Lactoferrin: Difference between revisions
LinkFA-Bot (talk | contribs) m Bot: Link GA +ru |
expand |
||
| Line 2: | Line 2: | ||
--> |
--> |
||
{{PBB|geneid=4057}} |
{{PBB|geneid=4057}} |
||
'''Lactoferrin''' (LF), also known as '''lactotransferrin''' (LTF), is a [[ |
'''Lactoferrin''' (LF), also known as '''lactotransferrin''' (LTF), is a multifunctional [[protein]] of the [[transferrin]] family. Lactoferrin is a [[globular proteins|globular]] [[glycoprotein]] with a molecular mass of about 80 [[Atomic mass unit|kDa]] which is widely represented in various secretory fluids, such as [[milk]], [[saliva]], [[tears]] and [[Mucus|nasal secretions]]. Lactoferrin is also present in secondary granules of [[Neutrophil granulocyte|PMN]] and is secreted by some [[acinar cells]]. Lactoferrin can be purified from milk or produced [[Recombinant DNA|recombinantly]]. Human [[colostrum]] (''"first milk"'') has the highest concentration, followed by human milk, then cow milk (150 mg/L).<ref name="pmid1599309"/> |
||
Lactoferrin is one of the components of the [[immune system]] of the body; it has antimicrobial activity ([[bacteriocide]], [[fungicide]]) and is part of the innate defense, mainly at mucoses.<ref name="pmid1599309">{{cite journal|author = Sánchez L, Calvo M, Brock JH|title = Biological role of lactoferrin|journal = Arch. Dis. Child.|volume = 67|issue = 5|pages = 657–61|year = 1992|pmid = 1599309|pmc = 1793702}}</ref> In particular, lactoferrin provides [[Antiseptic|antibacterial]] activity to human infants.<ref>{{cite book|url=http://books.google.com/books?id=E3bvD2jU4B0C&pg=PA1028|page=1028|title=Food biotechnology|author=Kalidas Shetty|publisher=CRC Press|year=2006|isbn=0824753291}}</ref><ref>{{cite book|url=http://books.google.com/books?id=Ts41TBTi9QMC&pg=PA191|page=191|title=Animal breeding: technology for the 21st century|author=A. John Clark|publisher=CRC Press|year=1998|isbn=9057022923}}</ref> Lactoferrin interacts with [[DNA]] and [[RNA]], [[polysaccharides]] and [[heparin]], and shows some of its biological functions in complexes with these [[ligand]]s. |
|||
| ⚫ | |||
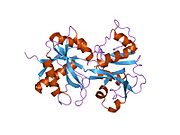
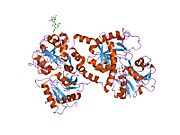
Lactoferrin belongs to the transferrin family proteins ([[transferrin|TF]], melanotransferrin, ovotransferin, etc.). Its molecular mass is 80,000 [[atomic mass unit|u]] (80 [[dalton (unit)|kDa]]). It generally contains two bound Fe<sup>+3</sup> ions. It contains 4 identical domains, with two surrounding each iron atom.<ref name="pmid18541155">{{cite journal | author = Baker EN, Baker HM | title = A structural framework for understanding the multifunctional character of lactoferrin | journal = Biochimie | volume = 91 | issue = 1 | pages = 3–10 | year = 2009 | month = January | pmid = 18541155 | doi = 10.1016/j.biochi.2008.05.006 | url = | issn = }}</ref> |
|||
== |
==History== |
||
Occurrence of iron-containing red protein in bovine milk was reported at least in 1939;<ref>M. Sorensen and S. P. L. Sorensen, Compf. rend. trav. lab. Carlsberg (1939) 23, 55, cited by Groves (1960)</ref> however, it could not be properly characterized due to insufficient purity. First detailed characterizations of this protein were reported around 1960. They documented the molecular weight, isoelectronic point, optical absorption spectra and presence of two iron atoms per protein molecule.<ref>{{cite journal|doi=10.1021/ja01498a029}}</ref><ref name=j60/> The protein was extracted from milk, contained iron and was structurally and chemically similar to [[Blood serum|serum]] [[transferrin]]. Therefore, it was named lactoferrin in 1961, though the name lactotransferrin was used in some earlier publications, and later studies demonstrated that the protein is not restricted to milk. Also in 1961 the antibacterial action of lactoferrin has been documented and associated with its ability to bind iron.<ref>{{cite book|url=http://books.google.com/books?id=2oTsweiwImAC&pg=PA2|pages=1-2|title=Lactoferrin: natural, multifunctional, antimicrobial|author=A. S. Naidu|publisher=CRC Press|year=2000|isbn=0849309093}}</ref> |
|||
Lactoferrin's antimicrobial activity is due partly to its high affinity for [[iron|Fe<sup>3+</sup>]] (ferric state). LF proteolysis produces the small peptides lactoferricin and kaliocin-1 with antimicrobial activity. The combination of iron and lactoferrin in mucosal secretions modulates the ability and aggregation of pathogenic bacteria, and inhibits both bacteria and viruses from binding to host cells. It is also an antifungal agent.{{Citation needed|date=October 2009}} |
|||
| ⚫ | |||
Lactoferrin receptors have been found on brush-border cells, PMN, monocytes, [[macrophages]] and activated lymphocytes.<ref name="pmid7672721">{{cite journal | author = Levay PF, Viljoen M | title = Lactoferrin: a general review | journal = Haematologica | volume = 80 | issue = 3 | pages = 252–67 | year = 1995 | pmid = 7672721 | doi = | url = | issn = }}</ref> |
|||
=== Molecular structure === |
|||
Lactoferrin is one of the transferrin proteins which transfer [[iron]] to the cells and control the level of free iron in the blood and external secretions. It is present in the milk of humans and other mammals,<ref name=j60>Johansson, B. Isolation of an iron-containing red protein from human milk. (1960) Acta Chem. Scand., 14, 510–512.</ref> in the [[blood plasma]] and [[neutrophil]]s and is one of the major proteins of virtually all exocrine secrets of mammals, such as [[saliva]], [[gall]], [[tears]] and [[pancreas]].<ref>{{cite journal|pmid=3858982}}</ref> Concentration of lactoferrin in the milk varies from 7 g/L in the [[colostrum]] to 1 g/L in mature milk. |
|||
[[X-ray crystallography|X-ray diffraction]] reveals that lactoferrin is based on one [[polypeptide]] chain which contains about 700 amino acids and forms two homologous globular [[domain]]s named N-and C-lobes. N-lobe corresponds to amino acid residues 1-333 and C-lobe to 345-692, and the ends of those domains are connected by a short α-helix.<ref> Baker, H. M., Anderson, B. F., Kidd, R. D., Shewry, S. C., and Baker, E. N. Lactoferrin three-dimensional structure: a framework for interpreting function. In Lactoferrin: Structure, Function and Application (Shimazaki, K., ed.). (2000) pp. 3 - 15, Elsevier Science, Amsterdam.</ref><ref>{{cite journal|pmid=16261257}}</ref> Each lobe consists of two subdomains, N1, N2 and C1, C2, and contains one iron binding site and one [[glycosylation]] site. The degree of glycosylation of the protein may be different and therefore the molecular weight of lactoferrin varies between 76 and 80 kDa. The stability of lactoferrin has been associated with the high glycosylation degree.<ref>{{cite journal|author=Hakansson, A., Zhivotovsky, B., Orrenius, S., Sabharwal, H., Svanborg, C. |title=Apoptosis induced by a human milk protein |year=1995|journal=Proc. Natl. Acad. Sci. U S A|volume=92|pages=8064-8068|pmc=41287}}</ref> |
|||
Lactoferrin inhibits dendritic cell-mediated HIV-1 transmission by blocking the binding of the HIV protein [[gp120]] to the cellular receptor [[DC-SIGN]], which is a critical binding interaction that never changes regardless of strain.<ref>{{cite journal |author=Groot F, Geijtenbeek TB, Sanders RW, ''et al.'' |title=Lactoferrin prevents dendritic cell-mediated human immunodeficiency virus type 1 transmission by blocking the DC-SIGN--gp120 interaction |journal=J. Virol. |volume=79 |issue=5 |pages=3009–15 |year=2005 |month=March |pmid=15709021 |pmc=548463 |doi=10.1128/JVI.79.5.3009-3015.2005 |url=}}</ref> |
|||
Lactoferrin belongs to the basic proteins, its [[isoelectric point]] is 8.7. It exists in two forms: iron-rich hololactoferrin and iron-free apolactoferrin. Their tertiary structures are different; apolactoferrin is characterized by "open" conformation of the N-lobe and the "closed" conformation of the C-lobe, and both lobes are closed in the hololactoferrin.<ref>{{cite journal|pmid=10089508}}</ref> |
|||
==Genetics== |
|||
In humans, the lactoferrin gene (LTF) is located on chromosome 3; location: 3q21-q23.<ref name="pmid3356163">{{cite journal | author = McCombs JL, Teng CT, Pentecost BT, Magnuson VL, Moore CM, McGill JR | title = Chromosomal localization of human lactotransferrin gene (LTF) by in situ hybridization | journal = Cytogenet. Cell Genet. | volume = 47 | issue = 1-2 | pages = 16–7 | year = 1988 | pmid = 3356163 | doi = | url = | issn = }}</ref> |
|||
Each lactoferrin molecule can reversibly bind two ions of iron, [[zinc]], [[copper]] or other metals.<ref>{{cite journal|pmid=7672721}}</ref> The binding sites are localized in each of the two protein globules. There, each ion is bonded with six ligands: four from the polypeptide chain (two [[tyrosine]] residues, one [[histidine]] residue and one [[aspartic acid]] residue) and two from [[carbonate]] or [[bicarbonate]] ions. |
|||
| ⚫ | |||
Lactoferrin forms reddish complex with iron; its affinity for iron is 300 times higher than that of [[transferrin]].<ref>Mazurier J., SpikG. Comparative study of the iron-binding properties of human transferring. Complete and sequential iron saturation and desaturation of the lactotransferrin. (1980) Biochim. Biophys. Acta, 629, 399-408.</ref> The affinity increases in weakly acidic medium. This facilitates the transfer of iron from transferrin to lactoferrin during [[inflammation]]s, when the pH of tissues decreases due to accumulation of [[lactic acid|lactic]] and other acids.<ref name=Sousa> Sousa, M., Brock, JH, Iron in immunity. Cancer and Inflammation (1989) John Wiley & Sons ISBN 0471921505</ref> The saturated iron concentration in lactoferrin in [[human milk]] is estimated as 10 to 30% (100% corresponds to all lactoferrin molecules containing 2 iron atoms). It is demonstrated that lactoferrin is involved not only in the transport of iron, zinc and copper, but also in the regulation of their intake.<ref>{{cite journal|doi=10.1021/bi00133a010}}</ref> Presence of loose ions of zinc and copper does not affect the iron binding ability of lactoferrin, and might even increase it. |
|||
| ⚫ | The human lung as well as saliva contain a wide range of antimicrobial compound including lactoperoxidase system producing [[hypothiocyanite]] and lactoferrin while hypothiocyanite is missing in [[cystic fibrosis]] patients.<ref name="pmid17082494">{{cite journal |
||
=== Polymeric forms=== |
|||
| ⚫ | Lactoferrin with hypothiocyanite has been granted [[orphan drug]] status by the [[European Medicines Agency|EMEA]]<ref name="urlwww.ema.europa.eu">{{cite web |
||
Both in blood plasma and in secretory fluids lactoferrin can exist in different polymeric forms ranging from [[monomer]]s to [[tetramer]]s. Lactoferrin tends to polymerize both ''in vitro'' and ''in vivo'', especially at high concentrations.<ref Name=Sousa /> Several authors found that the dominant form of lactoferrin in physiological conditions is a tetramer, with the monomer:tetramer ratio of 1:4 at the protein concentrations of 10<sup>−5</sup> M.<ref name=Bennett> Bennett, R.M., Davis, J. Lactoferrin interacts with deoxyribonucleic acid: a preferential reactivity with double-stranded DNA and dissociation of DNA-anti-DNA complexes. (1982) J. Lab. Clin. Med., 99, 127-138.</ref><ref>{{cite journal|pmid=6979357}}</ref><ref>{{cite journal|pmid=7762423}}</ref> |
|||
It is suggested that the [[oligomer]] state of lactoferrin is determined its concentration and that [[polymerization]] of lactoferrin is strongly affected by the presence of Ca<sup>2+</sup> ions. In particular, monomers were dominant at concentrations below 10<sup>−10</sup>−10<sup>−11</sup> M in the presence of Ca<sup>2+</sup>, but they converted into tetramers at lactoferrin concentrations above 10<sup>−9</sup>−10<sup>−10</sup> M.<ref name=Furmanski>{{cite journal|pmid=2754391}}</ref><ref name=Bennett /> [[Titer]] of lactoferrin in the blood corresponds to this particular "transition concentration" and thus lactoferrin in the blood should be presented both as a monomer and tetramer. Many functional properties of lactoferrin depend on its oligomeric state. In particular, monomeric, but not tetrameric lactoferrin can strongly bind to DNA. |
|||
| ⚫ | |||
| ⚫ | |||
== Biological functions == |
|||
| ⚫ | |||
Lactoferrin belongs to the [[innate immune system]]. Apart from its main biological function, namely binding and transport of iron ions, lactoferrin also has antibacterial, antiviral, [[antiparasitic]], catalytic, anti-cancer, anti-allergic and radioprotecting functions and properties.<ref>{{cite journal|url=http://www.vri.cz/docs/vetmed/53-9-457.pdf|title=Lactoferrin: a review|journal=Veterinarni Medicina|volume=53|year=2008 |volume=9|page=457}}</ref> |
|||
| ⚫ | |||
=== Antibacterial activity === |
|||
==Further reading== |
|||
Antibacterial activity of lactoferrin is best studied; it originates from the iron binding properties of lactoferrin which deprive the bacterial flora from an element necessary for its growth.<ref name=Farnaud>{{cite journal|pmid=14568385}}</ref> Antibacterial action of lactoferrin is also explained by the presence of specific [[receptor]]s on the cell surface of microorganisms. Lactoferrin binds to lipopolysaccharide of bacterial walls, and the oxidized iron part of the lactoferrin oxidizes bacteria via formation of [[peroxide]]s. This affects the membrane permeability and results in the cell breakdown ([[lysis]]).<ref Name=Farnaud /> |
|||
{{refbegin | 2}} |
|||
* {{cite journal | author = Shin K, Wakabayashi H, Yamauchi K, Teraguchi S, Tamura Y, Kurokawa M, Shiraki K | title = Effects of orally administered bovine lactoferrin and lactoperoxidase on influenza virus infection in mice | journal = J. Med. Microbiol. | volume = 54 | issue = Pt 8 | pages = 717–23 | year = 2005 | month = August | pmid = 16014423 | doi = 10.1099/jmm.0.46018-0 | url = | issn = }} |
|||
Although lactoferrin has also other, not related to iron, antibacterial mechanisms, such as stimulation of phagocytosis,<ref>{{cite journal|pmid=9691154}}</ref> the described above interaction with the outer bacterial membrane is the dominant and most studied.<ref>{{cite journal|pmid=8612745}}</ref> Lactoferrin not only disrupts the membrane, but even penetrates into the cell. Its binding to the bacteria wall is associated with the specific [[peptide]] [[lactoferricin]], which is located at the N-lobe of lactoferrin and is produced by ''in vitro'' cleavage of lactoferrin with another protein, [[trypsin]].<ref>{{cite journal|pmid=9588189}}</ref><ref name=Sojar>{{cite journal|pmid=9490007}}</ref> |
|||
* {{cite journal | author = Ganz T | title = Antimicrobial polypeptides in host defense of the respiratory tract | journal = J. Clin. Invest. | volume = 109 | issue = 6 | pages = 693–7 | year = 2002 | month = March | pmid = 11901174 | pmc = 150915 | doi = 10.1172/JCI15218 | url = | issn = }} |
|||
* {{cite journal | author = Britigan BE, Hayek MB, Doebbeling BN, Fick RB | title = Transferrin and lactoferrin undergo proteolytic cleavage in the Pseudomonas aeruginosa-infected lungs of patients with cystic fibrosis | journal = Infect. Immun. | volume = 61 | issue = 12 | pages = 5049–55 | year = 1993 | month = December | pmid = 8225581 | pmc = 281282 | doi = | url = | issn = }} |
|||
=== Antiviral activity=== |
|||
{{PBB_Further_reading |
|||
Lactoferrin acts, mostly ''in vitro'', on a wide range of human and animal viruses based on DNA and RNA [[genome]]s,<ref name=vander>{{cite journal|pmid=11675140|url=http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.104.745&rep=rep1&type=pdf}}</ref> including the [[herpes simplex virus]] 1 and 2,<ref>{{cite journal|pmid=7661698}}</ref><ref name=Giansanti>{{cite journal|pmid=11908636}}</ref> [[cytomegalovirus]],<ref>{{cite journal|pmid=7622881}}</ref> [[HIV]],<ref name=Giansanti /><ref name=Puddi>{{cite journal|pmid=9785469}}</ref> [[hepatitis C virus]],<ref>{{cite journal|pmid=17241377}} Erratum in: Liver Int. Apr; 27 (3): 421.</ref><ref name=Nozaki>{{cite journal|pmid=12522210}}</ref> [[hantavirus]]es, [[rotavirus]]es, [[poliovirus]] type 1,<ref name=Sojar /> [[adenovirus]]es,<ref name=Arnold>{{cite journal|pmid=11750941}}</ref> [[human respiratory syncytial virus]] and [[murine leukemia virus]]es.<ref Name=Sojar /> |
|||
| citations = |
|||
*{{cite journal | author=van der Strate BW, Beljaars L, Molema G, ''et al.'' |title=Antiviral activities of lactoferrin |journal=Antiviral Res. |volume=52 |issue= 3 |pages= 225–39 |year= 2002 |pmid= 11675140 |doi=10.1016/S0166-3542(01)00195-4 }} |
|||
The most studied mechanism of antiviral activity of lactoferrin is its diversion of virus particles from the target cells. Many viruses tend to bind to the [[lipoprotein]]s of the cell membranes and then penetrate into the cell.<ref Name=Nozaki /> Lactoferrin binds to the same lipoproteins thereby repelling the virus particles. Iron-free apolactoferrin is more efficient in this function than hololactoferrin; and lactoferricin, which is responsible for antimicrobial properties of lactoferrin, shows almost no antiviral activity.<ref name=vander /> |
|||
*{{cite journal | author=Weinberg ED |title=Human lactoferrin: a novel therapeutic with broad spectrum potential |journal=J. Pharm. Pharmacol. |volume=53 |issue= 10 |pages= 1303–10 |year= 2002 |pmid= 11697537 |doi=10.1211/0022357011777792 }} |
|||
*{{cite journal | author=Valenti P, Antonini G |title=Lactoferrin: an important host defence against microbial and viral attack |journal=Cell. Mol. Life Sci. |volume=62 |issue= 22 |pages= 2576–87 |year= 2006 |pmid= 16261253 |doi= 10.1007/s00018-005-5372-0 }} |
|||
Beside interacting with the cell walls, lactoferrin also directly binds to viral particles, such as the [[hepatitis]] viruses.<ref Name=Nozaki /> This mechanism is also confirmed by the antiviral activity of lactoferrin against rotaviruses,<ref name=Sojar /> which act on different cell types. |
|||
*{{cite journal | author=Ward PP, Paz E, Conneely OM |title=Multifunctional roles of lactoferrin: a critical overview |journal=Cell. Mol. Life Sci. |volume=62 |issue= 22 |pages= 2540–8 |year= 2006 |pmid= 16261256 |doi= 10.1007/s00018-005-5369-8 }} |
|||
}} |
|||
Lactoferrin also suppresses virus replication after the virus penetrated into the cell.<ref Name=Sojar /><ref name=Puddi /> Such an indirect antiviral effect is achieved by affecting [[natural killer cell]]s, [[granulocyte]]s and [[macrophage]]s – cells, which play a crucial role in the early stages of viral infections, such as [[severe acute respiratory syndrome]] (SARS).<ref>{{cite journal|author = Renji Reghunathan, Manikandan Jayapal, Li-Yang Hsu, Hiok-Hee Chng, Dessmon Tai, Bernard P Leung and Alirio J Melendez|title = Expression profile of immune response genes in patients with Severe Acute Respiratory Syndrome|journal = BMC Immunol|year = 2005|issue = 6|pages = 2|url = http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=15655079|doi=10.1186/1471-2172-6-2}}</ref> |
|||
{{refend}} |
|||
=== Antifungal activity=== |
|||
Lactoferrin and lactoferricin inhibit ''in vitro'' growth of ''Trichophyton mentagrophytes'', which are responsible for several skin diseases such as [[ringworm]].<ref>{{cite journal|pmid=11020258}}</ref> Lactoferrin also acts against the ''[[Candida albicans]]'' – a [[diploid]] [[fungus]] (a form of [[yeast]]) which causes [[Opportunistic infection|opportunistic]] oral and [[genital]] infections in humans.<ref name=Lupetti>{{cite journal|pmid=12499200}}</ref><ref name=Viejo>{{cite journal|pmid=15047526}}</ref> [[Fluconazole]] has long been used against ''Candida albicans'' that resulted in emergence of [[strain]]s resistant to this drug. |
|||
However, a combination of lactoferrin with fluconazole can act against fluconazole-resistant strains of ''Candida albicans'' as well as other types of [[Candida]]: ''C. glabrata, C. krusei, C. parapsilosis'' and ''C. tropicalis''.<ref name=Lupetti /> Antifungal activity is observed for sequential incubation of ''Candida'' with lactoferrin and then with fluconazole, but not vice versa. The antifungal activity of lactoferricin exceeds that of lactoferrin. In particular, synthetic peptide 1-11 lactoferricin shows much greater activity against ''Candida albicans'' than native lactoferricin.<ref name=Lupetti /> |
|||
Administration of lactoferrin through drinking water to mice with weakened immune systems and symptoms of [[aphthous ulcer]] reduced the number of ''Candida albicans'' strains in the mouth and the size of the damaged areas in the tongue.<ref>{{cite journal|pmid=12878528}}</ref> Oral administration of lactoferrin to animals also reduced the number of pathogenic organisms in the tissues close to the [[gastrointestinal tract]]. ''Candida albicans'' could also be completely eradicated with a mixture containing lactoferrin, [[lysozyme]] and [[introakonazol]] in HIV-positive patients who were resistant to other antifungal drugs.<ref>{{cite journal|pmid=11089630}}</ref> Such antifungal action when other drugs deem inefficient is characteristic of lactoferrin and is especially valuable for HIV-infected patients.<ref>{{cite journal|pmid=10543740}}</ref> |
|||
Contrary to the antiviral and antibacterial actions of lactoferrin, very little is known about the mechanism of its antifungal action. Lactoferrin seem to destroy the cell wall and binds the [[plasma membrane|plasma membrane]] of ''C. albicans''.<ref name=Viejo /> |
|||
=== Interaction with nucleic acids === |
|||
One of the important properties of lactoferrin is its ability to bind with nucleic acids. The fraction of protein extracted from milk, contains 3.3% RNA,<ref name=Bennett /> |
|||
besides, the protein preferably binds to the double-stranded then to the single-stranded DNA. The ability of lactoferrin to bind DNA is used for the isolation and purification of lactoferrin using [[affinity chromatography]] with columns containing immobilized DNA-containing [[sorbent]]s, such as [[agarose]] with the immobilized single-stranded DNA.<ref>{{cite journal|pmid=3827843}}</ref> |
|||
=== Enzymatic activity of lactoferrin === |
|||
Lactoferrin hydrolyzes [[RNA]] and exhibits the properties of [[pyrimidine]]-specific secretory [[ribonuclease|ribonucleases]]. In particular, by destroying the RNA genome, milk RNase inhibits reverse transcription of [[retrovirus]]es that cause [[breast cancer]] in mice.<ref>{{cite journal|pmid=4139659}}</ref> [[Parsi]] women in West [[India]] have the milk RNase level markedly lower than in other groups, and their [[breast cancer]] rate is three times higher than average.<ref>{{cite journal|doi=10.1038/262802a0}}</ref> Thus, [[ribonuclease]]s of milk, and lactoferrin in particular, might play an important role in [[pathogenesis]] of diseases caused by various [[retrovirus]]es. |
|||
== Genes of lactoferrin== |
|||
At least 60 gene sequences of lactoferrin have been characterized in 11 species of mammals.<ref Name=Jing>{{cite journal|author = Jing-Fen Kang, Xiang-Long Li, Rong-Yan Zhou, Lan-Hui Li, Fu-Jun Feng and Xiu -Li Guo|title = Bioinformatics Analysis of Lactoferrin Gene for Several Species|journal = Biochemical Genetics|year = 2008|volume = 46|issue = 5-6|pages = 312-322}}</ref> In most species, [[stop codon]] is TAA, and TGA in ''[[Mus musculus ]]''. Deletions, insertions and mutations of stop codons affect the coding part and its length varies between 2,055 and 2,190 [[nucleotide]] pairs. Gene polymorphism between species is much more diverse than the intraspecific polymorphism of lactoferrin. There are differences in amino acid sequences: 8 in ''[[Homo sapiens ]]'', 6 in ''[[Mus musculus ]]'', 6 in ''[[Capra hircus ]]'', 10 in ''[[Bos taurus]]'' and 20 in ''[[Sus scrofa ]]''. This variation may indicate functional differences between different types of lactoferrin.<ref Name=Jing /> |
|||
In humans, lactoferrin gene ''LTF'' is located on the third [[chromosome]] in the [[locus]] 3q21-q23. In [[ox]]en, the coding sequence consists of 17 [[exon]]s and has a length of about 34,500 [[nucleotide]] pairs. Exons of the lactoferrin gene in oxen have a similar size to the exons of other genes of the [[transferrin]] family, whereas the sizes of introns differ within the family. Similarity in the size of exons and their distribution in the domains of the protein molecule indicates that the evolutionary development of lactoferrin gene occurred by duplication.<ref>{{cite journal|author = Seyfert HM, Tuckoricz A, Interthal H, Koczan D, Hobom G|title = Structure of the bovine lactoferrin-encoding gene and its promoter|journal = Gene|year = 1994|volume = 143|issue = 2|pages = 265-9}}</ref> Study of polymorphism of genes which encode lactoferrin helps selecting livestock breeds that are resistant to [[mastitis]].<ref Name=Biochimie_2009> {{cite journal|author = O'Halloran F, Bahar B, Buckley F, O'Sullivan O, Sweeney T, Giblin L.|Title = Characterisation of single nucleotide polymorphisms identified in the bovine lactoferrin gene sequences across a range of dairy cow breeds|journal = Biochimie|year = 2009|volume = 91|issue = 1|pages = 68-75}}</ref> |
|||
== Lactoferrin receptor== |
|||
Lactoferrin receptor plays an important role in the [[internalization]] of lactoferrin; it also facilitates absorption of iron ions by lactoferrin. It was shown that [[gene expression]] increases with age in the [[duodenum]] and decreases in the [[jejunum]].<ref>{{cite journal|author = Liao Y, Lopez V, Shafizadeh TB, Halsted CH, Lönnerdal B|title = Cloning of a pig homologue of the human lactoferrin receptor: expression and localization during intestinal maturation in piglets|journal = Comp Biochem Physiol A Mol Integr Physiol|year = 2007|volume = 148|issue = 3|pages = 584-90|pmid=17766154}}</ref> |
|||
| ⚫ | |||
| ⚫ | The human lung as well as saliva contain a wide range of antimicrobial compound including lactoperoxidase system producing [[hypothiocyanite]] and lactoferrin while hypothiocyanite is missing in [[cystic fibrosis]] patients.<ref name="pmid17082494">{{cite journal|author = Moskwa P, Lorentzen D, Excoffon KJ, Zabner J, McCray PB, Nauseef WM, Dupuy C, Bánfi B|title = A novel host defense system of airways is defective in cystic fibrosis|journal = Am. J. Respir. Crit. Care Med.|volume = 175|issue = 2|pages = 174–83|year = 2007|pmid = 17082494|pmc = 2720149|doi = 10.1164/rccm.200607-1029OC}}</ref> Lactoferrin, a component of innate immunity, prevents bacterial [[biofilm]] development.<ref name="pmid11048725">{{cite journal|author = Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg EP|title = Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms|journal = Nature|volume = 407|issue = 6805|pages = 762–4|year = 2000|pmid = 11048725|doi = 10.1038/35037627}}</ref><ref name="pmid12037568">{{cite journal|author = Singh PK, Parsek MR, Greenberg EP, Welsh MJ|title = A component of innate immunity prevents bacterial biofilm development|journal = Nature|volume = 417|issue = 6888|pages = 552–5|year = 2002|pmid = 12037568|doi = 10.1038/417552a}}</ref> The loss of microbicidal activity and increased formation of biofilm due to decreased lactoferrin activity in patients with cystic fibrosis.<ref name="pmid15346334">{{cite journal|author = Rogan MP, Taggart CC, Greene CM, Murphy PG, O'Neill SJ, McElvaney NG|title = Loss of microbicidal activity and increased formation of biofilm due to decreased lactoferrin activity in patients with cystic fibrosis|journal = J. Infect. Dis.|volume = 190|issue = 7|pages = 1245–53|year = 2004|pmid = 15346334|doi = 10.1086/423821}}</ref> These findings demonstrate the important role of lactoferrin in human host defense and especially in lung.<ref name="pmid16503962">{{cite journal|author = Rogan MP, Geraghty P, Greene CM, O'Neill SJ, Taggart CC, McElvaney NG|title = Antimicrobial proteins and polypeptides in pulmonary innate defence|journal = Respir. Res.|volume = 7|pages = 29|year = 2006|pmid = 16503962|pmc = 1386663|doi = 10.1186/1465-9921-7-29}}</ref> |
||
| ⚫ | Lactoferrin with hypothiocyanite has been granted [[orphan drug]] status by the [[European Medicines Agency|EMEA]]<ref name="urlwww.ema.europa.eu">{{cite web|url = http://www.ema.europa.eu/pdfs/human/comp/opinion/39298409en.pdf|title = Public summary of positive opinion for orphan designation of hypothiocyanite/lactoferrin for the treatment of cystic fibrosis |date = 2009-09-07|work = Pre-authorisation Evaluation of Medicines for Human Use|publisher = European Medicines Agency|accessdate = 2010-01-23}}</ref> and the [[Food and Drug Administration (United States)|FDA]].<ref name="urlwww.bioalaxia.eu">{{cite web|url = http://www.bioalaxia.eu/content/meveol-orphan-drug-status-granted-fda-treatment-cystic-fibrosis|title = Meveol: orphan drug status granted by the FDA for the treatment of cystic fibrosis|date =2009-11-05|publisher = United States Food and Drug Administration|accessdate = 2010-01-23}}</ref> |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
==External links== |
==External links== |
||
*[http://ca.expasy.org/cgi-bin/niceprot.pl?P02788 Expasy Database, chemical structure] |
*[http://ca.expasy.org/cgi-bin/niceprot.pl?P02788 Expasy Database, chemical structure] |
||
*[http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene&cmd=retrieve&dopt=default&list_uids=4057 |
*[http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene&cmd=retrieve&dopt=default&list_uids=4057 LTF on the National Center for Biotechnology Information] |
||
*[http://www.fda.gov/bbs/topics/NEWS/2003/NEW00935.html FDA] Lactoferrin Considered Safe to Fight E. Coli. |
*[http://www.fda.gov/bbs/topics/NEWS/2003/NEW00935.html FDA] Lactoferrin Considered Safe to Fight E. Coli. |
||
| Line 63: | Line 97: | ||
[[Category:Proteins]] |
[[Category:Proteins]] |
||
{{protein-stub}} |
|||
{{Link GA|ru}} |
{{Link GA|ru}} |
||
[[de:Lactoferrin]] |
[[de:Lactoferrin]] |
||
[[es:Lactoferrina]] |
[[es:Lactoferrina]] |
||
Revision as of 01:39, 8 June 2010
Template:PBB Lactoferrin (LF), also known as lactotransferrin (LTF), is a multifunctional protein of the transferrin family. Lactoferrin is a globular glycoprotein with a molecular mass of about 80 kDa which is widely represented in various secretory fluids, such as milk, saliva, tears and nasal secretions. Lactoferrin is also present in secondary granules of PMN and is secreted by some acinar cells. Lactoferrin can be purified from milk or produced recombinantly. Human colostrum ("first milk") has the highest concentration, followed by human milk, then cow milk (150 mg/L).[1]
Lactoferrin is one of the components of the immune system of the body; it has antimicrobial activity (bacteriocide, fungicide) and is part of the innate defense, mainly at mucoses.[1] In particular, lactoferrin provides antibacterial activity to human infants.[2][3] Lactoferrin interacts with DNA and RNA, polysaccharides and heparin, and shows some of its biological functions in complexes with these ligands.
History
Occurrence of iron-containing red protein in bovine milk was reported at least in 1939;[4] however, it could not be properly characterized due to insufficient purity. First detailed characterizations of this protein were reported around 1960. They documented the molecular weight, isoelectronic point, optical absorption spectra and presence of two iron atoms per protein molecule.[5][6] The protein was extracted from milk, contained iron and was structurally and chemically similar to serum transferrin. Therefore, it was named lactoferrin in 1961, though the name lactotransferrin was used in some earlier publications, and later studies demonstrated that the protein is not restricted to milk. Also in 1961 the antibacterial action of lactoferrin has been documented and associated with its ability to bind iron.[7]
Structure and properties
Molecular structure
Lactoferrin is one of the transferrin proteins which transfer iron to the cells and control the level of free iron in the blood and external secretions. It is present in the milk of humans and other mammals,[6] in the blood plasma and neutrophils and is one of the major proteins of virtually all exocrine secrets of mammals, such as saliva, gall, tears and pancreas.[8] Concentration of lactoferrin in the milk varies from 7 g/L in the colostrum to 1 g/L in mature milk.
X-ray diffraction reveals that lactoferrin is based on one polypeptide chain which contains about 700 amino acids and forms two homologous globular domains named N-and C-lobes. N-lobe corresponds to amino acid residues 1-333 and C-lobe to 345-692, and the ends of those domains are connected by a short α-helix.[9][10] Each lobe consists of two subdomains, N1, N2 and C1, C2, and contains one iron binding site and one glycosylation site. The degree of glycosylation of the protein may be different and therefore the molecular weight of lactoferrin varies between 76 and 80 kDa. The stability of lactoferrin has been associated with the high glycosylation degree.[11]
Lactoferrin belongs to the basic proteins, its isoelectric point is 8.7. It exists in two forms: iron-rich hololactoferrin and iron-free apolactoferrin. Their tertiary structures are different; apolactoferrin is characterized by "open" conformation of the N-lobe and the "closed" conformation of the C-lobe, and both lobes are closed in the hololactoferrin.[12]
Each lactoferrin molecule can reversibly bind two ions of iron, zinc, copper or other metals.[13] The binding sites are localized in each of the two protein globules. There, each ion is bonded with six ligands: four from the polypeptide chain (two tyrosine residues, one histidine residue and one aspartic acid residue) and two from carbonate or bicarbonate ions.
Lactoferrin forms reddish complex with iron; its affinity for iron is 300 times higher than that of transferrin.[14] The affinity increases in weakly acidic medium. This facilitates the transfer of iron from transferrin to lactoferrin during inflammations, when the pH of tissues decreases due to accumulation of lactic and other acids.[15] The saturated iron concentration in lactoferrin in human milk is estimated as 10 to 30% (100% corresponds to all lactoferrin molecules containing 2 iron atoms). It is demonstrated that lactoferrin is involved not only in the transport of iron, zinc and copper, but also in the regulation of their intake.[16] Presence of loose ions of zinc and copper does not affect the iron binding ability of lactoferrin, and might even increase it.
Polymeric forms
Both in blood plasma and in secretory fluids lactoferrin can exist in different polymeric forms ranging from monomers to tetramers. Lactoferrin tends to polymerize both in vitro and in vivo, especially at high concentrations.[15] Several authors found that the dominant form of lactoferrin in physiological conditions is a tetramer, with the monomer:tetramer ratio of 1:4 at the protein concentrations of 10−5 M.[17][18][19]
It is suggested that the oligomer state of lactoferrin is determined its concentration and that polymerization of lactoferrin is strongly affected by the presence of Ca2+ ions. In particular, monomers were dominant at concentrations below 10−10−10−11 M in the presence of Ca2+, but they converted into tetramers at lactoferrin concentrations above 10−9−10−10 M.[20][17] Titer of lactoferrin in the blood corresponds to this particular "transition concentration" and thus lactoferrin in the blood should be presented both as a monomer and tetramer. Many functional properties of lactoferrin depend on its oligomeric state. In particular, monomeric, but not tetrameric lactoferrin can strongly bind to DNA.
Biological functions
Lactoferrin belongs to the innate immune system. Apart from its main biological function, namely binding and transport of iron ions, lactoferrin also has antibacterial, antiviral, antiparasitic, catalytic, anti-cancer, anti-allergic and radioprotecting functions and properties.[21]
Antibacterial activity
Antibacterial activity of lactoferrin is best studied; it originates from the iron binding properties of lactoferrin which deprive the bacterial flora from an element necessary for its growth.[22] Antibacterial action of lactoferrin is also explained by the presence of specific receptors on the cell surface of microorganisms. Lactoferrin binds to lipopolysaccharide of bacterial walls, and the oxidized iron part of the lactoferrin oxidizes bacteria via formation of peroxides. This affects the membrane permeability and results in the cell breakdown (lysis).[22]
Although lactoferrin has also other, not related to iron, antibacterial mechanisms, such as stimulation of phagocytosis,[23] the described above interaction with the outer bacterial membrane is the dominant and most studied.[24] Lactoferrin not only disrupts the membrane, but even penetrates into the cell. Its binding to the bacteria wall is associated with the specific peptide lactoferricin, which is located at the N-lobe of lactoferrin and is produced by in vitro cleavage of lactoferrin with another protein, trypsin.[25][26]
Antiviral activity
Lactoferrin acts, mostly in vitro, on a wide range of human and animal viruses based on DNA and RNA genomes,[27] including the herpes simplex virus 1 and 2,[28][29] cytomegalovirus,[30] HIV,[29][31] hepatitis C virus,[32][33] hantaviruses, rotaviruses, poliovirus type 1,[26] adenoviruses,[34] human respiratory syncytial virus and murine leukemia viruses.[26]
The most studied mechanism of antiviral activity of lactoferrin is its diversion of virus particles from the target cells. Many viruses tend to bind to the lipoproteins of the cell membranes and then penetrate into the cell.[33] Lactoferrin binds to the same lipoproteins thereby repelling the virus particles. Iron-free apolactoferrin is more efficient in this function than hololactoferrin; and lactoferricin, which is responsible for antimicrobial properties of lactoferrin, shows almost no antiviral activity.[27]
Beside interacting with the cell walls, lactoferrin also directly binds to viral particles, such as the hepatitis viruses.[33] This mechanism is also confirmed by the antiviral activity of lactoferrin against rotaviruses,[26] which act on different cell types.
Lactoferrin also suppresses virus replication after the virus penetrated into the cell.[26][31] Such an indirect antiviral effect is achieved by affecting natural killer cells, granulocytes and macrophages – cells, which play a crucial role in the early stages of viral infections, such as severe acute respiratory syndrome (SARS).[35]
Antifungal activity
Lactoferrin and lactoferricin inhibit in vitro growth of Trichophyton mentagrophytes, which are responsible for several skin diseases such as ringworm.[36] Lactoferrin also acts against the Candida albicans – a diploid fungus (a form of yeast) which causes opportunistic oral and genital infections in humans.[37][38] Fluconazole has long been used against Candida albicans that resulted in emergence of strains resistant to this drug. However, a combination of lactoferrin with fluconazole can act against fluconazole-resistant strains of Candida albicans as well as other types of Candida: C. glabrata, C. krusei, C. parapsilosis and C. tropicalis.[37] Antifungal activity is observed for sequential incubation of Candida with lactoferrin and then with fluconazole, but not vice versa. The antifungal activity of lactoferricin exceeds that of lactoferrin. In particular, synthetic peptide 1-11 lactoferricin shows much greater activity against Candida albicans than native lactoferricin.[37]
Administration of lactoferrin through drinking water to mice with weakened immune systems and symptoms of aphthous ulcer reduced the number of Candida albicans strains in the mouth and the size of the damaged areas in the tongue.[39] Oral administration of lactoferrin to animals also reduced the number of pathogenic organisms in the tissues close to the gastrointestinal tract. Candida albicans could also be completely eradicated with a mixture containing lactoferrin, lysozyme and introakonazol in HIV-positive patients who were resistant to other antifungal drugs.[40] Such antifungal action when other drugs deem inefficient is characteristic of lactoferrin and is especially valuable for HIV-infected patients.[41]
Contrary to the antiviral and antibacterial actions of lactoferrin, very little is known about the mechanism of its antifungal action. Lactoferrin seem to destroy the cell wall and binds the plasma membrane of C. albicans.[38]
Interaction with nucleic acids
One of the important properties of lactoferrin is its ability to bind with nucleic acids. The fraction of protein extracted from milk, contains 3.3% RNA,[17] besides, the protein preferably binds to the double-stranded then to the single-stranded DNA. The ability of lactoferrin to bind DNA is used for the isolation and purification of lactoferrin using affinity chromatography with columns containing immobilized DNA-containing sorbents, such as agarose with the immobilized single-stranded DNA.[42]
Enzymatic activity of lactoferrin
Lactoferrin hydrolyzes RNA and exhibits the properties of pyrimidine-specific secretory ribonucleases. In particular, by destroying the RNA genome, milk RNase inhibits reverse transcription of retroviruses that cause breast cancer in mice.[43] Parsi women in West India have the milk RNase level markedly lower than in other groups, and their breast cancer rate is three times higher than average.[44] Thus, ribonucleases of milk, and lactoferrin in particular, might play an important role in pathogenesis of diseases caused by various retroviruses.
Genes of lactoferrin
At least 60 gene sequences of lactoferrin have been characterized in 11 species of mammals.[45] In most species, stop codon is TAA, and TGA in Mus musculus . Deletions, insertions and mutations of stop codons affect the coding part and its length varies between 2,055 and 2,190 nucleotide pairs. Gene polymorphism between species is much more diverse than the intraspecific polymorphism of lactoferrin. There are differences in amino acid sequences: 8 in Homo sapiens , 6 in Mus musculus , 6 in Capra hircus , 10 in Bos taurus and 20 in Sus scrofa . This variation may indicate functional differences between different types of lactoferrin.[45]
In humans, lactoferrin gene LTF is located on the third chromosome in the locus 3q21-q23. In oxen, the coding sequence consists of 17 exons and has a length of about 34,500 nucleotide pairs. Exons of the lactoferrin gene in oxen have a similar size to the exons of other genes of the transferrin family, whereas the sizes of introns differ within the family. Similarity in the size of exons and their distribution in the domains of the protein molecule indicates that the evolutionary development of lactoferrin gene occurred by duplication.[46] Study of polymorphism of genes which encode lactoferrin helps selecting livestock breeds that are resistant to mastitis.[47]
Lactoferrin receptor
Lactoferrin receptor plays an important role in the internalization of lactoferrin; it also facilitates absorption of iron ions by lactoferrin. It was shown that gene expression increases with age in the duodenum and decreases in the jejunum.[48]
Cystic fibrosis
The human lung as well as saliva contain a wide range of antimicrobial compound including lactoperoxidase system producing hypothiocyanite and lactoferrin while hypothiocyanite is missing in cystic fibrosis patients.[49] Lactoferrin, a component of innate immunity, prevents bacterial biofilm development.[50][51] The loss of microbicidal activity and increased formation of biofilm due to decreased lactoferrin activity in patients with cystic fibrosis.[52] These findings demonstrate the important role of lactoferrin in human host defense and especially in lung.[53]
Lactoferrin with hypothiocyanite has been granted orphan drug status by the EMEA[54] and the FDA.[55]
See also
References
- ^ a b Sánchez L, Calvo M, Brock JH (1992). "Biological role of lactoferrin". Arch. Dis. Child. 67 (5): 657–61. PMC 1793702. PMID 1599309.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kalidas Shetty (2006). Food biotechnology. CRC Press. p. 1028. ISBN 0824753291.
- ^ A. John Clark (1998). Animal breeding: technology for the 21st century. CRC Press. p. 191. ISBN 9057022923.
- ^ M. Sorensen and S. P. L. Sorensen, Compf. rend. trav. lab. Carlsberg (1939) 23, 55, cited by Groves (1960)
- ^ . doi:10.1021/ja01498a029.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ a b Johansson, B. Isolation of an iron-containing red protein from human milk. (1960) Acta Chem. Scand., 14, 510–512.
- ^ A. S. Naidu (2000). Lactoferrin: natural, multifunctional, antimicrobial. CRC Press. pp. 1–2. ISBN 0849309093.
- ^ . PMID 3858982.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ Baker, H. M., Anderson, B. F., Kidd, R. D., Shewry, S. C., and Baker, E. N. Lactoferrin three-dimensional structure: a framework for interpreting function. In Lactoferrin: Structure, Function and Application (Shimazaki, K., ed.). (2000) pp. 3 - 15, Elsevier Science, Amsterdam.
- ^ . PMID 16261257.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ Hakansson, A., Zhivotovsky, B., Orrenius, S., Sabharwal, H., Svanborg, C. (1995). "Apoptosis induced by a human milk protein". Proc. Natl. Acad. Sci. U S A. 92: 8064–8068. PMC 41287.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ . PMID 10089508.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . PMID 7672721.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ Mazurier J., SpikG. Comparative study of the iron-binding properties of human transferring. Complete and sequential iron saturation and desaturation of the lactotransferrin. (1980) Biochim. Biophys. Acta, 629, 399-408.
- ^ a b Sousa, M., Brock, JH, Iron in immunity. Cancer and Inflammation (1989) John Wiley & Sons ISBN 0471921505
- ^ . doi:10.1021/bi00133a010.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ a b c Bennett, R.M., Davis, J. Lactoferrin interacts with deoxyribonucleic acid: a preferential reactivity with double-stranded DNA and dissociation of DNA-anti-DNA complexes. (1982) J. Lab. Clin. Med., 99, 127-138.
- ^ . PMID 6979357.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . PMID 7762423.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . PMID 2754391.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ "Lactoferrin: a review" (PDF). Veterinarni Medicina. 9: 457. 2008.
- ^ a b . PMID 14568385.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . PMID 9691154.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . PMID 8612745.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . PMID 9588189.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ a b c d e . PMID 9490007.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ a b . PMID 11675140 http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.104.745&rep=rep1&type=pdf.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . PMID 7661698.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ a b . PMID 11908636.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . PMID 7622881.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ a b . PMID 9785469.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . PMID 17241377.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) Erratum in: Liver Int. Apr; 27 (3): 421. - ^ a b c . PMID 12522210.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . PMID 11750941.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ Renji Reghunathan, Manikandan Jayapal, Li-Yang Hsu, Hiok-Hee Chng, Dessmon Tai, Bernard P Leung and Alirio J Melendez (2005). "Expression profile of immune response genes in patients with Severe Acute Respiratory Syndrome". BMC Immunol (6): 2. doi:10.1186/1471-2172-6-2.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ . PMID 11020258.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ a b c . PMID 12499200.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ a b . PMID 15047526.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . PMID 12878528.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . PMID 11089630.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . PMID 10543740.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . PMID 3827843.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . PMID 4139659.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ . doi:10.1038/262802a0.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ a b Jing-Fen Kang, Xiang-Long Li, Rong-Yan Zhou, Lan-Hui Li, Fu-Jun Feng and Xiu -Li Guo (2008). "Bioinformatics Analysis of Lactoferrin Gene for Several Species". Biochemical Genetics. 46 (5–6): 312–322.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Seyfert HM, Tuckoricz A, Interthal H, Koczan D, Hobom G (1994). "Structure of the bovine lactoferrin-encoding gene and its promoter". Gene. 143 (2): 265–9.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ O'Halloran F, Bahar B, Buckley F, O'Sullivan O, Sweeney T, Giblin L. (2009). Biochimie. 91 (1): 68–75.
{{cite journal}}: Missing or empty|title=(help); Unknown parameter|Title=ignored (|title=suggested) (help)CS1 maint: multiple names: authors list (link) - ^ Liao Y, Lopez V, Shafizadeh TB, Halsted CH, Lönnerdal B (2007). "Cloning of a pig homologue of the human lactoferrin receptor: expression and localization during intestinal maturation in piglets". Comp Biochem Physiol A Mol Integr Physiol. 148 (3): 584–90. PMID 17766154.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Moskwa P, Lorentzen D, Excoffon KJ, Zabner J, McCray PB, Nauseef WM, Dupuy C, Bánfi B (2007). "A novel host defense system of airways is defective in cystic fibrosis". Am. J. Respir. Crit. Care Med. 175 (2): 174–83. doi:10.1164/rccm.200607-1029OC. PMC 2720149. PMID 17082494.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg EP (2000). "Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms". Nature. 407 (6805): 762–4. doi:10.1038/35037627. PMID 11048725.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Singh PK, Parsek MR, Greenberg EP, Welsh MJ (2002). "A component of innate immunity prevents bacterial biofilm development". Nature. 417 (6888): 552–5. doi:10.1038/417552a. PMID 12037568.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rogan MP, Taggart CC, Greene CM, Murphy PG, O'Neill SJ, McElvaney NG (2004). "Loss of microbicidal activity and increased formation of biofilm due to decreased lactoferrin activity in patients with cystic fibrosis". J. Infect. Dis. 190 (7): 1245–53. doi:10.1086/423821. PMID 15346334.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rogan MP, Geraghty P, Greene CM, O'Neill SJ, Taggart CC, McElvaney NG (2006). "Antimicrobial proteins and polypeptides in pulmonary innate defence". Respir. Res. 7: 29. doi:10.1186/1465-9921-7-29. PMC 1386663. PMID 16503962.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ "Public summary of positive opinion for orphan designation of hypothiocyanite/lactoferrin for the treatment of cystic fibrosis" (PDF). Pre-authorisation Evaluation of Medicines for Human Use. European Medicines Agency. 2009-09-07. Retrieved 2010-01-23.
- ^ "Meveol: orphan drug status granted by the FDA for the treatment of cystic fibrosis". United States Food and Drug Administration. 2009-11-05. Retrieved 2010-01-23.
External links
- Expasy Database, chemical structure
- LTF on the National Center for Biotechnology Information
- FDA Lactoferrin Considered Safe to Fight E. Coli.