Glufosfamide
| |
| Names | |
|---|---|
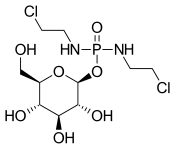
| IUPAC name
(2S,3R,4S,5S,6R)-2-bis(2-chloroethylamino)phosphoryloxy-6-(hydroxymethyl)oxane-3,4,5-triol
| |
| Other names
β-D-glucosylisophosphoramide mustard
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C10H21Cl2N2O7P | |
| Molar mass | 383.162702 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Glufosfamide, also known as glucophosphamide, D-glucose isophosphoramide mustard, D-19575[1] is an experimental cytotoxic chemotherapeutic agent for treatment of malignancies.
Chemical structure
Glufosfamide is, basically, a glycosidic conjugate between β-D-glucose and the active alkylating moiety of the well-known antineoplasic drug ifosfamide, so-called "isophosphoramide mustard".
Theoretical advantages
Glufosfamide, being a conjugate of glucose and active alkylating moiety of ifosfamide, has the better cell permeability than the parent compound — ifosfamide — or its metabolites. Glufosfamide utilizes the normal cell glucose transport mechanism (a sodium-dependent glucose/sodium co-transporter) for its own transport into the cell. And the glucose uptake mechanism is grossly overexpressed and upregulated in certain cancer cell lines, especially pancreatic cancer, non-small cell lung cancer, and glioblastoma multiforme. This, theoretically, should render them more sensitive to the alkylating effects of glufosfamide while relatively sparing (doing relatively little collateral damage) to the normal cells in which the glucose uptake mechanism is not so upregulated.[2]
Clinical trials
Glufosfamide has successfully passed Phase I and Phase II of clinical trials in the treatment of pancreatic cancer, non-small cell lung cancer and recurrent glioblastoma multiforme and began Phase III trials.[3] Subsequently, however, further development was discontinued by Baxter Oncology, without any clear explanation for the reason of discontinuation.
References
- ^ Mazur L, Opydo-Chanek M, Stojak M. Glufosfamide as a new oxazaphosphorine anticancer agent. Anticancer Drugs. 2011 Jul; 22 (6) :488-93. doi:10.1097/CAD.0b013e328345e1e0, PMID 21427562.
- ^ Glufosfamide: beta-D-Glc -IPM, D 19575.Drugs R D. 2005, 6 (1) :49-52, PMID 15801867.
- ^ Liang J, Huang M, Duan W, Yu XQ, Zhou S. Design of new oxazaphosphorine anticancer drugs. Curr Pharm Des. 2007, 13 (9) :963-78, PMID 17430192.
