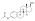Estradiol benzoate: Difference between revisions
No edit summary |
|||
| Line 20: | Line 20: | ||
| legal_US = |
| legal_US = |
||
| legal_status = Rx-only |
| legal_status = Rx-only |
||
| routes_of_administration = [[Intramuscular injection]] |
| routes_of_administration = [[Intramuscular injection]], [[subcutaneous injection]], [[vaginal administration|vaginal]] |
||
| class = [[Estrogen (medication)|Estrogen]]; [[Estrogen ester]] |
| class = [[Estrogen (medication)|Estrogen]]; [[Estrogen ester]] |
||
| Line 88: | Line 88: | ||
Estradiol benzoate is and has been available as an [[oil solution]] for intramuscular injection provided as [[vial]]s and [[ampoule]]s at concentrations of 0.167, 0.2, 0.33, 1, 1.67, 2, 5, 10, 20, and 25 mg/mL.<ref name="KleemannEngel2014">{{cite book|author1=A. Kleemann|author2=J. Engel|author3=B. Kutscher|author4=D. Reichert|title=Pharmaceutical Substances, 5th Edition, 2009: Syntheses, Patents and Applications of the most relevant APIs|url=https://books.google.com/books?id=fO2IAwAAQBAJ&pg=PA1167|date=14 May 2014|publisher=Thieme|isbn=978-3-13-179525-0|pages=1167–1174}}</ref><ref name="Muller1998">{{cite book|author=Muller|title=European Drug Index: European Drug Registrations, Fourth Edition|url=https://books.google.com/books?id=2HBPHmclMWIC&pg=PA150|date=19 June 1998|publisher=CRC Press|isbn=978-3-7692-2114-5|pages=150,349,370}}</ref><ref name="NNR1949" /> It is also available as a [[microcrystalline]] [[aqueous suspension]] for intramuscular injection under the brand name Agofollin Depot.<ref name="Avicenum1973">{{cite book|title=Review of Czechoslovak Medicine|url=https://books.google.com/books?id=odA0AQAAIAAJ|year=1973|publisher=Avicenum - Czechoslovak Medical Press|quote=Oestradiol benzoate, aqueous microcrystalline suspension (Agofollin Depot SPOFA).|page=5}}</ref><ref name="Agofollin-Depot-Label">https://web.archive.org/web/20190519104654/http://www.sukl.cz/download/pil/PI16221.pdf</ref><ref name="MarekJosef2010">{{cite book|author=Marek Josef a kolektiv|title=Farmakoterapie vnitřních nemocí - 4. zcela přepracované a doplněné vydání|url=https://books.google.com/books?id=Xc2OHeo0i0cC&pg=PA377|date=1 January 2010|publisher=Grada Publishing a.s.|isbn=978-80-247-2639-7|pages=377–|quote=Injection of estrogenic preparations - Injectable preparations are AGOFOLLIN, inj. 5 mg (estradiol dipropionate), AGOFOLLIN DEPOT, inj. 10 mg (estradiol benzoate), and NEOFOLLIN, inj. 5 mg (estradiol valerate). The producer of all these preparations is Biotika. Non-protracted AGOFOLLIN is used only for initiation of treatment, then it is continued with depot injections, which are administered three times: cycle day 4, 11 and 18. At the same time, [progesterone] (AGOLUTIN DEPOT, Biotika, amp. 2 ml / 50 mg, cycle day 18 and 25) is administered. Estrogen injection is not completely physiological - after application, the estrogen plasma concentration increases unnecessarily high and then decreases rapidly.}}</ref><ref name="HankaPetr2008" /> [[Sistocyclin]] was the brand name of a product containing 10 mg microcrystalline estradiol benzoate and 200 mg microcrystalline [[progesterone (medication)|progesterone]] in an aqueous suspension.<ref name="Ufer1968">{{cite book | last1 = Ufer | first1 = Joachim | chapter = Die therapeutische Anwendung der Gestagene beim Menschen | trans-chapter = Therapeutic Use of Progestagens in Humans | pages = 1026–1124 | doi = 10.1007/978-3-642-99941-3_7 | title = Die Gestagene | trans-title = Progestogens | year = 1968 | publisher = Springer-Verlag | isbn = 978-3-642-99941-3 | url = https://books.google.com/books?id=t8GpBgAAQBAJ&pg=PA1058 | quote = C. Dysfunktionelle Uterusblutungen. [...] 1. Depotinjektionen. 1. Originalmethode nach KAUFMANN und OBER. Es wird 1 Amp. mit 200 mg Progesteron und 10 mg Oestradiol-Monobenzoat als Kristallsuspension (Sistocyclin) injiziert [676, 678, 679, 295, 482, 365, 434, 563, 400]. [...] Beispiele. KAUFMANN et al. [485]: 400 mg Progesteron + 20 mg Oestradiolmonobenzoat Kristallsuspension. ELERT [224] U. HERRMANN [363]: 200 mg Progesteron + 10 mg Oestradiolmono benzoat Kristallsuspension.}}</ref><ref name="Ciba1957a">{{cite book | title = Ciba Symposium | url = https://books.google.com/books?id=KwkbAQAAMAAJ | year = 1957 | publisher = Ciba | quote = CIBA's range of hormone preparations has been increased with the advent of "Sistocyclin", one ampoule of which contains 200 mg progesterone and 10 mg oestradiol monobenzoate in crystalline suspension; it thus meets the requirements—in line with the most recent findings of the KAUFMANN Clinic—of cases marked by deficiency of corpus luteum hormone, e. g. in functional bleeding such as metropathia haemorrhagica.}}</ref><ref name="Ciba1957b">{{cite book | title = Ciba Zeitschrift | url = https://books.google.com/books?id=WgpOAQAAIAAJ | year = 1957 | page = 3001 | quote = Sistocyclin - a microcrystal suspension containing 200 mg progesterone and 10 mg oestradiol monobenzoate per ampoule - has become particularly useful in the treatment of so-called, functional [...]}}</ref><ref name="Pschyrembel2011">{{cite book|author=Willibald Pschyrembel|title=Praktische Gynäkologie: für Studierende und Ärzte|url=https://books.google.com/books?id=vVaTnHDFzZ0C&pg=PA601|date=15 June 2011|publisher=Walter de Gruyter|isbn=978-3-11-150424-7|pages=601–}}</ref> [[Follivirin]] (and previously Femandren M) is the brand name of a product containing 2.5 mg microcrystalline estradiol benzoate and 25 to 50 mg microcrystalline [[testosterone isobutyrate]] in aqueous suspension.<ref name="Folivirin-Label" /><ref name="Kubíková2014">{{cite journal | last1 = Kubíková | first1 = Drahomíra | title = Menopauzální symptomy a hormonální substituční terapie | trans-title = Menopausal symptoms and hormone replacement therapy | language = Czech | date = 2014 | journal = Praktické Lékárenství | volume = 10 | issue = 2 | pages = 68–73 | issn = 1801-2434 | doi = | pmid = | url = http://www.medvik.cz/link/bmc14059249 <!-- | url2 = http://www.solen.sk/pdf/95e898d92100a736e32110dd1b846a9e.pdf-->}}</ref><ref name="Josefkolektiv2010">{{cite book|author1=Marek Josef|author2=a kolektiv|title=Farmakoterapie vnitřních nemocí: 4., zcela přepracované a doplněné vydání|url=https://books.google.com/books?id=bUqECwAAQBAJ&pg=PA380|date=14 May 2010|publisher=Grada Publishing a.s.|isbn=978-80-247-9524-9|pages=380–|quote=In addition, testosterone isobutyrate in FOLIVIRIN, Biotika, an injection containing 25 mg testosterone isobutyrate and 2.5 mg estradiol benzoate is available. It is applied every 4-6 weeks depending on the effect.}}</ref><ref name="Ciba1953">{{cite book|title=Ciba Symposium: 1953/57:Index|url=https://books.google.com/books?id=yQgbAQAAMAAJ|year=1953|publisher=Ciba|page=197|quote=Femandren M. C'est le nom des nouvelles ampoules cristallines destinées au traitement associé œs- trogène-androgène. Elles renferment, sous forme de microcristaux, 2,5 mg de mono- benzoate d'œstradiol et 50 mg d'isobutyra- te de testostérone ; elles sont indiquées pour traiter les cas où il convient d'administrer simultanément de l'hormone femelle et de l'hormone mâle et où il importe aussi d'obtenir un effet prolongé, par exemple lors de symptômes d'insuffisance à la ménopause ou après castration. L'effet d'une injection se prolonge pendant 3-6 semaines.}}</ref> |
Estradiol benzoate is and has been available as an [[oil solution]] for intramuscular injection provided as [[vial]]s and [[ampoule]]s at concentrations of 0.167, 0.2, 0.33, 1, 1.67, 2, 5, 10, 20, and 25 mg/mL.<ref name="KleemannEngel2014">{{cite book|author1=A. Kleemann|author2=J. Engel|author3=B. Kutscher|author4=D. Reichert|title=Pharmaceutical Substances, 5th Edition, 2009: Syntheses, Patents and Applications of the most relevant APIs|url=https://books.google.com/books?id=fO2IAwAAQBAJ&pg=PA1167|date=14 May 2014|publisher=Thieme|isbn=978-3-13-179525-0|pages=1167–1174}}</ref><ref name="Muller1998">{{cite book|author=Muller|title=European Drug Index: European Drug Registrations, Fourth Edition|url=https://books.google.com/books?id=2HBPHmclMWIC&pg=PA150|date=19 June 1998|publisher=CRC Press|isbn=978-3-7692-2114-5|pages=150,349,370}}</ref><ref name="NNR1949" /> It is also available as a [[microcrystalline]] [[aqueous suspension]] for intramuscular injection under the brand name Agofollin Depot.<ref name="Avicenum1973">{{cite book|title=Review of Czechoslovak Medicine|url=https://books.google.com/books?id=odA0AQAAIAAJ|year=1973|publisher=Avicenum - Czechoslovak Medical Press|quote=Oestradiol benzoate, aqueous microcrystalline suspension (Agofollin Depot SPOFA).|page=5}}</ref><ref name="Agofollin-Depot-Label">https://web.archive.org/web/20190519104654/http://www.sukl.cz/download/pil/PI16221.pdf</ref><ref name="MarekJosef2010">{{cite book|author=Marek Josef a kolektiv|title=Farmakoterapie vnitřních nemocí - 4. zcela přepracované a doplněné vydání|url=https://books.google.com/books?id=Xc2OHeo0i0cC&pg=PA377|date=1 January 2010|publisher=Grada Publishing a.s.|isbn=978-80-247-2639-7|pages=377–|quote=Injection of estrogenic preparations - Injectable preparations are AGOFOLLIN, inj. 5 mg (estradiol dipropionate), AGOFOLLIN DEPOT, inj. 10 mg (estradiol benzoate), and NEOFOLLIN, inj. 5 mg (estradiol valerate). The producer of all these preparations is Biotika. Non-protracted AGOFOLLIN is used only for initiation of treatment, then it is continued with depot injections, which are administered three times: cycle day 4, 11 and 18. At the same time, [progesterone] (AGOLUTIN DEPOT, Biotika, amp. 2 ml / 50 mg, cycle day 18 and 25) is administered. Estrogen injection is not completely physiological - after application, the estrogen plasma concentration increases unnecessarily high and then decreases rapidly.}}</ref><ref name="HankaPetr2008" /> [[Sistocyclin]] was the brand name of a product containing 10 mg microcrystalline estradiol benzoate and 200 mg microcrystalline [[progesterone (medication)|progesterone]] in an aqueous suspension.<ref name="Ufer1968">{{cite book | last1 = Ufer | first1 = Joachim | chapter = Die therapeutische Anwendung der Gestagene beim Menschen | trans-chapter = Therapeutic Use of Progestagens in Humans | pages = 1026–1124 | doi = 10.1007/978-3-642-99941-3_7 | title = Die Gestagene | trans-title = Progestogens | year = 1968 | publisher = Springer-Verlag | isbn = 978-3-642-99941-3 | url = https://books.google.com/books?id=t8GpBgAAQBAJ&pg=PA1058 | quote = C. Dysfunktionelle Uterusblutungen. [...] 1. Depotinjektionen. 1. Originalmethode nach KAUFMANN und OBER. Es wird 1 Amp. mit 200 mg Progesteron und 10 mg Oestradiol-Monobenzoat als Kristallsuspension (Sistocyclin) injiziert [676, 678, 679, 295, 482, 365, 434, 563, 400]. [...] Beispiele. KAUFMANN et al. [485]: 400 mg Progesteron + 20 mg Oestradiolmonobenzoat Kristallsuspension. ELERT [224] U. HERRMANN [363]: 200 mg Progesteron + 10 mg Oestradiolmono benzoat Kristallsuspension.}}</ref><ref name="Ciba1957a">{{cite book | title = Ciba Symposium | url = https://books.google.com/books?id=KwkbAQAAMAAJ | year = 1957 | publisher = Ciba | quote = CIBA's range of hormone preparations has been increased with the advent of "Sistocyclin", one ampoule of which contains 200 mg progesterone and 10 mg oestradiol monobenzoate in crystalline suspension; it thus meets the requirements—in line with the most recent findings of the KAUFMANN Clinic—of cases marked by deficiency of corpus luteum hormone, e. g. in functional bleeding such as metropathia haemorrhagica.}}</ref><ref name="Ciba1957b">{{cite book | title = Ciba Zeitschrift | url = https://books.google.com/books?id=WgpOAQAAIAAJ | year = 1957 | page = 3001 | quote = Sistocyclin - a microcrystal suspension containing 200 mg progesterone and 10 mg oestradiol monobenzoate per ampoule - has become particularly useful in the treatment of so-called, functional [...]}}</ref><ref name="Pschyrembel2011">{{cite book|author=Willibald Pschyrembel|title=Praktische Gynäkologie: für Studierende und Ärzte|url=https://books.google.com/books?id=vVaTnHDFzZ0C&pg=PA601|date=15 June 2011|publisher=Walter de Gruyter|isbn=978-3-11-150424-7|pages=601–}}</ref> [[Follivirin]] (and previously Femandren M) is the brand name of a product containing 2.5 mg microcrystalline estradiol benzoate and 25 to 50 mg microcrystalline [[testosterone isobutyrate]] in aqueous suspension.<ref name="Folivirin-Label" /><ref name="Kubíková2014">{{cite journal | last1 = Kubíková | first1 = Drahomíra | title = Menopauzální symptomy a hormonální substituční terapie | trans-title = Menopausal symptoms and hormone replacement therapy | language = Czech | date = 2014 | journal = Praktické Lékárenství | volume = 10 | issue = 2 | pages = 68–73 | issn = 1801-2434 | doi = | pmid = | url = http://www.medvik.cz/link/bmc14059249 <!-- | url2 = http://www.solen.sk/pdf/95e898d92100a736e32110dd1b846a9e.pdf-->}}</ref><ref name="Josefkolektiv2010">{{cite book|author1=Marek Josef|author2=a kolektiv|title=Farmakoterapie vnitřních nemocí: 4., zcela přepracované a doplněné vydání|url=https://books.google.com/books?id=bUqECwAAQBAJ&pg=PA380|date=14 May 2010|publisher=Grada Publishing a.s.|isbn=978-80-247-9524-9|pages=380–|quote=In addition, testosterone isobutyrate in FOLIVIRIN, Biotika, an injection containing 25 mg testosterone isobutyrate and 2.5 mg estradiol benzoate is available. It is applied every 4-6 weeks depending on the effect.}}</ref><ref name="Ciba1953">{{cite book|title=Ciba Symposium: 1953/57:Index|url=https://books.google.com/books?id=yQgbAQAAMAAJ|year=1953|publisher=Ciba|page=197|quote=Femandren M. C'est le nom des nouvelles ampoules cristallines destinées au traitement associé œs- trogène-androgène. Elles renferment, sous forme de microcristaux, 2,5 mg de mono- benzoate d'œstradiol et 50 mg d'isobutyra- te de testostérone ; elles sont indiquées pour traiter les cas où il convient d'administrer simultanément de l'hormone femelle et de l'hormone mâle et où il importe aussi d'obtenir un effet prolongé, par exemple lors de symptômes d'insuffisance à la ménopause ou après castration. L'effet d'une injection se prolonge pendant 3-6 semaines.}}</ref> |
||
A [[vaginal administration|vaginal]] [[tablet (pharmacy)|tablet]] formulation containing 0.125 mg estradiol benzoate and 10 mg [[monalazone sodium]] has been marketed under the brand name Malun 25.<ref name="Leidenberger2013">{{cite book|author=Freimut A. Leidenberger|title=Klinische Endokrinologie für Frauenärzte|url=https://books.google.com/books?id=YTiuBgAAQBAJ&pg=PA527|date=17 April 2013|publisher=Springer-Verlag|isbn=978-3-662-08110-5|pages=527–}}</ref> |
|||
{{Available forms of estradiol}} |
{{Available forms of estradiol}} |
||
Revision as of 15:34, 10 June 2019
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌɛstrəˈdaɪoʊl ˈbɛnzoʊeɪt/ ES-trə-DY-ohl BEN-zoh-ayt |
| Trade names | Agofollin Depot, Benovocyclin, Benzofoline, Dimenformon, Progynon-B, many others |
| Other names | EB; E2B; Oestradiol benzoate; 17β-Estradiol-3-benzoate; NSC-9566; Benzhormovarine, Difollisterol, Follicormon, Follidimyl, Follidrinbensoat, Oestro-Vitis, Oestroform |
| Routes of administration | Intramuscular injection, subcutaneous injection, vaginal |
| Drug class | Estrogen; Estrogen ester |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | IM: High[1] |
| Protein binding | Estradiol: ~98% (to albumin and SHBG)[2][3] |
| Metabolism | Cleavage via esterases in the liver, blood, and tissues[4][5] |
| Metabolites | Estradiol, benzoic acid, and metabolites of estradiol[4][5] |
| Elimination half-life | IM: 48–120 hrs (2–5 days)[6] |
| Duration of action | IM (0.3–1.7 mg): 2–3 days[7][8] IM (5 mg): 4–6 days[9][10][11] |
| Excretion | Urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.040 |
| Chemical and physical data | |
| Formula | C25H28O3 |
| Molar mass | 376.488 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Estradiol benzoate (EB), sold under the brand name Progynon-B among others, is an estrogen medication which is used in hormone therapy for menopausal symptoms and low estrogen levels in women, in hormone therapy for transgender women, and in the treatment of gynecological disorders.[8][12][13] It is also used in the treatment of prostate cancer in men.[8] Estradiol benzoate is used in veterinary medicine as well.[14][15] When used clinically, the medication is given by injection into muscle usually two to three times per week.[8][12][16]
Side effects of estradiol benzoate include breast tenderness, breast enlargement, nausea, headache, and fluid retention.[17] Estradiol benzoate is a synthetic estrogen and hence is an agonist of the estrogen receptor (ER), the biological target of estrogens like estradiol.[4][5] It is an estrogen ester and a prodrug of estradiol in the body.[4][5] Because of this, it is considered to be a natural and bioidentical form of estrogen.[4]
Estradiol benzoate was discovered in 1933 and was introduced for medical use in 1936.[4][18][19][20] It was the first estradiol ester to be discovered, as well as marketed, and was one of the first estrogens to be introduced for medical use.[19][20] Along with estradiol dipropionate, it was among the most widely used esters of estradiol for many years following its introduction.[21] However, in the 1950s, longer-acting estradiol esters that necessitate less frequent injections, such as estradiol valerate and estradiol cypionate, were developed, and have since mostly superseded estradiol benzoate.[9] Nonetheless, estradiol benzoate remains widely available throughout the world.[15] It is not available for medical use in the United States, but is available in this country for use in veterinary medicine.[22][23]
Medical uses
The medical uses of estradiol benzoate are the same as those of estradiol and other estrogens.[8][12] Estradiol benzoate is used in hormone therapy for the treatment of menopausal symptoms such as hot flashes and vaginal atrophy and in the treatment of hypoestrogenism and delayed puberty due to hypogonadism or other causes in women.[8][12] It is also used in hormone therapy for transgender women.[13][24][25] Aside from hormone therapy, estradiol benzoate is used in the treatment of gynecological disorders such as menstrual disorders, dysfunctional uterine bleeding, and breast engorgement.[8][12] In addition, it is used as a form of high-dose estrogen therapy in the palliative treatment of prostate cancer in men.[8]
Estradiol benzoate has a relatively short duration of action, and is administered by intramuscular injection usually two to three times per week.[8][12] It is used in the treatment of menopausal symptoms at a dosage of 1 to 1.66 mg initially and 0.33 to 1 mg for maintenance two times per week, and in the treatment of hypoestrogenism and delayed puberty at a dosage of 1.66 mg two to three times per week.[8][26] The dosage used in hormone therapy for transgender women is 0.5 to 1.5 mg two to three times per week.[13] In the treatment of prostate cancer, estradiol benzoate is used at a dosage of 1.66 mg three times per week (for a total of 5 mg per week).[8]
| Route/form | Estrogen | Low | Standard | High | |||
|---|---|---|---|---|---|---|---|
| Oral | Estradiol | 0.5–1 mg/day | 1–2 mg/day | 2–4 mg/day | |||
| Estradiol valerate | 0.5–1 mg/day | 1–2 mg/day | 2–4 mg/day | ||||
| Estradiol acetate | 0.45–0.9 mg/day | 0.9–1.8 mg/day | 1.8–3.6 mg/day | ||||
| Conjugated estrogens | 0.3–0.45 mg/day | 0.625 mg/day | 0.9–1.25 mg/day | ||||
| Esterified estrogens | 0.3–0.45 mg/day | 0.625 mg/day | 0.9–1.25 mg/day | ||||
| Estropipate | 0.75 mg/day | 1.5 mg/day | 3 mg/day | ||||
| Estriol | 1–2 mg/day | 2–4 mg/day | 4–8 mg/day | ||||
| Ethinylestradiola | 2.5–10 μg/day | 5–20 μg/day | – | ||||
| Nasal spray | Estradiol | 150 μg/day | 300 μg/day | 600 μg/day | |||
| Transdermal patch | Estradiol | 25 μg/dayb | 50 μg/dayb | 100 μg/dayb | |||
| Transdermal gel | Estradiol | 0.5 mg/day | 1–1.5 mg/day | 2–3 mg/day | |||
| Vaginal | Estradiol | 25 μg/day | – | – | |||
| Estriol | 30 μg/day | 0.5 mg 2x/week | 0.5 mg/day | ||||
| IM or SC injection | Estradiol valerate | – | – | 4 mg 1x/4 weeks | |||
| Estradiol cypionate | 1 mg 1x/3–4 weeks | 3 mg 1x/3–4 weeks | 5 mg 1x/3–4 weeks | ||||
| Estradiol benzoate | 0.5 mg 1x/week | 1 mg 1x/week | 1.5 mg 1x/week | ||||
| SC implant | Estradiol | 25 mg 1x/6 months | 50 mg 1x/6 months | 100 mg 1x/6 months | |||
| Footnotes: a = No longer used or recommended, due to health concerns. b = As a single patch applied once or twice per week (worn for 3–4 days or 7 days), depending on the formulation. Note: Dosages are not necessarily equivalent. Sources: See template. | |||||||
| Route/form | Estrogen | Dosage | Ref(s) |
|---|---|---|---|
| Oral | Estradiol | 10 mg 3x/day AI-resistant: 2 mg 1–3x/day |
[27][28] [27][29] |
| Estradiol valerate | AI-resistant: 2 mg 1–3x/day | [27][29] | |
| Conjugated estrogens | 10 mg 3x/day | [30][31][32][33] | |
| Ethinylestradiol | 0.5–1 mg 3x/day | [31][27][34][33] | |
| Diethylstilbestrol | 5 mg 3x/day | [31][35][36] | |
| Dienestrol | 5 mg 3x/day | [34][33][36] | |
| Dimestrol | 30 mg/day | [30][33][36] | |
| Chlorotrianisene | 24 mg/day | [30][36] | |
| IM or SC injection | Estradiol benzoate | 5 mg 2–3x/week | [34][37][35][38] |
| Estradiol dipropionate | 5 mg 2–3x/week | [34][35][39][38] | |
| Estradiol valerate | 30 mg 1x/2 weeks | [37] | |
| Polyestradiol phosphate | 40–80 mg 1x/4 weeks | [40][41] | |
| Estrone | 5 mg ≥3x/week | [42] | |
| Notes: (1) Only in women who are at least 5 years postmenopausal.[27] (2) Dosages are not necessarily equivalent. | |||
| Route/form | Estrogen | Dosage | |
|---|---|---|---|
| Oral | Estradiol | 1–2 mg 3x/day | |
| Conjugated estrogens | 1.25–2.5 mg 3x/day | ||
| Ethinylestradiol | 0.15–3 mg/day | ||
| Ethinylestradiol sulfonate | 1–2 mg 1x/week | ||
| Diethylstilbestrol | 1–3 mg/day | ||
| Dienestrol | 5 mg/day | ||
| Hexestrol | 5 mg/day | ||
| Fosfestrol | 100–480 mg 1–3x/day | ||
| Chlorotrianisene | 12–48 mg/day | ||
| Quadrosilan | 900 mg/day | ||
| Estramustine phosphate | 140–1400 mg/day | ||
| Transdermal patch | Estradiol | 2–6x 100 μg/day Scrotal: 1x 100 μg/day | |
| IM or SC injection | Estradiol benzoate | 1.66 mg 3x/week | |
| Estradiol dipropionate | 5 mg 1x/week | ||
| Estradiol valerate | 10–40 mg 1x/1–2 weeks | ||
| Estradiol undecylate | 100 mg 1x/4 weeks | ||
| Polyestradiol phosphate | Alone: 160–320 mg 1x/4 weeks With oral EE: 40–80 mg 1x/4 weeks | ||
| Estrone | 2–4 mg 2–3x/week | ||
| IV injection | Fosfestrol | 300–1200 mg 1–7x/week | |
| Estramustine phosphate | 240–450 mg/day | ||
| Note: Dosages are not necessarily equivalent. Sources: See template. | |||
Available forms
Estradiol benzoate is and has been available as an oil solution for intramuscular injection provided as vials and ampoules at concentrations of 0.167, 0.2, 0.33, 1, 1.67, 2, 5, 10, 20, and 25 mg/mL.[43][44][8] It is also available as a microcrystalline aqueous suspension for intramuscular injection under the brand name Agofollin Depot.[45][46][47][24] Sistocyclin was the brand name of a product containing 10 mg microcrystalline estradiol benzoate and 200 mg microcrystalline progesterone in an aqueous suspension.[48][49][50][51] Follivirin (and previously Femandren M) is the brand name of a product containing 2.5 mg microcrystalline estradiol benzoate and 25 to 50 mg microcrystalline testosterone isobutyrate in aqueous suspension.[52][53][54][55]
A vaginal tablet formulation containing 0.125 mg estradiol benzoate and 10 mg monalazone sodium has been marketed under the brand name Malun 25.[56]
| Route | Ingredient | Form | Dose[b] | Brand names[c] |
|---|---|---|---|---|
| Oral | Estradiol | Tablet | 0.1, 0.2, 0.5, 1, 2, 4 mg | Estrace, Ovocyclin |
| Estradiol valerate | Tablet | 0.5, 1, 2, 4 mg | Progynova | |
| Transdermal | Estradiol | Patch | 14, 25, 37.5, 50, 60, 75, 100 µg/d | Climara, Vivelle |
| Gel pump | 0.06% (0.52, 0.75 mg/pump) | Elestrin, EstroGel | ||
| Gel packet | 0.1% (0.25, 0.5, 1.0 mg/pk.) | DiviGel, Sandrena | ||
| Emulsion | 0.25% (25 µg/pouch) | Estrasorb | ||
| Spray | 1.53 mg/spray | Evamist, Lenzetto | ||
| Vaginal | Estradiol | Tablet | 10, 25 µg | Vagifem |
| Cream | 0.01% (0.1 mg/gram) | Estrace | ||
| Insert | 4, 10 µg | Imvexxy | ||
| Ring | 2 mg/ring (7.5 µg/d, 3 mon.) | Estring | ||
| Estradiol acetate | Ring | 50, 100 µg/d, 3 months | Femring | |
| Injection[d] | Estradiol | Microspheres | 1 mg/mL | Juvenum E |
| Estradiol benzoate | Oil solution | 0.167, 0.2, 0.333, 1, 1.67, 2, 5, 10, 20, 25 mg/mL | Progynon-B | |
| Estradiol cypionate | Oil solution | 1, 3, 5 mg/mL | Depo-Estradiol | |
| Estradiol valerate | Oil solution | 5, 10, 20, 40 mg/mL | Progynon Depot | |
| Implant | Estradiol | Pellet | 20, 25, 50, 100 mg, 6 mon. | Estradiol Implants |
Notes and sources:
| ||||
Contraindications
Side effects
The side effects of estradiol benzoate are the same as those of estradiol. Examples of such side effects include breast tenderness and enlargement, nausea, bloating, edema, headache, and melasma.[17]
Overdose
Interactions
Pharmacology

Pharmacodynamics
Estradiol benzoate is an estradiol ester, or a prodrug of estradiol.[4][5] As such, it is an estrogen, or an agonist of the estrogen receptors.[4][5] Estradiol benzoate has very low affinity for the ERs, on the order of 100-fold less than that of estradiol.[66] As such, estradiol benzoate is regarded as essentially inactive in terms of estrogenic effect itself, acting solely as a prodrug to estradiol.[5] Estradiol benzoate is of about 38% higher molecular weight than estradiol due to the presence of its C3 benzoate ester.[67][15] Because estradiol benzoate is a prodrug of estradiol, it is considered to be a natural and bioidentical form of estrogen.[4]
| Estrogen | Form | Dose (mg) | Duration by dose (mg) | ||
|---|---|---|---|---|---|
| EPD | CICD | ||||
| Estradiol | Aq. soln. | ? | – | <1 d | |
| Oil soln. | 40–60 | – | 1–2 ≈ 1–2 d | ||
| Aq. susp. | ? | 3.5 | 0.5–2 ≈ 2–7 d; 3.5 ≈ >5 d | ||
| Microsph. | ? | – | 1 ≈ 30 d | ||
| Estradiol benzoate | Oil soln. | 25–35 | – | 1.66 ≈ 2–3 d; 5 ≈ 3–6 d | |
| Aq. susp. | 20 | – | 10 ≈ 16–21 d | ||
| Emulsion | ? | – | 10 ≈ 14–21 d | ||
| Estradiol dipropionate | Oil soln. | 25–30 | – | 5 ≈ 5–8 d | |
| Estradiol valerate | Oil soln. | 20–30 | 5 | 5 ≈ 7–8 d; 10 ≈ 10–14 d; 40 ≈ 14–21 d; 100 ≈ 21–28 d | |
| Estradiol benz. butyrate | Oil soln. | ? | 10 | 10 ≈ 21 d | |
| Estradiol cypionate | Oil soln. | 20–30 | – | 5 ≈ 11–14 d | |
| Aq. susp. | ? | 5 | 5 ≈ 14–24 d | ||
| Estradiol enanthate | Oil soln. | ? | 5–10 | 10 ≈ 20–30 d | |
| Estradiol dienanthate | Oil soln. | ? | – | 7.5 ≈ >40 d | |
| Estradiol undecylate | Oil soln. | ? | – | 10–20 ≈ 40–60 d; 25–50 ≈ 60–120 d | |
| Polyestradiol phosphate | Aq. soln. | 40–60 | – | 40 ≈ 30 d; 80 ≈ 60 d; 160 ≈ 120 d | |
| Estrone | Oil soln. | ? | – | 1–2 ≈ 2–3 d | |
| Aq. susp. | ? | – | 0.1–2 ≈ 2–7 d | ||
| Estriol | Oil soln. | ? | – | 1–2 ≈ 1–4 d | |
| Polyestriol phosphate | Aq. soln. | ? | – | 50 ≈ 30 d; 80 ≈ 60 d | |
Notes and sources
Notes: All aqueous suspensions are of microcrystalline particle size. Estradiol production during the menstrual cycle is 30–640 µg/d (6.4–8.6 mg total per month or cycle). The vaginal epithelium maturation dosage of estradiol benzoate or estradiol valerate has been reported as 5 to 7 mg/week. An effective ovulation-inhibiting dose of estradiol undecylate is 20–30 mg/month. Sources: See template. | |||||
Pharmacokinetics
Following administration, estradiol benzoate acts as a prodrug of estradiol via cleavage by esterases into estradiol and the natural fatty acid benzoic acid.[5] This cleavage occurs not only in the liver, but also in the blood and in tissues.[4][5] Esters of estradiol like estradiol benzoate are readily hydrolyzed to estradiol, but have an extended duration when administered in via intramuscular or subcutaneous injection due to a depot effect afforded by their fatty acid ester moiety and consequent high lipophilicity.[5] A long-lasting local tissue depot is formed by the injection that slowly releases estradiol benzoate into the circulation.[5]
Intramuscular injection of oil solution
The duration of action of estradiol benzoate in oil solution by intramuscular injection at typical clinical doses (e.g., 0.33–1.66 mg) is said to be 2 to 3 days.[7][8] A single dose of 2.5 mg estradiol benzoate in oil solution by intramuscular injection was found to produce plasma estradiol levels of greater than 400 pg/mL, measured 24 hours post-injection, in a group of patients with minimal baseline levels of estradiol (due to GnRH analogue therapy with triptorelin).[68] The elimination half-life of estradiol benzoate in oil solution by intramuscular injection has been reported to be 48 to 120 hours (2 to 5 days).[6]
A single intramuscular injection of 5 mg estradiol benzoate in oil solution has been found to result in peak circulating concentrations of 940 pg/mL estradiol and 343 pg/mL estrone, which occurred at about 2 days post-injection.[9] Compared to two other commonly used estradiol esters, estradiol benzoate had the shortest duration, at approximately 4 to 5 days, whereas estradiol valerate and estradiol cypionate were found to last for 7 to 8 days and 11 days, respectively.[9] This is because estradiol benzoate has a shorter and less bulky fatty acid chain, and in relation to this, is comparatively less lipophilic.[5] For a given estradiol ester, the shorter or less bulky the fatty acid chain is, the less lipophilic, shorter-lasting, and less uniform/plateau-like the resultant levels of estradiol are as well as the higher (and hence more spike-like) the peak/maximal levels are.[5]
| Estrogen | Dose | Cmax | Tmax | Duration |
|---|---|---|---|---|
| Estradiol benzoate | 5 mg | E2: 940 pg/mL E1: 343 pg/mL |
E2: 1.8 days E1: 2.4 days |
4–5 days |
| Estradiol valerate | 5 mg | E2: 667 pg/mL E1: 324 pg/mL |
E2: 2.2 days E1: 2.7 days |
7–8 days |
| Estradiol cypionate | 5 mg | E2: 338 pg/mL E1: 145 pg/mL |
E2: 3.9 days E1: 5.1 days |
11 days |
| Notes: All via i.m. injection of oil solution. Determinations via radioimmunoassay with chromatographic separation. Sources: See template. | ||||
Template:Hormone levels with intramuscular estradiol benzoate
Intramuscular injection of crystalline aqueous suspension
Microcrystalline estradiol benzoate in aqueous suspension (brand names Agofollin Depot alone and Follivirin in combination with testosterone isobutyrate)[46][52] has been found to have a longer duration of action than amorphous estradiol benzoate in oil solution when administered via intramuscular injection.[69][70][71][72][73][74][75][76]: 310 Whereas the duration of a single intramuscular injection of estradiol benzoate in oil solution is 6 days, the duration of a single intramuscular injection of microcrystalline estradiol benzoate in aqueous suspension is 21 to 39 days.[76][70] Its duration also surpasses that of estradiol valerate and estradiol cypionate.[76] The duration of microcrystalline aqueous suspensions administered by intramuscular injection is dependent both on concentration and on crystal size.[77][78][74][79]
Other routes
In contrast to the intramuscular route, the duration of estradiol benzoate is not prolonged if it is administered directly into the circulation via intravenous injection.[80] Similarly to estradiol valerate and estradiol acetate, estradiol benzoate is also active with oral and sublingual administration.[7][76]: 310 However, it is not marketed for use by these routes.[43] Subcutaneous pellet implantation of estradiol benzoate has been studied, but likewise no estradiol benzoate implants have been marketed.[81]
Chemistry
Estradiol benzoate is a synthetic estrane steroid and the C3 benzoate (benzenecarboxylate) ester of estradiol.[15][67][82] It is also known as estradiol 3-benzoate or as estra-1,3,5(10)-triene-3,17β-diol 3-benzoate.[15][67][82] Two estradiol esters that are related to estradiol benzoate are estradiol dipropionate, the C3,17β dipropionate ester of estradiol, and estradiol acetate, the C3 acetate ester of estradiol.
| Estrogen | Structure | Ester(s) | Relative mol. weight |
Relative E2 contentb |
log Pc | ||||
|---|---|---|---|---|---|---|---|---|---|
| Position(s) | Moiet(ies) | Type | Lengtha | ||||||
| Estradiol | – | – | – | – | 1.00 | 1.00 | 4.0 | ||
| Estradiol acetate | C3 | Ethanoic acid | Straight-chain fatty acid | 2 | 1.15 | 0.87 | 4.2 | ||
| Estradiol benzoate | C3 | Benzoic acid | Aromatic fatty acid | – (~4–5) | 1.38 | 0.72 | 4.7 | ||
| Estradiol dipropionate | C3, C17β | Propanoic acid (×2) | Straight-chain fatty acid | 3 (×2) | 1.41 | 0.71 | 4.9 | ||
| Estradiol valerate | C17β | Pentanoic acid | Straight-chain fatty acid | 5 | 1.31 | 0.76 | 5.6–6.3 | ||
| Estradiol benzoate butyrate | C3, C17β | Benzoic acid, butyric acid | Mixed fatty acid | – (~6, 2) | 1.64 | 0.61 | 6.3 | ||
| Estradiol cypionate | C17β | Cyclopentylpropanoic acid | Cyclic fatty acid | – (~6) | 1.46 | 0.69 | 6.9 | ||
| Estradiol enanthate | C17β | Heptanoic acid | Straight-chain fatty acid | 7 | 1.41 | 0.71 | 6.7–7.3 | ||
| Estradiol dienanthate | C3, C17β | Heptanoic acid (×2) | Straight-chain fatty acid | 7 (×2) | 1.82 | 0.55 | 8.1–10.4 | ||
| Estradiol undecylate | C17β | Undecanoic acid | Straight-chain fatty acid | 11 | 1.62 | 0.62 | 9.2–9.8 | ||
| Estradiol stearate | C17β | Octadecanoic acid | Straight-chain fatty acid | 18 | 1.98 | 0.51 | 12.2–12.4 | ||
| Estradiol distearate | C3, C17β | Octadecanoic acid (×2) | Straight-chain fatty acid | 18 (×2) | 2.96 | 0.34 | 20.2 | ||
| Estradiol sulfate | C3 | Sulfuric acid | Water-soluble conjugate | – | 1.29 | 0.77 | 0.3–3.8 | ||
| Estradiol glucuronide | C17β | Glucuronic acid | Water-soluble conjugate | – | 1.65 | 0.61 | 2.1–2.7 | ||
| Estramustine phosphated | C3, C17β | Normustine, phosphoric acid | Water-soluble conjugate | – | 1.91 | 0.52 | 2.9–5.0 | ||
| Polyestradiol phosphatee | C3–C17β | Phosphoric acid | Water-soluble conjugate | – | 1.23f | 0.81f | 2.9g | ||
| Footnotes: a = Length of ester in carbon atoms for straight-chain fatty acids or approximate length of ester in carbon atoms for aromatic or cyclic fatty acids. b = Relative estradiol content by weight (i.e., relative estrogenic exposure). c = Experimental or predicted octanol/water partition coefficient (i.e., lipophilicity/hydrophobicity). Retrieved from PubChem, ChemSpider, and DrugBank. d = Also known as estradiol normustine phosphate. e = Polymer of estradiol phosphate (~13 repeat units). f = Relative molecular weight or estradiol content per repeat unit. g = log P of repeat unit (i.e., estradiol phosphate). Sources: See individual articles. | |||||||||
History
Estradiol benzoate was one of the first estrogens to be developed and marketed.[19][20] In 1931, Adolf Butenandt found that the benzoic acid ester of estrone had a prolonged duration of action.[11][83] Schwenk and Hildebrant discovered estradiol via reduction of estrone in 1933, and they proceeded to synthesize estradiol benzoate from estradiol the same year.[4][18] Estradiol benzoate was patented by Schering in 1933, and then by Schering-Kahlbaum in 1936 in an oil preparation for injectable use, and was introduced later that year in 1936 under the brand name Progynon-B.[19][20][43] Following its introduction, estradiol benzoate, along with estradiol dipropionate, were the most widely used esters of estradiol for many years.[21] However, estradiol valerate and estradiol cypionate, which are longer-acting esters that require less frequent administration, were developed and introduced in the 1950s, and have since largely superseded estradiol benzoate and estradiol dipropionate.[9]
Society and culture
Generic names
Estradiol benzoate is the generic name of the drug and its INN, BANM, and JAN, while oestradiol benzoate was formerly its BANM.[14][15][67][82]
Brand names
The major brand name of estradiol benzoate is Progynon-B.[15][67][82] It has also been sold under a variety of other brand names including Agofollin Depot, Benovocyclin, Benzofoline, Dimenformon, Ovocylin Benzoate, and Ovocyclin M, among many others.[15][67][82]
Availability
Estradiol benzoate is available in Europe and in other parts of the world.[15][43] It was previously available for medical use in the United States, but is no longer marketed in this country.[15][23][43][22] However, it is approved and marketed in the United States for veterinary use as a subdermal implant both alone and in combination with the androgen/anabolic steroid trenbolone acetate (brand names Celerin and Synovex, respectively).[23][84][85] Outside of the United States, estradiol benzoate is also marketed in combination with progesterone for use as an intramuscular injection.[14][86]
Microcrystalline estradiol benzoate in aqueous suspension is available in the Czech Republic and Slovakia alone under the brand name Agofollin Depot and in combination with microcrystalline testosterone isobutyrate under the brand name Folivirin.[46][52][14]
Research
Estradiol benzoate has been studied in combination with norethisterone enanthate as a once-a-month combined injectable contraceptive, but ultimately did not complete development for this indication.[87]
See also
- Estradiol benzoate/hydroxyprogesterone caproate
- Estradiol benzoate/progesterone
- Estradiol benzoate/testosterone isobutyrate
References
- ^ Düsterberg B, Nishino Y (December 1982). "Pharmacokinetic and pharmacological features of oestradiol valerate". Maturitas. 4 (4): 315–24. doi:10.1016/0378-5122(82)90064-0. PMID 7169965.
- ^ Stanczyk, Frank Z.; Archer, David F.; Bhavnani, Bhagu R. (2013). "Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment". Contraception. 87 (6): 706–727. doi:10.1016/j.contraception.2012.12.011. ISSN 0010-7824. PMID 23375353.
- ^ Tommaso Falcone; William W. Hurd (2007). Clinical Reproductive Medicine and Surgery. Elsevier Health Sciences. pp. 22, 362, 388. ISBN 978-0-323-03309-1.
- ^ a b c d e f g h i j k Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens I: Physiology and Mechanisms of Action of Estrogens and Antiestrogens. Springer Science & Business Media. pp. 8–. ISBN 978-3-642-58616-3.
- ^ a b c d e f g h i j k l m Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- ^ a b Benno Runnebaum; Thomas Rabe (17 April 2013). Gynäkologische Endokrinologie und Fortpflanzungsmedizin: Band 1: Gynäkologische Endokrinologie. Springer-Verlag. pp. 86–. ISBN 978-3-662-07635-4.
- ^ a b c H.J. Buchsbaum (6 December 2012). The Menopause. Springer Science & Business Media. pp. 62–. ISBN 978-1-4612-5525-3.
- ^ a b c d e f g h i j k l m n "NNR: Products Recently Accepted by the A. M. A. Council on Pharmacy and Chemistry". Journal of the American Pharmaceutical Association (Practical Pharmacy ed.). 10 (11): 692–694. 1949. doi:10.1016/S0095-9561(16)31995-8. ISSN 0095-9561.
- ^ a b c d e Oriowo MA, Landgren BM, Stenström B, Diczfalusy E (April 1980). "A comparison of the pharmacokinetic properties of three estradiol esters". Contraception. 21 (4): 415–24. doi:10.1016/S0010-7824(80)80018-7. PMID 7389356.
- ^ Karl Knörr; Henriette Knörr-Gärtner; Fritz K. Beller; Christian Lauritzen (8 March 2013). Lehrbuch der Geburtshilfe und Gynäkologie: Physiologie und Pathologie der Reproduktion. Springer-Verlag. pp. 508–. ISBN 978-3-662-00526-2.
- ^ a b A. Labhart (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 512–. ISBN 978-3-642-96158-8.
- ^ a b c d e f Swyer GI (April 1959). "The oestrogens". Br Med J. 1 (5128): 1029–31. doi:10.1136/bmj.1.5128.1029. PMC 1993181. PMID 13638626.
- ^ a b c Gianna E. Israel (March 2001). Transgender Care: Recommended Guidelines, Practical Information, and Personal Accounts. Temple University Press. pp. 64–. ISBN 978-1-56639-852-7.
- ^ a b c d e "Estradiol". Drugs.com. Cite error: The named reference "Drugs.com" was defined multiple times with different content (see the help page).
- ^ a b c d e f g h i j Index Nominum 2000: International Drug Directory. Taylor & Francis US. 2000. p. 406. ISBN 978-3-88763-075-1.
- ^ IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer (2007). Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy. World Health Organization. pp. 388–. ISBN 978-92-832-1291-1.
- ^ a b Amit K. Ghosh (23 September 2010). Mayo Clinic Internal Medicine Board Review. OUP USA. pp. 222–. ISBN 978-0-19-975569-1.
- ^ a b Miescher K, Scholz C, Tschopp E (1938). "The activation of female sex hormones: Mono-esters of alpha-oestradiol". Biochem. J. 32 (8): 1273–80. PMC 1264184. PMID 16746750.
- ^ a b c d Enrique Raviña; Hugo Kubinyi (16 May 2011). The Evolution of Drug Discovery: From Traditional Medicines to Modern Drugs. John Wiley & Sons. p. 175. ISBN 978-3-527-32669-3. Retrieved 20 May 2012.
- ^ a b c d Folley SJ (December 1936). "The effect of oestrogenic hormones on lactation and on the phosphatase of the blood and milk of the lactating cow" (PDF). The Biochemical Journal. 30 (12): 2262–72. doi:10.1042/bj0302262. PMC 1263335. PMID 16746289.
- ^ a b Schwartz MM, Soule SD (1955). "Estradiol 17-beta-cyclopentylpropionate, a longacting estrogen". Am. J. Obstet. Gynecol. 70 (1): 44–50. doi:10.1016/0002-9378(55)90286-6. PMID 14388061.
- ^ a b "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 16 November 2016.
- ^ a b c Richard Witherspoon (1 June 1994). Presidents List of Articles Which May Be Designated Or Modified As Eligible Articles for Purposes of the U.S. Generalized System of Preferences. DIANE Publishing. pp. 64–. ISBN 978-0-7881-1433-5.
- ^ a b Fifková Hanka; Weiss Petr; Procházka Ivo; Cohen-Kettenis Peggy T., Pfäfflin Friedemann, Jarolím Ladislav, Veselý Jiří, Weiss Vladimír (4 August 2008). Transsexualita a jiné poruchy pohlavní identity. Grada Publishing a.s. pp. 95–. ISBN 978-80-247-6962-2.
Injection of estradiol benzoate is supplied as Agofollin Depot inj. 10 mg, Biotika and as estradiol valerate Neofollin inj., 5 mg, Hoechst-Biotika. Depot estrogen injections are not recommended due to side effects. Possibility "overdose" of the patient is higher (in some individuals receiving doses "the higher the better," and parenteral drug administration may in some instances these cause serious side effects). While misuse of the drug with peroral administration also occurs, the problems are not so extreme.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Weiss Petr a kolektiv (1 January 2010). Sexuologie. Grada Publishing a.s. pp. 452–. ISBN 978-80-247-2492-8.
- ^ American Medical Association. Dept. of Drugs; Council on Drugs (American Medical Association); American Society for Clinical Pharmacology and Therapeutics (1 February 1977). "Estrogens, Progestagens, Oral Contraceptives, and Ovulatory Agents". AMA drug evaluations. Publishing Sciences Group. p. 540–572. ISBN 978-0-88416-175-2.
Intramuscular: For replacement therapy, (Estradiol, Estradiol Benzoate) 0.5 to 1.5 mg two or three times weekly; (Estradiol Cypionate) 1 to 5 mg weekly for two or three weeks; (Estradiol Dipropionate) 1 to 5 mg every one to two weeks; (Estradiol Valerate) 10 to 40 mg every one to four weeks.
- ^ a b c d e Coelingh Bennink HJ, Verhoeven C, Dutman AE, Thijssen J (January 2017). "The use of high-dose estrogens for the treatment of breast cancer". Maturitas. 95: 11–23. doi:10.1016/j.maturitas.2016.10.010. PMID 27889048.
- ^ "ESTRACE® TABLETS (estradiol tablets, USP) FDA label" (PDF). 2005.
- ^ a b Palmieri C, Patten DK, Januszewski A, Zucchini G, Howell SJ (January 2014). "Breast cancer: current and future endocrine therapies". Mol. Cell. Endocrinol. 382 (1): 695–723. doi:10.1016/j.mce.2013.08.001. PMID 23933149.
- ^ a b c Green RB, Sethi RS, Lindner HH (July 1964). "Treatment of advanced carcinoma of the breast: Progress in therapy during the past decade". Am. J. Surg. 108: 107–21. doi:10.1016/0002-9610(64)90094-7. PMID 14182428.
- ^ a b c Thomas, John A.; Keenan, Edward J. (6 December 1986). "Estrogens and Antiestrogenic Drugs". Principles of Endocrine Pharmacology. Springer Science & Business Media. pp. 135–165. doi:10.1007/978-1-4684-5036-1_7. ISBN 978-1-4684-5036-1.
- ^ "Premarin® (conjugated estrogens tablets, USP) FDA label" (PDF). 2003.
- ^ a b c d Van Winkle, Walton (1949). "Council on Pharmacy and Chemistry. Estrogens and Androgens in Mammary Cancer". JAMA: The Journal of the American Medical Association. 140 (15): 1214. doi:10.1001/jama.1949.02900500022007. ISSN 0098-7484.
- ^ a b c d Kahr, Ernst (1966). "Die Allgemeinbehandlung" [General Treatment]. Der Inoperable Krebskranke: Möglichkeiten der Therapie in Klinik und Praxis [The Inoperable Cancer Patient: Possibilities of Therapy in Clinic and Practice]. Springer-Verlag. pp. 104–155. doi:10.1007/978-3-642-86140-6_4. ISBN 978-3-642-86140-6.
- ^ a b c Dao, Thomas L. (1975). "Pharmacology and Clinical Utility of Hormones in Hormone Related Neoplasms". In Alan C. Sartorelli; David G. Johns (eds.). Antineoplastic and Immunosuppressive Agents. pp. 170–192. doi:10.1007/978-3-642-65806-8_11. ISBN 978-3-642-65806-8.
- ^ a b c d Nathanson IT, Kelley RM (January 1952). "Hormonal treatment of cancer". N. Engl. J. Med. 246 (5): 180–9, concl. doi:10.1056/NEJM195201312460505. PMID 14890833.
- ^ a b Dobson L (August 1962). "The management of metastatic breast cancer". Surg. Clin. North Am. 42: 861–76. doi:10.1016/S0039-6109(16)36728-7. PMID 13886800.
- ^ a b Martz G (13 March 2013). Die hormonale Therapie maligner Tumoren: Endokrine Behandlungsmethoden des metastasierenden Mamma-, Prostata- und Uterus-Corpuscarcinoms. Springer-Verlag. pp. 39–. ISBN 978-3-642-86282-3.
- ^ Committee on Research, AMA (1960). "Androgens and estrogens in the treatment of disseminated mammary carcinoma. Retrospective study of nine hundred forty-four patients". Journal of the American Medical Association. 172 (12): 1271. doi:10.1001/jama.1960.03020120049010. ISSN 0002-9955.
- ^ "Estradurin® (polyestradiol phosphate) information and labels". Pharmanovia.
- ^ Ostrowski MJ, Jackson AW (1979). "Polyestradiol phosphate: a preliminary evaluation of its effect on breast carcinoma". Cancer Treat Rep. 63 (11–12): 1803–7. PMID 393380.
- ^ "Estrone suspension FDA review" (PDF). 1979.
- ^ a b c d e f A. Kleemann; J. Engel; B. Kutscher; D. Reichert (14 May 2014). Pharmaceutical Substances, 5th Edition, 2009: Syntheses, Patents and Applications of the most relevant APIs. Thieme. pp. 1167–1174. ISBN 978-3-13-179525-0. Cite error: The named reference "KleemannEngel2014" was defined multiple times with different content (see the help page).
- ^ a b Muller (19 June 1998). European Drug Index: European Drug Registrations, Fourth Edition. CRC Press. pp. 150, 349, 370. ISBN 978-3-7692-2114-5. Cite error: The named reference "Muller1998" was defined multiple times with different content (see the help page).
- ^ Review of Czechoslovak Medicine. Avicenum - Czechoslovak Medical Press. 1973. p. 5.
Oestradiol benzoate, aqueous microcrystalline suspension (Agofollin Depot SPOFA).
- ^ a b c https://web.archive.org/web/20190519104654/http://www.sukl.cz/download/pil/PI16221.pdf
- ^ Marek Josef a kolektiv (1 January 2010). Farmakoterapie vnitřních nemocí - 4. zcela přepracované a doplněné vydání. Grada Publishing a.s. pp. 377–. ISBN 978-80-247-2639-7.
Injection of estrogenic preparations - Injectable preparations are AGOFOLLIN, inj. 5 mg (estradiol dipropionate), AGOFOLLIN DEPOT, inj. 10 mg (estradiol benzoate), and NEOFOLLIN, inj. 5 mg (estradiol valerate). The producer of all these preparations is Biotika. Non-protracted AGOFOLLIN is used only for initiation of treatment, then it is continued with depot injections, which are administered three times: cycle day 4, 11 and 18. At the same time, [progesterone] (AGOLUTIN DEPOT, Biotika, amp. 2 ml / 50 mg, cycle day 18 and 25) is administered. Estrogen injection is not completely physiological - after application, the estrogen plasma concentration increases unnecessarily high and then decreases rapidly.
- ^ Ufer, Joachim (1968). "Die therapeutische Anwendung der Gestagene beim Menschen" [Therapeutic Use of Progestagens in Humans]. Die Gestagene [Progestogens]. Springer-Verlag. pp. 1026–1124. doi:10.1007/978-3-642-99941-3_7. ISBN 978-3-642-99941-3.
C. Dysfunktionelle Uterusblutungen. [...] 1. Depotinjektionen. 1. Originalmethode nach KAUFMANN und OBER. Es wird 1 Amp. mit 200 mg Progesteron und 10 mg Oestradiol-Monobenzoat als Kristallsuspension (Sistocyclin) injiziert [676, 678, 679, 295, 482, 365, 434, 563, 400]. [...] Beispiele. KAUFMANN et al. [485]: 400 mg Progesteron + 20 mg Oestradiolmonobenzoat Kristallsuspension. ELERT [224] U. HERRMANN [363]: 200 mg Progesteron + 10 mg Oestradiolmono benzoat Kristallsuspension.
- ^ Ciba Symposium. Ciba. 1957.
CIBA's range of hormone preparations has been increased with the advent of "Sistocyclin", one ampoule of which contains 200 mg progesterone and 10 mg oestradiol monobenzoate in crystalline suspension; it thus meets the requirements—in line with the most recent findings of the KAUFMANN Clinic—of cases marked by deficiency of corpus luteum hormone, e. g. in functional bleeding such as metropathia haemorrhagica.
- ^ Ciba Zeitschrift. 1957. p. 3001.
Sistocyclin - a microcrystal suspension containing 200 mg progesterone and 10 mg oestradiol monobenzoate per ampoule - has become particularly useful in the treatment of so-called, functional [...]
- ^ Willibald Pschyrembel (15 June 2011). Praktische Gynäkologie: für Studierende und Ärzte. Walter de Gruyter. pp. 601–. ISBN 978-3-11-150424-7.
- ^ a b c https://web.archive.org/web/20190520031642/http://www.sukl.cz/download/pil/PI15789.pdf
- ^ Kubíková, Drahomíra (2014). "Menopauzální symptomy a hormonální substituční terapie" [Menopausal symptoms and hormone replacement therapy]. Praktické Lékárenství (in Czech). 10 (2): 68–73. ISSN 1801-2434.
- ^ Marek Josef; a kolektiv (14 May 2010). Farmakoterapie vnitřních nemocí: 4., zcela přepracované a doplněné vydání. Grada Publishing a.s. pp. 380–. ISBN 978-80-247-9524-9.
In addition, testosterone isobutyrate in FOLIVIRIN, Biotika, an injection containing 25 mg testosterone isobutyrate and 2.5 mg estradiol benzoate is available. It is applied every 4-6 weeks depending on the effect.
- ^ Ciba Symposium: 1953/57:Index. Ciba. 1953. p. 197.
Femandren M. C'est le nom des nouvelles ampoules cristallines destinées au traitement associé œs- trogène-androgène. Elles renferment, sous forme de microcristaux, 2,5 mg de mono- benzoate d'œstradiol et 50 mg d'isobutyra- te de testostérone ; elles sont indiquées pour traiter les cas où il convient d'administrer simultanément de l'hormone femelle et de l'hormone mâle et où il importe aussi d'obtenir un effet prolongé, par exemple lors de symptômes d'insuffisance à la ménopause ou après castration. L'effet d'une injection se prolonge pendant 3-6 semaines.
- ^ a b Freimut A. Leidenberger (17 April 2013). Klinische Endokrinologie für Frauenärzte. Springer-Verlag. pp. 527–. ISBN 978-3-662-08110-5. Cite error: The named reference "Leidenberger2013" was defined multiple times with different content (see the help page).
- ^ "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 26 July 2018.
- ^ Lobo RA (5 June 2007). Treatment of the Postmenopausal Woman: Basic and Clinical Aspects. Academic Press. pp. 177, 217–226, 770–771. ISBN 978-0-08-055309-2.
- ^ Falcone T, Hurd WW (14 June 2017). Clinical Reproductive Medicine and Surgery: A Practical Guide. Springer. pp. 179–. ISBN 978-3-319-52210-4.
- ^ Becker KL (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 889, 1059–1060, 2153. ISBN 978-0-7817-1750-2.
- ^ Krishna UR, Sheriar NK (1996). Menopause. Orient Blackswan. pp. 70–. ISBN 978-81-250-0910-8.
- ^ "AERODIOL (Oestradiol hemihydrate 150 micrograms/actuation)" (PDF). Servier Laboratories (Aust) Pty Ltd.
- ^ Sahin FK, Koken G, Cosar E, Arioz DT, Degirmenci B, Albayrak R, Acar M (2008). "Effect of Aerodiol administration on ocular arteries in postmenopausal women". Gynecol. Endocrinol. 24 (4): 173–7. doi:10.1080/09513590701807431. PMID 18382901.
300 μg 17β-estradiol (Aerodiol®; Servier, Chambrayles-Tours, France) was administered via the nasal route by a gynecologist. This product is unavailable after March 31, 2007 because its manufacturing and marketing are being discontinued.
- ^ Plouffe Jr L, Ravnikar VA, Speroff L, Watts NB (6 December 2012). Comprehensive Management of Menopause. Springer Science & Business Media. pp. 271–. ISBN 978-1-4612-4330-4.
- ^ Hospital Formulary and Compendium of Useful Information. University of California Press. 1952. pp. 49–. GGKEY:2UAAZRZ5LN0.
- ^ Bovee TF, Helsdingen RJ, Rietjens IM, Keijer J, Hoogenboom RL (2004). "Rapid yeast estrogen bioassays stably expressing human estrogen receptors alpha and beta, and green fluorescent protein: a comparison of different compounds with both receptor types". J. Steroid Biochem. Mol. Biol. 91 (3): 99–109. doi:10.1016/j.jsbmb.2004.03.118. PMID 15276617.
- ^ a b c d e f J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 897–. ISBN 978-1-4757-2085-3.
- ^ Vizziello G, D'Amato G, Trentadue R, Fanizza G (1993). "[Estradiol benzoate test in the study of pituitary block induced by triptorelin]". Minerva Ginecol (in Italian). 45 (4): 185–9. PMID 8506068.
- ^ von Wattenwyl, H (1944), "Über eine neue Anwendungsart oestrogener Substanzen" [A new type of application of estrogenic substances], Schweiz. med. Wchnschr. (in German), 74: 159–161
- ^ a b Toppozada, Hussein K. (1950). "Oestrogenic Therapy with Prolonged Action". Obstetrical & Gynecological Survey. 5 (4): 531. doi:10.1097/00006254-195008000-00021. ISSN 0029-7828.
- ^ Ferin J (January 1952). "Relative duration of action of natural and synthetic estrogens administered parenterally in women with estrogen deficiency". J. Clin. Endocrinol. Metab. 12 (1): 28–35. doi:10.1210/jcem-12-1-28. PMID 14907837.
- ^ Ober, K. G.; Klein, Irmhild; Weber, Marianne (1954). "Zur Frage einer Progesteronbehandlung: Experimentelle Untersuchungen mit dem Hooker-Forbes-Test und klinische Beobachtungen mit Kristallsuspensionen" [On the question of progesterone treatment: experimental studies with the Hooker-Forbes test and clinical observations with crystal suspensions]. Archiv für Gynäkologie. 184 (5): 543–616. doi:10.1007/BF00976991. ISSN 0003-9128.
- ^ Field-Richards S (April 1955). "A preliminary series of cases of uterine hypoplasia treated by local injection of an oestrogenic emulsion". J Obstet Gynaecol Br Emp. 62 (2): 205–13. doi:10.1111/j.1471-0528.1955.tb14121.x. PMID 14368390.
Oestradiol monobenzoate or oestradiol diproprionate are slowly absorbed from oily solution after intramuscular injection and for this purpose are to be preferred to the unesterified form. As an even slower absorption of oestradiol monobenzoate can be obtained from an aqueous emulsion of this hormone (Lens, Overbeek and Polderman, 1949). Such a preparation for parenteral use was made available for this experiment by Messrs. Organon Laboratories Limited.
- ^ a b Lens J, Overbeek GA, Polderman J (1949). "The effect of sex hormones in some organic solvents; emulsified in water". Acta Endocrinol. 2 (4): 396–404. doi:10.1530/acta.0.0020396. PMID 18140399.
- ^ Ralph I. Dorfman (5 December 2016). Steroidal Activity in Experimental Animals and Man. Elsevier Science. pp. 40–. ISBN 978-1-4832-7299-3.
Ferin (1952) also studied duration of action in women with estrogen deficiency by recording the days of freedom from hot flushes. He rates estradiol-3-benzoate, estradiol-3-furoate, estradiol dipropionate, estradiol-17-caprylate, estradiol-3-benzoate-17-caprylate in oil, and finally estradiol-3-benzoate in emulsion or as microcrystals in that order of duration of action. After 10 mg. of each of the above preparations, a woman would typically remain free of symptoms for 10 days. This could, however, be as much as 50 days.
- ^ a b c d Horský, Jan; Presl, Jiří (1981). "Hormonal Treatment of Disorders of the Menstrual Cycle". In J. Horsky; J. Presl (eds.). Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. pp. 309–332. doi:10.1007/978-94-009-8195-9_11. ISBN 978-94-009-8195-9.
- ^ Shearman RP (June 1975). "The development of depot contraceptives". J. Steroid Biochem. 6 (6): 899–902. doi:10.1016/0022-4731(75)90323-4. PMID 1177432.
- ^ Edkins, Robert Patrick (1959). "The Modification of the Duration of Drug Action". Journal of Pharmacy and Pharmacology. 11 (S1): 54T–66T. doi:10.1111/j.2042-7158.1959.tb10412.x. ISSN 0022-3573.
- ^ Herrmann, U. (1958). "Abhängigkeit der durch Oestrogen- und Progesteron-Kristalle induzierten Abbruchblutung von der Korngröße". Gynecologic and Obstetric Investigation. 146 (4): 318–323. doi:10.1159/000306607. ISSN 1423-002X.
- ^ Parkes AS (February 1938). "Effective Absorption of Hormones". Br Med J. 1 (4024): 371–3. doi:10.1136/bmj.1.4024.371. PMC 2085798. PMID 20781252.
- ^ Bishop PM, Folley SJ (August 1951). "Absorption of hormone implants in man". Lancet. 2 (6676): 229–32. doi:10.1016/S0140-6736(51)93237-0. PMID 14862159.
- ^ a b c d e A. D. Roberts (1991). Dictionary of Steroids: Chemical Data, Structures, and Bibliographies. CRC Press. p. 415. ISBN 978-0-412-27060-4. Retrieved 20 May 2012.
- ^ Butenandt, A.; Hildebrandt, F. (1931). "Über ein zweites Hormonkrystallisat aus Schwangerenharn und seine physiologischen und chemischen Beziehungen zum krystallisierten Follikelhormon. [Untersuchungen über das weibliche Sexualhormon, 6. Mitteilung.]". Hoppe-Seyler's Zeitschrift für Physiologische Chemie. 199 (4–6): 243–265. doi:10.1515/bchm2.1931.199.4-6.243. ISSN 0018-4888.
- ^ http://www.fda.gov/downloads/AnimalVeterinary/Products/ApprovedAnimalDrugProducts/FOIADrugSummaries/ucm116143.pdf
- ^ http://www.fda.gov/downloads/AnimalVeterinary/Products/ApprovedAnimalDrugProducts/FOIADrugSummaries/UCM338208.pdf
- ^ "Progesterone". Drugs.com. Retrieved 2018-07-31.
- ^ Goldsmith, A., & Toppozada, M. (1983). Long-acting contraception. pp. 94-95 https://www.popline.org/node/423289