hyperuricemia and gout: diagnosis and therapy]. Der Internist (in German). 47 (5): 509–521. doi:10.1007/s00108-006-1578-y. PMID 16586130. S2CID 11480796.
- ^ a b Vitart, V.; Rudan, I.; Hayward, C.; et al. (April 2008). "SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout". Nature Genetics. 40 (4): 437–442. doi:10.1038/ng.106. PMID 18327257. S2CID 6720464.
- ^ "Hyperuricosuria". StatPearls. StatPearls. 2022. PMID 32965872.
- ^ Friedman, M. & Byers, S. O. (1 September 1948). "Observations concerning the causes of the excess excretion of uric acid in the Dalmatian dog". The Journal of Biological Chemistry. 175 (2): 727–735. doi:10.1016/S0021-9258(18)57191-X. PMID 18880769.
- ^ Hazard, L. C. (2004). Sodium and Potassium Secretion by Iguana Salt Glands. University of California Press. pp. 84–85. ISBN 978-0-520-23854-1.
{{cite book}}:|work=ignored (help) - ^ Zeeck, E.; Harder, T.; Beckmann, M. (1998). "Uric acid: the sperm-release pheromone of the marine polychaete Platynereis dumerilii". Journal of Chemical Ecology. 24 (1): 13–22. doi:10.1023/A:1022328610423. S2CID 42318049.
- ^ Major, T. J.; Topless, R. K.; Merriman, T. R. (2018). "Evaluation of the diet wide contribution to serum urate levels: meta-analysis of population based cohorts". The BMJ. 363: k3951. doi:10.1136/bmj.k3951. PMC 6174725. PMID 30305269.
- ^ Keenan, R. T. (2020). "The biology of urate". Seminars in Arthritis and Rheumatism. 50 (35): S2–S10. doi:10.4103/bc.bc_1_19. PMC 6611195. PMID 32620198.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Aringer, M.; Graessler, J. (December 2008). "Understanding deficient elimination of uric acid". Lancet. 372 (9654): 1929–1930. doi:10.1016/S0140-6736(08)61344-6. PMID 18834627. S2CID 1839089.
- ^ Kolz, M.; Johnson, T.; et al. (June 2009). Allison, David B. (ed.). "Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations". PLOS Genet. 5 (6): e1000504. doi:10.1371/journal.pgen.1000504. PMC 2683940. PMID 19503597.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Köttgen, A.; et al. (February 2013). "Genome-wide association analyses identify 18 new loci associated with serum urate concentrations" (PDF). Nature Genetics. 45 (2): 145–154. doi:10.1038/ng.2500. PMC 3663712. PMID 23263486.
- ^ Döring, A.; Gieger, C.; Mehta, D.; et al. (April 2008). "SLC2A9 influences uric acid concentrations with pronounced sex-specific effects". Nature Genetics. 40 (4): 430–436. doi:10.1038/ng.107. PMID 18327256. S2CID 29751482.
- ^ Mandal, Asim K.; Mount, David B. (February 2015). "The molecular physiology of uric acid homeostasis". Annual Review of Physiology. 77: 323–345. doi:10.1146/annurev-physiol-021113-170343. PMID 25422986.
- ^ "Harmonisation of Reference Intervals" (PDF). Pathology Harmony (UK). Archived from the original (PDF) on 2 August 2013. Retrieved 13 August 2013.
- ^ Zhao, J; Huang, Y (2015). "Salivary uric acid as a noninvasive biomarker for monitoring the efficacy of urate-lowering therapy in a patient with chronic gouty arthropathy". Clinica Chimica Acta. 450: 115–20. doi:10.1016/j.cca.2015.08.005. PMID 26276048.
- ^ Cirillo, P.; Sato, W.; Reungjui, S.; et al. (December 2006). "Uric acid, the metabolic syndrome, and renal disease" (PDF). Journal of the American Society of Nephrology. 17 (12 Suppl. 3): S165–S168. doi:10.1681/ASN.2006080909. PMID 17130256.
- ^ Angelopoulos, T. J.; Lowndes, J.; Zukley, L.; Melanson, K. J.; Nguyen, V.; Huffman, A.; Rippe, J. M. (June 2009). "The Effect of High-Fructose Corn Syrup Consumption on Triglycerides and Uric Acid". The Journal of Nutrition. 139 (6): 1242S–1245S. doi:10.3945/jn.108.098194. PMID 19403709.
- ^ "High uric acid level". Mayo Clinic. 11 September 2010. Retrieved 24 April 2011.
- ^ Howard, A. N. (1981). "The historical development, efficacy and safety of very-low-calorie diets". International Journal of Obesity. 5 (3): 195–208. PMID 7024153.
- ^ "Diuretic-Related Side Effects: Development and Treatment". Medscape. Retrieved 17 May 2013.
- ^ a b c d Howard, S. C.; Jones, D. P.; Pui, C.-H. (12 May 2011). "The Tumor Lysis Syndrome". The New England Journal of Medicine. 364 (19): 1844–1854. doi:10.1056/NEJMra0904569. ISSN 0028-4793. PMC 3437249. PMID 21561350.
- ^ Li, R.; Yu, K.; Li, C. (2018). "Dietary factors and risk of gout and hyperuricemia: a meta-analysis and systematic review". Asia Pacific Journal of Clinical Nutrition. 27 (6): 1344–1356. doi:10.6133/apjcn.201811_27(6).0022. PMID 30485934.
- ^ Heinig, M.; Johnson, R. J. (December 2006). "Role of uric acid in hypertension, renal disease, and metabolic syndrome". Cleveland Clinic Journal of Medicine. 73 (12): 1059–1064. doi:10.3949/ccjm.73.12.1059. PMID 17190309. S2CID 45409308.
- ^ Richette, P.; Bardin, T. (January 2010). "Gout". Lancet. 375 (9711): 318–328. doi:10.1016/S0140-6736(09)60883-7. PMID 19692116. S2CID 208793280.
- ^ a b Choi, H. K.; Atkinson, K.; Karlson, E. W.; Willett, W.; Curhan, G. (March 2004). "Purine-rich foods, dairy and protein intake, and the risk of gout in men". The New England Journal of Medicine. 350 (11): 1093–1103. doi:10.1056/NEJMoa035700. PMID 15014182.
- ^ "Gout diet: What's allowed, what's not". Mayo Clinic. 2 July 2020.
{{cite web}}: CS1 maint: url-status (link) - ^ Schrauzer, Gerhard N. (2002). "Lithium: Occurrence, Dietary Intakes, Nutritional Essentiality". Journal of the American College of Nutrition. 21 (1): 14–21. doi:10.1080/07315724.2002.10719188. PMID 11838882. S2CID 25752882.
- ^ "NHS Clinical Knowledge Summaries". UK National Health Service. Archived from the original on 4 March 2012.
- ^ Pacher, P.; Nivorozhkin, A.; Szabó, C. (2006). "Therapeutic effects of xanthine oxidase inhibitors: Renaissance half a century after the discovery of allopurinol". Pharmacological Reviews. 58 (1): 87–114. doi:10.1124/pr.58.1.6. PMC 2233605. PMID 16507884.
- ^ Luo, Y. C.; Do, J. S.; Liu, C. C. (October 2006). "An amperometric uric acid biosensor based on modified Ir–C electrode". Biosensors & Bioelectronics. 22 (4): 482–488. doi:10.1016/j.bios.2006.07.013. PMID 16908130.
- ^ Nyhan, W. L. (March 2005). "Lesch-Nyhan Disease". Journal of the History of the Neurosciences. 14 (1): 1–10. doi:10.1080/096470490512490. PMID 15804753. S2CID 37934468.
- ^ Borghi, C.; Verardi, F. M.; Pareo, I.; Bentivenga, C.; Cicero, A. F. (2014). "Hyperuricemia and cardiovascular disease risk". Expert Review of Cardiovascular Therapy. 12 (10): 1219–1225. doi:10.1586/14779072.2014.957675. PMID 25192804. S2CID 42023170.
- ^ Saito, Yuichi; Tanaka, Atsushi; Node, Koichi; Kobayashi, Yoshio (July 2021). "Uric acid and cardiovascular disease: A clinical review". Journal of Cardiology. 78 (1): 51–57. doi:10.1016/j.jjcc.2020.12.013. ISSN 1876-4738. PMID 33388217.
- ^ Cappuccio, F. P.; Strazzullo, P.; Farinaro, E.; Trevisan, M. (July 1993). "Uric acid metabolism and tubular sodium handling. Results from a population-based study". Journal of the American Medical Association. 270 (3): 354–359. doi:10.1001/jama.270.3.354. PMID 8315780.
- ^ Dehghan, A.; van Hoek, M.; Sijbrands E. J., Hofman A.; Witteman, J. C. (February 2008). "High serum uric acid as a novel risk factor for type 2 diabetes". Diabetes Care. 31 (2): 361–362. doi:10.2337/dc07-1276. PMID 17977935.
- ^ De Oliveira, E. P.; et al. (2012). "High plasma uric acid concentration: Causes and consequences". Diabetology & Metabolic Syndrome. 4: 12. doi:10.1186/1758-5996-4-12. PMC 3359272. PMID 22475652.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Wang, J. Y.; et al. (2012). "Predictive value of serum uric acid levels for the diagnosis of metabolic syndrome in adolescents". The Journal of Pediatrics. 161 (4): 753–6.e2. doi:10.1016/j.jpeds.2012.03.036. PMID 22575243.
- ^ Banach, K.; Bojarska, E.; Kazimierczuk, Z.; Magnowska, L.; Bzowska, A. (2005). "Kinetic Model of Oxidation Catalyzed by Xanthine Oxidase—The Final Enzyme in Degradation of Purine Nucleosides and Nucleotides". Nucleic Acids. 24 (5–7): 465–469. doi:10.1081/ncn-200060006. PMID 16247972. S2CID 42906456.
- ^ "What is Gout: What Causes Gout?". MedicalBug. 6 January 2012. Archived from the original on 2 April 2019. Retrieved 6 May 2012.
- ^ Worcester, E. M.; Coe, F. L. (2008). "Nephrolithiasis". Primary Care: Clinics in Office Practice. 35 (2): 369–391. doi:10.1016/j.pop.2008.01.005. PMC 2518455. PMID 18486720.
- ^ Pak, C. Y. (September 2008). "Medical stone management: 35 years of advances". The Journal of Urology. 180 (3): 813–819. doi:10.1016/j.juro.2008.05.048. PMID 18635234.
- ^ Hess, F. M.; King, J. C.; Margen, S. (1 December 1977). "Effect of low zinc intake and oral contraceptive agents on nitrogen utilization and clinical findings in young women". The Journal of Nutrition. 107 (12): 2219–2227. doi:10.1093/jn/107.12.2219. PMID 925768.
- ^ Garg, J. P.; Chasan-Taber, S.; Blair, A.; et al. (January 2005). "Effects of sevelamer and calcium-based phosphate binders on uric acid concentrations in patients undergoing hemodialysis: a randomized clinical trial". Arthritis and Rheumatism. 52 (1): 290–295. doi:10.1002/art.20781. PMID 15641045.
- ^ Wang, L.; Hu, W.; Wang, J.; Qian, W.; Xiao, H. (2016). "Low serum uric acid levels in patients with multiple sclerosis and neuromyelitis optica: An updated meta-analysis". Multiple Sclerosis and Related Disorders. 9: 17–22. doi:10.1016/j.msard.2016.05.008. PMID 27645338.
- ^ Umeki, S.; Ohga, R.; Konishi, Y.; Yasuda, T.; Morimoto, K.; Terao, A. (November 1986). "Oral zinc therapy normalizes serum uric acid level in Wilson's disease patients". The American Journal of the Medical Sciences. 292 (5): 289–292. doi:10.1097/00000441-198611000-00007. PMID 3777013. S2CID 39995735.