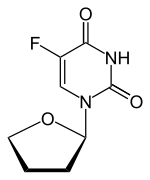
Tegafur
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 5-fluoro-1-(oxolan-2-yl)pyrimidine-2,4-dione |
| AHFS/Drugs.com | International Drug Names |
| License data | |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | 3.9-11 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.038.027 |
| Chemical and physical data | |
| Formula | C8H9FN2O3 |
| Molar mass | 200.169 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Tegafur is a chemotherapeutic prodrug of 5-fluorouracil (5-FU) used in the treatment of cancers. It is a component of the combination drug tegafur/uracil. When metabolised, it becomes 5-FU.[1]
It was patented in 1967 and approved for medical use in 1972.[2]
Medical uses
[edit]As a prodrug to 5-FU it is used in the treatment of the following cancers:[3]
- Stomach (when combined with gimeracil and oteracil)
- Breast (with uracil)
- Gallbladder
- Lung (specifically adenocarcinoma, typically with uracil)
- Colorectal (usually when combined with gimeracil and oteracil)
- Head and neck
- Liver (with uracil)[4]
- Pancreatic
It is often given in combination with drugs that alter its bioavailability and toxicity such as gimeracil, oteracil or uracil.[3] These agents achieve this by inhibiting the enzyme dihydropyrimidine dehydrogenase (uracil/gimeracil) or orotate phosphoribosyltransferase (oteracil).[3]
Adverse effects
[edit]The major side effects of tegafur are similar to fluorouracil and include myelosuppression, central neurotoxicity and gastrointestinal toxicity (especially diarrhoea).[3] Gastrointestinal toxicity is the dose-limiting side effect of tegafur.[3] Central neurotoxicity is more common with tegafur than with fluorouracil.[3]
Pharmacogenetics
[edit]The dihydropyrimidine dehydrogenase (DPD) enzyme is responsible for the detoxifying metabolism of fluoropyrimidines, a class of drugs that includes 5-fluorouracil, capecitabine, and tegafur.[5] Genetic variations within the DPD gene (DPYD) can lead to reduced or absent DPD activity, and individuals who are heterozygous or homozygous for these variations may have partial or complete DPD deficiency; an estimated 0.2% of individuals have complete DPD deficiency.[5][6] Those with partial or complete DPD deficiency have a significantly increased risk of severe or even fatal drug toxicities when treated with fluoropyrimidines; examples of toxicities include myelosuppression, neurotoxicity and hand-foot syndrome.[5][6]
Mechanism of action
[edit]It is a prodrug to 5-FU, which is a thymidylate synthase inhibitor.[3]
Pharmacokinetics
[edit]It is metabolised to 5-FU by CYP2A6.[7][8]
Interactive pathway map
[edit]Click on genes, proteins and metabolites below to link to respective articles.[§ 1]
- ^ The interactive pathway map can be edited at WikiPathways: "FluoropyrimidineActivity_WP1601".
See also
[edit]References
[edit]- ^ El Sayed YM, Sadée W (September 1983). "Metabolic activation of R,S-1-(tetrahydro-2-furanyl)-5-fluorouracil (ftorafur) to 5-fluorouracil by soluble enzymes". Cancer Research. 43 (9): 4039–4044. PMID 6409396.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 511. ISBN 9783527607495.
- ^ a b c d e f g Sweetman S, ed. (14 November 2011). "Martindale: The Complete Drug Reference". Pharmaceutical Press. Retrieved 12 February 2014.
- ^ Ishikawa T (May 2008). "Chemotherapy with enteric-coated tegafur/uracil for advanced hepatocellular carcinoma". World Journal of Gastroenterology. 14 (18): 2797–2801. doi:10.3748/wjg.14.2797. PMC 2710718. PMID 18473401.
- ^ a b c Caudle KE, Thorn CF, Klein TE, Swen JJ, McLeod HL, Diasio RB, Schwab M (December 2013). "Clinical Pharmacogenetics Implementation Consortium guidelines for dihydropyrimidine dehydrogenase genotype and fluoropyrimidine dosing". Clinical Pharmacology and Therapeutics. 94 (6): 640–645. doi:10.1038/clpt.2013.172. PMC 3831181. PMID 23988873.
- ^ a b Amstutz U, Froehlich TK, Largiadèr CR (September 2011). "Dihydropyrimidine dehydrogenase gene as a major predictor of severe 5-fluorouracil toxicity". Pharmacogenomics. 12 (9): 1321–1336. doi:10.2217/pgs.11.72. PMID 21919607.
- ^ Nakayama T, Noguchi S (January 2010). "Therapeutic usefulness of postoperative adjuvant chemotherapy with Tegafur-Uracil (UFT) in patients with breast cancer: focus on the results of clinical studies in Japan". The Oncologist. 15 (1): 26–36. doi:10.1634/theoncologist.2009-0255. PMC 3227888. PMID 20080863.
- ^ Matt P, van Zwieten-Boot B, Calvo Rojas G, Ter Hofstede H, Garcia-Carbonero R, Camarero J, et al. (October 2011). "The European Medicines Agency review of Tegafur/Gimeracil/Oteracil (Teysuno™) for the treatment of advanced gastric cancer when given in combination with cisplatin: summary of the Scientific Assessment of the Committee for medicinal products for human use (CHMP)". The Oncologist. 16 (10): 1451–1457. doi:10.1634/theoncologist.2011-0224. PMC 3228070. PMID 21963999.

