Wikipedia talk:WikiProject Chemistry: Difference between revisions
→Bystrite: new section |
Thecurran91 (talk | contribs) →Bystrite: Offer, but is it notable? |
||
| Line 379: | Line 379: | ||
Anyone knowledgeable able to clean this up? [[User:Casliber|Casliber]] ([[User talk:Casliber|talk]] '''·''' [[Special:Contributions/Casliber|contribs]]) 03:53, 6 January 2010 (UTC) |
Anyone knowledgeable able to clean this up? [[User:Casliber|Casliber]] ([[User talk:Casliber|talk]] '''·''' [[Special:Contributions/Casliber|contribs]]) 03:53, 6 January 2010 (UTC) |
||
*I'll be happy to clean it up/give it a rewrite, but is it [[Wikipedia:Notability|notable]]? I don't really have the experience to say yay or nay on this matter, although the intro says itself the mineral "has no special value or remarkable importance". [[User:Thecurran91|<font color="orange"><b>Thecurran</b></font>]]<font color="orange"><b><sup>91</sup></b></font> ([[user talk:Thecurran91|talk]]) 16:20, 6 January 2010 (UTC) |
|||
Revision as of 16:20, 6 January 2010
| This is the talk page for discussing WikiProject Chemistry and anything related to its purposes and tasks. |
|
| Archives: 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55 |
| Chemistry Project‑class | |||||||
| |||||||
| Article alerts |
|---|
|
Articles for deletion
Proposed deletions
Categories for discussion
Templates for discussion
Redirects for discussion
Featured article candidates Featured article reviews
Good article reassessments
Requests for comments
Requested moves
Articles to be merged
Articles to be split
Articles for creation
|
Discussion of the WikiProject Chemistry - Please add your comment and discussion here. Older discussions are archived.
This discussion page is about the Chemistry project itself, for detailed, in-depth discussions about specific topics, you'd be best served at the talk page of the specific subject, e.g., Chemicals, Chemical infoboxes, etc. There is also an image request page which might be of interest to you.
Bromantan vs. Bromantane
Houston, we've got a problem! --Yikrazuul (talk) 21:26, 30 July 2009 (UTC)
- Merge into one article, namely the one with the International Nonproprietary Name. Bromantane seems to be the better candidate for now. --Dirk Beetstra T C 21:47, 30 July 2009 (UTC)
may I find out where the wiki chem forum on irc is and how to join? It is tuesdays. Sbillinghurst (talk) 19:36, 6 October 2009 (UTC)
- Merge? Fvasconcellos (t·c) 21:47, 30 July 2009 (UTC)
- Merge per Beetstra. Bromantane is a long-existing article vs the quick'n'dirty new Bromantan. DMacks (talk) 21:56, 30 July 2009 (UTC)
Functional groups
I suggest that we try for some consensus on the structure and style of articles on functional groups. July statistics show that these articles are highly consulted (within Chemistry): ether at #23, ester at #28, amine at #39, ketone at #78, carboxylic acid at #121, amide at #141. So to get the conversation going, I (or others if they have inclination) will draft a manual of style within Wikipedia:Manual of Style (chemistry) and then invite other editors to comment. I had started to heavily edit amine and ketone articles, but I will desist until we have some sort of agreement on what these articles should look like. Apologies for not checking earlier.--Smokefoot (talk) 23:04, 5 August 2009 (UTC)
- That would be great! Alkane was largely translated from the German version, which is a featured article on dewiki, but it failed good article nomination: that might give you some some ideas if you're short of any (which I doubt!). Physchim62 (talk) 00:09, 6 August 2009 (UTC)
Hi Smokefoot, are you thinking of expanding Wikipedia:Manual_of_Style_(chemistry)/Compound_classes? Or a separate section altogether? --Rifleman 82 (talk) 01:53, 6 August 2009 (UTC)
- Thank you Rifleman. I completely overlooked that category, which should cover functional groups. The details of this particular MOS might be further edited bearing in mind the model of alkane, as pointed out by Physchim. Some debate may arise about the extent that we want bulleted or tabulated lists of reactions (these things all undergo many reactions and are formed by many reactions) vs the same information presented in paragraph form. Bulleted lists are easier for named reactions, but my sense is that paragraphs are more explanatory.--Smokefoot (talk) 04:52, 6 August 2009 (UTC)
- Wow, thanks for raising this! I've long felt that these articles were important but neglected, but I hadn't really appreciated that they were so popular with our users. I'll try to contribute. Walkerma (talk) 06:42, 6 August 2009 (UTC)
Good idea to overhaul these articles. The things I always wanted to know when learning functional groups were:
- what is the general connectivity of the FG (with R groups)?
- can any of those R groups be hydrogen, or would that make it a different FG?
- one or more simple examples (usually R = ethyl, methyl or phenyl)
- basic nomenclature with simple examples (e.g. [alkyl] + [carboxylate] for esters, example: ethyl acetate)
- 3D structure with reasons (usually v. relevant to reactivity as well), e.g.:
- amines are pyramidal due to lone pair on N
- amides are planar since lone pair is delocalised into electron-withdrawing carbonyl group
- N in anilines can range from pyramidal to planar as ring becomes more electron withdrawing
Ben (talk) 09:49, 6 August 2009 (UTC)
- it is so rewarding to read that the functional group pages appear neglected and need to be overhauled. Please bear in mind that the synthesis / reaction sections are linked from the Organic_reaction#Organic_reactions_by_functional_groups. The relationship between the functional group pages and the organic reaction page should be preserved. For some reason bullets are not popular within Wiki (why!). Tables are better than prose (it is still a list and trying to get to connect the dots will soon run into original research). I have constructed a table in the amine page so you can see for yourself what it would look like. V8rik (talk) 17:38, 6 August 2009 (UTC)
- Well tables of reactions might work, although I was dead set on the prose approach. If we go with tables, here are two challenges: (i) risk of undue weight to minor methods and (ii) risk of undue emphasis on "named reactions" that might scare off novice readers. One imperfect solution is to preface the Table (as is currently in amines) with a paragraph of prose overview. Another problem of course is that the artwork within the tables will eventually need to be more standardized to minimize the need for scrolling. It would be nice if we had pop-up art (e.g. that expanded with a mouse-over) and if we could upload editable chemdraw files (or can we?).--Smokefoot (talk) 02:19, 7 August 2009 (UTC)
- I'm not sure I agree with V8rik. Lists tend to attract a lot of trivia, and become redundant, repetitive, or untidy after a while. Sodium bicarbonate is one such example.[1] I personally feel that thematically similar reactions should be grouped together in prose, with examples as reaction schemes. This is more compact, and helps ensure that at least a little is said about each example. For the example of amine, perhaps the Delepine reaction, Gabriel synthesis, and maybe the benzylamine route be grouped together, because they all similarly involve the introduction of the amine group by alkylating a reagent, with the primary amine obtained by hydrolysis. For benzylamine, hydrogenation. --Rifleman 82 (talk) 02:06, 7 August 2009 (UTC)
- I agree collections of reactions (alkylations, reductions, isocyanate chem) could be aggregated into paragraphs that draw parallels and contrasts, and by definition, prose is the way to explain things. Wikipedia-chem, however, has a tradition of lists and tables. Not my cup of tea, but it's an evolutionary phase and democratic because amassing factoids invites participation, which is good. Eventually the information will be integrated and explained with increased use of prose.--Smokefoot (talk) 13:26, 7 August 2009 (UTC)
- agree with Smokefoot, why not start with proze and round up with an infobox (table listing all reactions). I rarely make claims in my edits about what is important and what is not (on whose authority?). If you see 5 items listed in a table , why would you infer these items are of equal importance? No need for artwork in the tables: keep that confined to chemical reaction page. Lists perhaps attract trivia but prose certainly makes content vulnerable to deletion (we need to break out introductory chemistry and advance into contemporary chemistry). I have changed the amine synthesis section a bit to get an idea what it would look like V8rik (talk) 16:15, 7 August 2009 (UTC)
I think this is close to GA status once the intro is expanded. Anyone want to help in make it a GA nominee? Burningview (talk) 15:01, 6 August 2009 (UTC)
Other Names
I recently created the article Dihydrocortisone and when I added its other names to the chembox, I saw its really hard to see where the first name ends and the second name starts (exept for a small space between them). I couldnt use a comma because the names already have commas in them so I used a semi colon but its still confusing and hard to see the different names, any suggestions? Pikiwyn talk 20:28, 7 August 2009 (UTC)
- For this particular case, I would suggest deleting the latter of the two "other names" that you added. The two names are equivalent: the second has an additional stereochemical descriptor - "17α" instead of just "17" - but this is unnecessary as the substituant at carbon-17 in a substituted pregnane must always go alpha. Incidentally, it is the name as a substituted pregnane which is the IUPAC name, not the name as a substituted phenanthrene.
- In general, there are no fixed guidelines for separating names on a list like this one in the infobox. Personally, I like to separate them with
<br/>tags, but sometimes this makes the list too long. The semi-colon solution you chose would be fine, except I think there's an even better solution. Physchim62 (talk) 09:52, 8 August 2009 (UTC)- I just thought of using a * infront of each name instead is that fine? Pikiwyn talk 15:28, 8 August 2009 (UTC)
Stubs
Hello Pikiwyn. I have observed that you have created a lot of hard-core stubs (e.g. Chlorostyrene; Chloroxuron; Chlorthiamide and so on...) Though it is nice to gain more articles here on Wiki, those stubs are impo not informative. Even the CAS is not listed, what are the substances used for, how can one make them. Many gaps are not close. Just writing e. g. "Chlorthiamide is an organic compound with the chemical formula C7H5Cl2NS." is not sufficiant. On de-Wiki, we would delete them cos they are not anything close to an "article".
First I thought you were a BOT, just pasting some ChemSpider Infos here on Wiki. This is - btw - not good, since on ChemSpider they calculate some physical data. If this is true or not, well, nobody knows. It would be very, very beneficial if you wrote something more about those "organic compounds". Thanks, --Yikrazuul (talk) 14:11, 9 August 2009 (UTC)
- Sorry, I was trying to help by creating pages for the "well known" chemicals on the list of organic compounds that didn't have one yet. I was hoping that other people would edit my articles later and that they'd become more informative, but I'll try find more information and add it to the articles. (by the way I'm not a bot) Pikiwyn talk 15:36, 9 August 2009 (UTC)
something to work on
I am not in this WikiProject, but I realized the page Iodotrifluoroethylene is extremly small.--JordanITP (talk) 20:14, 12 August 2009 (UTC)
Frost diagrams
FROST DIAGRAMS: This text from the first sentence is wrong and actually refers to "Pourbaix-diagrams": "A Frost diagram is an Oxidation State Free Energy Diagram (O.S.F.E), also known as an Eh-pH diagram". Instead a Frost-diagram is a plot of oxidation-numbers against oxidation-numbersXstandard potential? I amn not a specialist however... Mats —Preceding unsigned comment added by 130.238.7.35 (talk) 09:06, 18 August 2009 (UTC)
- The term "Frost diagram" seems more used in German than in English, although the diagrams themselves are used in English-language textbooks such as Greenwood & Earnshaw. I once had a Swiss-German boss who couldn't believe that I didn't know the name for the type of diagram I'd just shown on the screen! They are different from Pourbaix diagrams, and are very useful for showing the variation in oxidizing power as you descend a group. Physchim62 (talk) 18:54, 18 August 2009 (UTC)
- I also didn't know these diagrams had a name. For a moment I thought you were talking about the Frost mnemonic... --Itub (talk) 15:48, 19 August 2009 (UTC)
Molecular-weight redirect pages
First time I've seen this type of thing:
Strikes me as highly unlikely search-terms. DMacks (talk) 19:17, 20 August 2009 (UTC)
- In Chemistry laboratories, researchers could seek those informations. Nono64 (talk) 19:21, 20 August 2009 (UTC)
- I'm a big fan of redirects and follower of the "redirects are cheap" philosophy, but these are pushing it... Even if we assume for a moment that they are useful, there's the issue of significant figures, which means that instead of one redirect per molecular weight you need several. In short, redirects are not the best tool for doing "molecular weight searches". --Itub (talk) 19:42, 20 August 2009 (UTC)
- Agree with Itub. If there is an interest for this type of information, we could try to create a "molecular weight index" to Wikipedia but, for the time being, I'm not sure that that's useful. Exact molecular weights can be calculated routinely and simply using free software, so I am not convinced there is a real need. Physchim62 (talk) 19:51, 20 August 2009 (UTC)
- I'm a big fan of redirects and follower of the "redirects are cheap" philosophy, but these are pushing it... Even if we assume for a moment that they are useful, there's the issue of significant figures, which means that instead of one redirect per molecular weight you need several. In short, redirects are not the best tool for doing "molecular weight searches". --Itub (talk) 19:42, 20 August 2009 (UTC)
- Agree also. I am against those kind of redirs. One could then also start redirecting melting points. And I doubt that any lab will use wikipedia in order to search for a mass in that way. --Yikrazuul (talk) 19:48, 29 August 2009 (UTC)
- As the number is available in the chemboxes, I would expect that a google or Wikipedia search might come up with the answer as well. Does not seem too useful (next step: disambig pages for the molecular weight of Ethanol and Dimethyl ether?). --Dirk Beetstra T C 19:55, 20 August 2009 (UTC)
Image of single pentacene molecule
A team at IBM has imaged a single pentacene molecule creating some nice pretty pictures.[2][3] Do you guys think we should ask them nicely if we can use the image on the pentacene page? They have released it to the media after all, but I'm not sure what the procedure is for asking the copyright holders permission to use it here. Meodipt (talk) 11:54, 28 August 2009 (UTC)
chemical bonds? c=c c-c c-h
it's great to have chemistry (organic and inorganic), but some of us out here also just want more of an introduction. i'm having a hard time finding how chem bonds form. i took chemistry and remember how bonds work to begin with. i know a covalent bond shares 2 electrons, but not how it happens?
will still look, but can someone help on wikipedia? or do you have it already and i can't find it?
thanks much, alexa NYC 98.14.164.155 (talk) 15:49, 31 August 2009 (UTC)
Nanotechnology
I read a while ago in the Signpost that you guys were looking for more expertise in nanotechnology. How much response have you had yet? I've been editing the nanotech articles myself over the past three years or so; I've had some thoughts about starting a nanotech task force but haven't quite gotten to doing the necessary footwork yet. How many others here have an interest in nanotechnology? Has any planning been discussed for how best to improve the nano articles? Antony-22 (talk) 05:19, 1 September 2009 (UTC)
Attention needed
For the reasons given at User talk:Materialscientist#Another one that might interest you, attention is needed to Multiangle Light Scattering (MALS) and Differential Light Scattering (DLS). Uncle G (talk) 14:19, 2 September 2009 (UTC)
Sugar and sucrose
We ought to revisit the merger of sugar and sucrose. Almost all of sugar is about sucrose, as indicated by the opening paragraph and to the final bit. Parts that are not about sucrose duplicate the content in carbohydrates (saccharide redirects there). So readers are not getting the best presentation, and editors are duplicating effort. I worry also that we are confusing readers. I understand that many editors are wary of merges, but this sugar-carbohydrate-sucrose overlap appears to be a serious disservice. We'd leave carbohydrate alone. Based on the current sizes of the articles, I would propose to shift sucrose content to sugar.--Smokefoot (talk) 13:32, 3 September 2009 (UTC)
Digit grouping style (notice of discussion)
In case anyone is interested, a discussion about digit grouping styles is taking place at Village Pump (policy), related to this question:
On Wikipedia, should the selection of digit grouping styles depend upon regional and topical conventions used in the English language?
Please refer to that page for details and discussion. TheFeds 04:30, 9 September 2009 (UTC)
Combinatorial Chemistry
The Combinatorial Chemistry page is both very brief and limited almost exclusively to pharmaceutical chemistry. The part on Materials Science is only a two-sentence paragraph. I'm a newly registered Wiki user and I could certainly add extensively to that section. There are significant books out like Combinatorial Materials Science (Narasimhan et al, Wiley-Interscience, 2007. Jncawse (talk) 18:13, 11 September 2009 (UTC)
- Thanks! That page is so important, yet it's in pretty poor shape. Your contributions would be very welcome - just be sure to cite your sources. Leave a message here or on my talk page if you need any help - though it's not an area I'm really knowledgable on. Cheers, Walkerma (talk) 18:56, 11 September 2009 (UTC)
Merger proposal
I've proposed a merge of A type proanthocyanidin into proanthocyanidin, please see my reasoning here. Thanks Smartse (talk) 18:33, 19 September 2009 (UTC)
Strange editors and strange content
We have a group of closely inter-related editors (possibly the same person) who are writing strange or ultra-specialized articles.
- strange editors with similar interests: User:Whitenob, User:Jimjarvis, User:Tonyrail.
- strangeness created: Symbiosis (chemical), Nyholm-Rail reaction, Anthony Nicholl Rail.
My guess is that these people are Anthony Rail or are connected to him. Rail made an interesting (now unimportant) discovery during his PhD work in the early 60's working for R.S. Nyholm, the biography of whom two of these users have contribute glowing and detailed material. --Smokefoot (talk) 19:07, 19 September 2009 (UTC)
- Anthony Nicholl Rail seems like a strong candidate for AfD. In fact, so do Symbiosis (chemical), Nyholm-Rail reaction. None of these topics get many google hits; none of the references in Rail's bio are actually about him; the term "chemical symbiosis" is not really is use as far as I'm aware (but I could be wrong); the named reaction is not listed in my Merck Manual, nor does get any google scholar or google books hits. If someone AfD's these, please post a note here. Yilloslime TC 19:22, 19 September 2009 (UTC)
- "Symbiosis" could probably be merged into HSAB theory; "antisymbiosis" is simply the trans effect. I've never heard either term used. Nyholm–Rail reaction is another term that seems to have passed me by, dispite many years of RL research in similar fields. You'd be lucky to get a publication out of it these days, unless you could show some special significance: metal-mediated carbon-pnictogen bond cleavage in complexes of alkylphosphines and -arsines is well known, and a review on its significance in the deactivation of homogeneous catalysts appeared in 1985 (Chem. Rev. 85:171). Physchim62 (talk) 21:54, 19 September 2009 (UTC)
- Anthony Nicholl Rail seems like a strong candidate for AfD. In fact, so do Symbiosis (chemical), Nyholm-Rail reaction. None of these topics get many google hits; none of the references in Rail's bio are actually about him; the term "chemical symbiosis" is not really is use as far as I'm aware (but I could be wrong); the named reaction is not listed in my Merck Manual, nor does get any google scholar or google books hits. If someone AfD's these, please post a note here. Yilloslime TC 19:22, 19 September 2009 (UTC)
- One might add Urban thermal plume. Somebody needs to have a friendly word, because many of his contributions are useful (especially the historical ones) but the scientific and glodal warming ones veer towards self-promotion. Chris (talk) 05:54, 20 September 2009 (UTC)
What is the case with Template reaction. I now it in a different meaning, like in the article doi:10.1016/S0957-4166(99)00174-3--Stone (talk) 21:51, 20 September 2009 (UTC)
- Well User:Whitenob is responding, so we are making progress. As Itub suggested somewhere chemical symbiosis is connected to HSAB theory. The Template reaction is well known in coordination chemistry, e.g. metal-promoted macrocyclizations, so that article can be morphed and modernized. And thanks for Physchim62 for locating the review on P-C bond degradation, pertinent to the organoAs gymnastics. The contributions above were all well intentioned, although still strange. --Smokefoot (talk) 22:10, 20 September 2009 (UTC)
Since Yilloslime asked: chemical symbiosis was nominated for deletion; I posted my comments there. --Itub (talk) 17:36, 21 September 2009 (UTC)
- I've closed the AfD. Tim Vickers (talk) 19:23, 23 September 2009 (UTC)
Real? Accurate? Tim Vickers (talk) 17:17, 23 September 2009 (UTC)
- Looks like BS to me, but I don't have SciFinder access and my inorganic textbooks are elsewhere so I can't check for sure. Wouldn't Si + HOCl give sand and HCl is they reacted at all? Yilloslime TC 17:59, 23 September 2009 (UTC)
- And seems unusual at best for an active metal to reduce Si4+ to Si rather than redoxing with the hypochlorite! SciFinder doesn't find it by name or by chemical formula. DMacks (talk) 18:19, 23 September 2009 (UTC)
- I was able to look at the cited textbook on Amazon.com. "Silicon hypochlorite" only appears in the index where it refers to a page that discusses sodium hypochlorite - seems to just be a typo. Silicon hypochlorite does not appear in Chemical Abstracts, so it is not likely to have ever appeared in the chemical literature. Hoax or mistake, it should be deleted, so I have proposed deletion. -- Ed (Edgar181) 20:04, 23 September 2009 (UTC)
- Thanks. Tim Vickers (talk) 20:25, 23 September 2009 (UTC)
- I was able to look at the cited textbook on Amazon.com. "Silicon hypochlorite" only appears in the index where it refers to a page that discusses sodium hypochlorite - seems to just be a typo. Silicon hypochlorite does not appear in Chemical Abstracts, so it is not likely to have ever appeared in the chemical literature. Hoax or mistake, it should be deleted, so I have proposed deletion. -- Ed (Edgar181) 20:04, 23 September 2009 (UTC)
- And seems unusual at best for an active metal to reduce Si4+ to Si rather than redoxing with the hypochlorite! SciFinder doesn't find it by name or by chemical formula. DMacks (talk) 18:19, 23 September 2009 (UTC)
Add that fuse was extinguished.... I've started an AfD here. Yilloslime TC 23:58, 23 September 2009 (UTC)
- I would find it really disappointing if the faulty arguments at the AFD in favor of keeping this article won out. ChemNerd (talk) 01:11, 24 September 2009 (UTC)
- Those...are some really scarily-confused comments for keep! DMacks (talk) 02:25, 24 September 2009 (UTC)
Dictionary of chemical formulas/E
Dictionary of chemical formulas/E has been prodded for deletion. 76.66.197.30 (talk) 05:33, 28 September 2009 (UTC)
'Tiz the season: Nobel predictions?
To the successful guesser of anyone getting the 2009 Nobel for Chemistry, I pledge to find one obscure DOI. ISI Thompson ([[4]]) predicts Michael Grätzel. --Smokefoot (talk) 01:22, 6 October 2009 (UTC)
- I'm going to go for Zoltan G. Hajos – aka Zghajos (talk · contribs) – as the first Wikipedian to win a Nobel Prize, along with Benjamin List (only 41 years old, might count against him), for their work on organocatalysis. It the committee were really cunning, they could find a way to include Henri B. Kagan on the list, hence closing the controversy over the 2001 Prize. Physchim62 (talk) 08:15, 6 October 2009 (UTC)
- Well, my vote is for Self-assembled monolayers by Nuzzo and Whitesides.--Smokefoot (talk) 01:48, 7 October 2009 (UTC)
Atomic spectra
People here might be interested in this proposal which has come up at WP:PHYS to create images of atomic spectra. I'm not sure that it is feasible in the way that the proposer states it, but it is an interesting problem of relevance to chemistry. Physchim62 (talk) 11:48, 9 October 2009 (UTC)
FYI - My Graduate Physical Organic Class is Editing Chemistry Articles (Again)
Just an FYI - I am continuing to implement the editing of Wikipedia into my graduate course. This year I have made several changes to the project. One notable change is that the students will work on editing the site in their "sandbox" and only put it live/online in early December after getting approval from me and the graduate student instructor. A second major change is that I'm asking the students to add a "general" introductory paragraph explaining the concept to a more general (non-scientific) audience.
Here are the sites being edited:
- Host-guest_chemistry
- Hammett Equation (adding Swain-Lupton and other modifications)
- KIE Kinetic Isotope Effects (adding information on tunneling)
- Transannular_strain
- Walsh Diagrams - New Site!
- Salt_bridge_(protein) (modify to include other types of salt bridges, thermodynamics, etc)
- Cheletropic_reaction
- Hypervalent_molecule
- A_value
- Allylic_strain
- Self-healing_material (chemistry behind self-healing process, particularly using non-covalent interactions)
- Solvent and Solvent Effects
Questions/comments/concerns to Ajm mich (talk) 16:59, 12 October 2009 (UTC)
- Ha! finally the Walsh article! With respect to the other articles , will the focus be on expanding the articles or replacing the articles? V8rik (talk) 19:55, 12 October 2009 (UTC)
In most cases, the students are expanding the current articles. Ajm mich (talk) 23:02, 1 December 2009 (UTC)
Volatility, etc.
These basic and related (to Volatility (chemistry)) articles could use some help:
- Evaporation (tag asks for expert)
- Vaporization (looks decent but only one reference—from a non-hard science source)
- Boiling (needs references/cleanup)
- The disambigs Volatile and Volatility have merge? tags.
-Shootbamboo (talk) 16:09, 17 October 2009 (UTC)
Given I have been sloshing both this and iron sulfate around on my garden, I thought I'd expand it for DYK (which I have done). However I have not written chemical articles before so have no experience with infoboxes and where else to get chemical data. Input muchly appreciated :) Casliber (talk · contribs) 02:44, 19 October 2009 (UTC)
- Nifty topic. We need an article on Fe(edta), which is chemically complicated stuff because of the pH-dependent structure and redox. Eventually, one might think about renaming the article to something more specific than iron chelate. --Smokefoot (talk) 22:43, 22 October 2009 (UTC)
Polymer article names
I just noticed that Poly(methyl methacrylate) was changed to Poly (methyl methacrylate). I prefer the former, because it's what I'm used to reading in textbooks and journals. Is the latter (with a space between poly and the bracket) actually a recognised form of nomenclature, or is it made up?
Ben (talk) 18:40, 22 October 2009 (UTC)
- Well all kinds of crazy edits are made for all kinds of &%#$ reasons. I recommend renaming the article back to what most of us earthlings call the stuff.--Smokefoot (talk) 22:43, 22 October 2009 (UTC)
- Fixed. Cacycle (talk) 05:37, 23 October 2009 (UTC)
Some of you might want to check out what is going on when I try to contribute to the above mentioned article in order to boost it from its status as a "Stub". After swearing never to return, Paula Pilcher has reincarnated herself as Marie Poise.
The massive insults and blanket deletions continue, more aggressive and obsessive than ever. No one seems to mind -- except yours truly. PLEASE advise. I would sincerely like to contribute to more chemistry-related articles. I minored in Phys. Chem. @ UW, while majoring in Mat. Sci. & Engr. -- logger9 (talk) 09:55, 9 November 2009 (UTC)
The problem with your edits is that you appear to be trying to write a textbook. The article on Physical chemistry does indeed need your help, but you are not going about it the right way. Think how it would look if every bit of PChem was at the detail you have added. It would be far too long. This article should be a lead into others, many of which actually exist. Go to the talk page to discuss what you are trying to do, and let us see if we can make progress. --Bduke (Discussion) 10:26, 9 November 2009 (UTC)
- Same with your edits in organic chemistry. There is for example no need to explain what a reaction mechanism is in this page when another page called reaction mechanism already exists. Also try to use internal links and also try to add references to literature. V8rik (talk) 20:55, 9 November 2009 (UTC)
Silver updated
Hello, came across Silver and Palladium in the PR backlog. Despite the work Rifleman_82 did some time ago and others since, it needed an overhaul. The article may be a little light technically, but perhaps balanced. Another sexy picture or two might not hurt. So, any feedback before I tear into Palladium?--MornMore (talk) 09:10, 12 November 2009 (UTC)
Ergocalciferol
Can someone please fix the infobox for Ergocalciferol. Specifically, the last entry for the parameter "OtherNames" is too long, and stretches the infobox to a width greater than the width of my browser. I've placed several <br> tags for now to temporarily resolve the problem, though I'm not sure they're well-placed. The page has had this problem since last December. Mindmatrix 17:40, 13 November 2009 (UTC)
- done, that systematic name was crazy, everyone should know what ergosterol is —Preceding unsigned comment added by 65.107.1.165 (talk) 21:27, 23 November 2009 (UTC)
Sigmatropic Rearrangements
The Sigmatropic page has undergone some major revisions and has been recommended for rank and importance. It would be useful if others could read and comment on the page, and if the recommendation could be validated. Wtucker8891 (talk) 04:01, 14 November 2009 (UTC)
- The article looks very good, but ... Talk:Sigmatropic_reaction#Comments
Pronunciations
Input regarding the pronunciation of the chemical elements at Wikipedia_talk:WikiProject_Elements#Pronunciation_guides_-_getting_it_right would be appreciated. Thanks, --Cybercobra (talk) 03:44, 16 November 2009 (UTC)
Talk:Orbital hybridisation doesn't seem to have a strong consensus visible on whether to use "hybridisation" or "hybridization", and the topic has been raised. Time to get this resolved. DMacks (talk) 23:09, 16 November 2009 (UTC)
nomenclature articles
There's no article for polycyclic organics naming methods... could someone write atleast a rudimentary one up? 76.66.197.2 (talk) 08:07, 21 November 2009 (UTC)
authoritive density correction coefficient
Working in the field of custody transfer supervision I need to find a means of relating observed values to standard values for industrial sized amount of chemicals (range 10 000 l up to 10 000 000 l) this is traditionlayy done using density correction coefficemts so that observed volumes may be transformed in into the equvalent mass by adjusting the standard density to the apparent density. It is often difficult to obtain an authoritive value for the density correction coefficient and various values may be in use for the same material and even for a single parcel of one shipment one material. Would it be possible to include an authoritive density correction coefficeint with the other data listed for each chemical?
Bernard Stewart
¨¨¨¨ —Preceding unsigned comment added by 81.234.110.163 (talk) 11:16, 21 November 2009 (UTC)
- I'm not sure that we can, and certainly not for each chemical. For a start, Wikipedia doesn't offer authoritative values, our values are simply those we've found somewhere else… We try to find what we think is the best source, but that doesn't make it authoritative: other people would have to agree before it became an "authority".
- We have an article on flow measurement that illustrates the difficulties: there are many ways of measuring fluid flow, and many different density corrections. For a liquid, at the incompressible limit, you would need to know the density and the viscosity for ambient temperature work, and the coefficient of thermal expansion for low temperature work. For a gas or a supercritical fluid, forget even having a single value! Page 6 of this PDF shows the most important relationships for one design of flowmeter, but remember that different designs of meters will have different correction coefficients! Physchim62 (talk) 15:08, 21 November 2009 (UTC)
This article could use improvement. If you read a mineral handbook, you will realize that halides do not have to be simple compounds. The anion can be a charged halide molecule (polyatomic ion). Dana's System of Mineralogy and Handbook of Mineralogy are two good places to find the groups of halides. Johnny Mineralogist (talk) 04:49, 24 November 2009 (UTC)
- Not exactly sure what a "simple compound" is. Almost all inorganic halides contain polyatomic anions, the prime example being NaCl. It's a matter of perspective and to some extent semantics.--Smokefoot (talk) 01:54, 28 November 2009 (UTC)
- Halite is a simple ionic compound - mineralogical perspective. It does "not" contain polyatomic ions, rather simple Na+ and Cl+ ions arranged in a repeating cubic crystal lattice. And the halide mineral class is a mineralogical article - so we use mineralogical "semantics". Vsmith (talk) 02:10, 28 November 2009 (UTC)
Acidic oxide and oxoacid
What are the differences between the two terms acidic oxide and oxoacid? --Aushulz (talk) 15:23, 27 November 2009 (UTC)
- If you look at Acidic_oxide#Acidic_oxides_as_anhydrides, you'll see the acidic oxide is on the left, and the corresponding oxoacid is on the right. The latter is in effect the hydrated version of the former. Walkerma (talk) 17:07, 27 November 2009 (UTC)
- Now it's clear. Thank you. --Aushulz (talk) 02:58, 28 November 2009 (UTC)
request for comments: logger9 edits currently discussed at ANI
Edits by user:Logger9 have been discussed at numerous places, including this page (above, section "Physical Chemistry"). Now the conflict has reached a place where binding decisions can be taken: the admin noticeboard. At the end of the long thread Wikipedia:ANI#Repeated_Reverts_at_Solid, User:Xxanthippe has made the following proposal:
- I have expressed the view before that the edits of logger9 are of indifferent quality. They demonstrate little ability to synthesise the material into an effective overview and to distinguish between relevant and irrelevant material. They are sometimes verbose and rambling. While this was not important in the earlier stages of Wikipedia it is becoming more noticeable as WP matures and its overall quality improves. This is a content issue and administrative action is not needed to deal with it. It does explain, though, why other editors are attempting to improve the articles of logger9 and are becoming frustrated at the obstacles they find in doing so.
- What is more disturbing is logger9's reaction to those who attempt to develop and improve the articles that he identifies himself with on his web site http://www.wavesignal.com/. His standard operating procedure is to revert to his own version. He ignores, provokes, insults (parasite) and drives other editors away. I fear that there is only one way to deal with obsessive and recalcitrant behaviour of this sort (which unfortunately is not uncommon on WP). I suggest an indefinite ban on his editing the articles that he identifies with namely: Solid, Sol-gel, Liquid, Crystal growth, Crystal structure, Kinetic theory of solids, Transparent materials, Transparent ceramics, Ceramic engineering, Nanotechnology, Strength of glass, Physics of glass, Glass transition, Colloidal crystal, Light scattering, Spinodal decomposition, Transformation toughening, Plastic deformation in solids, Phase transformations in solids. Those of his edits that are found to be useful will be retained; those that are not can be improved without the threat of an edit war.
- An indefinite ban is not a permanent ban and when the articles have settled into a steady state after the efforts of other editors logger9 can appeal for release from the ban. Xxanthippe (talk) 11:31, 28 November 2009 (UTC).
If you want to comment, please go to Wikipedia:ANI#Repeated_Reverts_at_Solid. -- Marie Poise (talk) 16:43, 28 November 2009 (UTC)
Nitrocarboxylic acid
Nitrocarboxylic acid looks like a article for deletion or somebody must write something better than that. --Stone (talk) 21:38, 28 November 2009 (UTC)
- PROD'ed. DMacks (talk) 19:59, 14 December 2009 (UTC)
Book-class
Since this is one of the biggest WikiProject, and that a couple of Wikipedia-Books are chemistry related, could this project adopt the book-class? This would really help WikiProject Wikipedia-Books, as the WikiProject Chemistry people can oversee books like Chemistry much better than we could as far as merging, deletion, content, and such are concerned. Eventually there probably will be a "Books for discussion" process, so that would be incorporated in the Article Alerts. I'm placing this here rather than on the template page since several taskforces would be concerned.
There's an article in this week Signpost if you aren't familiar with Wikipedia-Books and classes in general. Thanks. Headbomb {ταλκκοντριβς – WP Physics} 20:43, 1 December 2009 (UTC)
- Just to let you know, I've implement the book-class for both the Chemistry Project and the Elements Project.
- As I mentionned on the Elements Projects, I've also created WP:Books/Hydrogen as an example book (took me about 4 minutes, so it's probably not perfect, and some stuff is probably irrelevant, while other is missing) based off Category:Hydrogen and Category:Isotopes of hydrogen.
- The easiest way to create books is to enable the "book-creator" (click on "Create a book" on the print/export toolbox on the left), then go to a category like Category:Helium and click on "Add this category to your book"). Give some structure (chapters), and you're pretty much done. (See Help:Books for more.)
- I think each elements could get their own books (container discovers biographies, isotopes, important reactions, ...) but there's also several topics in Chemistry that could get their own books as well. Anyway, happy-editing. If you have questions, just message me and I'll help you as best I can. Headbomb {ταλκκοντριβς – WP Physics} 06:15, 5 December 2009 (UTC)
Color of chemicals - no joke
I realize that great articles can spring from unpromising starts, but this one is pretty strange. "Optical spectroscopy," which should describes the "color of chemicals" redirects to Spectroscopy. Color could probably use a section that contains a section on "colored chemicals." Or the article on chemical compounds could have a section on their optical properties. My recommendation would be to convert Color of chemicals into an article on aquo complexes, which would feature a list of the colors of the common aquo ions, since that is in the current Color of chemicals. The metal aquo ions (e.g. [Ni(H2O)6]2+) are important, and we could explain their water exchange properties, pKa's, and possibly roles in everyday life (nutrition, corrosion, catalysis).--Smokefoot (talk) 17:05, 13 December 2009 (UTC)
- It might be a bit of an eclectic start, but I definitely think there's an article to be written here. shoy (reactions) 19:21, 13 December 2009 (UTC)
- Why would we not task spectroscopy or color to tackle this topic? I dont get it. The color of chemicals is exactly the theme of optical spectroscopy. No way around that, unless we are recommending that Wiki-chem develop two parallel tracks, articles for scientists and articles for non-scientists, both on the same subject? Seems like what we call a cop-out.--Smokefoot (talk) 20:13, 13 December 2009 (UTC)
- I agree, we should have an article about it, which goes from the very basic to something more advanced. I do think we should concentrate more on why certain chemicals are coloured, rather than just discussing chemistry which is unrelated to their colour. Physchim62 (talk) 19:47, 13 December 2009 (UTC)
- agree with Smokefoot, content belongs in color V8rik (talk) 20:40, 13 December 2009 (UTC)
As Smokefoot says, we don't have an article on optical spectroscopy, and UV/VIS spectroscopy describes the measurement technique more than anything else. I think the regular editors of color would have an issue if we just decided to devote a large section to why ions and chemical compounds appear colored. Would anyone like to suggest a better title where an article on this subject might reside? shoy (reactions) 19:35, 14 December 2009 (UTC)
Images of silicon dioxide
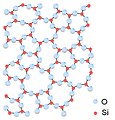
Could some other chemists please take a look at these images of silicon dioxide?
They all appear to depict silicon dioxide in which each silicon atom is connected to three oxygen atoms, where it should be four. One was marked as disputed on Commons by another editor, and I marked the others. These images have been widely used on different language projects for many years (including here at silicon dioxide, so I'm thinking that maybe I'm missing something. Are they correct? -- Ed (Edgar181) 17:39, 14 December 2009 (UTC)
- Yikes. As you infer, Si will always be tetrahedral in typical forms of SiO2. Several polymorphs are known I have heard, including interpenetrated things. The structures you show resemble As2S3. Cristobalite is a good source of images, although chemists dont typically like the format used. --Smokefoot (talk) 18:33, 14 December 2009 (UTC)
- These images are either misleading or ... As Smokefoot points out, SiO2 is tetrahedral and each silicon must have four oxygens connected for any of the polymorphs. The cristabolite images are far better, although the tetrahedrons may not appeal to chemists. The caption on the image in silicon dioxide refers to amorphous structure of tetrahedrons, but the image shows no tetrahedrons. I'll remove the image as misleading. I'd say those images should be avoided, no image is preferred to an erroneous or misleading one. Vsmith (talk) 04:14, 15 December 2009 (UTC)
- Would be nice to get a rotating 3-d image like the one at Mindat with choice of rotating unit cell, structure or polyhedra. Vsmith (talk) 04:30, 15 December 2009 (UTC)
- One could say that the images above are projections implying some invisible bonds normal to the viewing direction. The problem with drawing some complex unit cells is that a "3D" image becomes too messy. SiO2 is important and I will (kind of trying to convince myself :-) drop that bloody AIV patrol and make time to draw (or find) proper structures. Materialscientist (talk) 04:40, 15 December 2009 (UTC)
- Materialscientist is correct, they are tetrahedra, the fourth oxygen is just not visible in the current projection. Vsmith, I respect what you do here, however, if you consult many advanced text books on the structure of amorphous materials you will see structures drawn two-dimensionally in this way. It is possible to draw the structures in 3D and to show the tetrahedra fully, however as materialscientist hinted at, the unit cell for an amorphous solid is infinite so the model becomes very confusing. Besides, the current images are not misleading as long as they are described as being two-dimensional. I see you commented out the image in silicon dioxide. I added the image back in but added the fact that it is in two-dimensions, hope that is ok with you. Jdrewitt (talk) 09:45, 2 January 2010 (UTC)
- One could say that the images above are projections implying some invisible bonds normal to the viewing direction. The problem with drawing some complex unit cells is that a "3D" image becomes too messy. SiO2 is important and I will (kind of trying to convince myself :-) drop that bloody AIV patrol and make time to draw (or find) proper structures. Materialscientist (talk) 04:40, 15 December 2009 (UTC)
Some images are used in other WP's articles, e.g. nl:Glas. Are there any alternative images around? --Leyo 12:21, 23 December 2009 (UTC)
→ Commons:Commons:Deletion requests/Images of silica --Leyo 17:07, 29 December 2009 (UTC)
- As the author of the image in question for the amorphous structure of silica, the image is in two-dimensions. Of course silicon is 4-fold coordinated by oxygen in silica, however, in two-dimensions the fourth oxygen atom is hidden from view by the silicon atom. Jdrewitt (talk) 22:28, 31 December 2009 (UTC)
Perhaps someone here could help with this article on a chemist. The article creator, Hezimmerman, keeps just posing his CV. It has been explained to him repeatedly that you can't do that, and another editor and I made a two-sentence stub from some of the information in the CV. Hezimmerman seems not to be getting it though, and made a complaint today that "everytime I expand it as requested, it gets deleted". Anyone here want to take a crack at wrtiting a decent article on him and/or helping to explain WP to him? (He appears to pass our notability criterion easily.) LadyofShalott 15:45, 15 December 2009 (UTC)
- The CV seems to check out, from the small part I've verified. Howard E. Zimmerman is a photochemist, and his students have gone on in organic photochemistry. Zimmerman himself must be quite old by now, as he says he served in WW2 and many of his students seem to be close to retirement: for this reason, it is hard to quickly find Zimmerman's own publications. Physchim62 (talk) 22:08, 15 December 2009 (UTC)
New online tool for collecting ChemBox data
We have just connected a demo CGI to our portable Web sketcher at http://www.xemistry.com/edit/frame.html which we hope will be helpful for collecting chemical structure identifiers for the standard chemical infobox. Draw a compound (example: pyridine) in the sketcher, then press the "Compute Wikipedia Data" button in the form below. The tool will scan various Internet-accessible databases and format a cut&paste-ready chembox section for Wikipedia editing with the harvested data.
Feedback is appreciated! —Preceding unsigned comment added by 217.232.234.237 (talk) 20:26, 18 December 2009 (UTC)
- Looks really nice and helpful. Could you elaborate on which databases you scan for what data? Also, I'd like to mention that you display the ChEBI as "ChEBI = CHEBI:16716", IIRC, the 'CHEBI:' is there unnecessary. Thanks!!! --Dirk Beetstra T C 22:04, 18 December 2009 (UTC)
- The databases scanned are the ones listed in the chembox template - ChEBI, Chemspider, Pubchem, KEGG, Drugbank, and ESIS (for EC#). SMILES and InChI/InChIKey are computed locally by the CGI. Cas numbers are resolved by CSLS and PubChem, with an added reliability check by testing presence in commonchemistry.org. The entries in the individual databases can also conveniently recalled via the selection button left to the top text line of the sketcher - it allows you to access identifiers, database URLs or opened database result pages directly from the current contents of the drawing. It is a good idea to look at these pages for verification of the chembox results. In case the box template should get expanded, we already have the interface code for accessing EMolecules, and to compute the new IUPAC StdInChI/StdInChIKeys.
- If there is agreement, that the CHEBI: prefix should be removed in the output, this is of course trivial. Anybody with a different opinion on this? —Preceding unsigned comment added by 217.232.238.208 (talk) 09:30, 19 December 2009 (UTC)
- If it is all standard preceded by 'CHEBI:', then it is just as convenient to remove it there, and let the chembox generate the appropriate code.
- I am working myself with an off-wiki script getting and updating the identifiers in the boxes that are already there. I find many, many occasions where there are conflicting parameters (more than one CASNo as the least, completely conflicting CASNo's as the last; also multiple ChemSpiderID's are sometimes found for the 'same' compound, or there are differences in stereochemistry). What does the script do when it finds 'conflicting' parameters (for now, I press 'ignore' in my script, they need to be done by hand).
- OK, I have now removed the ChEBI prefix. As far as multiple CasNos etc are concerned: I am currently satisfied with the first found. Due to the nature of the data source (PubChem etc.) it is the one most likely to be correct (PubChem sorts names and identifiers according to a reliability rank, using for example repeat counts of identical names/IDs in multiple independent data submissions). You should (simple, because this can be done directly from the mode selector button in the sketcher) open the located database pages and cross-check the data, and omit lines you have doubts about.
- Multiple CasNos for the same compound actually do not even need to be wrong. There are cases when you have different IDs for an 'unidentified (but later to be found racemic)' vs. 'known racemic' stereo center, etc. All database queries run by this script use full stereochemistry matching where possible. Computed SMILES and InChIs, which take the data only from the current sketch, capture all specified stereochemistry, and these InChIs and SMILES are then used for further queries.
- Btw, since you are working on a batch script: Do NOT, NEVER EVER, use OpenBabel to compute SMILES and InChIs for Wikipedia. There are catastrophic stereochemistry (and, in case of SMILES, aromaticity) bugs in that software. —Preceding unsigned comment added by 217.232.238.208 (talk) 17:44, 19 December 2009 (UTC)
- I am only working from external databases, comparing 'by eye' the structures (ignoring the ones which are too difficult; see Special:Contributions/Beetstra, look for the 'script assisted update'-edits). It would be great if at some point someone could run a script which would be able to compare the images (at least those which can be machine read) with the InChI's and/or SMILES, and see where there are differences (and making lists of those). --Dirk Beetstra T C 17:58, 19 December 2009 (UTC)
We need a polymerbox!
Many polymer articles don't have an appropriate infobox. I found one with an inappropriate one too (sodium polystyrene sulfonate). It will be nice to aggregate information about these polymers in one place. Is it possible to extend the Chembox to have a polymerbox module? Or perhaps a new box which remains consistent with our Chembox? Not being a polymer chemist, I'm not very sure of what data is important, but at the very least, the formula and identifiers should be there. --Rifleman 82 (talk) 12:37, 23 December 2009 (UTC)
- On de-WP, we have Infobox Polymer (examples in use: Polyethylen, Ethylen-Propylen-Copolymer). --Leyo 13:04, 23 December 2009 (UTC)
Could this be a module in the current chembox? The main and the identifiers and properties box seem close enough (add a Tg e.g.), and some others are also appropriate (similar compounds module is useful). --Beetstra (public) (Dirk BeetstraT C on public computers) 17:08, 23 December 2009 (UTC)
Yes, would be a good idea. I'm wondering about the MW/formula, though. --Rifleman 82 (talk) 17:32, 23 December 2009 (UTC)
- I think if someone came up with a separate infobox specifically for polymers, it would end up very similar to {{chembox}} anyway. Adding a new module for polymers (or maybe just some new property parameters) to chembox seems like the best way to go to me. Properties such as
MWmolecular formula can still be accomodated: see polypropylene for an example. -- Ed (Edgar181) 18:31, 23 December 2009 (UTC)
Molecular is not a property of a polymer in the terms of the chembox, and the formula can go into the normal field for it. The rest generally should go into ranges, or be omitted as they are often dependent on Mw/Mn (not to speak of, e.g. for polypropyle, tacticity or similar). It needs some creativity, but it can be done. I think it would need a real polymer chemist (not one like me ..) to come up with some things that can't be done now (but if it can't be implemented in the chembox, then a polymer box will have the same problem), but I think our 'biggest' problem is going to be that isotactic polypropylene is not the same as atactic polypropylene which is not the same as syndiotactic polypropylene (e.g. I presume all three have different CASno's?), but that problem already exists for some cis/trans or stereoisomers as well (and the box also is not really capable to solve that problem). Someone up to adding Tg to the propertiesbox, I don't want to use my admin account here (but may change my mind). --Beetstra (public) (Dirk BeetstraT C on public computers) 22:06, 23 December 2009 (UTC)
If you could all give some feedback, I could make the bot request, and ~120 decent books would be created. Headbomb {ταλκκοντριβς – WP Physics} 17:49, 2 January 2010 (UTC)
Anyone knowledgeable able to clean this up? Casliber (talk · contribs) 03:53, 6 January 2010 (UTC)
- I'll be happy to clean it up/give it a rewrite, but is it notable? I don't really have the experience to say yay or nay on this matter, although the intro says itself the mineral "has no special value or remarkable importance". Thecurran91 (talk) 16:20, 6 January 2010 (UTC)