Pixantrone
| |
| Names | |
|---|---|
| IUPAC name
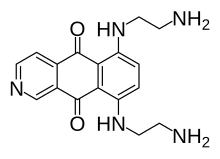
6,9-bis[(2-aminoethyl)amino]benzo[g]isoquinoline-5,10-dione
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C17H19N5O2 | |
| Molar mass | 325.365 g/mol |
| Pharmacology | |
| Intravenous | |
| Pharmacokinetics: | |
| 9.5–17.5 hours | |
| Fecal (main route of excretion) and renal (4–9%) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Pixantrone (rINN, codenamed BBR2778) is an immunosuppressant drug, an analogue of mitoxantrone with less toxic effects on cardiac tissue.[1]
It acts as a topoisomerase II poison and intercalating agent.[2]
Uses
Anthracyclines are important oncotherapeutics; however, their use is associated with irreversible and cumulative heart damage. Pixantrone was developed to reduce heart damage related to treatment while retaining efficacy. [3] It also can be administered through a peripheral vein rather than a central implanted catheter as required for other drugs in this class[4].
Cancer
It is being studied as an antineoplastic for different kinds of cancer, including solid tumors and hematological malignancies such as non-Hodgkin lymphomas.
Animal studies demonstrated that pixantrone does not worsen pre-existing heart muscle damage, suggesting that pixantrone may be useful in patients pretreated with anthracyclines. While only minimal cardiac changes are observed in mice given repeated cycles of pixantrone, 2 cycles of traditional anthracyclines doxorubicin or mitoxantrone result in marked or severe heart muscle degeneragion. [3]
Clinical trials substituting pixantrone for doxorubicin in standard first-line treatment of patients with aggressive non-Hodgkin's lymphoma (NHL), had a reduction in severe (grade 3/4) side effects when compared to patients treated with standard doxorubicin-based therapy. Despite pixantrone patients receiving more treatment cycles, a three-fold reduction in the incidence of severe heart damage (LVEF decline >15 percent) was seen as well as clinically significant reductions in infections and thrombocytopenia, and a significant reduction in febrile neutropenia. These findings could have major implications for treating patients with breast cancer, lymphoma, and leukemia, where debilitating cardiac damage from doxorubicin might be prevented. [5]
Multiple Sclerosis
It is also as potent as mitoxantrone in animal models of multiple sclerosis. [6] The potential efficacy in multiple sclerosis of immunosuppressants used in cancer therapy has received attention. However, no human trial results have yet been published. Pixantrone could be studied for it's ability to effect potent immunosuppression in MS similar to off-label treatments of MS with mitoxantrone, but with potentially less cardiotoxicity. Suggestions that early administration of potent immunosuppressants is definitely more effective than approved immunomodulators [7] such as interferon beta-1a[2] to delay or even reverse disability progression will need to be newly compared to the successful immunomodulator Fingolimod, which may demonstrate [8] the superiority of immunomodulation.
Myasthenia Gravis
Pixantrone reduces the severity of experimental autoimmune myasthenia gravis in Lewis rats.[9]
Alzheimer's disease
In vitro cell viability experiments indicated that pixantrone significantly reduces amyloid beta (A beta(1-42)) neurotoxicity. Soluble and toxic oligomers of A beta protein have been identified as the true neurotoxic species involved in Alzheimer's disease. [10]
Allergic Encephalomyelitis
Pixantrone has a similar mechanism of action as mitoxantrone on the effector function of lymphomonocyte B and T cells in experimental allergic encephalomyelitis but with lower cardiotoxicity. pixantrone inhibits antigen specific and mitogen induced lymphomononuclear cell proliferation, as well as IFN-gamma production[1].
Clinical Research
As of 2009, several phase III clinical trials of pixantrone are underway.[11][12]
Previous treatment options for multiply relapsed aggressive non-Hodgkin lymphoma had disappointing response rates. [13] The completed phase II RAPID (PIX203) trial compared the standard CHOP-R regimen of Cyclophosphamide, Doxorubicin, Vincristine, Prednisone, and Rituximab to the same regimen, but substituting Doxorubicin with Pixantrone. The objective was to show that Pixantrone was not inferior to CHOP-R and less toxic to the heart. [14] Pixantrone was shown to have potentially reduced cardiotoxicity and demonstrated promising clinical activity in these phase II studies in heavily pretreated non-Hodgkin lymphoma patients. [15]
A Potential Treatment for non-Hodgkin’s Lymphoma
The pivotal phase III EXTEND (PIX301) randomized clinical trial studied pixantrone to see how well it works compared to other chemotherapy drugs in treating patients with relapsed non-Hodgkin's lymphoma. [16] The complete response rate in patients treated with pixantrone has been significantly higher than in those receiving other chemotherapeutic agents for treatment of relapsed/refractory aggressive non-Hodgkin lymphoma.
| Pixantrone (n=70) | Control (n=70) | P value | |
|---|---|---|---|
| Complete Response (CR/CRu) | 20% (14) | 5.7% (4) | p=0.021 |
| Overall Response Rate (ORR) | 37% (26) | 14.3% (10) | p=0.003 |
Table 1. Response Rates and Number of Responses in treatement of non-Hodgkin lymphoma[17]
Regulatory Approval Process
U.S. Food and Drug Administration
Study sponsor Cell Therapeutics announced that Pixantrone achieved the primary efficacy endpoint, and in April 2009 began a rolling submission of a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) for pixantrone to treat relapsed or refractory aggressive NHL. They will request priority review by FDA. The FDA granted fast track designation for pixantrone in third-line (or more) treatment of relapsed or refractory aggressive NHL[18].
European Medicines Evaluation Agency
On May 5, 2009, Pixantrone became available in Europe on a Named-Patient Basis. A named-patient program is a compassionate use drug supply program under which physicians can legally supply investigational drugs to qualifying patients. Under a named-patient program, investigational drugs can be administered to patients who are suffering from serious illnesses prior to the drug being approved by the European Medicines Evaluation Agency. "Named-patient" distribution refers to the distribution or sale of a product to a specific healthcare professional for the treatment of an individual patient. In Europe, under the named-patient program the drug is most often purchased through the national health system. "Our experience with pixantrone has been positive with patients achieving a complete response where such a result was not achievable with other treatments," said Prof. Pier Luigi Zinzani, M.D., Institute of Hematology and Oncology, University of Bologna. "I am pleased that it is now available on a named-patient basis as it has the potential to address a significant unmet need in this heavily pretreated patient population." [19]
References
- ^ a b Mazzanti B, Biagioli T, Aldinucci A; et al. (2005). "Effects of pixantrone on immune-cell function in the course of acute rat experimental allergic encephalomyelitis". J. Neuroimmunol. 168 (1–2): 111–7. doi:10.1016/j.jneuroim.2005.07.010. PMID 16120465.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) Cite error: The named reference "pmid16120465" was defined multiple times with different content (see the help page). - ^ Evison BJ, Mansour OC, Menta E, Phillips DR, Cutts SM (2007). "Pixantrone can be activated by formaldehyde to generate a potent DNA adduct forming agent". Nucleic Acids Res. 35 (11): 3581–9. doi:10.1093/nar/gkm285. PMC 1920253. PMID 17483512.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Cavalletti E, Crippa L, Mainardi P, Oggioni N, Cavagnoli R, Bellini O, Sala F. (2007). "Pixantrone (BBR 2778) has reduced cardiotoxic potential in mice pretreated with doxorubicin: comparative studies against doxorubicin and mitoxantrone". Invest New Drugs. 25 (3): 187–95. PMID 17285358.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ http://news.prnewswire.com/DisplayReleaseContent.aspx?ACCT=104&STORY=/www/story/05-15-2009/0005026831
- ^ http://www.prnewswire.com/cgi-bin/stories.pl?ACCT=104&STORY=/www/story/07-11-2007/0004623260 Pixantrone Combination Therapy for First-line Treatment of Aggressive Non-Hodgkin's Lymphoma Results in Reduction in Severe Toxicities Including Heart Damage When Compared to Doxorubicin-based Therapy
- ^ Gonsette RE, Dubois B (2004). "Pixantrone (BBR2778): a new immunosuppressant in multiple sclerosis with a low cardiotoxicity". J. Neurol. Sci. 223 (1): 81–6. doi:10.1016/j.jns.2004.04.024. PMID 15261566.
- ^ Gonsette RE (2007). "Compared benefit of approved and experimental immunosuppressive therapeutic approaches in multiple sclerosis". Expert Opin Pharmacother. 8 (8): 1103–16. PMID 17516874.
- ^ http://www.novartis.com/newsroom/media-releases/en/2009/1309396.shtml
- ^ Ubiali F, Nava S, Nessi V, et al. Pixantrone (BBR2778) reduces the severity of experimental autoimmune myasthenia gravis in Lewis rats. J Immunol. 2008 Feb 15;180(4):2696-703.
- ^ Electrophoresis. 2009 Apr;30(8):1418-29. CE can identify small molecules that selectively target soluble oligomers of amyloid beta protein and display antifibrillogenic activity. Colombo R, Carotti A, Catto M, Racchi M, Lanni C, Verga L, Caccialanza G, De Lorenzi E.
- ^ BBR 2778 for Relapsed, Aggressive Non-Hodgkin's Lymphoma (NHL). ClinicalTrials.gov (2007-08-30). Retrieved on 2007-11-06.
- ^ Fludarabine and Rituximab With or Without Pixantrone in Treating Patients With Relapsed or Refractory Indolent Non-Hodgkin Lymphoma. ClinicalTrials.gov (2007-10-25). Retrieved on 2007-11-06.
- ^ http://www.abstract.asco.org/AbstView_65_35306.html
- ^ http://clinicaltrials.gov/ct2/show/NCT00268853 A Trial in Patients With Diffuse Large-B-Cell Lymphoma Comparing Pixantrone Against Doxorubicin (RAPID) Retrieved on 2009-05-16
- ^ http://www.abstract.asco.org/AbstView_65_35306.html Randomized phase III trial of pixantrone compared with other chemotherapeutic agents for third-line single-agent treatment of relapsed aggressive non-Hodgkin's lymphoma. J Clin Oncol 27:15s, 2009 (suppl; abstr 8523)
- ^ Pixantrone or Other Chemotherapy Drugs in Treating Patients With Relapsed Non-Hodgkin's Lymphoma. ClinicalTrials.gov (2007-10-25). Retrieved on 2007-11-06.
- ^ http://www.abstract.asco.org/AbstView_65_35306.html
- ^ http://www.celltherapeutics.com/pixantrone
- ^ http://news.prnewswire.com/DisplayReleaseContent.aspx?ACCT=104&STORY=/www/story/05-05-2009/0005019037
