Wikipedia talk:WikiProject Chemistry/Archive 25
| This is an archive of past discussions on Wikipedia:WikiProject Chemistry. Do not edit the contents of this page. If you wish to start a new discussion or revive an old one, please do so on the current talk page. |
| Archive 20 | ← | Archive 23 | Archive 24 | Archive 25 | Archive 26 | Archive 27 | → | Archive 30 |
![]() Template:SynthCompoundImage has been nominated for deletion. You are invited to comment on the discussion at the template's entry on the Templates for discussion page. DH85868993 (talk) 04:56, 18 August 2012 (UTC)
Template:SynthCompoundImage has been nominated for deletion. You are invited to comment on the discussion at the template's entry on the Templates for discussion page. DH85868993 (talk) 04:56, 18 August 2012 (UTC)
Element vs substance
There is a long-lasting ambiguity about what is a WP: primary meaning of element names, such as "hydrogen" and "carbon". There are, generally, from two up to three meanings associated with an element's name:
- An element proper: its atomic properties, its uses (as elementary substance and in compounds) and chemistry.
- An elementary substance, its allotropic modifications, or even, actually, chemically different compounds, such as in carbon.
- For some elements (especially in Groups 1, 2 and 17), the third meaning actually prevails: a ion, such as Na+, Ca2+ or F−.
The current pattern is chaotic: there is a distinct article octasulfur, for example. But there is no dioxygen article, although there are such articles as dioxygen in biological reactions, triplet oxygen and singlet oxygen.
Sometimes the discussion about the chemical element is separated in the "compounds of …" article (such as compounds of zinc), but only in best cases. We can see that such articles as Potassium, Chlorine, Phosphorus and even Silicon are unduly biased towards elementary substances and atomic properties at expense of chemistry: compounds of potassium, compounds of chlorine, compounds of phosphorus and compounds of silicon are red links, not even redirects.
This confusion also leads to a nasty disparateness in meaning of some article titles. For example, Potassium in biology certainly discusses the K+ ion. But Oxygen in biology was a redirect to dioxygen in biological reactions until very recently, when I made it a disambig page.
I am sure that most notable elementary substances, such as O2, must have own articles. It would allow to absorb such marginally notable articles as "singlet oxygen", for example. Incnis Mrsi (talk) 11:06, 19 August 2012 (UTC)
R-phrase template
I have concerns about the use and accessibility of {{R-phrase}}. Please join the discussion. Andy Mabbett (Pigsonthewing); Talk to Andy; Andy's edits 11:16, 20 August 2012 (UTC)
This article was moved recently from salt metathesis reaction due to no opposition. I'm hoping that you folks can take a look at it specifically. --Rifleman 82 (talk) 14:02, 21 August 2012 (UTC)
- Thank you for catching that lapse. Only so many pages one can watch! --Smokefoot (talk) 15:19, 21 August 2012 (UTC)
File:General reaction of the oxidative deamination of an amine, catalyzed by a monoamine oxidase.jpg
File:General reaction of the oxidative deamination of an amine, catalyzed by a monoamine oxidase.jpg has been nominated for deletion -- 76.65.128.252 (talk) 06:13, 31 August 2012 (UTC)
Chemical editors might want to offer advice and editing on this new article. Rather than letting this article become a forum for politic correctness verging on original research, the community here could help the article achieve balance with good WP:SECONDARY and WP:NOTNEWS referencing. I think chemists are often skeptical of the topic because the total energy inputs are often hidden from view.--Smokefoot (talk) 13:17, 8 September 2012 (UTC)
- I sourced the article from seven peer reviewed literature reviews in academic journals from international authors, and one APS peer reviewed research report. There are some additional peer reviewed journal articles to show the automobile exaust capture concept, for example, or the most recent seawater extraction work. Further replies at Talk:Carbon neutral fuel#Comments, Sept, 2012. —Cupco 18:18, 8 September 2012 (UTC)
Working with Org. Synth.
Thanks Martin, for reminding me. I've been in touch with the folks from Org. Synth. too. We reference that "journal" (book series, really) extensively. Their recent article URLs have deviated from their old convention, reducing the functionality of Organic Syntheses {{cite journal}}: Missing or empty |title= (help).. DOIs are out because Wiley owns them. I don't think there's resolution at present, but we do have an open channel of communication now. --Rifleman 82 (talk) 15:28, 8 September 2012 (UTC)
Decision tree for IUPAC polymer nomenclature.png
file:Decision tree for IUPAC polymer nomenclature.png is missing source, author, and license. According to the edit summaries, there was a problem with copyright on a previous version? -- 76.65.128.252 (talk) 04:07, 27 August 2012 (UTC)
- See this discussion below, which relates to this image and others like it. Walkerma (talk) 05:23, 10 September 2012 (UTC)
Lead electronegativity
Hello! I'm from it.Wikipedia, and i'm here to signal this talk. I noticed that lead electronegativity is 2,33 in the most part of Wikipedias but in a lot of books it's different: 1.8, 1.9, 2.33 or 3,33 (Pauling's scale). We chose 1.8 because in our opinion, according to Enciclopedia della Chimica (chemistry encyclopedia) 2.33 and 3,33 are lead(II) & lead(IV) ions value of electronegativity. We need your opinion, thanks. Bokuwa (talk) 13:44, 9 September 2012 (UTC)
MfD nomination of Portal:Xray Crystallography
Portal:Xray Crystallography, a portal in which you may be interested, has been nominated for deletion. Your opinions on the matter are welcome; please participate in the discussion by adding your comments at Wikipedia:Miscellany for deletion/Portal:Xray Crystallography and please be sure to sign your comments with four tildes (~~~~). You are free to edit the content of Portal:Xray Crystallography during the discussion but should not remove the miscellany for deletion template from the top of the page; such a removal will not end the deletion discussion. Thank you. ···日本穣? · 投稿 · Talk to Nihonjoe · Join WP Japan! 17:12, 15 September 2012 (UTC)
Hello. Today, I deleted an attack page claiming that the subject is the inventor of this process. Both the articles have the same creator. Could anyone check it for accuracy? Thanks. Vejvančický (talk | contribs) 06:43, 21 September 2012 (UTC)
- Looks completely bogus to me (not that I’m particularly qualified to comment). The only hit I get searching the Web for “aqua elemental analysis” is that page.—Odysseus1479 (talk) 11:40, 21 September 2012 (UTC)
- The article is complete nonsense and should be deleted. ChemNerd (talk) 12:09, 21 September 2012 (UTC)
- Thank you for your opinions. My knowledge of chemistry is almost non-existent. --Vejvančický (talk | contribs) 12:25, 21 September 2012 (UTC)
IUPAC collaboration on polymer terms
I've been in discussion with someone from the IUPAC Polymer Division, and they would like to work with us over the next few months to improve the IUPAC definitions in polymer articles. My contact notes: "there are Wikipedia pages that literally give the IUPAC definition but there are also places where there are deviations." This sounds like an excellent plan to me, because some of the IUPAC polymer people are happy to do the editing themselves - they just want to make sure that their work is in line with our protocols and style guide.
They are proposing that the dispersity article is a possible model for how the definitions could be included - see this diff to see how the pages might be edited. They wish to insert a small image file containing the definition, as well as editing the text accordingly. They are concerned that text alone might be vandalised, whereas a small image is much harder to vandalise; there is also a benefit that these definitions (posted in Commons) could also be used in other language Wikipedias to align them with IUPAC definitions. I would propose that the image approach is acceptable as long as (a) the definition is also included in text (to help with text searches), (b) the image files are posted in Commons and (c) the original source is properly cited in the article references section and in the Commons file description. Does this sound to be a good approach?
I consider IUPAC definitions to be the gold standard in chemistry, and our style guide states this: "Wikipedia editors strive to be mindful of IUPAC's advice but do not follow this advice rigidly, especially when the advice deviate from mainstream usage (see comments below on nomenclature)." We have previously agreed in these pages that IUPAC definitions are a good thing to include in appropriate places, and I strongly believe that we should not be advocating definitions that are outdated or "not recommended" according to IUPAC unless we have very good reason. I'm thrilled that this group is willing to do much of the editing themselves, and I would hope that we might be lucky enough to gain a couple of permanent chemistry contributors if the project goes well.
I am happy to work with the group, but then again I'm not a polymer chemist, so please jump in and help us if you can. If the consensus is to proceed, then we'll probably start in the next month or so. I'll be sure to post the article working list here so we can all keep an eye on the changes. Walkerma (talk) 13:58, 8 September 2012 (UTC)
- Seems school teacherish approach that emphasizes IUPAC vs explaining science & technology, which is our mission. At least that is my mission vs forcing nomenclature down readers' throats (that was User:Plasmic Physics' gig) To use your example: "the use of the term polydispersity index is strongly discouraged by IUPAC because it is not an index and the term polydispersity is tautologous." This kind of wording in an article seems reproving and reliant on the bureaucratease that readers cannot understand. We're supposed to look up "tautologous"? Let's focus on the science and leave the definitions to editors who dont have anything better to do. Jeesh.--Smokefoot (talk) 15:31, 8 September 2012 (UTC)

- I glanced at that article and didn't catch that image the first time. Um yeah. I don't think it's such a great idea. Firstly, text should be rendered as text, not as an image. If their entire definition should be included (and I'm ambivalent at present), it should be rendered as a text box. Secondly, we already reference the Gold Book formally, so I'm not sure that they need the extra prominence. Specific to this article, as a bit of a polymer chemist, I don't think the term "dispersity" with D-stroke is catching on at all. --Rifleman 82 (talk) 15:45, 8 September 2012 (UTC)
- Thanks for the feedback. I thought the image boxes were small enough, but maybe we can work with text only if that is the consensus - though I do think the image boxes are more vandal-resistant. I completely agree with Smokefoot in that I hate the focus of some on rule-following rather than explaining, but that's not the debate here, IMHO - the issue is (I think) how best to insert the polymer definitions into the articles, and to work with the IUPAC people in doing that. We cannot pretend that IUPAC definitions carry no authority; they are a part of an educational article just as much as the R/S numbers and CAS numbers in a substance article. One wouldn't think of writing an article on the metre without including the SI definition, and I think the same applies to the relevant articles on polymer terms. Nevertheless, I will report here the articles that are being edited, and I think this group should have the final say on which definitions are relevant. Are there any other opinions on the definitions being in image boxes? Thanks, Walkerma (talk) 05:26, 10 September 2012 (UTC)
- I feel like the use of an image to entrench a piece of text against editing kind of goes against the open nature of Wikipedia, and is probably against some guideline somewhere. A reference or an external link to the IUPAC website would be better as a means of increasing awareness of the stable IUPAC version.
- I don’t know about a general guideline, but I’m pretty sure that images like the sample should at least be in a vector format (viz SVG). Personally I agree with Rifleman that text should be text wherever possible, for searchability if nothing else. If these items were made into templates, if it turned out they really do attract vandals the templates could be semi-protected or whatever.—Odysseus1479 (talk) 02:10, 23 September 2012 (UTC)
- I feel like the use of an image to entrench a piece of text against editing kind of goes against the open nature of Wikipedia, and is probably against some guideline somewhere. A reference or an external link to the IUPAC website would be better as a means of increasing awareness of the stable IUPAC version.
- There's also the issue that Wikipedia should be descriptive rather than prescriptive; we have make it clear if there's a difference between the widely-used term and what is formally defined. All that being said, collaborating with IUPAC to improve these articles would certainly be helpful. Antony–22 (talk⁄contribs) 01:31, 23 September 2012 (UTC)
Organic
Hi my question be about the ingredients of the organic substance If protein be a ingredient of a orange what other type of ingredients are present — Preceding unsigned comment added by 74.93.125.153 (talk) 22:48, 22 September 2012 (UTC)
- Very briefly, the main organic components aside from proteins would be carbohydrates—sugars and cellulose—with some oxoacids, esters, and the like. The main inorganic compounds present would be water and electrolyte salts.
- This isn’t an appropriate forum for general information requests: it’s mainly for discussions about editing chemistry-related articles. If you have more questions, please ask at the Science Reference Desk.—Odysseus1479 (talk) 02:31, 23 September 2012 (UTC)
See Wikipedia talk:WikiProject Chemicals#Scandium trihydride. Plasmic Physics (talk) 12:59, 28 September 2012 (UTC)
I wanted to notify this WikiProject that this article has been nominated for deletion and I feel it needs urgent attention from chemistry experts. To voice your concerns or comments, visit Wikipedia:Articles for deletion/Adamantamine. Cheers! SwisterTwister talk 21:22, 29 September 2012 (UTC)
- I tried to change this article about CAS#768-94-5 into a redirect to Amantadine (CAS#768-94-5). I dont understand the hand-wringing. --Smokefoot (talk) 04:33, 30 September 2012 (UTC)
- As mentioned at the nomination page and per Wikipedia:Guide_to_deletion#You_may_edit_the_article_during_the_discussion, the deletion template should not be removed while the article's debate is continuing as this may confuse viewers and obstruct a proper consensus. I appreciate your efforts and you are free to improve the article if you wish. SwisterTwister talk
- You actions are amazingly bureaucratic.--Smokefoot (talk) 13:00, 30 September 2012 (UTC)
University of Georgia, Graduate Chemistry Course
This class again will be working on some contributions to transition metal chemistry topics. These projects generally were successful the last time (during Fall 2010), and we're aiming for even better products this year. Note that I allow the students to choose their own topics.
Plans for 2012 entries:
- Dirhenium decacarbonyl – article expansion
- metallocene – article expansion
- tropocoronand ligand – new entry
- NiFe nitrogenase – new entry
- carbido complexes – new entry
- Z-Ligand – new entry linked to covalent bond classification
- Ligand Bond Number – new entry
- Monometallic half sandwich compound – new entry linked to metallocene
2010 entries:
- Jacobsen's catalyst – successful article expansion
- started transition metal oxo complex
- photochemical carbon dioxide reduction – new entry
- bent metallocenes – new entry (to be integrated into metallocene)
- aurophilicity – article expansion
- tuck-in complex – new entry
I welcome your feedback. Regards, ChemPunk (talk) 15:49, 1 October 2012 (UTC)
Phenylethanolamine
Another editor created Phenylethanolamine, which was quickly tagged for speedy deletion. I added a reference and removed the speedy tag, but the article is now just a stub with a page on ChEBI as the only reference. Could someone expand the article, if the article is worth expanding? Eastmain (talk • contribs) 05:14, 10 October 2012 (UTC)
I have nominated the new article dioxandrolon for deletion at Wikipedia:Articles for deletion/Dioxandrolon. Please feel free to contribute to the discussion. ChemNerd (talk) 19:30, 10 October 2012 (UTC)
A different image exists under the same name on Commons (Commons:File:Hmd-reaction.PNG), but seems to show the same thing. Since the images are a bit different, does it mean that one of them is wrong, or do they show two different valid representations of the same thing? Also, do we really need both images? Ideally, the files would, of course, be in SVG format instead of PNG. --Stefan2 (talk) 13:55, 13 October 2012 (UTC) The former color-coded one I think is correct. The reaction delivers H- to only one face of the cation. It is unfortunate that the ChemDraw settings are so poor though. It's a step in the methanogenesis process. That part of Wikipedia needs help. --Smokefoot (talk) 15:57, 13 October 2012 (UTC)
Merging of the articles polyiodide and polyhalogen ions
I have created the article polyhalogen ions, and much of the contents in the article polyiodide have already been covered in the former one. Therefore I propose either to delete the polyiodide article, or move the polyiodide-related contents from polyhalogen ions to retain it, to avoid redundancy in both articles. Anyone can give me some suggestions? LHcheM (talk) 12:36, 15 October 2012 (UTC)
- Thanks! Good work.
- Polyiodide ions are by far the most known, and especially I3- has enough notability by itself to warrant at least that article. For the other halides the ions are less known, and could be merged into one I would say. My suggestion: move/merge the section on polyiodide from the polyhalogen ions into polyiodide, leaving a section header for it, a {{main}} pointing to polyiodide, and a 3-4 sentence paragraph taking the most important points. I hope this helps. --Dirk Beetstra T C 13:42, 15 October 2012 (UTC)
Elements and molecules as separated articles
I propose to do the same change we did in Spanish wikipedia: separate elements from molecules. They are different things (one compound from the other), with different properties. The element article has an element infobox, with element's information (like mass), and each molecule of that element has a separated article with the IUPAC name, with a molecule/compound infobox and with molecule information (like mass, which is different).
So we have an article Hydrogen with the element information, the atomic mass, the reference to metallic hydrogen, etc. And on the other hand we have the dihydrogen (or molecular hydrogen, H2) with the molecular information, like the molecular mass, and the molecular properties. So, it is clear when we talk about an element and an atom, and when we talk about a molecule.
The same with the element oxygen, and the molecules like dioxygen (or molecular oxygen, O2), ozone, etc.
When put all together in one single article, it is not clear when some info refers to the element or to the most common molecule.
- I support such a move. Plasmic Physics (talk) 10:22, 17 October 2012 (UTC)
- Interesting idea for sure, but I dont support this idea. That elements and atoms/ions of the element share the same names can be the source of confusion, but only for nonchemists who have no reason to consult an article about exotica. I worry that this change would lead to the opposite problem - nonchemist-readers who want to read about oxygen end up reading about the extremely exotic oxygen atom, thinking that this has anything to do with the air they breath. Instead, I recommend that we figure out a way within each element article to have a section on the atomic form of the element (ditto for the metals, where the magnetic and electronic properties are so different). Textbook authors, who are far more experienced that our collective editorship, have voted with their pens - all forms of elements are discussed in one chapter. But I look forward to being educated by the views of others.--Smokefoot (talk) 13:35, 17 October 2012 (UTC)
- They don't have to share the same name, see atomic carbon, octasulfur, triplet oxygen.
- Having both Triplet oxygen and singlet oxygen is a bit much. I recommend that singlet oxygen is merged into triplet oxygen in the form of a section, and moving the article to Dioxygen. This would create the opportunity to move all data from Oxygen, pertaining to the dioxygen molecule be moved to Dioxygen, while leaving a summary of Dioxygen in Oxygen. Plasmic Physics (talk) 22:18, 17 October 2012 (UTC)
- I think this goes a bit far. What we know as elemental hydrogen is H2, the dihydrogen molecule. Same goes for iron, what we know as Fe is the lustrous grey metal chunks. In fact, the element box there does not only describe the element iron, it also describes the metal iron. H · is not something that you encounter in daily life, as you also do not encounter Na · or Fe (pains my brain too much to think what would be the electron configuration of a gaseous Fe atom in complete isolation at ambient conditions at this time of the morning). Let alone that we have to consider elements for which different allotropes are known at room temperature (the yellow S8, the metastable polymer Sn .. or elemental sulfur). The element pages should clearly describe how the element is encountered in daily life. The less-common allotropes can be split out, like has been done for e.g. sulfur (allotropes of sulfur - note, that octasulfur is then a fork of that seems overkill, it is just a small article, which duplicates the information in both sulfur and allotropes of sulfur, it could easily be a redirect into the allotropes article on the right section).
- So simply, to my opinion, how 'the man in the street' would think of an element, should be extensively explained in the element page itself (i.e. the forms normal or related to what would be a 'normal' human temperature range, say from the temperatures of the north pole, to about the boiling of water, this would cover e.g. the two main allotropes of tin, gaseous H2, but only the yellow octasulfur allotrope for sulfur). If there are other allotropes, too many to keep in one article, then that may need an own article (in part duplicating the main element article, but to split it out completely would not be a service to the reader). But there are no hard rules to this. --Dirk Beetstra T C 06:21, 18 October 2012 (UTC)
- We have to think outside of the box -- in the elemental hydrogen article, there is more to write about than just the diatomic molecule, like what role it plays in other molecules. The elemental article should be more general, reserving the intricate detail on the diatomic molecule for a dedicated article Dihydrogen. Of course, it only makes sense if the molecule is notable enough to warrant its own article. Consider the articles water and properties of water, I'm using the same logic. Plasmic Physics (talk) 07:48, 18 October 2012 (UTC)
- Interesting idea for sure, but I dont support this idea. That elements and atoms/ions of the element share the same names can be the source of confusion, but only for nonchemists who have no reason to consult an article about exotica. I worry that this change would lead to the opposite problem - nonchemist-readers who want to read about oxygen end up reading about the extremely exotic oxygen atom, thinking that this has anything to do with the air they breath. Instead, I recommend that we figure out a way within each element article to have a section on the atomic form of the element (ditto for the metals, where the magnetic and electronic properties are so different). Textbook authors, who are far more experienced that our collective editorship, have voted with their pens - all forms of elements are discussed in one chapter. But I look forward to being educated by the views of others.--Smokefoot (talk) 13:35, 17 October 2012 (UTC)
- I see where you are coming from here, Plasmic Physics, and for certain elements there is certainly a good reason to do so. But I would not put it into a hard rule. E.g. Sulfur. The 'common' allotrope there is the yellow S8. That is the yellow stuff, and I think that if you ask 'the man in the street', that is what they would describe sulfur to look like, and that is what I then would put into the article sulfur, not 'force' it out of there. Same for carbon, the 'common' allotropes of the pure element as one would normally encounter it, are diamond and graphite. Keep those quite far expanded in the article. It is the information that people expect to find there, they should not be forwarded through specialised links ('for more information about the allotropes of carbon, see ..'). Water does describe ice, water and steam. If there is enough left over, then surely, create an article separate for that, and for the properties of water that is the case. For dihydrogen and dioxygen, there may indeed be enough, but that is not necessarily true for all, and I still would keep the main information in the element article, not a forced split for all 'because we also do it for H2', and taking in account the non-chemical reader, who we want to present the main info where they expect it, not sending them around Wikipedia on a quest to find it. --Dirk Beetstra T C 10:03, 18 October 2012 (UTC)
- I agree with Beetstra. Plasmic makes the point that hydrogen is in lots of molecules, but that hydrogen has no more to do with H atoms than with H2 so the content is appropriate for our main article. I worry that this direction would confuse the nonchemical reader, all for the sake of satisfying our own tendencies to classify. Its a messy world, and good editors, IMHO, cope with messiness rather than respond by spawning articles on what one might call semantic distinctions. One could imagine future editors subdividing iron according to polymorphs (very different properties, and splat cooled material, and the atomic Fe). In fact I recommend that we try to achieve some consensus and incorporate that consensus into our MOS.--Smokefoot (talk) 12:06, 18 October 2012 (UTC)
- I see where you are coming from here, Plasmic Physics, and for certain elements there is certainly a good reason to do so. But I would not put it into a hard rule. E.g. Sulfur. The 'common' allotrope there is the yellow S8. That is the yellow stuff, and I think that if you ask 'the man in the street', that is what they would describe sulfur to look like, and that is what I then would put into the article sulfur, not 'force' it out of there. Same for carbon, the 'common' allotropes of the pure element as one would normally encounter it, are diamond and graphite. Keep those quite far expanded in the article. It is the information that people expect to find there, they should not be forwarded through specialised links ('for more information about the allotropes of carbon, see ..'). Water does describe ice, water and steam. If there is enough left over, then surely, create an article separate for that, and for the properties of water that is the case. For dihydrogen and dioxygen, there may indeed be enough, but that is not necessarily true for all, and I still would keep the main information in the element article, not a forced split for all 'because we also do it for H2', and taking in account the non-chemical reader, who we want to present the main info where they expect it, not sending them around Wikipedia on a quest to find it. --Dirk Beetstra T C 10:03, 18 October 2012 (UTC)
{{Chemical engineering}} has been nominated for deletion -- 65.92.181.190 (talk) 20:27, 20 October 2012 (UTC)
Showing lone pairs in chemical structures
I wonder if we should be showing lone pairs of electrons in chemical structures. The information from theory is not so tidy. The VSEPR rules are built on the role of l.p.s but I dont think that there are localized pairs of electrons in the way that is often depicted. Compare these two images:

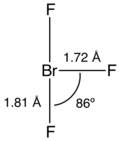 A number of "VSEPRy" compounds, mainly interhalogens, show lone pairs (e.g., ClF5, IF5) but some (IF3, HOCl, SO2) do not. I guess my recommendation is that within the article, when discussing rationales for structures we might show lone pairs, but the chembox structures would not, but others might have differing views. --Smokefoot (talk) 12:02, 28 October 2012 (UTC)
A number of "VSEPRy" compounds, mainly interhalogens, show lone pairs (e.g., ClF5, IF5) but some (IF3, HOCl, SO2) do not. I guess my recommendation is that within the article, when discussing rationales for structures we might show lone pairs, but the chembox structures would not, but others might have differing views. --Smokefoot (talk) 12:02, 28 October 2012 (UTC)
Please keep an eye on this article and look at the discussion on Talk:Quantum chemistry and the user talk page given there. This editor's only edits are on Quantum chemistry and Molecule insisting that Quantum chemistry is a branch of physics not chemistry. I'm on holiday and away from my books. It needs a discussion of sources. --Bduke (Discussion) 08:34, 29 October 2012 (UTC)
WikiProject Women in science
Sarah Stierch (talk) and Keilana|Parlez ici have started a new WikiProject, Wikipedia:WikiProject Women scientists. If you have any questions, feel free to ask one of us on our talk page. RockMagnetist (talk) 16:46, 5 November 2012 (UTC)
There are many pages linking here. I have created a redirect to Benzylpiperazine. Please retarget if there is a better destination. Rich Farmbrough, 18:47, 5 November 2012 (UTC).
- The redirect isn't appropriate because that target is a different chemical compound. I have deleted the redirect and removed the link to 2-Benzylpiperazine from one large template (which had provided the hundreds of unhelpful red links). -- Ed (Edgar181) 17:55, 8 November 2012 (UTC)
Wikichem textbook
People here may be interested in this paper on the successful implementation of a wiki approach to an online chemistry textbook. This paper is published as part of the CONFCHEM - a free, open access online conference for people interested in computers in education. I'm unconnected with CONFCHEM and chemwiki; I'm only passing it along because WP:CHEMISTRY participants may want to read the paper and perhaps comment. Walkerma (talk) 17:45, 8 November 2012 (UTC)
Historical details at Combinatorial chemistry
Additional opinions/eyes would be welcome regarding alterations of the claimed originator of combi-chem. DMacks (talk) 19:52, 14 November 2012 (UTC)
Pasting crystal structure images from journals
Check out this discussion here about pasting in ORTEPs. Our articles would change dramatically if we could do paste in such images from journals.--Smokefoot (talk) 14:30, 15 November 2012 (UTC)
New Asymmetric Hydrogenation Page
Hey Everyone, I've been working on getting a page on Asymmetric hydrogenation up and running for a couple of months now. I'm now into the finishing stages (just some schemes/images, a section on industrial applications, and general editing to do) and would very much like your feedback. Jump in if you want! Bmalbrecht (talk) 23:14, 29 October 2012 (UTC)
- Well done and a huge amount of work. It is more helpful if you reloaded your ChemDraw without the wording, and then make real captions - or someone can do this for you. By following this guideline your nice artwork is more versatile (see Wikipedia:Manual of Style/Chemistry/Structure drawing#General). For example, this figure would be more useful without the wording:

The references are impeccable - DOIs and capitalization. Personally, I think that the references are too numerous and too specialized, but others like this style of technical review. One question - where's Josiphos? Overall, really nice work.--Smokefoot (talk) 00:45, 30 October 2012 (UTC)
- Looks great! in general, more internal linking would be helpful. Go easy on words like excellent etc. I agree the images themselves should not contain text. Would make translations into other languages easier. A minor point on one of the images, the depiction of the barf counterion may confuse people. V8rik (talk) 21:45, 30 October 2012 (UTC)
Sorry for being slow to respond, everyone. I appear to have forgotten to "watch" the page :S I have put in captions that are not built in to most of the images, though I'm not sure how to do it in cases where I would need two separate captions (as in the example above). The references I think I will keep simply because Wikipedia likes referenced info, though I've tried to keep my actual writing as approachable as possible. Lots of internal linking has been added and I'll do some scouting out now for that BArF couterion and any hyperbole. Bmalbrecht (talk) 14:28, 27 November 2012 (UTC)
- I should add, I've nominated the page as a Featured Article candidate, so future comments there are most useful.Bmalbrecht (talk) 14:33, 27 November 2012 (UTC)
- I added an illustrative image to show you how to avoid wording in the image. Also be careful about ChemDraw settings and uniform fonts. Ask colleagues to look at the work to minimize mistakes such as nonprochiral substrates being reduced "asymmetrically", Ir(II) complexes, "Important Monophosphine Ligands" etc. Newer and non-practicing chemists tend toward primary references, which are always better than no references - so what you have is fine. But the best stuff is always the reviews and books, per our WP:SECONDARY guideline that sets a high standard. --Smokefoot (talk) 15:01, 27 November 2012 (UTC)
I did see it. I don't like it at all I'm afraid (the border that wikipedia forces you to put on things to do it that way seems quite hideous to me) but if everyone agrees that it should be done that way I suppose I could conform (and will change over those where it makes sense to do so). I used the ACS chem draw settings uniformally (this includes fonts). I confess that there is some variation due to scaling and I will try to find a way to fix this. I have had several people look through and no one has pointed out an asymmetric reduction of a substrate that is not prochiral, nor do I see one now. Let me know what you're looking at and I'll take a look. I fixed the 'monophosphines' so all now bind through P (I'm assuming that this was the problem), and I will attempt to hunt down that Ir(II). Thanks for letting me know.Bmalbrecht (talk) 02:23, 28 November 2012 (UTC)
- I agree about the framing around images is semi-hideous, maybe someone here can suggestion an alternative. It is the way we decouple words from graphics, which is more important in my view. We can conduct the rest of this conversation on Talk:Asymmetric hydrogenation. --Smokefoot (talk) 13:22, 28 November 2012 (UTC)
Editor review
Just so project members are aware, there is an editor than has been making quite a few unreferenced edits to chemboxes, many of which are dubious or clearly erroneous. There is a list of the IPs used at User talk:ChemNerd/temp. I see that User:Rifleman 82 has reverted most the edits, but if other chemists could review any remaining edits or keep an eye out for additional similar edits, that would be helpful. Thank you. ChemNerd (talk) 12:23, 24 November 2012 (UTC)
New or revised articles from some students
Here is a group of articles that will be appearing in the next month. This team tends to emphasize short articles on chemical compounds, but they are also focusing on article improvement. The list: POCOP (a pincer ligand), Diiminopyridines (another popular pincer ligand), 2,6-Diacetylpyridine (precursor to diiminopyridines), Phosphinoimidate (ligands of the type R3P=N-) Transition metal benzyne complex organometallic theme that has received attention from 3-4 big groups, Brookhart's acid ([H(OEt2)2][B(C6H3(CF3)2)4]), naphthalene tetracarboxylic dianhydride, building block in supramolecular chem, , 6-Nonenal (component of some spoiled foodstuffs), Triacetic acid lactone (so called "green platform chemical"), Imidazolines (article improvement), dihydropyridine (not much content, so we'll see), Biuret and Biuret test (article improvement), Barium manganate (BaMnO4, reagent for organic synthesis, long overdue), Heterogeneous catalysis (article improvement), oxychlorination (article improvement for halogenation), viscose (article improvement), Tris(triphenylphosphine)rhodium carbonyl hydride (HRh(CO)(PPh3)3, a traditional catalyst for hydroformylation -thanks to Project Osprey for help), bis(bis(trimethylsilyl)amido)iron, Fe(Ntms2)2 (article improvement for Metal bis(trimethylsilyl)amides), Rubber#biosynthesis (article improvement for rubber), carbon disulfide#carbon disulfide hydrolase, a recently discovered enzyme (article improvement for carbon disulfide), selenenic acid (RSe-O-H, functional group in glutathione peroxidase), Methionine sulfoxide (oxidized form of methionine), Borane dimethylsulfide (popular reagent, overdue), electrocrystallization (specialized technique for growing crystals), nanorod (article improvement - with nice locally produced TEM). --Smokefoot (talk) 00:37, 1 November 2012 (UTC)
- I've made valinol quite a lot over the years, so give me a nudge when the page is up and I'll see what I can add. -- Project Osprey (talk) 12:57, 28 November 2012 (UTC)
- It should be out this week, looking forward to your help. After the students insert their content, you are always welcome to tidy up, slash-and-burn, rephrase, or whatever. --Smokefoot (talk) 02:23, 4 December 2012 (UTC)
- What do you mean with "article improvement"? In the aryne article I can only see article replacement and content destruction. Who is gong to clean up the mess? V8rik (talk) 17:07, 8 December 2012 (UTC)
- A student wrote an essay on metal benzyne complexes. It was inserted into Aryne and was removed by the editor:Alevina89. So I reinstated the student work, then removed it, creating Transition metal benzyne complex since aryne article is rather long (too long and too detailed in my view). I re-edited the student work. If you wish to edit further, go for it. I also edited some of the aryne article, which, like all articles, could benefit from copy editing from someone with experience in technical writing. In terms of your accusation of "content destruction", there was no content on metal benzyne complexes before we started, and now this niche but nifty area is decently represented with a reasonably encyclopedic overview. We will be uploading further content as foretold above, including what we see as "article improvement". --Smokefoot (talk) 17:59, 8 December 2012 (UTC)
- I was too hasty, I seem to have a problem with Alevina89 and this editors edits on the aryne page and not with one of your students V8rik (talk) 19:55, 10 December 2012 (UTC)
Oxaziridine
Is there anyone here with the skills and interest to fix the Oxaziridine article? It basically had a lead, then someone converted it to a history section. The trouble is that now the lead is too short and the history section contains more than just information on history. It can't really be reverted as far as I can tell as the lead will then contain too much information not in the article. I feel it is an easyish fix if you have some idea about the subject, but a nightmare for someone who doesn't (i.e. me). It was a good article, but in it's current shape it doesn't meet the criteria. AIRcorn (talk) 11:28, 30 November 2012 (UTC)
Help in a couple of areas
Hi... I recently posted at Wikipedia talk:Chemical infobox#Template Problem about a problem with the info box on most chemicals pages, but have received no response. I was wondering if a chemically knowledgable editor who understands wiki-templates might be willing to have a look? Thanks.
Also, I was doing some referencing / wikilink work and spent some time on the metallocene article. I notice that a recent addition roughly doubled the length of the article, and it now has many one sentence paragraphs and errors and other issues. I have made a few changes but (at best) they were nibbling at the edges. If someone wants to have a look and do a proper copy edit, that would be great.
Many Thanks, EdChem (talk) 14:34, 9 December 2012 (UTC)
- I replied to the Chembox problem on that talkpage. I will try to get the User who improved the german version of the metallocene article have a look at it. --Saehrimnir (talk) 08:48, 10 December 2012 (UTC)
- Thanks for the reply about the chembox. As for the metallocene article, any and all improvements are welcome! Regards, EdChem (talk) 10:50, 10 December 2012 (UTC)
Discussion at WP:ENB
There has been an ongoing discussion at the Education Noticeboard (WP:ENB) that has included many areas of student editing of Wikipedia. I have just posted a section Wikipedia:Education noticeboard/Archive3#Some experiences on chemistry articles describing some of my observations and experiences. In it, I have made mention of articles on allylcyclopentane, insertion reaction, isolobal principle, metallocene, niobium(V) ethoxide, and oxaziridine. I have also mentioned work from both Smokefoot and Rifleman 82. Any and all comments, experiences, or additional thoughts are welcome in the discussion, as of course are any corrections or criticisms of my comments. Regards, EdChem (talk) 04:58, 16 December 2012 (UTC)
- Thanks for the heads up. Sounds like you communicated pretty well with that group. Not sure there is anything they or anyone can do. The pain with unsupervised student work is balanced by more positive developments. I always encourage editors to focus on WP:SECONDARY, which minimizes problems with WP:COI, WP:UNDUE, WP:RECENTISM, and WP:NOTABLE. --Smokefoot (talk) 19:37, 16 December 2012 (UTC)
Assessment guidelines for chemistry banner?
WikiProject Chemistry currently has a backlog of +1000 articles which are unassessed in some form or another. I was wondering if it might be useful to generate a guidelines page, similar to that done for Wikipedia:WikiProject_Chemicals/Assessment, to facilitate their grading. This would also help ensure a uniform assessment of articles as a whole, making the system a better tool for identifying articles in need of improvement. In theory the page could also contain guidance notes on common issues encountered within WikiProject Chemistry, such as the whole WP:PRIMARY WP:SECONDARY debate (I actually doubt we'll ever agree a definition of 'acceptable' but perhaps we could at least define 'unacceptable'). Project Osprey (talk) 13:52, 18 December 2012 (UTC)
Oxidation number and oxidation state shouldn't these be merged
Hello again, I have been reading the two articles, Oxidation number and Oxidation state and trying to make sense of them for a young but confused student.
The articles seem to start from the IUPAC definition then fall between two stools- the IUPAC definitions and the contradictory inorganic text book and exam board definitions. IUPAC defines oxidation state with a set of rules but text books define something almost identical as oxidation number. IUPAC says oxidation number relates to coodination chemistry, with a different rule from their oxidation state definition for the apportioning of electrons , but text books say oxidation number is synonymous with oxidation state and are quiet on the coordination chemistry angle. So after much well intentioned editing driven by the contradiction between what is taught and what IUPAC define, both articles have ended up as being full of good information but are confusing.
I suggest that Oxidation number and Oxidation state are merged and the main emphasis is on the text book / exam board definitions (which follow IUPAC oxidation state definition- but with some local differences) and a note that IUPAC define oxidation number differently and give some meaningful coordination chemistry examples. If they aren't merged I believe that eventually well intentioned editing will occur again and we'll be back at the current position.
I suspect during my long absence from Wikipedia this will have been discussed and that some folk may have views so I am putting it up here first. I am also starting to hear bees in my bonnet whispering about the Phosphite article but I'll address that ne another day. Axiosaurus (talk) 10:29, 19 December 2012 (UTC)
- The two terms are different, the classic example being osmium tetroxide (OsO4), where the osmium has an oxidation number of VIII but clearly can't have the same oxidation state as this would imply a stable 8+ charge (just checked and it's wiki page and it has this wrong). It's an issue of whether the coordination is ionic or covalent. When it's ionic then the oxidation state and number will likely be the same, where it’s covalent then they likely won’t. It is easy to see how the two terms can be confused, the difference is subtle - but I'd argue it is important. As such I don't think they should be merged but a clear and prominent explanation of the differences is needed and should be present on both pages Project Osprey (talk) 11:29, 19 December 2012 (UTC)
- That is an interesting take on oxidation number/state. I would be interested to know which text book says that and where that idea is taught as that may need to go into the article.Axiosaurus (talk) 14:04, 19 December 2012 (UTC)
- Oh god, that's going back a few years. I'm not sure I can help you find a text book but I may be able to help with the article overall. What’s been written there isn’t strictly speaking wrong but... well, it’s just awful. It also seems to have resulted in confusion in a number of pages on transition metal complexes. Once the oxidation number page is fixed it may be necessary to review all of these pages and correct any errors. My problem however, is that I'm working on a re-write already, does anyone know if it's possible to have 2 sandboxes? Project Osprey (talk) 10:01, 20 December 2012 (UTC)
- That is an interesting take on oxidation number/state. I would be interested to know which text book says that and where that idea is taught as that may need to go into the article.Axiosaurus (talk) 14:04, 19 December 2012 (UTC)
Proposed changes for three articles on Si-O chemistry
Big changes have been proposed by me in two large topics in organosilicon chemistry. No articles would disappear and the changes should help specialized and general readers alike, but since these topics are of high visibility, it is useful to serve notice lest other editors have suggestions.
- Propose to refocus Siloxane into an article about the Si-O-Si functional group. The main compounds that have siloxane functional groups are silicones ([R2SiiO]n]). The difference between siloxane polymers and silicone polymers is a source of confusion, even by experts. Some content would migrate from siloxane to silicones with appropriate "see also's" on both sides. We would then build up the discussion of the synthesis, structures, and reactions of Si-O-Si groups within Siloxane.
- Propose to change Silanol into an article on the functional group Si-O-H. Silanols are hugely important to surface science people, and it seems that we owe this readership an article on this critical functional group. Retained within the revised article would be a section on the parent species SiH4-x(OH)x. What we need is a good discussion of synthesis, structures, and reactions of Si-OH groups.
Suggestions or disagreements are welcome.--Smokefoot (talk) 18:56, 31 December 2012 (UTC)
Wanted articles on chemical compounds
If anyone is looking to create new chemical compound articles, there are many listed in this November 2012 report on red links within articles tagged under WikiProject Medicine. I thought it might be of interest. Thanks. Biosthmors (talk) 22:47, 30 November 2012 (UTC)
- I think the number of red links are more of a measure of how bloated some templates are rather than a measure of how "wanted" those articles are. :/ ChemNerd (talk) 19:05, 3 January 2013 (UTC)
Nonmetal categories
In WP:ELEMENTS we have been having a long-running discussion, here, about how we categorize the nonmetals. At the moment we have the following categories:
- Other nonmetals
- Halogens
- Noble gases
Partly the discussion was started to see if the term 'other nonmetals' could be dispensed with or improved upon.
We are now looking at a proposal to change the categories to:
- Reactive nonmetals
- Noble gases
The current discussion about this proposal has now come down to whether or not to have one category of reactive nonmetals (as shown above) or two, for example:
- Reactive nonmetals
- Highly reactive nonmetals
- Noble gases
There is a table here, which shows the eight proposals that have been presented to date for categorizing the non-noble nonmetals.
Your comments would be gratefully appreciated. Sandbh (talk) 21:36, 1 January 2013 (UTC)
Organometal “logos”
We have several “logos” in articles on organometal compounds. This may be problematic, because a lay reader may understand that e.g. C–Pb is an actual molecule. Opinions? --Leyo 18:57, 3 January 2013 (UTC)
- What an interesting perspective. I just installed such a logo on siloxane. My own feeling is that the risk of confusion is offset by the advantages to summarizing a topic quickly to chemists. Taking the potential problem to the extreme, I cannot foresee negative consequences of a few people holding in their mind that Pb-C is an organolead compound. --Smokefoot (talk) 00:06, 4 January 2013 (UTC)
- I would prefer if you added 6× an R to the image in Siloxane. This would avoid any potential confusion IMO. --Leyo 09:12, 4 January 2013 (UTC)
Periodic Table: proposed rename (move) from group 3 element to group 3 &tc, and periods alike
Originally from WP:ELEMENTS. See Talk:Group_3_element#Requested_move for a central discussion. -DePiep (talk) 21:08, 15 January 2013 (UTC)
Request for feedback
I recently made a reversion to Dissociation constant. Could someone let me know if I should have done so, or revert what I have done if I should not have? It seems to me that the content does not fit the article as being very specific to a particular branch of research, but I would like to be double-checked. --Izno (talk) 16:42, 13 January 2013 (UTC)
- I took a look at the talk page but didn't see any comments by you about the reversion, so I'll post my response here. I think the reversion was warranted. The "TCR" information, in my opinion, deserves its own page and could be mentioned on the page, but in my experience with chemical pedagogy, I don't think it fit the page well at all. Do you think we should leave a comment on the talk page so that the author knows why the reversion was done? Sean Egan(talk) 20:57, 18 January 2013 (UTC)
- I've no objection to leaving a comment. --IznoRepeat (talk) 21:05, 18 January 2013 (UTC)
Willard Gibbs
Hi. I've been working for a while on the bio. of Josiah Willard Gibbs. First, I'd like to mention that I think the article should be rated as of Top Importance in both chemistry and physics, and High Importance in math (it's currently High/High/Mid). It has often been pointed out that Gibbs essentially created modern physical chemistry (see the article for references). As a theoretical physicist he coined the term "statistical mechanics" and gave use the key concepts of the microcanonical, canonical, and grand canonical ensembles. And as a mathematician we owe him the concept of the cross product, the formulation of vector calculus in terms of div, grad, curl, and all that, and many of the ideas of what later became convex analysis.
My other concern is that I'd like to attract other editors who might want to edit and improve the article. Last Nov. I nominated it for FA. It got significant support, but some of the FA review regulars kept asking for independent copyediting and in the end the discussion just stalled and was archived. Gibbs's work as a theoretical scientist is extremely interesting and important, and I think his wikibio deserves more attention. - Eb.hoop (talk) 23:41, 17 January 2013 (UTC)
Alkyl-lead
I have redirected Alkyl-lead to Organolead compound. Is that cool? -- Alan Liefting (talk - contribs) 02:16, 25 January 2013 (UTC)
Should the section Genomic DNA Isolation from plants by modified CTAB method be removed completely? --Leyo 09:24, 25 January 2013 (UTC)
Overcategorisation?
Check the subcategories within Category:Phenolic compounds in food. Plasmic Physics (talk) 07:58, 26 January 2013 (UTC)
TAFI
 Hello, |
Typography question: italicize atomic-orbital subshells?
Should the labels associated with Azimuthal quantum numbers--"s", "p", "d", etc.--and the X-ray analogs--"K", "L", "M", etc.--be set in italic or plain text? Our articles that use them are not consistent, and even in the articles about the topics themselves. DMacks (talk) 03:13, 27 January 2013 (UTC)
- Finally found it in ACS Style Guide (6th ed). Explicitly says roman-type for both cases. Should this be noted in Wikipedia:Manual of Style/Text formatting? DMacks (talk) 03:17, 27 January 2013 (UTC)
- You can put it in WP:MOSCHEM? --Rifleman 82 (talk) 16:11, 27 January 2013 (UTC)
Descriptors r and s
Pentane-2,3,4-triol (see structure and stereochemistry on p. 397) with all OH groups up is named (2R,3r,4S)-pentane-2,3,4-triol by ChemBioDraw. Granisetron is an another example that has an r in its IUPAC name. It seems that there is no reference to r (and s, which is the counterpart) in the Gold Book. The rule is described under Cahn–Ingold–Prelog priority rules#Stereocenters: R/S (last paragraph), but no reference is cited. Does anyone know of a quotable publication? --Leyo 16:54, 29 January 2013 (UTC)
- "BASIC TERMINOLOGY OF STEREOCHEMISTRY (IUPAC Recommendations 1996)" says:
- r,s -- Stereodescriptors of pseudo-asymmetric atom. For references see under R, S.
- with those later references as:
- R.S. Cahn, C.K. Ingold and V. Prelog, Angew. Chem. 78,413-447 (1966), Angew. Chem. Internat. Ed. Eng. 5 , 385-415, 511 (1966); and V. Prelog and G. Helmchen,Angew. Chem. 94,614-631 (1982), Angew. Chem Internat. Ed. Eng. 21,567-583 (1982).
- "RULES FOR THE NOMENCLATURE OF ORGANI CHEMISTRY. SECTION E: STEREOCHEMISTRY (RECOMENDATIONS 1974)" says:
- Pseudoasymmetric atoms. A sub-rule decrees that R groups have preference over S groups and this permits pseudoasymmetric acid, as in Cab(c-R)(c-S') to be treated in the same way as chiral centres; but as such a molecule is achiral (not optically active) it is given the lower-case symbol r or s.
- DMacks (talk) 18:00, 29 January 2013 (UTC)
- Thanks a lot! I found these publications online: p. 2214, p. 29. Based on your information, I found out that I just did not have the right keyword for searching the Gold Book. There actually is an entry:
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "pseudo-asymmetric carbon atom". doi:10.1351/goldbook.P04921
- I added this reference to Cahn–Ingold–Prelog priority rules#Stereocenters: R/S. Would it be worth drawing (2R,3r,4S)-pentane-2,3,4-triol or another compound with r or s (which one?) as an illustrative example? --Leyo 22:22, 29 January 2013 (UTC)
- Thanks a lot! I found these publications online: p. 2214, p. 29. Based on your information, I found out that I just did not have the right keyword for searching the Gold Book. There actually is an entry:

- I drew (1R,2s,3S)-1,2,3-trichlorocyclopentane since it might be one of the easiest possible examples. --Leyo 13:26, 31 January 2013 (UTC)
Naming of functional groups × free "radicals" × functional families
Hi, I need suggestions for a nomenclature problem. Please forgive the boring long explanation below.
I have been working on methylene and related articles. There are three fairly common meanings for the word:
- (1)the free molecule :CH
2, officially called "carbene"; - (2)the 'terminal' functional group =CH
2, connected to a single atom by a double bond; - (3)the 'bridging' functional group -CH
2-, connected to two different atoms by single bonds.
Until recently there was one article called [[methylene]] for sense (1), with chembox and everything, that briefly mentioned senses (2) and (3); and a page called [[methylenes]] that was basically a disambiguation page (only a couple of lines for each sense) but not tagged as such.
Now there are separate pages for senses (2) methylene group and (3) methylene bridge. Those pages are only stubs now but I believe that there is enough material to write books on them (bond geometry, electronic structure, reactions, spectroscopy, etc.)
There is also a methylene (disambiguation), that can be reached via the link "for other uses see..." in [[methylene]].
Then I went through almost all links to [[methylene]] and [[methylenes]] to resolve them to the proper page. Of ~130 links, I found 91 that were actually for the bridge, 22 for the terminal group, about 10 (lost the exact count) that were indeed for the free molecule, and 5 that I could not figure out (Cyclophane Johnson–Corey–Chaykovsky reaction Organoselenium chemistry Persistent carbene Tröger's base).
So it seems that
- (A) as long as the article on the molecule is called just [[methylene]], we can expect that ~90% of the [[methylene]] links that will be created in the future by busy or lazy editors will be wrong. The reader who clicks on the link because he doesn't know the term will be quite confused; and since experts will not click there, the link will never be corrected. My conclusion: the article about the molecule must be renamed to a more specific name.
- (B) Renaming [[methylene bridge]] to be just [[methylene]] would be convenient and result in correct link in ~70% of the cases. However, perhaps 30% of the new links may still end up pointing to the wrong sense of the word. My conclusion: neither sense should be the "primary" sense.
- (C) The remaining alternative is to let [[methylene]] be the disambiguation page. Then new links [[methylene]] created by busy or lazy editors will end up pointing to a disamb page. This sounds bad, but such links can be detected by bots and will soon get fixed. Misdirected links will not.
I hope this plan is OK. Note that there are already two articles for CH3, methyl group and methyl radical. My question is what to call the current [[methylene]] article (about the molecule). Here are some ideas:
- (a) [[Methylene radical]]. This would follow the precedent of [[Methyl radical]]. Unfortunately "radical" my mean "bound group", so the confusion would continue.
- (b) [[Methylene (free radical)]]. Very clear and unambiguous, and fits Wikipedia general rules. However, I have been told that "radical" is now defined by the IUPAC and depends on spin state, and :CH
2 does not qualify. Is it so? - (c) [[Methylene molecule]] Never used in Wikipedia for any other page on chemical species.
- (d) [[Methylene (molecule)]] Ditto.
- (e) [[Methylene (carbene)]] This would be doubly correct since methylene is a carbene and also "carbene" is its quasi-official name. But in Wikipedia one does not call articles [[name (another name)]]. Also that title would be obscure to many readers and editors.
- (f) Carbene Very correct, but unfortunately it is taken by the carbene family
- (g) [[Methylene (chemical species)]] Too long, and maybe obscure to editors.
Any other suggestions? My favorite is (b) [[Methylene (free radical)]]. If "radical" is OK for methyl, why not for methylene? IUPAC be damned!
What do you say? All the best, --Jorge Stolfi (talk) 03:35, 29 January 2013 (UTC)
- How about Methylidene for the carbene molecule, Alkylidene for the double bonded functional group, and Methylene to redirect to methane.
- Alkylidene is chosen as it encompasses all doubly bonded alkane functional groups (ethylidene, propylidene, butylidene, etc.). Plasmic Physics (talk) 04:16, 29 January 2013 (UTC)
- I think that all of the content on methylene should be combined into one article that deals the various meanings. I fear that talented editors sometimes force semantics upon readers. If some of the suggestions above are followed, otherwise cohesive and realitively simple concepts will be splintered in order to be highly precise. So I hope that we can drop some precision that us pointy headed types think about and serve readers vs our fetishes. --Smokefoot (talk) 10:21, 29 January 2013 (UTC)
- That is fiddle-faddle, we have many articles on functional groups, as well as the corresponding radicals, eg. Hydroxyl v.s. Hydroxyl radical. Plasmic Physics (talk) 11:14, 29 January 2013 (UTC)
- I think that all of the content on methylene should be combined into one article that deals the various meanings. I fear that talented editors sometimes force semantics upon readers. If some of the suggestions above are followed, otherwise cohesive and realitively simple concepts will be splintered in order to be highly precise. So I hope that we can drop some precision that us pointy headed types think about and serve readers vs our fetishes. --Smokefoot (talk) 10:21, 29 January 2013 (UTC)
- Well, the concepts *are* quite distinct, and there is much to be said about each one that has is irrelevant for the other two. Merging back would result in one messy article that will frustrate readers and editors alike. (Imagine combining pipe (fluid conveyance) and pipe (smoking) into one pipe article. Think of the reader who follows the link in the sentence "Pipes often burst in cold winters".)
By the way, there is already a section in Substituent that lists all possible CHn functional groups, with their IUPAC nomenclature. So, any info that is common to both groups =CH2 and -CH2- could go there, together with similar info about -CH3, ≡CH, =CH-, etc.. (Perhaps that section should be split to [[methane substituents]], but that is another discussion.) However, that section (or any section/article with more than one CH2 meaning) would not satisfy the needs of readers and editors that need the link "[[methylene]]", as in "step III adds a methylene to the main ring".
This last example shows that splitting the topics is not just pedantism: the meaning of the text changes completely depending on which "methylene" the link points to, and readers who follow the link will be quite confused and frustrated if the target describes both. That happened to me on the 5 articles above. --Jorge Stolfi (talk) 16:36, 29 January 2013 (UTC)
- I'd support separate pages for the 'carbene' and 'methylene bridge' aspects with a disambiguous for 'methylene' on its own. I am, however, confused by Methylene_group, is this not just a Vinyl_group?Project Osprey (talk) 12:16, 30 January 2013 (UTC)
- I understand that (as functional groups) methylene is =CH
2, vinyl is -(CH)=CH
2. Thus every molecule that has a vinyl also has a methylene (and a methine -(CH)=), but not the other way around. See methylpropene. (Is this correct?) --Jorge Stolfi (talk) 12:30, 30 January 2013 (UTC)- Ah! Well in that case it all seems good to me Project Osprey (talk) 12:41, 30 January 2013 (UTC)
- I understand that (as functional groups) methylene is =CH
- Well, the concepts *are* quite distinct, and there is much to be said about each one that has is irrelevant for the other two. Merging back would result in one messy article that will frustrate readers and editors alike. (Imagine combining pipe (fluid conveyance) and pipe (smoking) into one pipe article. Think of the reader who follows the link in the sentence "Pipes often burst in cold winters".)
- Since there were no complaints, [[methylene]] is now methylene radical, and methylene is now a disambiguation page. --Jorge Stolfi (talk) 08:22, 6 February 2013 (UTC)
Proposed rewrite of head section in "radical (chemistry)"
Hi again. I felt tempted to rewrite the head section of radical (chemistry) to make it a bit more accessible to non-chemists, but maybe I got the concept wrong. Would please someone have a look at this draft and comment on it? Many thanks... --Jorge Stolfi (talk) 02:08, 30 January 2013 (UTC)
- Since there were no complaints, the head was rewritten. --Jorge Stolfi (talk) 08:19, 6 February 2013 (UTC)
RfC input needed
Input would be appreciated at an RfC regarding the Nobel prize in Chemistry. --Noleander (talk) 18:56, 5 February 2013 (UTC)
Chemistry education
There has been a merge tag on General chemistry proposing it be merged to Chemistry education for nearly a year, with only one comment at Talk:Chemistry education#Merge of General chemistry and that in the last day. Could we resolve this before the year anniversary comes up on 24 February? --Bduke (Discussion) 21:11, 6 February 2013 (UTC)
Charles Tanford (Cross-posted to WT:MCB and WT:Chemistry)
Looks like this article has been somewhat neglected. I happened to notice it was tagged with a "ref improve" tag, so I turned to the most logical source for citations.. the Protein Science "In Memoriam" article from 2009. Unfortunately, it appears that much of the current page is copied verbatim from the "In Memoriam" article. Tanford's a pretty significant figure in protein chemistry, so it would probably be worth expanding the article beyond just being a carbon copy of the obituary. Does anyone have time to work on such an expansion? Thanks. (+)H3N-Protein\Chemist-CO2(-) 18:33, 10 February 2013 (UTC)
Alkyl merger
We need to close a merger discussion at talk:alkyl#merger proposal. Plasmic Physics (talk) 03:51, 14 February 2013 (UTC)
Wikidata: phase II live
Since phase II (infoboxes) of Wikidata is now live, there is a need to define the implementation for chemistry articles. There is currently a just draft: d:Wikidata:Infoboxes task force/terms#Chemistry / Chemie / Chimie. --Leyo 20:19, 5 February 2013 (UTC)
- Recently created: d:Wikidata:Chemistry task force. Is there a good place in the project page to add a link? --Leyo 22:55, 14 February 2013 (UTC)
Wikidata
Wikidata is still under construction (there is no possibility to add values, text or references right now) but there is a big interest to prepare the elements which will be useful for chembox , elements or others data consumers in WP. If there people who are interested to work in that kinf of project, feel free to have a look here. Biglama (talk) 19:40, 13 February 2013 (UTC)
- Creation of a task force for chemical topic: here Biglama (talk) 15:08, 14 February 2013 (UTC)
Category:Articles without InChI source
I think Category:Articles without InChI source should be a hidden category and in Category:WikiProject Chemistry rather than Category:Chemistry. -- Alan Liefting (talk - contribs) 04:40, 20 February 2013 (UTC)
 Agree That sounds like a sensible idea. Aloneinthewild (talk) 21:17, 21 February 2013 (UTC)
Agree That sounds like a sensible idea. Aloneinthewild (talk) 21:17, 21 February 2013 (UTC)
Template up for deletion
See Wikipedia:Templates for discussion/Log/2013 February 28. -- Alan Liefting (talk - contribs) 21:29, 28 February 2013 (UTC)
Proposed move of Chlorination to Water chlorination
I propose that we move Chlorination to Water chlorination. The article is about chlorinating water for disinfection. For hard core chemists, Chlorination does not discuss additions to double bonds etc - that content is found in halogenation. So there is no problem in that respect. We currently have Water fluoridation, so Water chlorination would represent a parallel title on a related process, which seems desirable and logical. Water chlorination presently redirects to chlorine, which seems like a bad idea.
So that is the proposal. Alternative views are welcome. If no alternative views are expressed after a few days, I am hoping that some administrator can assist with this change. --Smokefoot (talk) 19:58, 10 March 2013 (UTC)
- As you note there now exists an article solely on water chlorination (as sanitation) called chlorination with a differentiate tag at the top directing people looking for chemical chlorination reactions to halogenation, which is fine by me but could be changed. The worst problem is the hidden redirect (I really hate those) that (as you note) directs water chlorination to the chlorine article, instead of to the main article on the topic, which (presently) is chlorination. That unfixable redirect has to go. I don't really care what the water sanitation article is ultimately called, but if it's not water chlorination the redirect from chlorination should be put in plain sight, and indeed directed to whatever we call the sanitation article (which would still include the differentiate tag on top to halogenation). Hopefully we can get along without a chlorination dab page, with just these two main uses of the term. SBHarris 21:42, 16 March 2013 (UTC)
- The move makes perfect sense to me and I support it. I think it would be best to get consensus on what to do with the title "chlorination" after the move, though. If it is to become a redirect to the article at "water chlorination", then there will be some cases where the incoming links to "chlorination" are actually meant for the halogenation meaning and those will have to be found and fixed. If "chlorination" becomes a disambig page, then there will be some disambiguation necessary for all the incoming links. I'll gladly help with either of those issues after the move. The move as suggested should take care of Sbharris' concern about the current bad redirect at "chlorination". Finally, this move should be able to be done by anyone, not just an administrator. Even though the target page currently exists, non-admins can move pages over redirects with no history (see WP:MOR). -- Ed (Edgar181) 11:28, 17 March 2013 (UTC)
- Ok, thanks for the clarification. I made the switch. Chlorination now redirects to Halogenation and Water chlorination has its own article, no longer directing to chlorine.--Smokefoot (talk) 13:57, 17 March 2013 (UTC)
- The page should have been moved using the WP:MOVE function instead of copying text from one title and pasting into the other. (Moving instead of copying preserves the edit history that is required by Wikipedia licensing policy.) I think I have the page histories sorted out correctly now. Also, I converted chlorination into a disambiguation page since roughly half of the incoming links were intended for each of the two meanings. I'm going through those thinks now and pointing them to the appropriate article. After those links are fixed, if "chlorination" should be a redirect instead of a disambig page (I don't care which) that change can be made. -- Ed (Edgar181) 15:59, 17 March 2013 (UTC)
Proposing title change of "Zinc finger protein 804A" to "Zinc fingers and Schizophrenia"
I would like to request a change in the existing article titled "Zinc finger protein 804A" to "Zinc Fingers and Schizophrenia". My reason for doing so is because the existing article has been classified as a "stub" article and I would like to expand on it with recent research. Also I feel that the new title will be more accessible to a wider audience, and allow other informed users to make high quality contributions. Ashkv (talk) 19:16, 16 March 2013 (UTC) March 16, 2013
These stubs were created yesterday by Jü (talk · contribs) as Secundary (chemistry) and Quartary (chemistry). Would anyone care to have a look—perhaps flesh them out, add some links so they won't be orphaned, assess whether they might best be merged into existing articles, etc.? Fvasconcellos (t·c) 14:38, 10 March 2013 (UTC)
- Those stubs included the unrelated ideas of covalent bonding patterns ("how many C attached to some atom of interest") with the biopolymer levels of organization ("sequence, helix/strand, full molecular geometry, multimolecular forms"). The latter doesn't seem like "chemistry", so it doesn't belong in a specifically "(chemistry)" page. We already have Primary structure, etc. as redirects to good content on those topics. I ripped the commentary about those out of the (chemistry) articles, instead leaving hatnotes to send readers to the redirects about these specific meanings.
- I don't think these (chemistry) need their own articles at all though. It's a very nice table comparing them, but is there really anything else to say about them in general. There is probably something to be said about some of them (pattern of carbocationic stability, quat-ammonium compounds, special reactions of quat-ammoniums and quat-phosphoniums, etc.), but again, that wouldn't be in this general a page. I do like the comparison chart, but all we really have in any of these articles is this same chart and the definition that restates "this article's" column in it. I !vote merge (not sure what to call the target though). DMacks (talk) 00:54, 17 March 2013 (UTC)
How about IUPAC_nomenclature_of_organic_chemistry as the merge target? --Rifleman 82 (talk) 03:00, 20 March 2013 (UTC)
Chemical Heritage Foundation wikipedian in residence
The Chemical Heritage Foundation has a wikipedian in residence opening for those interested.
Cross-posted form GLAM mailing list, sent by User:Dominic:
I am excited to announce that the Chemical Heritage Foundation[1]—a non-profit repository in Philadelphia, USA with collections relating to the history and heritage of chemistry, chemical engineering, and related sciences and technologies—is seeking an experienced Wikipedian to serve as their Wikipedian in Residence[2]. The Wikipedian in Residence will work as a community coordinator and strengthen the relationship between CHF and the Wikipedian community through a range of activities, similar to past GLAM-Wiki[3] residencies. This is a full-time, temporary position with a small stipend with an immediate opening.
The Wikipedian in Residence's potential activities includes organizing on-site events for the local Wikipedian community, adding digital content from CHF's holdings to Wikimedia Commons and Wikisource, encouraging increased content or quality of Wikipedia articles using CHF resources, teaching Wikipedia skills to the CHF staff or researcher community, and documenting their experiences in blogs, social media, or elsewhere.
To learn more or apply, please visit http://www.chemheritage.org/about/careers/wikipedian-in-residence.aspx. Questions relating to the position can be sent to Jeffery Guin, Manager of Emerging Media, at <removed>, or to me if they are general queries about Wikipedians in Residence or GLAM-Wiki. Please forward this message to other lists or or contacts you think would be interested. Thanks!
- See http://www.chemheritage.org/
- To learn more about Wikipedians in Residence, please visit <http://outreach.wikimedia.org/wiki/Wikipedian_in_Residence>.
- If you are interested in learning more about Wikipedia's work with cultural instituions generally, see <http://en.wikipedia.org/wiki/Wikipedia:GLAM/US>.
Smallman12q (talk) 01:27, 20 March 2013 (UTC)
New topics
There are two new issues on Wikipedia talk:WikiProject Chemicals. Plasmic Physics (talk) 03:13, 20 March 2013 (UTC)
Oxaziridine, an article that you or your project may be interested in, has been nominated for an individual good article reassessment. If you are interested in the discussion, please participate by adding your comments to the reassessment page. If concerns are not addressed during the review period, the good article status will be removed from the article. AIRcorn (talk) 11:57, 3 April 2013 (UTC)
Moving silenes.
Requesting discussion input at Talk:Silenes. Plasmic Physics (talk) 20:39, 4 April 2013 (UTC)
Notability of Chemical
How a notability of a chemical can be assessed? i.e. whether chemical qualifies for a wiki article?--Vigyani (talk) 09:25, 10 April 2013 (UTC)
- In general, the notability of chemical is proportional to its mentions in publications. Usual notable chemicals would be mentioned in hundreds of publications (= papers, patents, books). --Smokefoot (talk) 12:15, 10 April 2013 (UTC)
- do u have a good knowledge in chemistry? can u comment if these articles are notable [1] --Vigyani (talk) 12:20, 10 April 2013 (UTC)
- Importing the results of that lookup...
- Pages created by User:Ellis O'Neill:
- Aminoadipate aminotransferasea
- Homoaconitate
- Homoisocitrate
- Myceliophthorab (moved to Myceliophthora thermophila)
- Bathycoccus prasinosb
- Glucuronamide
- Chattonellab
- Gonyostomumb
- Ichthyotoxinc
- Euglena sanguineab
- Euglenophycin
- Prymnesin
- Thiocarbohydrazide
- Silver proteinate
- Base J
- Acarviosin
- Validamycin
- Actinoplanesb
- Pages created by User:Ellis O'Neill:
- Some are simple chemicals we can address here, but others are enzymes/proteins or plant/animal species that would be better handled by other wikiprojects (Wikipedia:WikiProject Molecular and Cellular Biology/Wikipedia:WikiProject Biophysics or Wikipedia:WikiProject Molecular and Cellular Biology for example). DMacks (talk) 12:53, 10 April 2013 (UTC)
- aI don't know whether WP-Biochem is trying to build a comprehensive set of enzymes.
- bI don't know whether WP-Bio is trying to build a comprehensive set of species.
- cThis is a type/class of chemicals. But it seems like a dict-def or even a synonym of another existing article.
- Others are specific chemicals that are in-scope to ponder here. DMacks (talk) 13:21, 10 April 2013 (UTC)
- Importing the results of that lookup...
- do u have a good knowledge in chemistry? can u comment if these articles are notable [1] --Vigyani (talk) 12:20, 10 April 2013 (UTC)
- You might want to talk to the editor before running around tagging all his articles for deletion! DMacks (talk) 12:55, 10 April 2013 (UTC)
- i did left a message on his talk page, but he is not responding and just keep on creating articlesVigyani (talk) 13:05, 10 April 2013 (UTC)
- There is no notability criteria specific for chemical compounds so the general notability guideline is the prevailing policy. I have looked at these articles and none of them stand out as obviously non-notable, but I haven't done a thorough search in the chemical literature. I've done a little cleanup of the newest articles to start with. -- Ed (Edgar181) 13:46, 10 April 2013 (UTC)
- I should note too that I have declined speedy deletion of a couple of these articles. AFD would be better if notability is questioned. -- Ed (Edgar181) 14:10, 10 April 2013 (UTC)
- Its okay with me. --Vigyani (talk) 14:21, 10 April 2013 (UTC)
2,3 Sigmatropic rearrangement http://en.wikipedia.org/wiki/2,3-sigmatropic_rearrangement
Has anyone noticed the error in the third diagram involving the sulfur containing ring expansion. The Me group has to be a CH2 anion for the mechanism to work. Also the diagram breaks the law of conservation of charge. You start with overall positive charge on the reactant side and end up with neutrality on the product side. Oops. So who fixes these things?
Thanks,
Ed Neeland — Preceding unsigned comment added by 142.231.67.19 (talk) 16:56, 18 April 2013 (UTC)
- if only we had the original source V8rik (talk) 20:22, 18 April 2013 (UTC)
- SciFinder says that specific example comes from Journal of Organic Chemistry. 43 (25): 4826–31. 1978.
{{cite journal}}: Missing or empty|title=(help). DMacks (talk) 00:42, 19 April 2013 (UTC)- DOI: 10.1021/jo00419a024 - Looks like the S-methyl is deprotonated to give the ylide. Project Osprey (talk) 08:12, 19 April 2013 (UTC)
- Thanks, I will work on a replacement image V8rik (talk) 19:28, 19 April 2013 (UTC)
- Image replaced V8rik (talk) 13:52, 21 April 2013 (UTC)
- Thanks, I will work on a replacement image V8rik (talk) 19:28, 19 April 2013 (UTC)
- DOI: 10.1021/jo00419a024 - Looks like the S-methyl is deprotonated to give the ylide. Project Osprey (talk) 08:12, 19 April 2013 (UTC)
- SciFinder says that specific example comes from Journal of Organic Chemistry. 43 (25): 4826–31. 1978.
Enantioselective synthesis re-write
I've drafted a re-write of the enantioselective synthesis page (available here). It's gone down ok on the talk page. However, as:
- I suspect many people would consider it a core page
- A total re-write of any page goes against our manual of style
I thought I would not be bold and actually make sure people were broadly ok with this before I pushed the edit across. Project Osprey (talk) 21:35, 22 April 2013 (UTC)
VisualEditor is coming
The WP:VisualEditor is designed to let people edit without needing to learn wikitext syntax. The articles will look (nearly) the same in the new edit "window" as when you read them (aka WYSIWYG), and changes will show up as you type them, very much like writing a document in a modern word processor. The devs currently expect to deploy the VisualEditor as the new site-wide default editing system in early July 2013.
About 2,000 editors have tried out this early test version so far, and feedback overall has been positive. Right now, the VisualEditor is available only to registered users who opt-in, and it's a bit slow and limited in features. You can do all the basic things like writing or changing sentences, creating or changing section headings, and editing simple bulleted lists. It currently can't either add or remove templates (like fact tags), ref tags, images, categories, or tables (and it will not be turned on for new users until common reference styles and citation templates are supported). These more complex features are being worked on, and the code will be updated as things are worked out. Also, right now you can only use it for articles and user pages. When it's deployed in July, the old editor will still be available and, in fact, the old edit window will be the only option for talk pages (I believe that WP:Notifications (aka Echo) is ultimately supposed to deal with talk pages).
The developers are asking editors like you to join the alpha testing for the VisualEditor. Please go to Special:Preferences#mw-prefsection-editing and tick the box at the end of the page, where it says "Enable VisualEditor (only in the main namespace and the User namespace)". Save the preferences, and then try fixing a few typos or copyediting a few articles by using the new "Edit" tab instead of the section [Edit] buttons or the old editing window (which will still be present and still work for you, but which will be renamed "Edit source"). Fix a typo or make some changes, and then click the 'save and review' button (at the top of the page). See what works and what doesn't. We really need people who will try this out on 10 or 15 pages and then leave a note Wikipedia:VisualEditor/Feedback about their experiences, especially if something mission-critical isn't working and doesn't seem to be on anyone's radar.
Also, if any of you are involved in template maintenance or documentation about how to edit pages, the VisualEditor will require some extra attention. The devs want to incorporate things like citation templates directly into the editor, which means that they need to know what information goes in which fields. Obviously, the screenshots and instructions for basic editing will need to be completely updated. The old edit window is not going away, so help pages will likely need to cover both the old and the new.
If you have questions and can't find a better place to ask them, then please feel free to leave a message on my user talk page, and perhaps together we'll be able to figure it out. WhatamIdoing (talk) 01:12, 7 May 2013 (UTC)
- Correction: Talk pages are being replaced by mw:Flow, not by Notifications/Echo. This may happen even sooner than the VisualEditor. WhatamIdoing (talk) 14:43, 7 May 2013 (UTC)
IP editor whose edits may need a closer look
While checking my watchlist, I discovered 145.48.182.105 making a tediously pedantic edit to the Body weight article (which I reverted). This user made similar edits to medical articles like Low birth weight, which I've also undone, and several chemistry articles. I initially wholesale reverted the chemistry edits until I discovered that Sbharris (talk · contribs) had modified this IP's edits at Atomic mass, which made me realize that they may have some merit. The only chemistry-related revert I've let stand is this one. Could some editors who are familiar with the subject check out the edits more closely? I only did chemistry up to year 10 in high school. Thanks! Graham87 06:04, 7 May 2013 (UTC)
- Edits shouldn't be reverted because they are "tediously pedantic". They should be reverted because they vandalism, or because they are detrimental to the quality of the article in some way or another. 'Tediously pedantic edits' fall into neutral territory. Plasmic Physics (talk) 07:02, 7 May 2013 (UTC)
- Yes, what I meant was "so tediously pedantic that they're detrimental to the article". For example, have you ever heard of low birth mass? With most of the edits to chemistry articles, they seem pedantic at a glance (perhaps tediously so), but I can't tell whether this pedanticism harms the articles in these cases. That's why I notified this project. Graham87 10:45, 7 May 2013 (UTC)
- Yes, that is an example of a wreckless edit. Such wrecklessness merits concern. Though I don't agree with "so tediously pedantic that they're detrimental to the article", in that the two ideas aren't related. Although, I can clearly determine that it is besides your point. Plasmic Physics (talk) 11:47, 7 May 2013 (UTC)
- I've been asked to "mass in" on this conversation. ;) The IP editor in question is not a vandal and may not even be that pedantic. Mass is slowly starting to replace weight in most science appliations, and if you google "low birth mass" with the quotes in place you will find that it is used in very many places and much literature. Not quite as many as "low birth weight" but gaining. The same is true of body weight and body mass. Atomic weight is likewise slowly (very slowly) giving way to relative atomic mass.
Personally, there's no problem reverting people who make such bold changes, but if they aren't vandals of course you don't revert them like vandals. Personally I try to revert recent changes always by simply saving an old copy, so I can put a curt RVV for vandals, but add a polite edit summary suitable for good faith but clueless users, like Reverting; you might be right, but this is tricky, so please discuss it on TALK. Everybody's blood pressure goes up on being reverted (happens still to me here, even after seven years) so that edit summary is important for good-will users.
In this case it would give us a chance to explain to our IP editor that although mass and weight are of course not the same, and yes) weight is a force and mass is not, and (yes) "mass" not "weight" is what is actually meant in most science applications, still the terminology hasn't caught up, and "weight" remains the most common term, and the one we must use on WP until mass becomes more commonly used. But since weight and mass differ by a (nearly) constant factor at rest on Earth, for those applications, weight can be safely used as a proxy for mass, as it often has been historically. (Although a mass balance in any kind of accelerated frame does actually measure mass against a standard mass, not weight-- a mass balance would work as well on the Moon]. For applications where great precision isn't needed (like baby masses) no harm is done by measuring weight with a spring scale and saying "mass" or "weight". To measure high precision atomic weights, metrologists actually do use mass balances, correct for buoyancy or use vacuum, and all kinds of other things to make sure they are measuring masses not weights, so no harm is done there, either. But see the atomic weight page for the history.
Interestingly, while we all agree easily that there is a crucial difference between mass and weight, it is astonishingly hard to define either one of them in science, as they have slightly different definitions even at the upper division text level, which haven't been agreed on. Take a look at the wars on the TALK pages of both pages. The articles and their TALK pages should be required reading, whenever anybody blithely decides to change one to the other in other WP articles. SBHarris 01:53, 8 May 2013 (UTC)
- Thanks for your massy and weightive comment. I admit I do have an itchy trigger finger on that rollback button, sometimes. Graham87 02:50, 8 May 2013 (UTC)
- I've been asked to "mass in" on this conversation. ;) The IP editor in question is not a vandal and may not even be that pedantic. Mass is slowly starting to replace weight in most science appliations, and if you google "low birth mass" with the quotes in place you will find that it is used in very many places and much literature. Not quite as many as "low birth weight" but gaining. The same is true of body weight and body mass. Atomic weight is likewise slowly (very slowly) giving way to relative atomic mass.
Racemates and angry anons
There's an anon on talk:Cypenamine who's very angry about a particular phrase in the article; I tried to mediate the issue by suggesting another way to interpret what the original editor may have intended, and the anon blew up at me for Using Big Words I Clearly Don't Understand. I'll admit it's been a few years since I worked in a lab, and I may have had somewhat low blood sugar when I wrote that, so I'd appreciate a less furious set of eyes. DS (talk) 02:55, 7 May 2013 (UTC)
- I tried, he 'ditoed' me. Perhaps there is a behavioural warning of some sort we can post on his talkpage? Plasmic Physics (talk) 03:35, 7 May 2013 (UTC)
- He also just posted on my talkpage calling me stupid in no uncertain terms. Plasmic Physics (talk) 05:02, 7 May 2013 (UTC)
- This looks like it is the same person as the one who was using 122.109.127.168 (who is blocked). Same use of language, also active on same article. I'll keep an eye, quick blocks to curb this may be in order, this is not the way other editors are to be addressed. --Dirk Beetstra T C 06:23, 7 May 2013 (UTC)
- Having looked (briefly) at the reference, there may actually be an issue with this statement - but I don't think it's the one he's kicking-up a fuss over. The statement is "[the] trans isomer is reported to be more active than the racemate", I think most people would assume this refered to its effectiveness as a drug. However the reference is a paper on the kinetic resolution of the cis and trans isomers, where the greater reactivity of the trans isomers was expoited to allow their seperation. The seperated and modified isomers were then tested against a single enzyme, with the trans again being more reactive. So, the statement isn't wrong but it may be misleading and the ref is a very narrow one. The anon is still very rude and the extent of his rage over some poor wording is a little baffling. Project Osprey (talk) 08:47, 7 May 2013 (UTC)
Hello I am the culprit anon in question (now OneAngryScientist) and this is all organic chem 101. The statment in question "[the] trans isomer is reported to be more active than the racemate" has no meaning unless a (+ or -) enantiomer is specified. So as I said to Rifleman82 it was probably a miss quote accidently dropping this from the designation of the trans isomer enantiomer they were pro-porting to be most active (as a stimulant?). Now @ Project Osprey (Phd chem)!, (where do I begin?)
- 1) My issue was obviously nothing to do with biological activity.
- 2) Resolution is a term applied to the separation of optical isomers (enantiomers) NOT (cis-trans isomers), which are (Diastereomers) and may be separated much more easily.
- 3) My rage stemmed from a long history of trying to explain this (VERY BASIC) concept to many editors that obviously did not understand the simple logic of the issue. Maybe I assumed a higher degree of understanding than I should have when trying to express my point.--OneMadScientist (talk) 02:20, 8 May 2013 (UTC)
- I know you're acting out of good faith so let me offer some advice: being rude will make it harder for you to have any possitive effect here. Just look at how much effort you've had to put in so far; you've been at this 2 weeks and haven't really gotten anywhere. I appreciate that must make things fustrating for you - but if you had simply clarified your point to begin with, rather than criticising pople, this would likely have been sortd by now. The statement might mean that one trans-enantiomer is more reactive than another or that the trans-enantiomers are more reactive than the cis-enantiomrs. My point was that it doesn't matter, because the paper isn't talking about the drug's biological activity, which is the only kind of activity anyone is likely to be interested in. Let me see if I can find some better references and hopefully we can sort this all out. Project Osprey (talk) 09:35, 8 May 2013 (UTC)
- Osprey, I advise you for your peace of mind, not to contend with him any longer. You are civil and wise, but he is intent on disregarding your and my council to his own destruction. As a consequence, neither of our proposals will be regarded either. I'm only saying this out of respect for you, but do as you think is right. Plasmic Physics (talk) 13:31, 9 May 2013 (UTC)
Thanks for bullet pointing my statements to you Osprey, However it appears that all the clarification in the world would be wasted on trying to reason with, (or provide understanding of my totally coherent point, to), you. I, with out a doubt, have made and supported my argument with consistently 100% correct SIMPLE logic. There is so much wrong and/or incoherent within your statements, but I will say (NOW!)one thing only to you. How do You Believe that you are making a scientifically logical (meaningful in ANY WAY) statement When you say "The statement is "[the] trans isomer is reported to be more active than the racemate", I think most people would assume this referred to its effectiveness as a drug." and then, next in relation to that quote " So, the statement isn't wrong but it may be misleading". I assure you that the bit in between that I removed does not in any way lend calcification or support in favor of your statement having meaning (in-particular that the statment I had issue with is, in fact, not meaning less - WHICH IT IS - full stop). Please keep your response, if any, simple (KISS principle) and logical if possible and not divert (what you perceive as) my issue with this statement (which should be as simple as 2+2=4 for a Bsc chem) into something more "complex!??" than it actually is. Sorry if I appear rude but I am now actually very tired and sick, literally ,and a bit foggy in the head, and I feel like I am being nibbled to death by ducks here for want of a more suitable analogy! I am surprised DS and plasma physics have not also chimed in with there 2 cents worth (which would also appear a highly overvalued currency in this issue atleast) sorry couldn't help my self. --OneMadScientist (talk) 10:39, 9 May 2013 (UTC)
da,da - He blinded me with Science!!!--OneMadScientist (talk) 11:27, 9 May 2013 (UTC)
- I'm not going to dignify that with a response. Plasmic Physics (talk) 12:17, 9 May 2013 (UTC)
Good idea--OneMadScientist (talk) 12:21, 9 May 2013 (UTC)
Hello again Osprey - I Just checked out your homepage again (talk). I through skim reading, and the references to articles, interests etc, thought you were saying that you have completed a phd thesis in chemistry, but from the way that it is actually worded your educational qualifications could have nothing to do with chemistry!??. so sorry if I assumed a level of traditionally acquired knowledge that you do not actually possess.--OneMadScientist (talk) 12:32, 9 May 2013 (UTC) intelectual
As you're only talking to me, I think we should continue this conversation on my talk page. I don't think the other editors are interested in picking through another one of your 10 post replies. Project Osprey (talk) 13:15, 9 May 2013 (UTC)
Could not be bothered--OneMadScientist (talk) 13:36, 9 May 2013 (UTC)
I will however here make one more (crazy) post for you to wade through. Would you tell us all (on your homepage at least) what was the subject of your PHD thesis (so scary) and what was your bsc in (if you have one) maybe a (bachelor of arts) perhaps. I would presume It is not good form for an editor to misrepresent their academic qualifications (even through carefully constructed wording giving a plausible deniability type defense). This is somewhat of a de-facto talk page of mine (in the actual subject in question specifically) I suppose (it all about me :) and my huge intellectual ego and arrogant behavior!?) AND UNINTELAGEABLE POSTS? so I don't understand your reluctance to discuss these issues here at least the directly related statement one/s. I shudder at the fact that you are involved in the Enantioselctive Synthesis page and something to do with Enantioselctive Catalysis and chiral ligands. I have not checked them out as yet Hopefully others have corrected all your mistakes allready. when I was studying Wikipedia was not acceptable as a source for information (and hopefully still is not) and I can obviously understand why. Anyway mostly all these posts of mine stem from replies to ignorant half cocked posts of others and are I think (obviously due to my huge ego) mine are the most intelligible informative and accurate and to the point/s. And the disciples came, and said unto him, Why speakest thou unto them in parables? And he said, it is given unto you to know the mysteries of the kingdom of heaven, but to them it is not (it is in riddles!!)!--OneMadScientist (talk) 15:00, 9 May 2013 (UTC)
@Plasma Physics - I posted a comment (including an apology for my rudeness to you) on your talk page at 13:37 which I will not repet here This was before I saw your comment to project osprey posted at 13;31 above (beacause I thought you had wisely realized you were out of your depth and decided not to comment on the science, civility is another issue).and I probably would not have But I will once again forgive your ignorance in this issue. Ouote " Osprey, I advise you for your peace of mind, not to contend with him any longer. You are civil and wise, but he is intent on disregarding your and my council to his own destruction. As a consequence, neither of our proposals will be regarded either. I'm only saying this out of respect for you, but do as you think is right. Plasmic Physics (talk) 13:31, 9 May 2013 (UTC)"
I can pretty much 100% guarantee you (from the basis of my interaction with project ospery) that I am both wiser and more knowledgeable(in this area) (and apparently more intelligent in general) than project osprey (although when roused to anger by repeated blind ignorance less civil sure probably). Osprey consistently misses the point entirely. I don't know what point you were trying to make was/is but my humble advice to you is to carefully read my first post in this section (where this whole thing should have ended) with oh I was blind but now I see and study the definitions in the links which upon glancing at appear at least in general definition correct. And without meaning to be rude, I am quite confident that my ability to avoid the "destruction" of which you speak (in this subject at the very, very least) will be much better served by not relying on either of your councils.--OneMadScientist (talk) 15:48, 9 May 2013 (UTC)

