Eszopiclone: Difference between revisions
Dockingman (talk | contribs) Reverted 1 edit by 67.163.212.185 (talk): Undo rm comment without justification. (TW) |
|||
| Line 156: | Line 156: | ||
==Availability in Europe== |
==Availability in Europe== |
||
On September 11, 2007, Sepracor signed a marketing deal with British pharmaceutical company [[GlaxoSmithKline]] for the rights to sell Eszopiclone (under the name '''Lunivia''' rather than Lunesta) in [[Europe]].<ref name=autogenerated1>[http://www.gsk.com/media/pressreleases/2007/2007_09_11_GSK1114.htm GlaxoSmithKline and Sepracor Inc. announce international alliance for commercialisation of Lunivia]</ref> Sepracor was expected to receive approximately 155 million dollars if the deal went through.<ref name=autogenerated1 /> In 2008 Sepracor submitted an application to the [[European Medicines Agency|EMA]] (the European Union's equivalent to the US [[Food and Drug Administration|FDA]]) for authorization to market the drug in the EU, and initially received a favourable response.<ref>[http://www.ema.europa.eu/docs/en_GB/document_library/Other/2010/02/WC500070840.pdf COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USEm SUMMARY OF POSITIVE OPINION for LUNIVIA] - [[European Medicines Agency]]/[[Committee for Medicinal Products for Human Use]], 23 Oct 2010</ref> However Sepracor withdrew its authorization application in 2009 after the EMA stated it would not be granting eszopiclone 'new active substance' status, as it was essentially pharmacologically and therapeutically too similar to [[zopiclone]] to be considered a new patentable product.<ref>[http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2009/11/news_detail_000083.jsp&jsenabled=true Sepracor Pharmaceuticals Ltd withdraws its marketing authorisation application for Lunivia (eszopiclone)] - [[European Medicines Agency]], 15 May 2009</ref> Since zopiclone's [[patent]] has expired, this ruling would have allowed rival companies to also legally produce cheaper [[Generic drug|generic]] versions of eszopiclone for the European market.<ref>[http://www.twobirds.com/English/News/Articles/Pages/Data_exclusivity_definition_new_active_substance.Aspx Data exclusivity and definition of a new active substance: suspension of generic escitalopram-containing medicines by CHMP] - Bird and Bird Commercial Law 23 Apr 2010</ref> {{As of|2011|1}}, Sepracor has not resubmitted its authorization application and Lunesta/Lunivia is not available in Europe. The deal with GSK has fallen through,{{Citation needed|date=January 2011}} and GSK have instead launched a $3.3 billion deal to market Actelion's [[Almorexant]] sleeping tablet.{{Citation needed|date=January 2011}} |
On September 11, 2007, Sepracor signed a marketing deal with British pharmaceutical company [[GlaxoSmithKline]] for the rights to sell Eszopiclone (under the name '''Lunivia''' rather than Lunesta) in [[Europe]].<ref name=autogenerated1>[http://www.gsk.com/media/pressreleases/2007/2007_09_11_GSK1114.htm GlaxoSmithKline and Sepracor Inc. announce international alliance for commercialisation of Lunivia]</ref> Sepracor was expected to receive approximately 155 million dollars if the deal went through.<ref name=autogenerated1 /> In 2008 Sepracor submitted an application to the [[European Medicines Agency|EMA]] (the European Union's equivalent to the US [[Food and Drug Administration|FDA]]) for authorization to market the drug in the EU, and initially received a favourable response.<ref>[http://www.ema.europa.eu/docs/en_GB/document_library/Other/2010/02/WC500070840.pdf COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USEm SUMMARY OF POSITIVE OPINION for LUNIVIA] - [[European Medicines Agency]]/[[Committee for Medicinal Products for Human Use]], 23 Oct 2010</ref> However Sepracor withdrew its authorization application in 2009 after the EMA stated it would not be granting eszopiclone 'new active substance' status, as it was essentially pharmacologically and therapeutically too similar to [[zopiclone]] to be considered a new patentable product.<ref>[http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2009/11/news_detail_000083.jsp&jsenabled=true Sepracor Pharmaceuticals Ltd withdraws its marketing authorisation application for Lunivia (eszopiclone)] - [[European Medicines Agency]], 15 May 2009</ref> Since zopiclone's [[patent]] has expired, this ruling would have allowed rival companies to also legally produce cheaper [[Generic drug|generic]] versions of eszopiclone for the European market.<ref>[http://www.twobirds.com/English/News/Articles/Pages/Data_exclusivity_definition_new_active_substance.Aspx Data exclusivity and definition of a new active substance: suspension of generic escitalopram-containing medicines by CHMP] - Bird and Bird Commercial Law 23 Apr 2010</ref> {{As of|2011|1}}, Sepracor has not resubmitted its authorization application and Lunesta/Lunivia is not available in Europe. The deal with GSK has fallen through,{{Citation needed|date=January 2011}} and GSK have instead launched a $3.3 billion deal to market Actelion's [[Almorexant]] sleeping tablet, currently undergoing stage three medical trials.{{Citation needed|date=January 2011}} |
||
==See also== |
==See also== |
||
Revision as of 12:05, 29 March 2012
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Lunesta |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a605009 |
| License data |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 52-59% |
| Metabolism | Hepatic oxidation and demethylation (CYP3A4 and CYP2E1-mediated) |
| Elimination half-life | 6 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.149.304 |
| Chemical and physical data | |
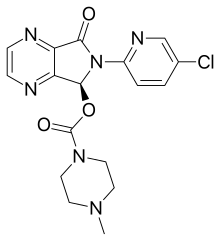
| Formula | C17H17ClN6O3 |
| Molar mass | 388.808 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Eszopiclone, marketed by Sepracor under the brand-name Lunesta, is a nonbenzodiazepine hypnotic used as a treatment for insomnia. Eszopiclone is the active dextrorotatory stereoisomer of zopiclone, and belongs to the class of drugs known as cyclopyrrolones.
Eszopiclone is a short acting nonbenzodiazepine sedative hypnotic. According to its manufacturer, it has been shown to be safe and effective for the short term treatment of primary insomnia in the elderly and safe in younger adults for 6–12 months. All clinical trials of eszopiclone published so far have been funded by the manufacturer of eszopiclone, Sepracor.[3] There were 43 million prescriptions issued for insomnia medications during 2005 in the USA which generated a total of $2.7 billion for pharmaceutical companies.[4] Eszopiclone (Lunesta) along with other "Z Drugs" including zolpidem (Ambien), zaleplon (Sonata) are the most commonly prescribed sedative hypnotics in the USA.
Eszopiclone is not marketed in the European Union following a 2009 decision by the EMA denying it new active substance status,[5] in which it ruled that eszopiclone was too similar to zopiclone to be considered a new patentable product.[6]
Pharmacology
Eszopiclone acts on benzodiazepine binding site situated on GABAA neurons as an agonist.[7] Eszopiclone is rapidly absorbed after oral administration, with serum levels peaking between 1 and 1.3 hours.[8] The elimination half-life of eszopiclone is approximately 6 hours and it is extensively metabolized by oxidation and demethylation. Approximately 52% to 59% of a dose is weakly bound to plasma protein. Cytochrome P450 (CYP) isozymes CYP3A4 and CYP2E1 are involved in the biotransformation of eszopiclone; thus, drugs that induce or inhibit these CYP isozymes may affect the metabolism of eszopiclone. Less than 10% of the orally administered dose is excreted in the urine as racemic zopiclone.[9] In terms of benzodiazepine receptor binding and relevant potency, 3 mg of eszopiclone is equivalent to 10 mg of diazepam.[10]
Indications
Eszopiclone's mechanism of action is via the benzodiazepine receptor-GABA complex. Other drugs which are similar to eszopiclone and also work via the benzodiazepine receptor-GABA complex include benzodiazepines, zaleplon, and zolpidem.
Dosages
For treatment to improve sleep onset and/or sleep maintenance the recommended dose is 2 mg–3 mg for adult patients (aged 18–64 years) and 2 mg for older adult patients aged 65 years or older. The 1 mg dose is for older adult patients whose problems are related to sleep onset.[11]
Side effects
Eszopiclone has fewer anticholinergic side effects than racemic zopiclone.[12] The following side effects may occur from usage of eszopiclone (Lunesta):[13]
Common side effects can include:
- unpleasant bitter or metallic taste
- headaches
- chest pain
- cold-like symptoms
- pain
- dry mouth
- daytime drowsiness
- lightheadedness
- dizziness
- upset stomach
- decreased sexual desire
- painful menstruation (periods)
- heartburn
- Breast enlargement in males
Less common side effects can include:
- rashes
- itching
- incoordination
- swelling of the hands, feet, ankles, or lower legs
- painful or frequent urination
- back pain
neuropsychiatric adverse effects reported include;[14]
- aggressive behavior
- confusion
- agitation
- auditory and visual hallucinations
- worsening of depression
- suicidal thoughts
- depersonalisation
- amnesia
If a person does not sleep immediately after taking eszopiclone or if they get up shortly after taking the medication they may experience dizziness, lightheadedness, hallucinations (seeing things or hearing voices that are not there), as well as problems with coordination and memory.
Increased risk of depression
Some researchers have claimed that insomnia causes depression and hypothesized that insomnia medications may help to treat depression. However, an analysis of data of clinical trials submitted to the FDA concerning the drugs zolpidem, zaleplon and eszopiclone found that these sedative hypnotic drugs more than doubled the risks of developing depression compared to those taking placebo pills. Hypnotic drugs therefore may be contraindicated in patients suffering from or at risk of depression. Hypnotics were found to be more likely to cause depression than to help it. Studies have found that long term users of sedative hypnotic drugs have a markedly raised suicide risk as well as an overall increased mortality risk.
Elderly
Sedative hypnotic drugs including eszopiclone are more commonly prescribed to the elderly than to younger patients despite benefits of medication being generally unimpressive. Care should be taken in choosing an appropriate hypnotic drug and if drug therapy is initiated it should be initiated at the lowest possible dose to minimise side effects.[15] An extensive review of the medical literature regarding the management of insomnia and the elderly found that there is considerable evidence of the effectiveness and durability of non-drug treatments for insomnia in adults of all ages and that these interventions are underutilized. Compared with the benzodiazepines, the nonbenzodiazepine sedative-hypnotics, including eszopiclone appeared to offer few, if any, significant clinical advantages in efficacy or tolerability in elderly persons. It was found that newer agents with novel mechanisms of action and improved safety profiles, such as the melatonin agonists, hold promise for the management of chronic insomnia in elderly people. Long-term use of sedative-hypnotics for insomnia lacks an evidence base and has traditionally been discouraged for reasons that include concerns about such potential adverse drug effects as cognitive impairment (anterograde amnesia), daytime sedation, motor incoordination, and increased risk of motor vehicle accidents and falls. In addition, the effectiveness and safety of long-term use of these agents remain to be determined. It was concluded that more research is needed to evaluate the long-term effects of treatment and the most appropriate management strategy for elderly persons with chronic insomnia.[16]
Dependence
In the United States Eszopiclone is a schedule IV controlled substance under the Controlled Substances Act. Use of benzodiazepines and similar benzodiazepine-like drugs such as eszopiclone may lead to physical and psychological dependence. The risk of abuse and dependence increases with the dose and duration of usage and concomitant use of other psychoactive drugs. The risk is also greater in patients with a history of alcohol or drug abuse or history of psychiatric disorders. Tolerance may develop after repeated use of benzodiazepines and benzodiazepine-like drugs for a few weeks. Eszopiclone was studied for up to 6 months in a group of patients which showed no signs of tolerance or dependence in a study funded and carried out by Sepracor. [17]
Abuse
A study of abuse potential of eszopiclone found that in persons with a known history of benzodiazepine abuse, eszopiclone at doses of 6 and 12 mg produced effects similar to those of diazepam 20 mg . The study found that at these doses which are two or more times greater than the maximum recommended doses, a dose-related increase in reports of amnesia and hallucinations was observed for both eszopiclone (lunesta) as well as for diazepam (Valium).[13]
Withdrawal symptoms
If a person has taken Eszopiclone for longer than 1–2 weeks they should not stop taking the medication abruptly[citation needed] and should consult their doctor. Usually doctors will direct a slow reduction in dosage to minimise withdrawal symptoms. Particularly after abrupt cessation of medication, withdrawal symptoms may include:[citation needed]
- anxiety
- unusual dreams
- dizziness
- stomach and muscle cramps
- upset stomach
- vomiting
- sweating
- shakiness
- insomnia
- seizures (rare)
Overdose
Eszopiclone is dangerous in overdose. Signs of eszopiclone overdose reported included dulled mental status, ST-elevation coronary vasospasm, troponemia, ventricular fibrillation arrest and prolonged coma (lasting up to 48 hours).[18][19] Texas poison control centers reported that during 2005-2006 there were 525 total eszopiclone overdoses recorded in the state of Texas, the majority of which were intentional suicide attempts.[20]
Controversy
The Journal of Clinical Sleep Medicine published a paper which had carried out a systematic review of the medical literature concerning insomnia medications including eszopiclone. The review found that almost all trials of sleep disorders and drugs are sponsored by the pharmaceutical industry. It was found that the odds ratio for finding results favorable to industry in industry-sponsored trials was 3.6 times higher than non-industry-sponsored studies. The paper found that there is little research into hypnotics that is independent from the drug manufacturers. The author was concerned that there is no discussion of adverse effects of sedative hypnotics discussed in the medical literature such as significant increased levels of infection, cancers and increased mortality in eszopiclone and other sedative hypnotic drugs and an overemphasis on the positive effects. The author concluded by stating that "major hypnotic trials are needed to more carefully study potential adverse effects of hypnotics such as daytime impairment, infection, cancer, and death and the resultant balance of benefits and risks."[21]
In a 2009 article in the New England Journal of Medicine, "Lost in Transmission — FDA Drug Information That Never Reaches Clinicians", it was reported that the largest of three Lunesta trials found that compared to placebo Lunesta "was superior to placebo" while it only shortened initial time falling asleep by 15 minutes on average. "Clinicians who are interested in the drug’s efficacy cannot find efficacy information in the label: it states only that Lunesta is superior to placebo. The FDA’s medical review provides efficacy data, albeit not until page 306 of the 403-page document. In the longest, largest phase 3 trial, patients in the Lunesta group reported falling asleep an average of 15 minutes faster and sleeping an average of 37 minutes longer than those in the placebo group. However, on average, Lunesta patients still met criteria for insomnia and reported no clinically meaningful improvement in next-day alertness or functioning."[22]
Availability in Europe
On September 11, 2007, Sepracor signed a marketing deal with British pharmaceutical company GlaxoSmithKline for the rights to sell Eszopiclone (under the name Lunivia rather than Lunesta) in Europe.[23] Sepracor was expected to receive approximately 155 million dollars if the deal went through.[23] In 2008 Sepracor submitted an application to the EMA (the European Union's equivalent to the US FDA) for authorization to market the drug in the EU, and initially received a favourable response.[24] However Sepracor withdrew its authorization application in 2009 after the EMA stated it would not be granting eszopiclone 'new active substance' status, as it was essentially pharmacologically and therapeutically too similar to zopiclone to be considered a new patentable product.[25] Since zopiclone's patent has expired, this ruling would have allowed rival companies to also legally produce cheaper generic versions of eszopiclone for the European market.[26] As of January 2011[update], Sepracor has not resubmitted its authorization application and Lunesta/Lunivia is not available in Europe. The deal with GSK has fallen through,[citation needed] and GSK have instead launched a $3.3 billion deal to market Actelion's Almorexant sleeping tablet, currently undergoing stage three medical trials.[citation needed]
See also
References
- ^ WHO International Working Group for Drug Statistics Methodology (August 27, 2008). "ATC/DDD Classification (FINAL): New ATC 5th level codes". WHO Collaborating Centre for Drug Statistics Methodology. Archived from the original on 2008-05-06. Retrieved 2008-09-05.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ McCrae CS (2007). "Eszopiclone for late-life insomnia". Clin Interv Aging. 2 (3): 313–26. PMC 2685268. PMID 18044182.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ McKenzie WS (2007). "What every dentist should know about the "z-sedatives"". J Mass Dent Soc. 56 (53): 44–5. PMID 18069595.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Sepracor Pharmaceuticals Ltd withdraws its marketing authorisation application for Lunivia (eszopiclone) - EMA, 15 may 2009
- ^ End of Sepracor-GSK Deal Raises Question in Lunesta Patent Fight - CBS/BNet, 13 Jun 2009
- ^ Jufe GS (2007). "[New hypnotics: perspectives from sleep physiology]". Vertex. 18 (74): 294–9. PMID 18265473.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Halas CJ (January 1, 2006). "Eszopiclone". Am J Health Syst Pharm. 63 (1): 41–8. doi:10.2146/ajhp050357. PMID 16373464.
- ^ Najib J (2006). "Eszopiclone, a non-benzodiazepine sedative-hypnotic agent for the treatment of transient and chronic insomnia". Clin Ther. 28 (4): 491–516. doi:10.1016/j.clinthera.2006.04.014. PMID 16750462.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Professor Ashton (2007). "BENZODIAZEPINE EQUIVALENCE TABLE". Retrieved 21 March 2008.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ &Na;, (2005). "Eszopiclone: esopiclone, estorra, S-zopiclone, zopiclone–Sepracor". Drugs R D. 6 (2): 111–5. doi:10.2165/00126839-200506020-00006. PMID 15777104.
{{cite journal}}: CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ Patil PA, Kothekar MA (2006). "Development of safer molecules through chirality". Indian J Med Sci. 60 (10): 427–37. doi:10.4103/0019-5359.27676. PMID 17006031.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: unflagged free DOI (link) - ^ a b rxlist. "Lunesta". Retrieved 22 March 2008.
- ^ Duggal HS (2007). "New-onset transient hallucinations possibly due to eszopiclone: a case study" (PDF). Prim Care Companion J Clin Psychiatry (PDF). 9 (6): 468–9. doi:10.4088/PCC.v09n0611e. PMC 2139930. PMID 18185832.
- ^ Tariq SH, Pulisetty S (2008). "Pharmacotherapy for insomnia". Clin Geriatr Med. 24 (1): 93–105, vii. doi:10.1016/j.cger.2007.08.009. PMID 18035234.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Bain KT (2006). "Management of chronic insomnia in elderly persons". Am J Geriatr Pharmacother. 4 (2): 168–92. doi:10.1016/j.amjopharm.2006.06.006. PMID 16860264.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Brielmaier BD (2006). "Eszopiclone (Lunesta): a new nonbenzodiazepine hypnotic agent". Proc (Bayl Univ Med Cent). 19 (1): 54–9. PMC 1325284. PMID 16424933.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Lovett B (2007). "Prolonged coma after eszopiclone overdose". Am J Emerg Med. 25 (6): 735 (e5–6). doi:10.1016/j.ajem.2006.12.021. PMID 17606111.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Miller AH, Bruggman AR, Miller MM (2006). "Lunesta overdose: ST-elevation coronary vasospasm, troponemia, and ventricular fibrillation arrest". Am J Emerg Med. 24 (6): 741–6. doi:10.1016/j.ajem.2006.02.001. PMID 16984850.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Forrester MB (2007). "Eszopiclone ingestions reported to Texas poison control centers, 2005 2006". Hum Exp Toxicol. 26 (10): 795–800. doi:10.1177/0960327107084045. PMID 18025051.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Kripke DF (December 15, 2007). "Who should sponsor sleep disorders pharmaceutical trials?". J Clin Sleep Med. 3 (7): 671–3. PMC 2556906. PMID 18198797.
- ^ Schwartz, Lisa M. (2009). "Lost in Transmission — FDA Drug Information That Never Reaches Clinicians" (Online). New England Journal of Medicine. 361 (18). NEJM.org: 1717–1720. doi:10.1056/NEJMp0907708. PMID 19846841. Retrieved 2010-12-06.
{{cite journal}}: Cite has empty unknown parameters:|laysource=,|laydate=, and|laysummary=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ a b GlaxoSmithKline and Sepracor Inc. announce international alliance for commercialisation of Lunivia
- ^ COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USEm SUMMARY OF POSITIVE OPINION for LUNIVIA - European Medicines Agency/Committee for Medicinal Products for Human Use, 23 Oct 2010
- ^ Sepracor Pharmaceuticals Ltd withdraws its marketing authorisation application for Lunivia (eszopiclone) - European Medicines Agency, 15 May 2009
- ^ Data exclusivity and definition of a new active substance: suspension of generic escitalopram-containing medicines by CHMP - Bird and Bird Commercial Law 23 Apr 2010
