Wikipedia:Reference desk/Science: Difference between revisions
→Does water expand when heated?: water does expand |
|||
| Line 696: | Line 696: | ||
::I haven't looked at a lot of old mirrors to be familiar with the tarnished look Steve has in mind, but I would have thought that a "yellowish look" in an old mirror was due to the use of a cheaper reflecting metal that was yellowish to begin with. (Why would the reflecting metal tarnish if it's covered with glass?) But I was in the Hall of Mirrors last week, and what I noticed was that a lot of the mirrors have a gray or cloudy appearance. I didn't take too close a look (the place was chock-full with other tourists), but I certainly assumed that they were the originals, and this coloring was introduced during manufacture. --Anonymous, 16:26 UTC, October 1, 2007. |
::I haven't looked at a lot of old mirrors to be familiar with the tarnished look Steve has in mind, but I would have thought that a "yellowish look" in an old mirror was due to the use of a cheaper reflecting metal that was yellowish to begin with. (Why would the reflecting metal tarnish if it's covered with glass?) But I was in the Hall of Mirrors last week, and what I noticed was that a lot of the mirrors have a gray or cloudy appearance. I didn't take too close a look (the place was chock-full with other tourists), but I certainly assumed that they were the originals, and this coloring was introduced during manufacture. --Anonymous, 16:26 UTC, October 1, 2007. |
||
:::(The reflecting metal tarnishes because only the front face of the metal is covered with glass - the back is not. [[User:SteveBaker|SteveBaker]] 19:07, 1 October 2007 (UTC) |
:::(The reflecting metal tarnishes because only the front face of the metal is covered with glass - the back is not. [[User:SteveBaker|SteveBaker]] 19:07, 1 October 2007 (UTC) |
||
Don't get your panties in a wad, Stevie. I was just wondering if the possibility of dangerous levels of mercury vapor in the hall has ever been considered. |
|||
== Penis question == |
== Penis question == |
||
Revision as of 02:09, 2 October 2007
Wikipedia:Reference desk/headercfg
September 25
database software for idea storage
so lately I have been using GMail to store information that I would like to be searchable. For instance I want to store the name of walmarts Green technology activist so I email his name to myself with some keywords. This is obviously not ideal- is their a piece of software out there already that does something similar? I have a mac and considered creating a text document with names and keyword near each-other and using spotlight but that seems clunky, and possibly unworkable...
Other thoughts??
Ebenbayer 00:46, 25 September 2007 (UTC)
- Hmm, yes there are a variety of programs out there that try to do things like this. Here, for example, is a review of various note taking applications, any of which can likely do something as simple as creating a searchable list of notes and information. --24.147.86.187 01:53, 25 September 2007 (UTC)
Housing economics
I tried reading Real estate economics and housing bubble but they didn't help much... Construction locally has caused a major surplus in houses, to the point that 90% of the houses in some neighborhoods remain unoccupied, even after a few years. I see it's not desirable to sell a house for less than the value of its land/labor/materials, but prices aren't dropping to an equilibrium as they should (?). What is keeping the houses' prices from dropping? Are the developers (or whoever controls the prices) just holding out until the remaining 90% of houses are eventually bought? Because right now, those houses are sitting debts. HYENASTE 01:30, 25 September 2007 (UTC)
- Obviously the developers want to avoid selling at a loss. Thus they'll hold onto the properties while they consider it's of greater economic advantage to them not to sell, than to sell at a loss. There's some recognised economic principle (that I wish I could remember its name), possibly more based on stockmarkets (or sharemarkets depending where you are) that explains how investors are extremely reticient to 'lock in' a loss; they'll therefore sit on often high-risk loss-making stock for years rather than selling, in the hope that the stock will eventually at least regain the initial value that they paid for it. This is despite the fact that more often than not they'd be better to sell when the stock first starts crashing and invest in something else, even it means an initial loss. Anyway, the real estate you write about sounds something similar.
- Also what we don't know is the nature of the investments. It's possible for example that what sounds like a housing glut has been encouraged - either overtly or surreptitiously - by the government from wherever you're from. What often happens is that the government will give some type of encouragement to investors to invest in some particular area of the economy, then back it by guaranteeing them that they won't make a loss. If they do make a loss then the government (read the tax-payers) make up for the shortfall, either through direct bailouts of the investors, or through tax breaks. Thus the investors are encouraged to invest by what is essentially a government backed guaranteed profit - the investors take all the profit, while the government, and therefore tax-payers and society, take all the risk. But we don't really know enough about your particular situation to say for sure what's happening. --jjron 09:30, 25 September 2007 (UTC)
Intron degradation
Hi
The classic statement that people tend to make is "in one review, someone wrongly said that introns were degraded quickly, and everyone picked up on this and quoted this review for ever". My question is: what was this review? Does anyone know when, where or by whom it was published?
Cheers, Aaadddaaammm 07:38, 25 September 2007 (UTC)
- In lieu of actually answering the question, I'll at least provide some relevant information. This paper (which describes the process near the bottom) suggests the process was barely studied until recently, and that the half-lives of mammalian introns have been measured to lie between 6 and 29 minutes. Someguy1221 07:56, 25 September 2007 (UTC)
When you say degraded quickly are you referring to the splicing of RNA or their presence in the genome? David D. (Talk) 22:39, 26 September 2007 (UTC)
I'm talking about the intronic RNA after it's been spliced out of the pre-mRNA. Aaadddaaammm 23:09, 26 September 2007 (UTC)
- I have never heard of the review but a quick search reveal a paper from 1992 (L Qian, M N Vu, M Carter, and M F Wilkinson (1992) A spliced intron accumulates as a lariat in the nucleus of T cells. Nucleic Acids Res. 1992 October 25; 20(20): 5345–5350. ) that states "RNA splicing is a process whereby exons are joined and intervening sequences (introns) are removed in the form of a lariat structure" and cites the following review. Sharp PA, Konarksa MM, Grabowski PJ, Lamond AI, Marciniak, R and Seiler, SR (1987) Cold Spring Harbor Symposia on Quanititative Biology. LII, 277-285. That review sounds like a good candidate.
- The claimed first paper on this topic in eukaryotes is titled The stability and fate of a spliced intron from vertebrate cells, published in RNA in 1999. They cite half lives of less than 5 minutes for the intro they studied. David D. (Talk) 04:55, 28 September 2007 (UTC)
What's the problem with Boltzmann brains?
A couple of weeks ago, Newscientist magazine ran a doom and gloom article about Boltzmann brains.
As far as I can tell, the argument goes something like:
- We are typical observers
- If we're not typical, then the part of our universe we can see isn't typical
- Some time in the future, infinite expansion of the universe will lead to an infinite number of Boltzmann brains popping out of vacuum.
- The number of normal brains (like us) is going to tend towards "damn big" but not infinite.
- "Infinity" outnumbers "damn big"
- Over the course of the universe, they will outnumber us (on average)
- Given that we're outnumbered, on average, we can't be typical, therefore all our science falls around our feet in a horrible mess of untypicality!
- Therefore the world must end before we're outnumbered because obviously we're typical.
How does that matter to us? Why do we have to be typical for our observations to be useful? Is this something to do quantum wierdness? Human egocentricity?
Did I misunderstand the article?
Article is here, sorry, it's "premium" content.
--203.22.236.14 09:33, 25 September 2007 (UTC)
- The point is that there's a "noise floor" for scientific explanations of phenomena. I like to talk about Han Wavel instead. If your evolutionary explanation of Han Wavel's hotels (a civilization evolved that was capable of providing lemon-soaked paper napkins) depends on events less probable than the random formation of the hotels, then you haven't explained anything. For the same reason, you can't just say "well, the observable universe has amazingly low entropy, but that was bound to happen somewhere." You can't just compound improbability upon improbability; you have to come up with something more plausible than dumb luck. -- BenRG 12:24, 25 September 2007 (UTC)
- No! This kind of argument is complete crap. Let's take this carefully:
- We are typical observers - we don't know that. But I'll let that slide.
- If we're not typical, then the part of our universe we can see isn't typical - we do know that. Most of the universe appears to be vacuum - we are on a tiny blue-green planet. But, let's let this one slide too.
- Some time in the future, infinite expansion of the universe will lead to an infinite number of Boltzmann brains popping out of vacuum. - It's not widely agreed that the universe will undergo infinite expansion...but OK, let's run with this assumption too. If the universe is infinite and if a Boltzman brain is physically possible then there are certainly an infinite number of them. There are also an infinite number of anything else that's physically possible too. Infinite numbers of black holes, infinite numbers of cute little white kittens, infinite numbers of everything.
- The number of normal brains (like us) is going to tend towards "damn big" but not infinite. - No. If the universe is infinite then no matter how rare, there are still an infinite number of planets like ours inhabited with people like us. An infinite number of them have internets. An infinite number of those have Wikipedias with reference desks and an infinite number of those have questions identical to this one being asked right now. Infinity is infinite...once you have it, nothing within that infinity can be finite anymore - you either have zero of something or an infinite number of them. You can't cut corners.
- "Infinity" outnumbers "damn big" - it does - but the previous premise was wrong - so this is irrelevent to the argument.
- Over the course of the universe, they will outnumber us (on average) - Not possible, in an infinite universe there is an infinite number of everything that's physically possible. So averaged over the entire universe the ratio of us to them is infinity to infinity...which could be anything. Check out Hilbert's paradox of the Grand Hotel or Balls and vase problem to get some idea of why this assertion is nonsense.
- Given that we're outnumbered, on average, we can't be typical, therefore all our science falls around our feet in a horrible mess of untypicality! - the concept of 'average' is difficult when infinities are involved...but it's irrelevent here because we can't take the fact that we might be outnumbered as a 'given'. There are infinite numbers of both things...you can deduce nothing from that. There are an infinite number of elephants and there are an infinite number of humans - but humans outnumber elephants in the local region of space that we care about. With ants, the opposite is the case. You can't tell anything about the relative proportions of things when there are infinite numbers of both spread out over infinite volume.
- Therefore the world must end before we're outnumbered because obviously we're typical. - Nonsense.
- These kinds of bogus arguments come about from a lack of understanding about what infinite really means. There can be an infinite number of X's (whatever X is) within an infinite universe. But the distance between one X and the next can also be infinite, or it can be very large - or very small - you don't know. The average number of X's per cubic meter is a completely undefined number - it's infinity divided by infinity - which can be any number you like except (perhaps) zero. So in an infinite universe, the number of Boltzmann brains can be infinite but they might be 1000 billion lightyears apart on the average - and the number of human-like species on earth-like planets can be infinite but they might be 500 billion lightyears apart on the average - so for every one Boltzman brain, there are 8 human planets. We win. But then the average distances could be the other way around - and we'd lose. As soon as you get an infinity somewhere, you have to shut off 'normal' mathematical and logical concepts because they simply don't work anymore. A correct version of your argument (without resorting to infinities) is this:
- The universe might be infinite - we don't know.
- We believe the universe to be fairly uniform - at least to the scale of galactic clusters.
- Within any given volume of the universe, there is a probability Pb of a Boltzman brain spontaneously forming out of nothing. Mathematically, Pb is a spectacularly small number - and the probability of one forming anywhere within the observable universe is close to zero. The number of Boltzman brains that could ever possibly come in contact with humanity (Nb) is therefore overwhelmingly likely to be either one or (much more likely) zero.
- Within any given volume of the universe, there is a probability Ph of human-like creatures evolving through normal stellar/planetary formation mechanisms. Using the Drake equation, with our best estimate for the various parameters, we believe that Nh (the number of such civilisations within the observable universe) to be vast. The best knowledge we have says that there is (on average) between 2 and 5,000 human-level intelligent species per galaxy - which makes Nh be a very large number indeed for the observable universe.
- So, with the best knowledge we have, normal human-like intelligences VASTLY outnumber Boltzman brains within the observable universe. We can make a reasonable assumption that this is true in the remainder of the universe too...be it infinite or finite.
- Ergo, we have nothing to worry about.
- SteveBaker 13:56, 25 September 2007 (UTC)
- No! This kind of argument is complete crap. Let's take this carefully:
- Well summarized, Steve. Also, I take offense to the notion that a simpler form of self-aware organization could exist. Most of the humans I meet are barely self-aware. Humans are almost the simplest system that can possibly be self aware - whether you compare us to similar biological creatures like chimpanzees, squids, or microbes - or if you compare us to geological or astronomical formations like salt crystals or solar fusion convection cells... To postulate that a simpler system than our biological brain could exist is silly - we are entirely self-organizing, on time-scales of geologic and evolutionary systems. What could be more simple than a system which naturally unfurls itself according to basic physical law? It sounds like the Boltzmann brain is implicitly suggesting that some strange arrangement of hydrogen molecules in interstellar space will ever have the capacity to be "self-aware." In any case, we can barely define what it means to be sentient in the first place, so how can we start calculating statistical distribution of sentience? Nimur 14:37, 25 September 2007 (UTC)
- Firstly I think chimps are probably self aware (minor point) and secondly point out that 'new scientist' typically publishes absolute rubbish - don't mistake it for a scientific journal that will make any sense.87.102.10.190 14:49, 25 September 2007 (UTC)
- Of course - but chimps evolved similarly to us, so it's almost expected that they have a comparable level of intelligence (more or less). They didn't "pop out" due to statistical quantum fluctuation. Nimur 15:01, 25 September 2007 (UTC)
- Firstly, there have been experiments that show that (at least) chimps and dolphins are aware of their own existance. Paint a red dot onto the forehead of a chimp while it's asleep - then wake it up and show it a mirror. It'll notice that the chimp in the mirror has a red dot on it's forehead and (realising that the image is 'self') will try to rub the mark off it's own forehead...self-awareness, I believe.
- Secondly, we don't have a clue what it takes to make a system be 'self aware' - we don't even have a good definition of such a thing. We can pretty much assume that our computers are not yet self aware - but we know we could program one to tell us that it is - or to fake the results of an experiment to try to prove it. When the classic 'ELIZA' program was first shown to uninitiated people, they believed it was self-aware. The only reason I suspect that other humans are self-aware is because I make the rash presumption that since you guys seem to be a lot like me - you are probably as self-aware as I am - but in other regards, I have no more proof that you are self-aware than that my dogs are self-aware (I'm pretty sure they are). But I don't have any way to know that some other simpler system than a dog is not self-aware. In the end, I suspect that 'self-awareness' is merely an attribute of a sufficiently complex system.
- Thirdly, in defense of the OP - in an INFINITE universe, very complex things can (indeed, must) just pop into existance. If it's physically possible for something to exist - it must do so - and in an infinite number of places and times. Such is the nature of infinity combined with quantum randomness. However, the probability of it happening is so astronomically small, we can say with some confidence that such a thing will (essentially) never happen in any part of the universe that we can observe. SteveBaker 16:59, 25 September 2007 (UTC)
- Of course - but chimps evolved similarly to us, so it's almost expected that they have a comparable level of intelligence (more or less). They didn't "pop out" due to statistical quantum fluctuation. Nimur 15:01, 25 September 2007 (UTC)
It's the 'boltzmann brains popping out of the vacuum bit' that concerns me - arising from random fluctuation - without any supporting structure to maintain the 'brain' it will cease to exist as soon as it is made - it's like claiming 'clouds can think' - what?87.102.10.190 14:52, 25 September 2007 (UTC)
- Sure - but in an infinite universe, it doesn't matter how improbable an event is, it WILL happen someplace. So a mechanical brain 1000 miles across...complete with supporting structure can (and indeed, will) pop into existance. Now, whether computation is even possible with tenuous gaseous objects - I doubt - but who says it has to be gaseous. In an infinite universe, an exact clone of you - in a pink space-suit with pale blue stripes - just popped into existance in an infinite number of places and thought "Funny? How did I get here?". Such is the nature of true infinity. SteveBaker 16:59, 25 September 2007 (UTC)
- I disagree with SteveBaker's confidence that infinite sample sets defy the laws of causality and determinism. Nimur 23:08, 25 September 2007 (UTC)
- I tend to slightly doubt that 'things can appear from nowhere' at any probability, (even though that seems to have happened to the universe) - that aside - this is the science desk "Science (from the Latin scientia, 'knowledge'), in the broadest sense, refers to any systematic knowledge or practice.."etc and not the philosophy desk.. Current questions suggest that a philosophy desk would get as much questions as a science desk - maybe we should have one..87.102.23.3 01:45, 26 September 2007 (UTC)
- Precisely what do you mean by "with any probability"? What I mean is that in a truly infinite universe, any event with a probability greater than zero (even if the odds are a Googolplexgoogolplexgoogolplex:1 against) not only can happen - but will happen - and will happen an infinite number of times in an infinite universe. You can't deny that. If an infinite number of opportunities arise for a random event then if the odds of the event happening are greater than zero then the number of times the event will happen on the average is infinity multiplied by the probability of the event - and infinity times any number bigger than zero is still infinity. As for Nimur's scepticism: Determinism and causality are not violated - we merely have to invoke the Schrödinger equation. Since every particle in the universe has a very, very tiny (but definitely non-zero) probability amplitude of being at any point in space, the probability of all of the electrons, protons and neutrons required to make (say) a rather nice grand piano spontaneously appear out of nowhere is non-zero. If it's non-zero then this isn't just some hypothetical wild idea in my head: it is literally true that in the time it's taken me to write this reply, an infinite number of grand pianos have spontaneously appeared in the universe. That's not a joke - it's quite literally true. If we agree that the universe is infinite (which I personally doubt) then there is no way to deny that fact. If the universe is merely finite - then no, the probability of a grand piano spontaneously arising is far too small for it to ever happen. What keeps us sane is the fact that the observable universe (the part that could ever possibly affect us or be observed by us) is not infinite - so our little corner of an infinite universe has the same probability of crazy things happening as a finite universe and we don't have to worry too much about spontaneous piano formation. SteveBaker 13:09, 26 September 2007 (UTC)
- If we've got probability density functions that are non zero throughout all space and an infinite universe then I have to agree with you..
- However if I ask what's the probability of something appearing apparently at random and staying there for a meaningful length of time - ie being real then the situation changes - because if we take time to be infinitely divisible then the position of an object becomes fixed about a given point (with a wave equation representing how strongly it interacts with other objects)
- Consider a fixed proton - according to a probability density interpretation there is a non-zero probability of it's being anywhere.
- However if I measure it's position over time it becomes more and more clear that it has a central point about which it's density function drops off - over infinite time - it becomes more clear that the proton is point like and the 'probability density function' is really equivalent to a 'field strength' with which it interacts with other things.
- I think you are just considering moments in time and not taking a 'over all time view'?87.102.32.155 13:56, 26 September 2007 (UTC)
- NOTE if you take time to be infinitely divisible then you don't even need an infinte universe for these unlikely things to happen - as in the 1sec it takes me to press "X" there have been an infinite number of opportunities for odd things to happen - I'm suggesting that only the time averaged situation is meaningful..87.102.32.155 14:06, 26 September 2007 (UTC)
- Precisely what do you mean by "with any probability"? What I mean is that in a truly infinite universe, any event with a probability greater than zero (even if the odds are a Googolplexgoogolplexgoogolplex:1 against) not only can happen - but will happen - and will happen an infinite number of times in an infinite universe. You can't deny that. If an infinite number of opportunities arise for a random event then if the odds of the event happening are greater than zero then the number of times the event will happen on the average is infinity multiplied by the probability of the event - and infinity times any number bigger than zero is still infinity. As for Nimur's scepticism: Determinism and causality are not violated - we merely have to invoke the Schrödinger equation. Since every particle in the universe has a very, very tiny (but definitely non-zero) probability amplitude of being at any point in space, the probability of all of the electrons, protons and neutrons required to make (say) a rather nice grand piano spontaneously appear out of nowhere is non-zero. If it's non-zero then this isn't just some hypothetical wild idea in my head: it is literally true that in the time it's taken me to write this reply, an infinite number of grand pianos have spontaneously appeared in the universe. That's not a joke - it's quite literally true. If we agree that the universe is infinite (which I personally doubt) then there is no way to deny that fact. If the universe is merely finite - then no, the probability of a grand piano spontaneously arising is far too small for it to ever happen. What keeps us sane is the fact that the observable universe (the part that could ever possibly affect us or be observed by us) is not infinite - so our little corner of an infinite universe has the same probability of crazy things happening as a finite universe and we don't have to worry too much about spontaneous piano formation. SteveBaker 13:09, 26 September 2007 (UTC)
- I tend to slightly doubt that 'things can appear from nowhere' at any probability, (even though that seems to have happened to the universe) - that aside - this is the science desk "Science (from the Latin scientia, 'knowledge'), in the broadest sense, refers to any systematic knowledge or practice.."etc and not the philosophy desk.. Current questions suggest that a philosophy desk would get as much questions as a science desk - maybe we should have one..87.102.23.3 01:45, 26 September 2007 (UTC)
So, in some parallel universe there is a hydnjo that is one tP younger than myself :-) and another that is that much older :-( (assuming that time frames are as arbitrary as anything else). And in each case we have a left-handed me, the usual right-handed me and an ambidextrous me. Now, for each of those universes there is a good me and an evil me, a tall me and a short me, a cool me and a dorky me, a smart me and a stupid me etc (I don't know what the dividing interval is for those attributes but it sure makes for a bunch of us). After delving into those myriad universes we can then explore what's going on around two tPs, Geesh! - hydnjo talk 23:35, 26 September 2007 (UTC)
- The funniest thing with the Boltzmann Brains article in New Scientist has been the huge number of letters they received saying basically "this is humanocentric rubbish". For weeks they've been printing them, along with comments form the editor trying to defend the article. Almost every letter seems to say "Why assume we're typical? You can't base a theory on that.", and still they come... Skittle 15:33, 29 September 2007 (UTC)
Pull-top cans
I've noticed a problem with all pull-tops, which is worse on full pull-top cans than on pull-tabs (such as on beer cans). The curved piece of metal acts as a spring, such that, when the last piece breaks off, it launches any food or beverage bits on the tab onto you or the wall or whatever else is inconvenient. How could this problem be cured ? (The workaround is to never completely open it, but that makes it more difficult to access the contents.) Perhaps a different material would be less "springy" or perhaps the seal needs to be weaker near the end ? StuRat 14:36, 25 September 2007 (UTC)
- The difficulty is making it sturdy while closed, and easily-separable when opening it. I would guess that the manufacturers have decided to err on the side of "tightly sealed" rather than "deeply perforated" seals, despite the extra difficulty involved with opening the can. Of course, there's statistical variation in any manufacturing process, so if the seal is overly-perforated for ease of use, there will be many more cans with broken seals. Nimur 14:56, 25 September 2007 (UTC)
- This was described on some Discovery channel program or other about a year ago. It is indeed a tricky problem to manufacture something that's solid enough to keep the food fresh 100% of the time (even one can of rotted tuna could put them out of business). So they have to err on the 'perhaps accidentally harder to open 1% of the time' side. This is greatly complicated by the requirement for the cans to be dirt cheap - and because the machine tools that stamp the groove into the metal lid tend to wear out over time making the precise depth and width of the stamping change gradually over time. SteveBaker 17:04, 25 September 2007 (UTC)
- Pull it most of the way in the usual fashion, and then at the end, pull it sideways so that you're only applying a much-smaller peeling force to the edge of the top. The last bit will detach with a whimper rather than a "dang!". --Sean 15:43, 25 September 2007 (UTC)
- A way I found that works is to bend the lid back and forth at the end, so it breaks due to metal fatigue. You hardly get any spring effect when it breaks, that way. BlindMoglin 19:57, 25 September 2007 (UTC)
- I understand you're not talking about the cans as depicted in beverage can, which is called a 'stay tab'. Are pull tabs still used for beverages in the US? (They aren't in the Netherlands, nor in the rest of Europe afaik.) I used to use Sean's solution. Btw, stay tabs have another disadvantage, namely that an external part is pushed into the beverage, which can be unhygienic, depending on 'where the can has been'. DirkvdM 08:48, 27 September 2007 (UTC)
- Yes, those pull-tabs are still used here in the US, but I don't buy cans with those, because of their unhygienic nature. The cans I'm talking about are used for foods, like canned soup, and the full lid peels back, not into the food. I do use those, but don't like being splattered by the food when the lid breaks off. StuRat 12:54, 27 September 2007 (UTC)
- I don't think we're using the terminology the same way. I took the words from the beverage can article, where the 'stay tab' is the unhygienic one. Or do you mean to say 'pull tabs' are unhygienic as well? DirkvdM 18:55, 27 September 2007 (UTC)
- I've never heard anything called a "stay-tab", that must be British English. I call the unhygienic ones pull-tabs and the OK ones pull-tops. StuRat 00:32, 29 September 2007 (UTC)
- Ah, ok. Like I said, I got the terminology from the article, which I looked up to find out what you meant with by 'pull-top' in your original question. But now I'm confused again. In your previous post you said you still use the pull-tabs (stay tabs) in the US. But surely, they came later - the pull-tops (as you call them) were the originals, right? DirkvdM 10:03, 29 September 2007 (UTC)
- Not from my experience. The unhygienic pull-tabs have been on all canned soda-pop and beer as long as I can recall, while cans of food were mostly opened with can openers until recently. They are now changing many of the cans to have pull-tops. One exception I recall is Pringles canned potato chips (crisps), which came with a pull-top as far back as I can remember. The "splash" problem didn't occur there because the contents were solid. StuRat 16:25, 29 September 2007 (UTC)
- Funny, my experience is just the opposite. I've seen pull-tops for food such as fish (and Planter's peanuts, I believe) all my life, but stay-tabs for beverages only since the early eighties or thereabouts. But an age difference between us might also explain this (am I finally going to find out your age? :) ).
- HOLD ON, there are three things we're talking about. The pull-tops, where the entire lid comes off, the pull-tabs, where only a section comes off, and (in the terminology of the article) the stay-tabs, which (as the name indicates) stay attached to the can. Nothing comes off at all, and the reason for their invention was to reduce littering. For example the beverage can article only shows stay-tabs. What would you call those three and which are and were in use in the US?
- And again, looking at your original question, I'm confused. Pull-tops for beverages? That sounds like a horribly bad idea. At first I thought you were exaggerating, but if I understand you correctly now, you've got all the reason in the world to complain. To get this straight, you're talking about beverage cans where the entire top comes off? DirkvdM 18:03, 30 September 2007 (UTC)
- If you look at the caption in the picture at the top of that article, it is labeled as a pull-tab, not a stay-tab. That caption must have been added by an American, because we don't distinguish between the two cases, calling them both pull-tabs (I suspect that industry insiders distinguish between them, but the public does not). Note that I consider both to be unhygienic because you are expected to put your lips on the top and side of the can.
- And no, I don't know of any beverage where the entire top comes off as a pull-top, only foods. However, many such foods contain liquids, like canned pasta, for example (yes, I know any European will wretch in disgust at the thought of this). The fish containers, like sardines, have another mechanism where you have a metal opener around which you twist and wrap the metal top. I don't believe these typically come entirely off, but stay attached at the end. The containers are elongated so that this doesn't block access to the food. StuRat 15:26, 1 October 2007 (UTC)
Plastic pull-tops, like on an oatmeal container, don't seem to suffer from this problem. Could such plastic pull-tops be put on aluminum cans economically ? StuRat 13:02, 27 September 2007 (UTC)
doctor's question
Ordehimaria 16:18, 25 September 2007 (UTC)a doctor's usually contains all of the following items except ultrasonic scaler, high speed handpiece, air-water syringe, alginator
- does it really? —Preceding unsigned comment added by 88.110.206.28 (talk) 00:40, 26 September 2007 (UTC)
I would go with the ultrasonic scaler. Sounds like dentist stuff to me. --Click me! write to me 07:59, 27 September 2007 (UTC)
- All of those are dental tools: Ultrasonic Scaling (removal of calculus), high speed handpiece: cavity preparation, air-water syringe: AKA tripple syring/triplex (the little water gun/air gun), alginator: A very expensive mixing bowl for mixing alginate (the material used to take dental impressions). —Preceding unsigned comment added by 60.242.173.55 (talk) 08:08, 27 September 2007 (UTC)
Atomic theory
- What is an abundant isotope
- A sample of Argon exists as a mixture of three isotopes
- mass number 36, relative abundance 0.337%
- mass number 38, relative abundance 0.0630%
- mass number 40, relative abundance, 99.6%
How do I calculate the relative atomic mass of argon, please?
- Lastly how do i states the number of electrons, protons and neutrons in the ion 563+
Sorry to bombard you with these questions but they haven't been adequately explained by our chemistry teacher, sorry. --Hadseys 17:18, 25 September 2007 (UTC)
1. In your q.2 the abundant isotope is Ar40 - it means an isotope that occurs in significant amounts
2. The relative atomic mass is the average atomic mass - now the answer should be clearer.87.102.10.190 17:31, 25 September 2007 (UTC)
- 1) An abundant isotope is one that exists in large quantities on Earth. That means it can't be radioactive with a short half-life, because those don't last long. It must either be stable or radioactive with a very long half-life.
- 2) Do the math: 36×0.337/100 + 38×0.0630/100 + 40×99.6/100.
- 3) The atomic number is the number of protons and electrons in a neutral atom. If you have an ion, since electrons each have a charge of -1, add or subtract the opposite of that number to get the number of electrons (so, a +3 ion, has 3 fewer electrons, while a -3 ion has 3 more electrons). The atomic weight of an atom minus it's atomic number is the number of neutrons. Your question is messed up, do you mean it has an atomic number of 56 (barium) and a charge of +3 ? If so, we would still need to know the isotope or atomic weight to get the number of neutrons. BlindMoglin 17:33, 25 September 2007 (UTC)
- To amplify point (3) - the atomic number is the number of protons in the nucleus, it's the same for all atoms of that element. The atomic mass is the number of protons PLUS the number of neutrons - for most elements, there is one combination of protons+neutrons that is stable, or at least only mildly radioactive, the others are unstable and either shed protons or neutrons until they become stable - hence (mostly) there is one form of the atom that's stable and abundant and other forms that are unstable and rare - but that's not a hard-and-fast rule. The number of electrons (in a lone, uncharged atom) is the same as the number of protons - but when the atom is electrically charged or in a chemical compound, the number will be different.SteveBaker 17:42, 25 September 2007 (UTC)
Medical and Scientific effects of high voltage low amperage high frequency electric current on the skin
Please explain in simple terms the way electric current works specifically voltage versus amperage as pertains to devices sold as skin tightening devices. Ellisar —Preceding unsigned comment added by Ellisar (talk • contribs) 18:51, 25 September 2007 (UTC)
- [1] Someguy1221 19:16, 25 September 2007 (UTC)
- It's an interesting analysis they got there…current will go through a high-resistance region instead of around it but "everyone knows" that electricty takes the path of least resistance. And higher resistance for a given voltage and current creates higher temperature but power=I2R and V=IR so power is proportional to inverse resistance? DMacks 19:39, 25 September 2007 (UTC)
- I believe they point they were getting at was that, in whatever circuit the current ends up taking, the most heat is generated in the region of highest resistance within that circuit. Someguy1221 19:44, 25 September 2007 (UTC)
- It's an interesting analysis they got there…current will go through a high-resistance region instead of around it but "everyone knows" that electricty takes the path of least resistance. And higher resistance for a given voltage and current creates higher temperature but power=I2R and V=IR so power is proportional to inverse resistance? DMacks 19:39, 25 September 2007 (UTC)
- That takes math: I=E/R. Ohm's law. That's for DC, but the principle is the same. Raise the volts (E), you get more amps (I). Raise the resistance (R), you get less amps. This law is just basic physics, though. The device these doctors use undoubtedly supplies a predictable current, so the law doesn't apply at the tip of their probe. I read the website Someguy linked to, and the electronics part was sound. Everything the current passes through will heat up, but they cool the surface of the skin enough to keep it from burning much. The heating comes from power (P): P=EI. The resistance of the skin is constant for our purposes, and the current therefore depends solely on the applied voltage, so raising the voltage will increase both the current and power linearly. Hope this helps. P.S. My electronics teacher would slap me for using the word "voltage". --Milkbreath 20:48, 25 September 2007 (UTC)
- It's not going to be that simple though - as a part of you gets hotter, your blood supply adjusts to keep the temperature constant - and you start sweating. Since both sweat and blood are excellent conductors of electricity - I doubt you could get enough of a differential heating effect to make a difference. I'm betting this is junk science. I'd be very surprised if it really worked as advertised. SteveBaker 14:59, 26 September 2007 (UTC)
Pictures of brains by Wernicke and Broca
I have been unable to find drawings or photos of the brains upon which Broca and Wernicke based their descriptions of motor and sensory aphasias.72.75.96.28 21:18, 25 September 2007 (UTC)superiorolive
electronics project
hi, friends i am in the final year of engineering form electronics and communication field, i will be very thankful to you .if u can help me out in prep the project.. from my side i want to make some project that can highlight my intrest in: 1.communication field 2.electronics fdield 3.robotronics
hope to recive the answer soon and thanks in advance kamran ali —Preceding unsigned comment added by 59.178.114.98 (talk) 21:25, 25 September 2007 (UTC)
- How about a remote controlled robot ? That alone probably wouldn't be very impressive, therefore why not add some intelligence to the robot, so you can tell it where to go (say, the kitchen), but not the path, and it can figure out the path ? StuRat 00:32, 26 September 2007 (UTC)
- How about a bot to filter out all StuRats comments? —Preceding unsigned comment added by 88.110.206.28 (talk) 00:39, 26 September 2007 (UTC)
- Or perhaps a reverse filter for Google, which can only return the sexually explicit web sites which match a given search term. (I get sick of getting all those silly sites listed with cats on them.) :-) StuRat 00:55, 26 September 2007 (UTC)
- How about a remote control moving hand controlled by a glove with tactile feedback? Graeme Bartlett 15:05, 26 September 2007 (UTC)
How about a non remote controlled robot running wild on its own until its batteries run out. Do you think you can build something that can sense walls and stuff, not crash into windows or any glass, and not fall off the stairs? Does not have to do any work ... if it does move around on its own, you are ahead of Honda. --Click me! write to me 07:56, 27 September 2007 (UTC)
Prion transmission in food products made from animal tissues: the safety of gelatine and related products
Prions are thought to be responsible for Creutzfeldt-Jakob disease in humans, a fatal and degenerative form of transmissible spongiform encephalopathy. The CJD article says normal medical sterilization techniques do not deactivate the prions, and transmission from contaminated instruments is possible (if not proven). The Prion article says "Prions can be denatured by subjecting them to a temperatures of 134 degrees Celsius for 18 minutes in a pressurised steam autoclave." The similar prion-mediated Bovine spongiform encephalopathy is thought to have been transmitted from processed animal tissues (http://www.defra.gov.uk/animalh/bse/controls-eradication/causes.html) such as meat and bone meal used as a protein supplement in cattle feed, even theough the tissues were steam heated and theoretically rendered sterile in rendering plants.
Web sources of uncertain reliability [2] imply that gelatine production techniques could pass prions right through intact to the consumer, and that only animals known to be healthy should be used for gelatine production (a hard thing to verify). That same websource cites a study saying thatinfected brain tissue was "autoclaved during 1 hour at 134°C or for at least 18 minutes at temperatures of 135oC or 134-138oC. This was not sufficient either to inactivate scrapie- and BSE agents" which were shown to still be infectious inanimal studies.The Gelatine article references a study behind a paywall as to the safety of gelatine. The chondroitin article says "Most chondroitin appears to be made from extracts of cartilaginous cow and pig tissues (cow trachea and pig ear and nose)but other sources such as shark, fish and bird cartilage are also used." Thus one could theoretically seek chondroitin not derived from cattle. My question is: if animal tissues are used in products such as animal tissue derived Gelatine, in collagen casings (related to gelatine)for sausages, or in Chondroitin sulfate which many people as a dietary supplement to treat osteoarthritis, is the processing done at a temperature which would deactivate any prions which might be in the cattle tissue? Edison 22:04, 25 September 2007 (UTC)
- I'm shocked to see such a low temperature for denaturing prions. Richard Rhodes' book on the subject says you basically have to reduce tissue to ash to get rid of them. --Sean 23:45, 25 September 2007 (UTC)
- After the Mad Cow Disease outbreak in the UK I have been avoiding anything which may come from beef including gelatin and soap containing sodium tallowate. For years I avoided milk and milk products also. I'd be interested to know just how much mad cow disease is present in the british herd and elsewhere. 80.2.206.123 23:54, 26 September 2007 (UTC)
cigarette butts
What are cigarette butts made of. —Preceding unsigned comment added by 124.186.47.5 (talk) 22:12, 25 September 2007 (UTC)
- Cellulose. Have a read of Cigarette_filter#Manufacture. --Kurt Shaped Box 22:41, 25 September 2007 (UTC)
- Tobacco, in my case. Plus hemp paper. And a bit of glue. And some saliva, I suppose. But you probably mean filters. Filthy habit, filter-smoking. DirkvdM 08:51, 27 September 2007 (UTC)
What exactly is the relationship between muscle contractions (excercise & metabolism), and dilation/constriction of blood vessels (vasoconstriction & thermoregulation) ?
I'm kind-of confused about how vasoconstriction and muscle movements relate to each other, and just how big a role superficial veins have in thermoregulation (as opposed to deep veins or arteries).
It is fairly obvious that muscular activity results --or is accompanied by-- inceased arterial blood flow. Does exercise of skeletal muscles also cause superficial veins to engorge, and if so, is this due to thermoregulation?
Also, how does vasoconstriction cool internal organs, if (supposedly) it is due largely to superficial vein activity?
Thank you for reading this. Pine 23:14, 25 September 2007 (UTC)
- Exercise will cause veins to engorge, largely due the fact that they need to return the blood that has been pumped to the muscles in the arteries. The familar 'pump' that you get from weight training and other high load activities is essentially a result of the extra blood in the muscles. In general any vein transporting blood back from an exercised muscle will engorge, those at the surface may help to transfer excess heat from the body; I don't actually know if there's any variation in the degree of engorgement of the different veins depending on whether or not you need to loose heat though.
- I'm not sure what you mean by your last question. My presumption would be that if an internal organ needed to be cooled then you should get vasodilation in order to get more blood passing through the organ so that more heat could be transferred out of the organ. That blood could then pass near the skin surface in order to allow heat to escape. The only possible benefit I could see of vasoconstriction in this case would be to limit the blood flow, and therefore limit oxygen and to a lesser degree nutrient supply to the organ, which may then potentially lead to a drop in metabolic activity, and thus a drop in heat production. But that's a bit hypothetical. --jjron 09:21, 26 September 2007 (UTC)
September 26
What is this phychological behavior called?
I read something a while ago about a certain psychological quirk that can be used to get a feel for a person's personality or behavior. In essence it goes like this: Propose a hypothetical question "Do you think that most people do <x>?". A person who does <x> is more likely to answer Yes to that question. E.g. A person who cheats on their taxes is more likely to answer yes to "Do you think most people cheat on their taxes?", and so on. This tendency has a name... anyone know? The context I read it in was that companies would ask similar questions of prospective hires relating to behavior on the job (stealing from the company, harassing other workers, etc...). Thanks! Arakunem 00:29, 26 September 2007 (UTC)
- leading question,implicit assumption, begging the question ? 87.102.23.3 01:36, 26 September 2007 (UTC)
- None of those seem relevant here. —Tamfang 15:32, 30 September 2007 (UTC)
- Of course, honest people who are pessimistic about their fellow humans will also answer that way. (Just more bad news for us pessimists, apparently the world is against us.) StuRat 01:38, 26 September 2007 (UTC)
- Projection? --71.175.68.224 03:32, 26 September 2007 (UTC)
- I think you are looking for one of the cognitive biases, probably False consensus effect fits best, if you go through the List of cognitive biases you may find one that fits better. Vespine 06:44, 26 September 2007 (UTC)
- Cognative bias! That was the term! *Awards Vespine one pie* And thanks to all who responded! Arakunem 13:23, 26 September 2007 (UTC)
- I think you are looking for one of the cognitive biases, probably False consensus effect fits best, if you go through the List of cognitive biases you may find one that fits better. Vespine 06:44, 26 September 2007 (UTC)
- Sorry, but "cognitive bias" is a more general term. As the existence of a "list of cognitive biases" suggests, there are many of them. You asked about a specific one. --Anonymous, 22:51 UTC, September 29, 2007.
Runaway Global Warming
How far are we from runaway global warming? Twenty years? Ten years? Is there any way to find out? 210.49.155.132 13:16, 26 September 2007 (UTC)
- The most reliable way to find out is to wait and see. Otherwise, the only answers that exist are suppositions ranging from "never" to "it's already started", and there is no one agreed-upon best estimate. — Lomn 13:43, 26 September 2007 (UTC)
- That's a pretty silly answer. You might as well throw out all prediction in science and anything else. You make it sound like all suppositions are equal, like they are all equally valid guesses, even though some are no doubt based on better reasoning and better evidence than others. --24.147.86.187 13:49, 26 September 2007 (UTC)
- I think his point was that there is no consensus even among both "sides" of the issue. Arakunem 14:03, 26 September 2007 (UTC)
- I believe there is a general agreement among scientists that human activities have affected world climate and will continue to do so (with the exception of a few scientists, mainly paid off by oil companies). There is not, however, a consensus that we will reach a point where climate change will accelerate out of control. Some scientists think it will, but many others think we are only likely to see a steady, reversible increase in global temps. So, not only is there no agreement when "runaway" global warming will occur, but even that it will occur. StuRat 14:23, 26 September 2007 (UTC)
- I agree - "We don't know" is the best answer. There are several positive feedback effects (which we discussed earlier - and presumably triggered this question):
- Albedo reduction due to ice sheet retreat is clearly already happening. Both polar ice caps are shrinking ever faster so we are already in 'runaway' ice sheet shrinkage. The North pole will become completely open ocean within just a few years. The Northwest passage is already much more open to shipping than it once was and the geopolitical consequences of this are already unfolding (the Russians are starting to claim more terratorial waters on various tenuous grounds so that they may 'own' the rights of shipping to travel that way - the Panamanians have started a panic move to widen and deepen the Panama canal in order that they don't lose business to ships taking the NorthWest passage). Polar Bears and going to go extinct within our lifetimes.
- The terrifying prospect of deep ocean frozen methane melting has not started yet - so that's not yet a problem. If it does happen, we're in for a very rough ride.
- Sudden ocean current reversals are also 'threshold' events - but they don't affect global warming - only local weather patterns...however, there is a point of no return with those things too. If a major ocean current 'flips' and runs backwards, formerly temperate northern countries could be in deep trouble - and places that are currently frozen wastelands would become nice places to live. Not good for Europe - not so terrible for Canada...for example.
- There are other nasty ones that are more theoretical than real - the idea that when the air temperature increases, more water evaporates from the oceans, which has two side effects - one is that you get more cloud - which reflects sunlight and actually helps to keep the planet cool - but the other is that water vapour is in itself a greenhouse gas - which makes the planet hotter. Which of these effects 'wins' is not an easy question to answer.
- There no doubt that increased CO2 and heat levels along with shifting weather patterns will do serious things to plantlife. Some species will do better some will do worse. We don't really know whether the ones that will do better will be able to convert CO2 to Oxygen at a higher or lower rate than the ones that will go extinct. Hence we MIGHT get lucky and get some help from new plant distributions - or we might trigger another positive feedback that would make things yet worse still.
- Rising sea levels are not a good thing. Ocean is generally darker than land - so again, the higher sea levels rise, the more lighter-coloured land disappears under darker coloured water - and the more heat the planet absorbs - so the more ice melts and cooler water undergoes thermal expansion which makes yet more sea level rise. The precise rate at which this feedback loop kicks in depends on the exact shape of the land - how much land is covered by a 1 meter sea level rise (and how much the albedo changes as a result) is hard to measure exactly.
- But in the end, it is precisely that these effects are NOT known well that is the cause of the most worry. If we knew exactly what the consequences of our behaviour was, we could nail down exactly the costs and benefits of every action we took. However, we don't know that - and the science is just WAY too difficult. A prudent civilisation would say that the risk of a total global catastrophy is significant - and the consequences are about as serious as anything we could imagine - so we have to act quickly to minimise any further change. But we're not a prudent civilisation - so we're guaranteed to be in trouble - it's just that we don't know when exactly. SteveBaker 14:49, 26 September 2007 (UTC)
- Runaway global warming obviously hasn't happened yet. It would seem logical that it would have to be hotter than it's ever been in order to start. Wasn't the earth originally part of the sun? — Daniel 23:07, 26 September 2007 (UTC)
- Er - well, I guess you could say that - the earth formed in orbit around the sun from dust and such like and was much, much hotter then - but a lot has happened between then and now. It's not a reasonable comparison - the earth didn't have plantlife or even oceans back then. It's possible that runaway global warming has happened before - but then been reversed by (for example) a large meteor smashing into the earth and throwing up a large enough blanket of dust into the atmosphere to block the suns rays...that would chill temperatures significantly - perhaps dropping the planet back into a more stable, lower temperature. So I don't think that if the world was baked by global warming that it would stay that way forever - but even if it just stuck that way for a few centuries, it would be a total disaster. SteveBaker 01:54, 27 September 2007 (UTC)
- If the combined effects of an atmosphere containing huge amounts of CO2 and the total absence of reflective ice caps aren't enough to so much as keep the world from cooling, what could possibly make it heat at that point? Was there some cooling effect I don't know about? Am I combining things that didn't occur at the same time? I know they didn't all occur when the earth was formed, but where they ever all there at once? The probability of a large meteor impact, volcanic eruption, or other major cooling event occurring while it is still possible to save the environment is extremely low, implying that if something like that happened, all the runaway global warming did was rise the temperature to a different equilibrium. — Daniel 03:42, 27 September 2007 (UTC)
- Oh - yeah - OK. Well back then the earth had no water - water vapour is a tremendous greenhouse gas. With no water vapour in the air, the earth cooled off over millions of years. Water only arrived much later (from cometary collisions) - and by that time the earth was much cooler. But there have been huge changes over that time - vulcanism was much greater back then. The atmosphere was much less dense. There are way too many differences between then and now...and we don't have millions of years! SteveBaker 23:23, 27 September 2007 (UTC)
- If the combined effects of an atmosphere containing huge amounts of CO2 and the total absence of reflective ice caps aren't enough to so much as keep the world from cooling, what could possibly make it heat at that point? Was there some cooling effect I don't know about? Am I combining things that didn't occur at the same time? I know they didn't all occur when the earth was formed, but where they ever all there at once? The probability of a large meteor impact, volcanic eruption, or other major cooling event occurring while it is still possible to save the environment is extremely low, implying that if something like that happened, all the runaway global warming did was rise the temperature to a different equilibrium. — Daniel 03:42, 27 September 2007 (UTC)
Clearly, global warming has been happening since the last century, to the tune of less than one degree Celsius during that time. No measurements so far demonstrate anything definitely abnormal. There's an argument going on about how much of global warming is man-made, and the only answer almost everyone agrees on is "some of it". The consensus ("most of it") may or may not be right. There are some astonishing debates about the Medieval Warm Period, and a lot more work needs to be done on the variations in solar activity. As a non-scientist who tries to read the news on all this intelligently, I wouldn't say "Wait and see what happens", but I do say "Let's see what the scientific consensus is in ten years' time, and panic only a little for now". One thing which amuses me is that only thirty years ago, many climatologists were predicting a new Ice Age. If we had to choose between the two, I'd go for the global warming. Xn4 06:08, 27 September 2007 (UTC)
- 30 years ago, we didn't have global warming - the climate was within a normal range. The historical CO2 level (from a few hundred years ago) was around 270ppm. 30 years ago, it had risen to 310ppm - a significant rise to be sure - but not enough to be a problem. This year, we're up at 390ppm. So 30 years ago, the excess CO2 was around 40ppm - over the last 30 years, the excess has gone up to 120ppm above historical levels. Worse still, the annual increase in CO2 in the 1970's was only perhaps 8ppm per decade - now the rate of increase is around 15ppm per decade - indicating a dangerous exponential trend. The reason they weren't predicting a problem in 1977 is that we didn't HAVE a problem in 1977. Bear in mind that the natural annual 'swing' in CO2 levels is about 10ppm - seeing a difference of just 40ppm against that background swing wasn't a trivial matter with 1970's technology. Furthermore, we had only just started collecting routine CO2 level data back in the 1970's - so they didn't have much data to go on. Now we have 30+ years of accurate readings and (via ice cores and such) we have reasonably accurate data going back thousands of years. Now that we have the data - and the trend is much clearer, we can be very certain this time around. SteveBaker 12:41, 28 September 2007 (UTC)
- Oh - jeez..."lets wait and see". That's *so* lame. Let's put our heads back under the blankets and hope it all goes away. That way we don't have to work hard and we can carry on driving our Ford Expeditions. GET REAL!
- There is ALREADY an utterly clear scientific consensus - well over 95% of all scientists and over 99.9% of all atmospheric scientists agree that this is a problem that humans caused and humans have to fix if we don't want to trash the planet. You'll never get a better consensus on any topic than that. If you ask scientists whether black is white, 5% of them will argue about it. There are minor disagreements about the extent of the problem - but almost zero difference on what we have to do to fix it and when. IIRC, if we wait 10 years it's going to cost
10020 times as much to fix (according to that British government paperwhich I can't find right now, the Stern review) - if we wait 20 years, we're doomed.
- There is ALREADY an utterly clear scientific consensus - well over 95% of all scientists and over 99.9% of all atmospheric scientists agree that this is a problem that humans caused and humans have to fix if we don't want to trash the planet. You'll never get a better consensus on any topic than that. If you ask scientists whether black is white, 5% of them will argue about it. There are minor disagreements about the extent of the problem - but almost zero difference on what we have to do to fix it and when. IIRC, if we wait 10 years it's going to cost
- As for "nothing abnormal" - let me give you a simple thought experiment that even you can do. It is VERY clear that the North polar ice cap is going to disappear real soon now - check out photos of it over the last 10 years and that's utterly indisputable - look at the photos - you don't need a scientist to interpret them for you. That in turn will (for 100% sure) cause the extinction of the polar bears - if the average distance between ice-floes gets bigger than the distance they can swim, they will all die - and that's not going to take 10 years, it's going to happen in 5 years. Now, if this were a 'normal' event that happens every few hundred years, then how come the polar bears didn't go extinct the last time this happened? If this were 'normal' then Polar bears would not exist...and they do. So we know FOR SURE that if this has happened before, it was a VERY long time ago - certainly before the evolution of polar bears...which was also before mankind evolved from the great apes - before most of the other plant and animals that we know existed in their present forms. So if you call something that hasn't happened for millions of years 'normal' then we shouldn't worry about the total destruction of 95% of life on earth either - after all, that's what happened when the dinosaurs went extinct, that was 'normal' too. SteveBaker 15:25, 27 September 2007 (UTC)
- British government paper = Stern review. DuncanHill 15:29, 27 September 2007 (UTC)
- Thank-you! That's the one I was thinking of. As our article says: Its main conclusions are that one percent of global gross domestic product (GDP) per annum is required to be invested in order to avoid the worst effects of climate change, and that failure to do so could risk global GDP being up to twenty percent lower than it otherwise might be....I believe that was "Invest 1% now - or after 10 years of doing nothing it's going to cost 20%". Personally, I'd be very happy to give up 1% of my earnings every year to get this problem behind us. SteveBaker 17:43, 27 September 2007 (UTC)
- To Daniel, not sure what you mean, but this might help: In the beginning (to paraphrase a rather well-known book) most of the heat on planets came from the planets themselves. Then they started colling down and over time the Sun became the most prominent heat source. For Mars, that was not enough and it cooled down too much. For Venus, it was too much and it got a runaway warming effect. But for Earth, the temperature of the
porridgesurface was 'just right' for life (as we know it) to evolve. There were some positive and some negative feedbacks, which caused the temperature (and other variables) to go up and down over time. So life had to be adaptable (which can be seen as a definition of life). But the time-spans were very different. Adaptable life needs a shorter life-cycle than the changes, so it can adapt. It's the survival of life itself (or, rather, species) that matters - nature doesn't care much about the well-being of individuals. Every now and then, however, a change was so quick (for whatever reason) that life on Earth barely survived. Luckily, there are also always tiny critters like bacteria that evolve very quickly and can survive 'extremes' because they found a niche somewhere where conditions are uncommon. The 'new equilibrium' you talk about would then have been one that was more like those previously uncommon condition for some of these critters, in which they could thrive. So then life 'rebooted' and re-evolved from whatever survived. DirkvdM 09:38, 27 September 2007 (UTC)
- To Daniel, not sure what you mean, but this might help: In the beginning (to paraphrase a rather well-known book) most of the heat on planets came from the planets themselves. Then they started colling down and over time the Sun became the most prominent heat source. For Mars, that was not enough and it cooled down too much. For Venus, it was too much and it got a runaway warming effect. But for Earth, the temperature of the
- To Xn4: 'just one degree' is a whole lot for global (average) climate change, especially for such an extremely short timespan (such global changes usually take place over (hundreds of) thousands of years). Don't forget that just a 10 C rise can wipe out most life on Earth. As for that ice-age, as Steve pointed out, a local ice age is just possible. In general, the fact that we don't know what might happen is one of the scariest aspects. But we do know there will be some effect.
- As for the effect so far being small, consider this. We have to rule out variations on a smaller time-scale, the seasons, so we only get one measurement per year (that year's average). So over the last century, we've only had 100 measurements. For scientific certainty, that is not a lot. So if we do get (near) certainty that a change has taken place, then the reason must be that there is a strong effect. With this in mind, look at this page of the Dutch meteorological institute KNMI. The table at the top left shows the ten hottest years measured in the Netherlands since 1901 (the one below it shows the coldest years). All of those fall in the last 18 years. The chance of one such hottest year falling in that period is 18/106 = roughly 1/6. So the chance of throwing a 6 with a dice. So what is the chance of throwing 10 sixes in a row? (1/6)10 = 1/60,466,176 (there's a slight inaccuracy there, but that barely affects the end result). So there's a chance of one in sixty million that nothing is happening. For me (and science) that constitutes more than sufficient 'certainty' that something is going on. Such extremely strong certainty (given the subject at hand) with so few measurements means there must be a very strong and rapid effect. Given Earth's extinction history, we should do everything in our power to counter that.
- Note that even if human actions weren't the cause, this would be reason to do something very fast. But lucky us, we are probably the major cause, so we've got a very simple solution - just stop doing what we're doing. Or at least reduce it as much as we can. Or at least try. But we're not even doing that. We must be very stupid. DirkvdM 10:01, 27 September 2007 (UTC)
- That's true (and often missed by the critics). Even if this was caused by volcanos, forest fires, cow farts, solar cycles or whatever other 'natural' theory - it's happening too fast for evolution to keep up with it and life on our planet will change drastically - perhaps too drastically for humanity to survive at it's present level of comfort. Sure, eventually everything will evolve to cope with the new conditions - but it took us 4,000 years to evolve lactose tolerance - and quite a few people aren't there yet...so expect to have a rough ride for a few thousand years before we get over this one.
- So even if the causes are natural, we should fight it. A massive meteor bearing down on us would also be a 'natural' event - but that doesn't mean we shouldn't deploy spacecraft and nuclear weapons to deflect it. If indeed global warming were a natural event, we should count ourselves luck that we can fight nature by deliberately cutting back our CO2 usage.
- However, if you look at a graph of historical CO2 levels (as measured in tiny air bubbles buried in ice cores from the antarctic for example) and compare them to average global temperatures (from historical weather records and things like tree rings) - you find that they match up extremely well - and if you compare CO2 levels to our consumption of oil, coal and natural gas, you find that those curve match up extremely well too. Does one cause the other? Well - as DirkvdM so elegantly demonstrated - the odds are millions to one against it being some other cause. The problem is that most scientists are trained not to say that A causes B until they have actual 100% proof - and this is only 99.999% proof - which is why there too many of us have been cautious about speaking out about it. SteveBaker 17:43, 27 September 2007 (UTC)
- To DirkvdM: You wrote "To Xn4: 'just one degree' is a whole lot...". Those weren't my words, which were (see above) "less than one degree Celsius". Wikipedia's global warming article says that the average measured global air temperature near the Earth's surface has risen 0.74 ± 0.18 °C during the last hundred years. However, in the US (where records of temperature are probably as good as anywhere and better than most countries) the accepted figure is only 0.3°C. AccuWeather says the figure worldwide was 0.45°C. The grandiose Intergovernmental Panel on Climate Change (IPCC) in its last report took the bidding up to 0.6°C, Stern implies a higher figure, Wikipedia has 0.74 ± 0.18°C, and you now tell me (claiming to quote me) that it is 'one degree'. Where does this inflation end?
- As I read these debates, much of the hysteria about global warming is based on misquotation and evasion of the evidence. The IPCC produced a report in 1996 which included a graph showing that the Middle Ages were warmer than the present day. Its second assessment report in 2001 had a new graph which made the Medieval Warm Period disappear, by a crafty use of averaging and by silently leaving out the data showing the medieval warm period. (That material went into a file called 'Censored Data'). If the case is so overwhelming, why do people behave this way? Xn4 02:23, 28 September 2007 (UTC)
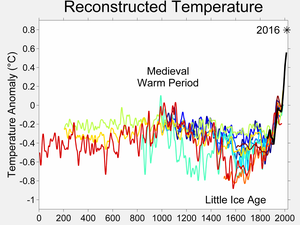
- Ok, let's say half a degree then. Still a whole lot for such a small time period. Like I said, climate change usually goes very slow by human standards, more in the order of thousands of years and just a few degrees over such a time period spells disaster for life on Earth (it has in the past). Half a degree in a mere century is a hell of a lot. But the IPCC says 0.74 C (which is where Wikipedia got that figure from of course). Who is misquoting now? :) And that is worldwide, not just the US. That's what the IPCC is about; collecting findings of scientists from all over the world, including the US. No matter how good your equipment is, having it in just one country is not going to tell you much about world climate. You need results from weather stations all over the world. And of course findings change over time as more data comes in. So far, the predictions (from previous assessment reports) have mostly turned out to be too conservative, especially concerning the melting of the Arctic ice cap.
- Do you have a source for the 'censored data' bit? Preferably one from outside the US, because that's where all the dissent seems to come from. And have you had a look at that graph in the Mediaeval Warm Period article you linked to? Note that that warm period is a thousand years ago and it is a slight bump, as opposed to the sudden spike in our time period. DirkvdM 06:10, 28 September 2007 (UTC)
- Yep - I agree. Just because one report did something 'iffy' doesn't allow you do dismiss the dozens and dozens of others that come to the same conclusion. 99.99% of atmospheric scientists agree...this is not a hotly debated topic amongst experts. Essentially all of the dissent comes from non-expert people who desperately wish this wasn't true and have decided to take the 'ignore it and hope it'll go away' approach. Sadly, the media (who love dissent and disagreement) are giving equal air time to the 0.01% of scientists and the other 99.99% - giving an entirely biassed view of actual scientific opinion. SteveBaker 11:27, 28 September 2007 (UTC)
- Also, oil is a multi trillion euro industry. It would be surprising if that extremely powerful industry didn't throw it's weight around, such as by
bribingfunding dissenting scientists and reporters. DirkvdM 14:50, 28 September 2007 (UTC)
- Also, oil is a multi trillion euro industry. It would be surprising if that extremely powerful industry didn't throw it's weight around, such as by
- I'd like Xn4 to respond to my argument about the Polar Bears (above). SteveBaker 11:27, 28 September 2007 (UTC)
Hi, Steve. You said "Polar Bears and going to go extinct within our lifetimes." I can't say it won't happen, I'm not a climatologist, I'm just a lay sceptic, but it seems to me a very categorical prediction, considering how little we know about several key factors, such as the unexpected variations in solar activity over the past fifty years. We can say that the polar bears survived the medieval warm period, which for hundreds of years saw higher temperatures than we have now (and which has been all but vanished from the graph above). You said "The terrifying prospect of deep ocean frozen methane melting has not started yet - so that's not yet a problem. If it does happen, we're in for a very rough ride." As I understand it, deep water temperature measurements haven't changed at all since the 1930s, and sea temperatures generally have not so far responded to any of the predictions made by climatologists. If and when they do so, it will surely be more rational to treat those predictions as having some authority. Xn4 19:42, 28 September 2007 (UTC)

- You're a layman. We are laymen. (Goo-goo-ga-choo.) So who should we trust? What about scientists? Better still, what about all the best scientists in the world putting their findings together? Hey, we've got that: IPCC. And they have looked into the solar activity and concluded that it's probably a minor contributor, if at all. Basically, you're saying you only believe scientists when it suits you. Similarly, first, you come up with a link to an article, and then when I use that too, you dismiss it.
- What do you mean by 'sea temperatures haven't responded'? Do you mean they haven't risen? Then what do you think has been the major cause for the rise in sea levels? You really should do more reading on the subject before you declare the world's scientific community nuts. Anyway, now you know, so will you, like you said, "treat those predictions as having some authority"?
- I've added another graph. Note that the mediaeval bump you make such a big deal of is just the last of many. Its the spike at our present time that's worrying. Note that on that time-scale it would be a vertical line, which I suppose is the reason the present temperature is marked with a arrow, showing what the average temperature was in 2004 - well above what it was 8000 years ago, despite the fact that it has been dropping gradually ever since - until about 100 years ago. DirkvdM 10:44, 29 September 2007 (UTC)
DirkvdM, would you please try harder to stay calm and avoid hurling insults? You ask "What do you mean by 'sea temperatures haven't responded'?" Please see what I said above - "...sea temperatures generally have not so far responded to any of the predictions made by climatologists". If you disagree with that, please refer us to one such prediction which was remotely correct, instead of changing the subject to ask about the remarkably small rise in sea levels so far observed. That rise, by the way, is also hugely less over any period you care to name than the climatologists have predicted it would be. Look (for instance) at the predictions published ten years ago and compare them with the out-turn. In my view, there is no reason to award a high level of trust to the forecasts of people whose record so far is that they have got nothing right, and I include the IPCC in that. And when it comes to graphs, they are no more than a simplistic way of presenting sets of figures. In this area, I don't blindly trust any of the figures produced by anyone: on both sides of the debate, they all come from people with an agenda. Xn4 22:49, 29 September 2007 (UTC)
- You can be skeptical and still strongly advocate for CO2 emission reductions...you simply have to approach this with cold rational logic.
- Even if you are skeptical of the science you have to agree that there are basically four ways this can play out:
- If the majority of climatologists are right and we don't act within about 10 years - the consequences are vast - unrecoverable - disasterous - end-of-the-world things - and doing the best we can to minimise the damage costs 20% of GDP - our children and grandchildren go through hell - and the planet is a very different place for our great grandchildren.
- If they are right and we act quickly - we lose some species, things are not good - but we survive. It costs us 1% of GDP and humanity comes away bruised and chastened - but the planet lives.
- If they are wrong and we don't act - then nothing much happens except a few scientists are very embarrassed.
- If they are wrong and we go ahead and cut CO2 emissions because we believe them - then we waste 1% of GDP cutting back on fossil fuel consumption and upgrading our infrastructure. This would be a fairly serious matter - but not disasterous by any means. As a side-effect we'd have dramatically cut fossil fuel consumption - and that's a good thing for other reasons (not relying on overseas oil from countries we'd prefer not to deal with - not running out of non-renewable fuels - not putting other noxious substances into the air, cutting back on acid rain and ozone-destroying gasses).
- This is a classic 'game theory' type of problem - and the solution to those is elementary mathematics that is totally beyond debate. You multiply the consequence 'score' by the probability for each outcome - and compare the results for the options you have before you. Whichever option gets the highest score is the one you should choose.
- OK - let's run the numbers.
- Let's assume (per User:Xn4) an ultra-skeptical 50/50 split of reliable evidence and assign global warming a probability of 0.5 of being correct.
- Let's assign the consequence score purely on monetary outlay - ignoring the other losses to our planet. You wind up with consequence scores of -20, -1, 0 and -1 respectively (using percentage of GDP outlay) for the four outcomes.
- You weight those by the probabilities - and (with a total-skeptic point of view) you have -10, -0.5, 0, -0.5 respectively. The average outcome of "Do nothing" is (-10 + 0)/2 which is -5 and the average outcome of "cut CO2 emissions immediately" is (-0.5 + -0.5)/2 which is -0.5. The game-theoretic response to the utterly devastating (1) outcome and the relatively mild (4) outcome is quite clear - you act as if the science is correct and you cut CO2 emissions. There is simply no contest.
- The only way to rationally advocate doing nothing for another 10 years is to change the probabilities of the two outcomes and say that the probability that 99.99% of climate scientists being wrong is at least 20 times higher than them being right. That would be enough to balance the decision and allow you to rationally decide to do nothing. But really - is it at all likely that 99.99% of people who have devoted their lives to studying this problem have only a 5% chance of being correct?
- But it doesn't end there. We only considered the cold, hard monetary cost. We didn't add in the somewhat nebulous cost of loss of biodiversity loss and the likely economic problems for some nations due to sea level rise. Those figures only make outcome (1) more expensive. We also have some benefits to outcome (4) that we decided not to take into account - so the cost score for (4) should be less than 0.5.
- Redo the math with whatever price you put on the quality of life and now the final decision matrix favors "fix the problem immediately" to an even greater degree. In order for a rational person to advocate "do nothing" with a clear conscience - they'd have say that the probability of the science being wrong is 100 or 1000 times more likely than it being correct.
- Do we really have a pile of evidence that global warming isn't true that is 100 to 1000 times more credible than the evidence that it is true? Wouldn't that massive pile of evidence convince at least a few more of the climatologists? I doubt that even User:Xn4 would be able to say that.
- So advocating that we wait 10 years before we consider fixing the problem is beyond mere 'skepticism' - it's mathematically provable outright stupidity. QED. SteveBaker 02:45, 30 September 2007 (UTC)
That's a much better line, Steve, though (if I may say so) you're allowing unscientific rhetoric and numbers plucked out of the air to creep into that presentation. It wasn't I who "advocate[d] doing nothing for another 10 years" (see above), nor have I "declare[d] the world's scientific community nuts", as claimed by DirkvdM. There are a lot of climatologists out there who expect us to believe their every word when there is absolutely no reason to do so, and on this subject we have a lot of hysterical people and politicians. The gamble we're being asked to make a judgement on is the biggest game in town, and we need to approach it calmly and analytically. We know there is global warming, and also that there has been global warming in the past. Nothing persuades me that we know the causes of it yet, and there seems to be a real possibility that natural causes will prove to be the biggest factor. We shouldn't forget that "there was a slight downward trend of temperatures from the 1940s to the early 1970s" (see our global cooling article) which remains to be explained. Even if human activities are the main cause of climate change (which, for all I know, they may be) changing our behaviour dramatically isn't going to reverse the situation in a few years. As a sceptic, my question is whether we need to put our greatest efforts into objective scientific research or into huge economic and environmental gambles based on numbers we don't have. Xn4 04:20, 30 September 2007 (UTC)
- Xn4 (three posts up), sorry if my last post came across a bit harsh. Then again, the 'harshest' thing I said was that you should do some more reading on the subject. And I certainly didn't insult you in any way, nor did I hurl anything. I just meant precisely what I said. Hell, I even started it with a little joke. Also, I'm totally calm. :)
- Anyway, you ask about a prediction of 10 years ago that was even remotely correct. That would be the second assessment report then (halfway down this page). I haven't read that (only the science part of the fourth report, about which I wrote the Dutch Wikipedia article) but I can't find any predictions for today in there - most predictions in that report are about 2100 and 2050. It's more about what has happened than what will happen. It's a bit odd to ask me to come up with a prediction you claim to be wrong. You say the rise in sea levels is remarkably small, but if you live in the Netherlands then 17 cm so far (!) sounds rather scary. If this keeps up, we'll probably have to give up some polders this century. And then there's Bangladesh, who don't have our technology nor the money to buy it. Remember, what matters is not so much what has happened so far, but what indication that gives for the future - not just our future, but the next few centuries and millennia.
- Steve, your game theory story is very good at illustrating the problem. But, as you also indicated, it's a huge oversimplification. Predicting climate change is next to impossible. Predicting the economic effects is like looking into a crystal ball. Also, if we lose 20% of our income then we're left with 80%. During my travels I've seen lots of people live quite happy lives on something like 10% of what people in the West have. But the major risk of climate change, which is oddly rarely mentioned, is the effect on agriculture. We've already seen major starvation as a result of unpredictable weather. And now the climate is starting to become unpredictable. And not locally, but worldwide. This is not a prediction but a risk we are running, the risk that we will have worldwide mass starvation. The chances are almost impossible to calculate, but the risk is huge. No matter how much money you have, if there is not enough food for everyone, people are going to die.
- Xn4, you say there has been global warming before as if that makes it ok. Yes there has been, and look at the results. In its most extreme form, almost all life on Earth died, probably as a result of a 'mere' 10 C rise in global temperatures. But in recent history there have also been plenty of examples of weather not behaving like it used to, leading to massively failing crops, often as a result of a volcanic eruption, local phenomena that on occasion have had worldwide effects. This shows us how much we rely on predictable weather for our survival. So we really should be extremely careful about changing the climate permanently and worldwide. Actually, it should scare the shit out of us, irrespective of what the actual change turns out to be. That we know there is likely to be a change (hell, it has already started) but don't know how big or even of what kind makes it even scarier because we can't prepare for it and any responses will be ad hoc, meaning much of the damage will be done before we can do anything about it.
- Where we should put our efforts (action now or more research first)is indeed a good question. But science needs data and those data come in very slowly (for any variable there is basically only one measurement per year due to the seasonal nature of climate). Finding that there already is an effect on climate (and therefore likely will be in the future) is a lot easier than estimating what, how much and where. That is simply going to take way too long so we have to do with what we know now, and that is that CO2-emissions (and methane) have an important effect.
- Strangely, you call mitigation a gamble. But it's what we're doing now that is a gamble, so we should stop doing it as much as we can. That requires quite a change in lifestyle, but humans have proven to be highly adaptable. All that is needed is the will. We can change quite a lot about ourselves, but not our need for food. DirkvdM 08:48, 30 September 2007 (UTC)
More time or more space?
Which is there more of in the universe? More time or more space? —Preceding unsigned comment added by 146.95.224.67 (talk) 13:23, 26 September 2007 (UTC)
- If Einstein is correct, then there is spacetime and the question holds no meaning. Otherwise, how do you propose to quantitatively compare space with time? — Lomn 13:44, 26 September 2007 (UTC)
- I'm not sure the question makes sense. You can measure how much "space" there is, but you can't measure how much "time" there is. Even with space and time being importantly linked (spacetime continuum), I'm still not sure the question makes sense. Is there more or less time today than there was a million years after the Big Bang? One could make an argument (depending on your definition of "space") that there was less space then, but I don't think you could argue that there was less time then. Anyway, if you could "measure" them I'm not sure you could reasonably compare them, as they use totally different units and all (is there more height or more volume in me? It's a silly question too). --24.147.86.187 13:48, 26 September 2007 (UTC)
You question on the surface seems meaningless - eg "which is greater, 1 mile or 100 minutes?"87.102.32.155 13:57, 26 September 2007 (UTC)
- Well, there might be an argument that the speed of light provides the appropriate conversion factor - so one lightyear of distance and one year of time might be considered equivalent. However, space is a three-dimensional thing and time is one-dimensional - so "more" is a tough concept. This is really an unanswerable one. Sorry. SteveBaker 14:28, 26 September 2007 (UTC)
- If you believe space to be finite, and time to be infinite, then there's more time. --Sean 15:26, 26 September 2007 (UTC)
- Here is a not entirely serious answer. Measured in natural units, the age of the Universe is roughly 8×1060 Planck times, whereas the radius of the observable universe is roughly 2.7 × 1061 Planck lengths. So the universe contains about 3 times as much space as time. (Since Planck length / Planck time = c, I suspect this is not coincidence). Gandalf61 15:59, 26 September 2007 (UTC)
- But you are comparing just the distance from one side to the other - the "amount" of space is it's volume surely? That would give you a volume of maybe 10184plancklength3...but how you compare a volumetric measure to a time is still unclear to me. SteveBaker 16:40, 26 September 2007 (UTC)
- How do you compare energy to mass? e=mc^2. You'll get the same answer if you use the system Gandalf used. Of course, I don't think the rest of that system has near as much evidence, but once you believe c^2, and therefore c, = 1, the rest isn't that much of a stretch. That system doesn't explain why people say gravity is the weakest force, as all four forces are equal to 1. — Daniel 22:57, 26 September 2007 (UTC)
- Where does mass come into this? We're talking about volume aren't we? Nil Einne 15:36, 28 September 2007 (UTC)
- Even if space and time are infinite, one could argue that space is "bigger" (depending on definitions of infinity). For example, if you spent every second counting (Countable set) a point in space (Uncountable set), you'd never finish. -- JSBillings 18:23, 27 September 2007 (UTC)
- The amounts of points in space and moments in time are both cardinality of the continuum. The number of seconds in an eternity and cubic meters in an infinite volume are countably infinite. Either way, they're the same size. I'd use the second one as it doesn't say a nanosecond is as long as an eternity. — Daniel 22:43, 27 September 2007 (UTC)
chiral universe
It seems likely that the universe is chiral (I'm assuming that based on local observations)
Question: Are there any theories etc that look into or try to draw conclusions from or about this 'fact'. eg such as what about the other enantiomer, and how can I possibly distinguish which enantiomeric form it is (it seems I can't), or does this overall chirality have any meaning or consequences -
I'd also be interested in any philosophical analysis of the impications of this if anyone knows someone who has studied it. Thanks87.102.32.155 14:01, 26 September 2007 (UTC)
- Does chirality (physics) shed any light on this ? StuRat 14:15, 26 September 2007 (UTC)
- Not much as far as I can tell - the article deals with small things and I was thinking about the overall effect - thanks for your concern anyway.87.102.32.155 14:40, 26 September 2007 (UTC)
- Thought the stuff about left and right handed fermions behaving differently looks interesting it's inherently flawed since this means that they are not true enantiomers. I'd be interested if someone can help to expand on this though.87.102.32.155 14:46, 26 September 2007 (UTC)
- I'm no expert. Are you sure that the universe is chiral? I thought the effect was limited to biologically active compounds here on Earth? If it's the latter then it's easily explained. When the first lifeform (some kind of replicating protein/RNA thing) formed from random chemical events on early earth, it had one particular chirality. Since the whole of life on Earth is descended from that one original molecule, we all have to have the same chirality - and all biochemicals in our environment (which have formed from descendents of that first molecule) have to follow suite. If we're seeing similar bias in chiral molecules from space (eg carried here on meteorites) then perhaps this is evidence for panspermia - in which case a similar argument might apply on a wider scale than just the earth. If we're seeing that bias in clouds of dust and gas out beyond the solar system...then I'd start to suspect a subtle chemical bias.
- I hope someone else can come up with a better argument because the effect is seen beyond the Earth, then this reply is rather unsatisfying! SteveBaker 14:22, 26 September 2007 (UTC)
- Chirality means that a thing lacks a mirror plane of symmetry (or that inversion converts it to the other enantiomer) - that property isn't confined to organic molecules.87.102.32.155 14:42, 26 September 2007 (UTC)
- Check out the earth (geology) or the solar system, or the galaxy - all are chiral as far as I can tell87.102.32.155 14:48, 26 September 2007 (UTC)
- StuRat's on the right track, especially the Chiral theories section. Certain parts of the universe (i.e., forces and theories at the lowest levels) don't seem to be equally mirrored. We don't even need to look to the cosmos to see chiral non-organic stuff: some minerals form chiral crystals (the mineral itself may be achiral, but crystallizes into an asymmetric matrix—the direction is arbitrary, but each crystal is a single direction). IIRC, quartz and calcite are two like this, can't remember off-hand. DMacks 14:52, 26 September 2007 (UTC)
- Thanks.. but that wasn't really my question. You are right that quartz (it is chiral) but equal amounts of L and R forms exist - so that doesn't come in here..
- Also if you think about it - if a theory claims that two supposedly left and right handed forms of a particle interact differently with their own handed forms then they are not actually left and right handed forms - and not enantiomers (as I mentioned above with repects to fermions)87.102.32.155 15:10, 26 September 2007 (UTC)
- If I could clarify my question - I assumed the universe the (whole thing) was chiral, and wanted to know more about a. its enantiomer (any theories about this) b. any theories that explain the overall chirality - ie a chiral beginning to the universe - an asymmetric start state, or a surplus of one form of 'chiral particles' over another. (As I understand it chirality cannot be created from an achiral state.)87.102.32.155 15:15, 26 September 2007 (UTC)
- If you follow my link, you'll see that certain physical forces aren't chiral "by accident and the mirror image would be equally likely/valid/etc", but rather have a specific chirality by their very nature. That is, (apparently) in a mirrored universe, they wouldn't work the same. Going back to the original "how to determine chirality at all" (as opposed to "same vs opposite" relative to some other known), there was an interesting discussion of that here a few months ago. DMacks 16:34, 26 September 2007 (UTC)
- It's an interesting link - but my worry is (I assume the fermions are a good example as any) - is that the two types of 'handed' fermions are not enatiomers - because the enantiomers would behave the same with respect to themselves. eg the article says F(r)F(r) interactions are not the same as F(l)F(l) (hope my terminology is obvious) - but if this is the case then in a 'mirror' world F(l) does not become F(r) because they are not enantiomers. So the description 'left handed fermions' is erroneous.
- Please explain more if I've missed something else, .. (apologies if I'm picking up on the wrong topic)87.102.32.155 18:50, 26 September 2007 (UTC)
- I might be getting a little confused - if a particle is intrinsically chiral (no problems here) then in a 'mirror world' it has the opposite chirality just like everything else, and so the two 'mirror worlds' are indistinguishable from an internal frame of reference.87.102.32.155 18:58, 26 September 2007 (UTC)
- If you follow my link, you'll see that certain physical forces aren't chiral "by accident and the mirror image would be equally likely/valid/etc", but rather have a specific chirality by their very nature. That is, (apparently) in a mirrored universe, they wouldn't work the same. Going back to the original "how to determine chirality at all" (as opposed to "same vs opposite" relative to some other known), there was an interesting discussion of that here a few months ago. DMacks 16:34, 26 September 2007 (UTC)
- According to quantum physics, the universe isn't deterministic. If there is any randomness in a symmetric system, asymmetry can be created. For example, make a symmetric pattern of dots on a grid, then move each one in a random direction. Chances are, it's chiral now. By the way, how can you tell the universe is chiral from local observations? How do you know there isn't a mirror earth in a mirror galaxy on the other side of the solar system? — Daniel 22:47, 26 September 2007 (UTC)
- Somebody would have spotted the mirror image wouldn't they using a telescope - (or maybe not) - you tell me?
- (sorry)
And why do I increasingly tire of answers that start "according to quantum physics"?83.100.254.236 11:20, 27 September 2007 (UTC)- Heh, see Poincaré sphere. —Tamfang 15:45, 30 September 2007 (UTC)
- "quantum theories" are theories that matter/energy/whatever is quantised - you (I think) are thinking of wave theories of matter (some of which have quantisation assumptions as well) - specifically quantum mechanics.83.100.254.236 11:39, 27 September 2007 (UTC)
- If the universe isn't deterministic then how did you manage to respond to this question? wouldn't you just be random dots...83.100.254.236 11:21, 27 September 2007 (UTC)
- It's not entirely random. It's more like there is some randomness added to an otherwise deterministic system. You can get a general idea of what will happen in the future, but there is a limit to how accurate you can be. — Daniel 22:37, 27 September 2007 (UTC)
- A system can be entirely random - yet exhibit remarkable stability. Take a bucket, fill it with 100 dice and roll them all. Add up the total. It will be remarkably close to 350 (percentage-wise that is) - almost certainly within 10% of that number. Roll a million dice and the total will be very close indeed to 3,500,000 - to within a tenth of a percent maybe. The things that are utterly random at the scale of an electron or an atom are utterly stable and predictable at the scale of a human being. The statistical nature of things means that a few atoms doing utterly crazy things simply doesn't effect large scale phenomena. There is an astronomically tiny chance of weird and wild things happening - but they are vastly less likely than rolling a million sixes on our million dice. SteveBaker 23:15, 27 September 2007 (UTC)
- It's not entirely random. It's more like there is some randomness added to an otherwise deterministic system. You can get a general idea of what will happen in the future, but there is a limit to how accurate you can be. — Daniel 22:37, 27 September 2007 (UTC)
- Who here knows General Relativity? I seem to remember reading that one of the solutions for the shape of the universe under GR has it rotating. —Tamfang 15:45, 30 September 2007 (UTC)
doubt regarding source and sink
can any one explain what are source/sink inputs/outputs? their connections in the electrical network? difference in the two networks of sourcing and sinking. ex: if the inputs or outputs of an i/o device are connected in the devicenet or controlnet network what does the input/output of that particular device mean? does it mean in teh following way.. input --input to the network output--output from th network
if not so explain me in detail. —Preceding unsigned comment added by Nag183raj (talk • contribs) 14:22, 26 September 2007 (UTC)
- What kind of network? Are you talking about electronics, river systems, atmospheric chemistry? You need to clarify your question quite a lot before we can answer it! SteveBaker 14:24, 26 September 2007 (UTC)
- I think we can give a general "systems" answer. The item in question (water, for example) comes from the source, and goes to the sink. In the view of that system, there are no inputs to the source or outputs from the sink. However, when considering the larger universe, there do need to be inputs to the source and outputs from the sink. If we consider a lake, for example, we can consider one river to be the source, and where it drains into the ocean to be the sink. From the POV of controlling water levels in the lake, those two are the only thing which needs to be considered. If we construct dams at the rivers that flow in and out (to the ocean), we can control the water level quite nicely, assuming the source and sink continue to function properly. However, if we view the "universe", which is the Earth's ecosystem (plus sunlight), water also evaporates from the sink (oceans) and precipitates into the source (watershed leading to upstream rivers). StuRat 14:35, 26 September 2007 (UTC)
- Read the opening paragrpah of our article on flow networks. Gandalf61 15:35, 26 September 2007 (UTC)
- In computer systems and business process analysis Data flow diagrams are often used to document and analyze procedures. A data source would be any process that brings data into the documented process or system ... for a example a customer filling out a purchase request is a source of data. A data sink removes data from the process. It could refer to throwing away old tax or payroll records from a filing system or deleting it from hard drive storage. Another example could be a water flow monitoring system. A flow meter taking measurements would be a source of data to the system while a non recording status monitoring panel would be a sink ... the data is displayed and then forgotten. Lazyquasar 13:55, 27 September 2007 (UTC)
Honesty in research grant applications
When people apply for grants for research, do they include their wages and the wages of all their staff? Do they ensure that the money granted goes where it's supposed to be (i.e. research proposal says 40 mice and they recieve enough money to do research with 40 mice but actually only use 30 and use the rest of the money elsewhere not stated in the research proposal). --Seans Potato Business 14:29, 26 September 2007 (UTC)
- Depends on the granting agency. Sometimes the grant is meant to be specifically for exactly the costs detailed in the grant. Sometimes the grant is for overall research but they want an estimate of what that will be (but recognize that these things can change). Sometimes the granting agency gives all the money at once and never checks up on it; sometimes they reimburse based on receipts. I'm not sure there's a single answer for this. --24.147.86.187 14:45, 26 September 2007 (UTC)
- It can vary depending on the source of funding and the country, but in years past I have certainly seen grant renewal documents filled out with lists of equipment that sound good and relevant to the research program, then the money was spent for otheer useful things that are more obscure. There was at that time no one for one auditing to see that 40 mice were purchased rather than 30 mice and 10 goldfish, to use your example. In one lab, rather than a 16 mm movie camera which was in the funding request, the money went for a reclining chair and a shotgun (don't ask). A string of publications in good journals trumps a lot of spending changes. Edison 15:32, 26 September 2007 (UTC)
- It depends on the grant, the granting agency, the applicant, and the project. Some grants are designed to cover capital costs only: major pieces of equipment, lab construction and renovation, etc. Other grants are meant for operating costs: salary support for staff, consumables like reagents and disposable equiment, etc. Some grants will include components for both capital and operating expenses. Many research institutions will take anywhere from ten to forty percent off the top of each grant as 'overhead' before anything goes to the research group; this is to cover the cost of construction, heat, light, and shared equipment and services (deionized water, house vacuum, autoclaving and dishwashing, etc.).
- There is an expectation from most granting agencies that the money granted will be spent in some way that bears at least a passing resemblance to what was specified in the grant application; how close that resemblance needs to be depends on the grant. Capital grants tend to be less flexible. If a medical researcher receives a $3 million capital grant for an MRI suite, then there will be hell to pay if he instead uses the money to build a blood chemistry lab. On the other hand, if the same researcher gets a small operating grant – say fifty or a hundred thousand dollars of seed money – to perform a basic research study and later finds that his proposed methods aren't going to work or that an early hypothesis is false, it will usually be acceptable to use the funds to perform related research using alternate methods or to prove a new (but relevant) hypothesis.
- Granting agencies generally understand that research will sometimes produce unexpected results—if we always knew how a study was going to turn out, there wouldn't be any reason to do the experiments in the first place. Larger operating grants often divide money out over two or more years of work, and scientists typically have to specify (and reach) goals or milestones in order to receive continued funding. A competent researcher will have contingency plans in place (if Method A doesn't work, we will try Method B; if Hypothesis X turns out to be false, we can investigate Hypothesis Y) for when an original plan of study doesn't turn out precisely as expected. (Such plans are often presented and required as part of a grant application.)
- There may also be a certain amount of forgiveness as long as a scientist is continuing to publish relevant, productive papers. A granting agency will often be pleased to see a tangential result published in a high-impact journal, even if the work done wasn't technically part of the original grant application. TenOfAllTrades(talk) 16:24, 26 September 2007 (UTC)
- In my experience, funders get a little more upset when money is redirected to completely different types of spending. If your budget includes $10k to hire an undergrad, but in reality you don't hire anyone and spend the money on an instrument or travel, the funder might well feel that you are not holding up your end of the agreement. Depending on the situtation though, the funder might not know how you are spending. ike9898 18:49, 26 September 2007 (UTC)
Typhoon in Hong Kong
Where can I find out about the typhoon that badly damaged Hong Kong around Christmas-time 1936? Jasper33 14:51, 26 September 2007 (UTC)
- The Typhoons of 16-17 August 1936 and 1-2 September 1937 (Appendix II to Hong Kong Meteorological Results 1937) C. W. Jeffries 1937.
- Hmm, not sure either of those covers it. I have a photo taken on the west coast of Borneo on Christmas morning 1936 that has The huge seas are caused by a typhoon further north - the one that damaged Hong Kong so badly written on the back. But thanks for trying. Jasper33 15:20, 26 September 2007 (UTC)
- The typhoon in August was a very severe one. It may be a not quite true label on your photo. Storm waves would not hang around for months, and the storm itself would have made a land fall and dissapated. I will look a bit further! Graeme Bartlett 15:24, 26 September 2007 (UTC)
- I think the caption is correct as it is one in a series showing Christmas celebrations on the beach (including a breakfast of curry puffs). Thanks for your help Jasper33 15:29, 26 September 2007 (UTC)
- The date could be right, but the beleif that the storm damaged Hong Kong may not be correct. If you have access ( look at TYPHOONS AND DEPRESSIONS OVER THE FAR EAST, AUGUST 1936 Rev.BERNARD F. OUCETTE, S. J. and TYPHOON AND DEPRESSIONS OVER THE FAR EAST, DECEMBER 1936 Rev.BERNARD F. DOUCETTE, S. J. . This last one should have your typhoon. But it only reports a depression at this time - with heavy rain. Graeme Bartlett 15:36, 26 September 2007 (UTC)
- Hmm, curioser and curioser. Perhaps the caption wirter was exaggerating for dramatic effect! Thanks for going to all the trouble Jasper33 15:44, 26 September 2007 (UTC)
- The date could be right, but the beleif that the storm damaged Hong Kong may not be correct. If you have access ( look at TYPHOONS AND DEPRESSIONS OVER THE FAR EAST, AUGUST 1936 Rev.BERNARD F. OUCETTE, S. J. and TYPHOON AND DEPRESSIONS OVER THE FAR EAST, DECEMBER 1936 Rev.BERNARD F. DOUCETTE, S. J. . This last one should have your typhoon. But it only reports a depression at this time - with heavy rain. Graeme Bartlett 15:36, 26 September 2007 (UTC)
- More on the August typhoon The reference desk would have to go to some effort to find what happens in 1936. Not many people on the desk would ahve been alive then! Graeme Bartlett 15:48, 26 September 2007 (UTC)
- I think the caption is correct as it is one in a series showing Christmas celebrations on the beach (including a breakfast of curry puffs). Thanks for your help Jasper33 15:29, 26 September 2007 (UTC)
- What a beautifully illustrated journal. They don't make 'em like that any more! Thanks again - lots of interesting details Jasper33 16:01, 26 September 2007 (UTC)
- The typhoon in August was a very severe one. It may be a not quite true label on your photo. Storm waves would not hang around for months, and the storm itself would have made a land fall and dissapated. I will look a bit further! Graeme Bartlett 15:24, 26 September 2007 (UTC)
- Hmm, not sure either of those covers it. I have a photo taken on the west coast of Borneo on Christmas morning 1936 that has The huge seas are caused by a typhoon further north - the one that damaged Hong Kong so badly written on the back. But thanks for trying. Jasper33 15:20, 26 September 2007 (UTC)
- How sure are you about the year? Our List of notable tropical cyclones mentions "The Great Hong Kong Typhoon, 1937 - killed 11,000" SteveBaker 16:01, 26 September 2007 (UTC)
- I know the photos have to be taken pre May 1937, as some of the people in them left Borneo at that date. Maybe what I've identified as Christmas celebrations in the photos aren't; the captions are in ink and some in blue biro and the blue biro ones are the ones which say Christmas 1936. I have a hunch that the biro captions are later additions, with the writer mis-remembering the date but not the typhoon. Who knows? Has anyone invented a time machine yet - that'd sort it! Jasper33 16:16, 26 September 2007 (UTC)
- Something written with a ballpoint pen would have to have been added later - they weren't mass-produced until the early 1940's - and they weren't at all common until the 1950's. OK - so the dates could be off - making this the famous September 1937 Typhoon. So check out [3]. SteveBaker 01:44, 27 September 2007 (UTC)
- Bloody hell. But not that one, as the photos have to pre-date May 1937. I'm thinking the Aug 1936 one sounds about right. Thanks for the link though - scary stuff. Jasper33 07:42, 27 September 2007 (UTC)
- Something written with a ballpoint pen would have to have been added later - they weren't mass-produced until the early 1940's - and they weren't at all common until the 1950's. OK - so the dates could be off - making this the famous September 1937 Typhoon. So check out [3]. SteveBaker 01:44, 27 September 2007 (UTC)
- I know the photos have to be taken pre May 1937, as some of the people in them left Borneo at that date. Maybe what I've identified as Christmas celebrations in the photos aren't; the captions are in ink and some in blue biro and the blue biro ones are the ones which say Christmas 1936. I have a hunch that the biro captions are later additions, with the writer mis-remembering the date but not the typhoon. Who knows? Has anyone invented a time machine yet - that'd sort it! Jasper33 16:16, 26 September 2007 (UTC)
Number of protons in Chromium isotope
1. Isotopes of chromium include 54Cr and 52Cr
- (i) Give the number of protons present in an atom 54Cr
- Is my answer of thirty protons correct?
- (ii)Deduce the number of neutrons present in an atom of 52Cr
- Is my answer of twenty eight neutrons correct?
- (iii) Apart from the relative atomic mass of each isotope what else would need to be known for the relative atomic mass of chromium tobe calculated. [This is the question I can't do]
Thanks guys--Hadseys 15:13, 26 September 2007 (UTC)
- 1. See chromium protons=24. No.
- 2. Yes 52-24=28
- 3. Correction "Apart from the
relativeatomic mass of each isotope.." the isotopes themselves don't have a relative atomic mass.. The other piece of info you need is the relative abundance87.102.32.155 15:20, 26 September 2007 (UTC)
- Question 1: Where did you get "thirty protons?" The atomic number of Chromium is 24. If it had thirty protons, it would be Zinc.
- Question 2. Chromium 52 would have a total of 52 protons and neutrons, and given the answer from Question 1, you can determine the number of neutrons by subtraction. Edison 15:25, 26 September 2007 (UTC)
2. A meteorite was found to contain three isotopes of element X. A mass spectromoter gave the following information about the isotopes
- Isotope 1: m/z is 24.0, its relative abundance is 64.2
- Isotope 2: m/z is 25.0, its relative abundance is 20.3
- Isotope 3: m/z is 26.0, its relative abundance is 15.5
- Could somebody help me figure out the relative atomic mass of X please --Hadseys 15:31, 26 September 2007 (UTC)
- The relative abundance is a percentage - so the percentages should add up to 100 (they do)
- Simply take an average of the m/z using the percentages as weights to get an average m/z
- eg average m/zaverage = (24.0*64.2 + 25.0*20.3 + 26.0*15.5)/100
- The value of m average depends on z - you don't know that but usually z=1 is the major peak so assume z=187.102.32.155 15:43, 26 September 2007 (UTC)
- Therefore, is X most likely to be magnesium? —Preceding unsigned comment added by Hadseys (talk • contribs) 15:49, 26 September 2007 (UTC)
Collared Doves - how much do they eat per day anyway?
I tossed out three very large handfuls of sunflower seed kernels for the collared doves this afternoon. Six doves landed almost immediately and began to chow down. Within ten minutes, all the seed was gone. This got me thinking - just how much can these particular birds eat in a single day? Based on past experience, they seem to be capable of eating as much food as I am willing to provide - I've seen individual birds eating non-stop at my bird table for half an hour or more. They never seem to be full. Heh, I thought that the gulls were greedy until I met these fellas... --Kurt Shaped Box 15:37, 26 September 2007 (UTC)
- There are probably 2 different answers, how much they should eat and how much they will eat, given an unlimited food supply. I suspect that, like many humans, they will overeat when given free food. StuRat 17:04, 26 September 2007 (UTC)
- People get full. Not all animals do. People will certainly overeat, but if you feed some animals enough, they'll eat until it kills them. — Daniel 22:35, 26 September 2007 (UTC)
- Tell me about it :-((( Arghhh!!! - hydnjo talk 00:18, 27 September 2007 (UTC)
My Sun conure again
How can I tell if she's happy?
No, I don't mean if she's contented living with me as my pet, I mean how do I tell by looking at her if she's actually feeling happy at a particular moment in time?
It's easy to tell if she's angry, scared, curious, hungry, hyper, tired or even "sexually frisky" but general happiness is more difficult. Any bird owners here got any tips? --84.64.198.245 15:56, 26 September 2007 (UTC)
- 'Happy' is always a bit tough. How do we define happiness for humans? While there are flaws with this approach, you might want to start with Maslow's hierarchy of needs (adapted suitably for your pet) and see how far up the pyramid you get. TenOfAllTrades(talk) 16:04, 26 September 2007 (UTC)
- I think my bird has most of the lower three levels of the pyramid, except the sexual stuff. She's a lone bird and I'm certainly not mating with her. :) Above that, it's hard to tell. Does a pet bird really need sexual intimacy anyway? --84.64.198.245 19:39, 26 September 2007 (UTC)
- I'd just assume she's happy, unless you have some reason to think otherwise. StuRat 16:56, 26 September 2007 (UTC)
- I've never owned a Sun Conure - but based on my experiences with other psittacines, standing on one foot and making clicking/grinding noises with the beak (i.e. 'chewing the cud', as I've heard some aviculturists refer to it) is a pretty good sign that the bird is chilled out and in a good mood. --Kurt Shaped Box 17:41, 26 September 2007 (UTC)
- I generally agree with Kurt; when our sun conure is "chilled out", she makes small muttering noises ("Merp merp, merp, merp."). In this mood, ours doesn't stand on one foot, but instead often nestles in among her humans' clothing. Another "happy" indication seems to be a fluffy head. Also, a long burst of complicated parrot-chatter seems to mean "I'm happy to see you guys! Here's everything that's happened since the last time I saw you ten minutes ago..."
- When you say "chewing cud", is that used in a comparative way, or is the bird actually re-chewing previously ingested food? Nimur 18:03, 29 September 2007 (UTC)
- To be honest, I'm not sure. I've watched my budgies at it and wondered this myself. The similarity in jaw movements to a cud-chewing cow is uncanny, though they could also merely be grinding the lower beak against the upper in order to smooth off any cracked edges. It's a tricky one to investigate - if you get too close to the birds while they're doing it, they stop doing it and look at you as though to say "What are you looking at?". --Kurt Shaped Box 18:19, 29 September 2007 (UTC)
Chromium
In order to obtain a mass spectrum of a gaseous sample of chromium, sample must first be ionised. Can somebody explain to me two reasons why this is necessary? —Preceding unsigned comment added by 82.36.182.217 (talk) 16:23, 26 September 2007 (UTC)
- What are the units on the values you get on a mass spectrum—what do those symbols mean? Would you get actual numbers if the charge were zero? See our detailed article about how Mass spectrometry works to see what role charge plays in the operation. DMacks 16:47, 26 September 2007 (UTC)
- Also the unionised gas would not be accelerated through the mass spectrometer.. really recommend reading mass spectrometry specifically with a view to looking at what must happen to the sample to register a spectrum -
- Answer:the atoms/molecules need to have an electric charge, not only to be separated, but also to be detected.87.102.32.155 18:40, 26 September 2007 (UTC)
DNA testing
If semen lives for about an hour outside the body, does that also mean it could only be positively tested to identify a rapist within that hour? —Preceding unsigned comment added by 216.136.112.25 (talk) 16:44, 26 September 2007 (UTC)
- Not at all, sperm doesn't need to be live to contain DNA. StuRat 16:49, 26 September 2007 (UTC)
- Scientists have even discovered DNA on the order of 300,000 to 400,000 years old[4]. I regularly work with DNA from tissue that's months if not years old from the death of all its cells. — Scientizzle 18:31, 26 September 2007 (UTC)
Niels Bohr
Can you tell me what Niels Bohr's problem with his model of the atom was?
Alexander Bell
why did he invent the telephone? —Preceding unsigned comment added by 206.168.195.171 (talk) 19:52, 26 September 2007 (UTC)
- All is revealed in Alexander Graham Bell. Someguy1221 20:17, 26 September 2007 (UTC)
- why did he also invent a telephone? Philipp Reis had the first (although not suitable for mass market) telephone.--Stone 09:19, 27 September 2007 (UTC)
- He "invented" the telephone to make a buck, although Elisha Gray in truth probably beat him to it, except for the bribing of a Patent Office employee to assist Bell's application. Reis' telephone could be adjusted to adequately reproduce speech, by means of an imperfect metallic contact, although Reis' theory did not include undulatory currents of electricity to perfectly reproduce the quality of speech. Edison 04:35, 28 September 2007 (UTC)
- Oh, you're a fine one to talk, pretending to have invented the light bulb! :-) --Anon, 22:55 UTC, Sep. 29, 2007.
- He "invented" the telephone to make a buck, although Elisha Gray in truth probably beat him to it, except for the bribing of a Patent Office employee to assist Bell's application. Reis' telephone could be adjusted to adequately reproduce speech, by means of an imperfect metallic contact, although Reis' theory did not include undulatory currents of electricity to perfectly reproduce the quality of speech. Edison 04:35, 28 September 2007 (UTC)
- why did he also invent a telephone? Philipp Reis had the first (although not suitable for mass market) telephone.--Stone 09:19, 27 September 2007 (UTC)
On Facebook, when I scroll to the bottom of a page and press a link, then press "back" on my browser, the page goes back to the top of the page, instead of where my last spot on the page (bottom) was. Why does this happen? Is it because Facebook is written mostly in, for example, javascript? Thanks. Acceptable 21:48, 26 September 2007 (UTC)
- Not quite sure what this has to with Science, but whatever.
- Likely it's because you clicked a link somewhere on the page that took you to another part of the page (those little icons below the profile picture perhaps?). This happens in Wikipedia too, when you click on the table of contents to go to a certain section. These commands use anchor tags. You'll only go back to another part of the page is you clicked a link that took you to another part of a page. There's not much you can do about this. --YbborTalk 22:16, 26 September 2007 (UTC)
- That is called an anchor element. This question probably belongs on the Computing desk. Nimur 18:05, 29 September 2007 (UTC)
Fecal material
Can anyone tell me why the fecal material of carnivors is brown? Does it have to do with Bile? Birds and Fowel seem to have grey or green (Geese) feces. If, indeed, it does have to do with Bile, would the color be different in a person who's gallbladder has been removed? Thanks, WSC —Preceding unsigned comment added by 66.74.110.121 (talk) 23:17, 26 September 2007 (UTC)
- Bilirubin is the chemical that gives it that color. I don't know about why it's different in different animals. --Sean 23:39, 26 September 2007 (UTC)
- See also here (though it doesn't do much more than corroborate Sean's answer). —Steve Summit (talk) 03:04, 27 September 2007 (UTC)
- Also, it's not always brown, but rather depends on what you eat. Mostly the mixture of things we eat doesn't have a very strong color, and the mix is often brown to begin with. Therefore, the effect of the bilirubin in the bile makes it even more of a pronounced brown. However, if you eat things with a single strong color, like blueberries, this can overpower the bilirubin and change the "output color". You might find the third bullet here (dealing with a lack of bile production) to be interesting: Bile#Abnormal_conditions_associated_with_bile. StuRat 12:32, 27 September 2007 (UTC)
- Removal of your gallbladder doesn't stop your bile production, it just means you can't store it up for those occasional blasts of fat that we introduce into our bodies.
- On another note, beets and squid ink pasta can have interesting effects on the color.
- Atlant 19:05, 27 September 2007 (UTC)
- As well as blue Kool-Aid. Drink a bunch of it and you'll see. Cool and safe science that kids find fascinating! Dismas|(talk) 19:10, 27 September 2007 (UTC)
- Atlant 19:05, 27 September 2007 (UTC)
- Ahh, Grossology! A great way to get kids into things scientific and yuchy!
- In addition to bilirubin, bile also contains biliverdin that imparts colour. David Ruben Talk 02:31, 28 September 2007 (UTC)
Bariatric surgery
I am trying to find any articles etc on anyone having bariatric surgery more than once.Tammyfirman 23:49, 26 September 2007 (UTC)
- If you mean operations like lap-band surgery or other similar methods, they almost always "work", in that they reduce the weight of the patient over time. (They do, however, have a high rate of side effects, including death.) So, there shouldn't be a need to repeat the surgery. Doctors do sometimes need to undo the surgery, though, as some people's weight drops dangerously low or dangerously quickly. Also, surgery to remove loose skin may be needed.
- If you mean surgery to actually remove excess quantities of fat, however, they may often be repeated. StuRat 12:17, 27 September 2007 (UTC)
September 27
eicosanoids
Are Eicosanoids considered fat soluble hormones? —Preceding unsigned comment added by Cgilcher (talk • contribs) 00:03, 27 September 2007 (UTC)
- Technically a hormone is any substance carried by the blood that acts on a distant target cell. According to that definition, eicosanoids are technically (fat soluble) hormones. However, in my experience (I'm but a lowly medical student), they're rarely called hormones. --David Iberri (talk) 00:13, 27 September 2007 (UTC)
- Fat soluble? Yes. They're even derived from omega-3 & omega-6 fatty acids. Hormones? You'll find them listed at Hormone#Chemical_classes_of_hormones. — Scientizzle 03:54, 27 September 2007 (UTC)
- I think Eicosanoids are considered lipids, so they are technically fat.128.163.224.198 00:46, 4 October 2007 (UTC)
- Fat soluble? Yes. They're even derived from omega-3 & omega-6 fatty acids. Hormones? You'll find them listed at Hormone#Chemical_classes_of_hormones. — Scientizzle 03:54, 27 September 2007 (UTC)
Surgery
In order to become a surgeon what type of schools do you have to go to? Would a medical school be enough? I am thinking about pursuing a career in this field so any help would be nice. Thank you —Preceding unsigned comment added by 24.242.119.35 (talk) 01:38, 27 September 2007 (UTC)
- In the United States (I see your IP is registered in Texas) a surgeon must complete medical school the same as a physician. This would then be followed by two or more years (I believe) of surgical residency and/or fellowship. Someguy1221 02:33, 27 September 2007 (UTC)
- Having said that, surgery is a highly prestigious occupation and it typically attracts the highest salary of any profession. If you are really serious about pursuing it, you should be seeking the advice of a professional career counsellor. That shouldn't be very hard, you should have easy access to one through your school or university. And you generally should be getting marks in the top 5 percent to even be considering it an option. Vespine 06:51, 27 September 2007 (UTC)
Virial Theorem esp. GPE
I am doing an assignment on Dark Matter and I need to know about the Virial Theorem. Most of the derivation of it is too complex for the assingment and my mathematics skills (which are probably about first year uni level) so I really only need to know that it states that KE = -1/2*GPE. However I would like to know how there is an easy way to quickly derive (using as many assumptions as required about the homegeneity/similarity of objects in the group) the mass of the cluster of galaxies. This website: [5] gives a fairly good formula except I dont understand the reason for the value of the total GPE, especially the factor of 1/2. Just in addition, I was wondering whether the tangential velocities of the galaxies is required for the formula for KE or is the radial velocity satisfactory? --AMorris (talk)●(contribs) 02:19, 27 September 2007 (UTC)
- I guess you have already looked at Kepler orbits earlier in your course. Just take the simplest case, that of a planet on circular orbit around a star. What is the kinetic energy associated with the orbital velocity and what is the potential energy associated with the planet's distance to the star (as compared to infinite distance)? You'll find that they have opposite signs and the kinetic energy is just one half of the potential one. It s as easy to see that it also holds for elliptic orbits. The factor is still precisely one half for cloud of many small particles of comparable size which all hold fast to each other by gravitation, but to see this you need indeed first learn a bit about Lagrangian and Hamiltonian mechanics. And to your ther question: Yes, for the kinetic energy you need to look at the full velocity, and actually, the tangential velocity is the dominant part. And it is precisely this tangential velocity that is too big to make the kinetic energy only half of the potential one if one calculates the potential energy from the gravitation of the visible galaxies. This is the very reason to infer dark matter halos. Simon A. 08:59, 28 September 2007 (UTC)
second law of thermodynamics
Heat is transfered from a very large mass of water at 91°C to 5kg of air that expands irreversibly in a cylinder-piston device fron 5bars, 42°C to 1bar, 41°C. the air does 2kj of work. determine the heat interaction in the process and entropy growth in the universe. —Preceding unsigned comment added by Fonkynet (talk • contribs) 03:05, 27 September 2007 (UTC)
- Please tell me that this isn't ... well, you know. ;-) (Click here for a more detailed explanation) - hydnjo talk 03:46, 27 September 2007 (UTC)
- <smacks forehead>Do your own homework! Second law of thermodynamics and many other articles may be useful, be we're not going to do your math and physics problems. — Scientizzle 03:50, 27 September 2007 (UTC)
- I agree - we're not doing your homework for you. But if you have a particular part of the problem that you don't understand, explain what it is - and we're definitely going to be here for you. SteveBaker 15:05, 27 September 2007 (UTC)
The continuation of the world as we know it?
With the possibility of humanity destroying itself on one hand, and a technological singularity or immersive virtual reality on the other, what's the probability that the world will continue as we know it for an extended period? — Daniel 03:52, 27 September 2007 (UTC)
- Indeed! Infinity rules! - hydnjo talk 03:59, 27 September 2007 (UTC)
- We know that the "world" (as in the planet Earth) can't continue indefinitely because the Sun will give up and die in a billion years or so. I find it hard to imagine humanity destroying itself completely - I can't really imagine any catastrophy that we could suffer that would wipe us all out. There are enough deep bunkers and remote communities with stores of food, water and power to let enough of us to survive most things - albeit with a very reduced population size. The 'singularity' is a slightly different matter - it's very nature means that we can't predict beyond it - but it's not entirely clear that it will actually happen at any time in the future. I would feel much more comfortable about the future of humanity if we had a 'backup' planet - a population of a few thousand people in a self-sustaining community on (say) Mars would be a great way to ensure we could survive most things. But in the long term (beyond about hundred million years) we really need to start thinking about having a backup star or two as well as a backup planet. SteveBaker 15:01, 27 September 2007 (UTC)
- If civilization is destroyed, even if humanity survives, I'd consider that the world ending as we know it. How about this: what's the general consensus on the idea that within a few centuries A) civilization will be destroyed, or B) humanity will be able to change their environment, which may be real or virtual, to pretty much anything they want it to be? How about if you change B to being so technologically advanced that any more advancement beyond that time is all but unnoticeable?
- By the way, I understand Steve fine, but what are hydnjo and Xn4 talking about? — Daniel 22:09, 27 September 2007 (UTC)
- If civilisation collapsed - yet there were enough people left to preserve genetic diversity and retain knowledge - I think we'd have civilisation back on the road again within a few generations. Human population can easily double every generation if we want it to - a thousand survivors could become a million people within 10 generations - 200 years maybe. But as for the time until humanity is destroyed, how could be possibly answer that? We can't predict that planet-killing meteor strike - we don't know when a pandemic disease might strike - we have no clue about the various weird deaths like a black hole eating our sun or a nearby star going nova. These things aren't very predictable...or we'd be predicting them. If we can get a colony started, self-sufficient and viable on Mars, then I'd say that nothing will annihilate our civilisation. If we can't then it's a totally unpredictable thing - so no answer is possible. SteveBaker 23:07, 27 September 2007 (UTC)
- I don't think it could start back up so soon. The rise of civilization as we know it depended on things like large deposits of metals with low melting points at or near the earth's surface; we have used a lot of those up. And of course we have gone trhough millions of years worth of fossil fuels. Eran of Arcadia 20:45, 1 October 2007 (UTC)
- If civilisation collapsed - yet there were enough people left to preserve genetic diversity and retain knowledge - I think we'd have civilisation back on the road again within a few generations. Human population can easily double every generation if we want it to - a thousand survivors could become a million people within 10 generations - 200 years maybe. But as for the time until humanity is destroyed, how could be possibly answer that? We can't predict that planet-killing meteor strike - we don't know when a pandemic disease might strike - we have no clue about the various weird deaths like a black hole eating our sun or a nearby star going nova. These things aren't very predictable...or we'd be predicting them. If we can get a colony started, self-sufficient and viable on Mars, then I'd say that nothing will annihilate our civilisation. If we can't then it's a totally unpredictable thing - so no answer is possible. SteveBaker 23:07, 27 September 2007 (UTC)
photon and magnet .
does magnetic or electric field can affect photon in any way ? photon consist of a electro-magnetic field but it does not seems to by effected either by electric or by magnetic field ; why ? please explain clearly . place interesting external links (if possible) . —Preceding unsigned comment added by Shamiul (talk • contribs) 06:31, 27 September 2007 (UTC)
- Static electromagnetic fields do not effect each other or passing photons (electromagnetic waves). This is thanks to the superposition principle, which, while a mathematical concept, happens to apply to electromagnetic fields. However, when an electromagnetic wave passes through matter, the electrons within are disturbed by the field, and will generate their own electromagnetic waves. The net result of these waves is what accounts for reflection and refraction, as well as more complex phenomena such as birefringence. Under cetain conditions, external magnetic fields can also perturb the passing of an EM wave through matter, as in the Faraday effect. I've deliberately taken a Maxwellian approach to this question and ignored quantum electrodynamics, because it scares me. Someguy1221 07:51, 27 September 2007 (UTC)
Sodium potassium pump
Exactly during which phase of the action potential does the sodium potassium pump start working? Lova Falk 07:02, 27 September 2007 (UTC)
- The sodium potassium pump never stops working. However, its action is sufficiently slow as to not interfere with the actions of the channels. Someguy1221 07:53, 27 September 2007 (UTC)
- Thank you! Does that mean that already during the falling phase of the action potential, lots of sodium ions are pumped back into the extracellullar fluid? Or is the pump so slow (or inefficient) that during hyperpolarization there are still lots of sodium ions in the cell and lots of potassium ions on the outside? Lova Falk 08:13, 27 September 2007 (UTC)
- You may be confusing hyperpolarization and repolarization. Regardless, during each the sodium potassium pump is, correct, far too slow affect either of these processes in the short term. Interestingly enough, the diagrams in my biology notes show the pump to be inactive during the action potential, but make utterly no mention of this in text (neither do our articles), and I'm willing to chalk it up to an inexplicable error...unless someone wants to point out that these diagrams are actually right. Someguy1221 08:36, 27 September 2007 (UTC)
- I meant hyperpolarization as the undershoot phase in the action potential (before it returns to the resting phase). The reason why I ask this question is because, in a couple of pictures and animations such as this one, they show an influx of sodium ions into the cell during the rising phase (picture 5/8 in the animation), less sodium ions inside the cell during the falling phase (picture 6/8), and none at all during the hyperpolarization (picture 7/8). So I wonder how the sodium ions got out again. From your answer, I understand it's not the pump. Or is it a mistake (or simplification) in the animation? Should there still be quite a few sodium ions inside the cell during hyperpolarization that eventually are pumped out by the pump? Lova Falk 12:11, 27 September 2007 (UTC)
- There's a lot of simplifications used to explain the action potential. In that animation, I think they just made the ions disappear to unclutter the image. The Na+/K+-ATPases are responsible for "reseting" the membrane potential during the refractory period after the undershoot. The pumps should essentially remain on as long as there's sufficient ATP.
- One of the common misconceptions about action potentials is that the depolarization and repolarization are the result of large changes in ion concentration. In fact, the important element is actually the electrical current generated by moving charges (ions) that creates a local voltage drop. This change in local voltage activates nearby voltage-gated ion channels to continue directional conduction. The fact that action potentials don't need wide swings in ion concentrations means that the pumps can keep chugging along and reset the membrane potential in relatively short order. — Scientizzle 15:37, 27 September 2007 (UTC)
- Thank you both of you! Lova Falk 16:46, 27 September 2007 (UTC)
- I meant hyperpolarization as the undershoot phase in the action potential (before it returns to the resting phase). The reason why I ask this question is because, in a couple of pictures and animations such as this one, they show an influx of sodium ions into the cell during the rising phase (picture 5/8 in the animation), less sodium ions inside the cell during the falling phase (picture 6/8), and none at all during the hyperpolarization (picture 7/8). So I wonder how the sodium ions got out again. From your answer, I understand it's not the pump. Or is it a mistake (or simplification) in the animation? Should there still be quite a few sodium ions inside the cell during hyperpolarization that eventually are pumped out by the pump? Lova Falk 12:11, 27 September 2007 (UTC)
- You may be confusing hyperpolarization and repolarization. Regardless, during each the sodium potassium pump is, correct, far too slow affect either of these processes in the short term. Interestingly enough, the diagrams in my biology notes show the pump to be inactive during the action potential, but make utterly no mention of this in text (neither do our articles), and I'm willing to chalk it up to an inexplicable error...unless someone wants to point out that these diagrams are actually right. Someguy1221 08:36, 27 September 2007 (UTC)
- Thank you! Does that mean that already during the falling phase of the action potential, lots of sodium ions are pumped back into the extracellullar fluid? Or is the pump so slow (or inefficient) that during hyperpolarization there are still lots of sodium ions in the cell and lots of potassium ions on the outside? Lova Falk 08:13, 27 September 2007 (UTC)
Central Valley result of impact event...?
Is there a theory that the Central Valley (in California) is the remnant of an impact event, perhaps as old as when the Moon was created? I have heard that this was investigated to the point where surveys have been done to look for signs of impact. yet without positive results.
The reason for my curiosity is based on the following:
- The current shape of Central Valley being due to geological activity which as compressed a more or less round or elliptical impact site into a relatively narrow elongated site.
- The moon resulted from such an impact.
- As you travel toward LA and leave the Central Valley you can see very large rock strata that is turned upward as you would expect along the periphery of an impact site.
- Throughout Montana and the Southwest, all the way from LA to Texas especially in Arizonian and New Mexico, there are great piles of homogeneous rock and dirt which are of course large enough to be called mountains but which fit the pattern of a large amount of Earth which was ejected by impact landing in great piles as it returned.
What knowledge do you have of such a theory and what conclusions do you know about that have been reached? Clem 08:41, 27 September 2007 (UTC)
- Searching through google and half a dozen astronomical journals, I have found all of nothing on anything like this theory. Someguy1221 08:53, 27 September 2007 (UTC)
- I'm pretty sure the theory is not original since I recall posting a question about it several years ago to a geo at Stanford and he responded that nothing to indicate such an impact occured has ever been found, after numerous drillings and the like. Clem 10:08, 27 September 2007 (UTC)
- Also, please note (if you take the Giant impact hypothesis as a given) that the impact that created the moon likely reduced the Earth into a molten ball, in addition to throwing up far more material than would a meteor that leaves only a few hundred mile wide crater, and Central Valley (California) offers a non-impact way of forming it. Now, my first two points don't preclude the possibility of millions of tons of debris raining down on a solid Earth for some time after the moon's formation, but I have no idea on that one. Someguy1221 09:03, 27 September 2007 (UTC)
- Perhaps not the Moon but certainly a large body... the upturned strata can be explained by subduction forces but not the great piles of rock and dirt debris throughout the Southwest. Only comparison with the underlying material, I suppose, can determine if these piles rained down, grew up or whatever. Clem 10:08, 27 September 2007 (UTC)
- Next time, please be more specific - I had to read halfway through the question to get an idea which Central Valley you were talking about. I was thinking Costa Rica (because I've been there and that's the only place where I've ever heard the term). DirkvdM 10:30, 27 September 2007 (UTC)
- Three problems with this theory:
- Our section on geology of the Central Valley describes a very different, non-impact origin for the Central Valley.
- The Central Valley is much younger than the hypothetical impact event that created the Moon, which would have happened more than 4.5 billion years ago.
- An impact event large event to form the Moon would have re-formed the whole surface of the Earth - it wouldn't leave any identifiable local features. Gandalf61 10:54, 27 September 2007 (UTC)
quanta
1. If N atoms have equally spaced energy levels (separation = q) and energy (total energy =E) is distributed randomly amongst the atoms in packets of energy q .. what is the name (if any) of the corresponding distribution. (as an average of all the possible combinations equally weighted) (I'm not asking for the equation)
2. Alternatively. If N atoms have equally spaced energy levels (separation = q) and energy (total energy =E) is distributed randomly amongst the atoms so that each possible distinct distribution is counted only once.. What is the name for this distribution (again as an average of the different possible energy distributions each equally weighted) (eg the atoms can be considered labelled so that energy distributions of E=3 over 3 atoms (q=1) has possible distributions including (3,0,0) and (0,3,0) and (0,0,3) that all count once.)
2b. Also does anyone know (link?) what happens when I don't 'label' the atoms so that in the above example (3,0,0) and (0,3,0) and (0,0,3) are only counted as one possible contribution?83.100.254.236 13:11, 27 September 2007 (UTC)
I'm looking for distributions (science or maths) that correspond to either of the two above when the energy levels have constant difference (even if this condition is a specific case within the distribution). Thanks.83.100.254.236 13:11, 27 September 2007 (UTC) (and no degenerate energy levels in my examples if you were wondering)
- I believe this is all answered by the Boltzmann distribution, my personal favorite physics equation. The question of whether distinct distributions (2 and 2b) are counted once or degenerately (the same as saying all particles are identical or all particles are distinct) merely affects how many energy states are included in the Boltzmann distribution. Someguy1221 18:16, 27 September 2007 (UTC)
- Erm the ditribution I got from 2 wasn't the same as the Boltzmann distribution.. I don't actually know what the distribution is like for 2b - I haven't been able to work it out yet.. (the difference between 2 and the Boltzmann distribution is that in 2 the average of microstates is taken whereas bolztmann takes the microstate with the highest 'entropy' (a micro state is (3,0,0) or (2,1,0) etc in the example I gave) see boltzmann for his definition of entropy in this case)87.102.83.163 17:00, 28 September 2007 (UTC)
- Anyone recognise distributions (including continuous ones) that at least match the shapes of the above.87.102.83.163 16:37, 28 September 2007 (UTC)
- These are particular cases of Maxwell-Boltzmann statistics and Bose-Einstein statistics. Just plug in the energy levels. If you further require that no two atoms be in the same state, you get Fermi-Dirac statistics instead. I don't understand the difference between (1) and (2a), since the atoms are distinguishable in both cases. --Reuben 17:54, 28 September 2007 (UTC)
- The difference between 1 and 2 is in the case given above- for the state (3,0,0) there is one way to obtain the state in 1, but for the state (1,1,1) there are 6 (3!) ways to obtain the state in 1. So in 1 each state is not equally likely.87.102.83.163 19:37, 28 September 2007 (UTC)
- Are you saying that in (1), you mean not only the atoms but also the quanta of energy to be labeled and distinguishable? In that case, 2(a) and 2(b) are Maxwell-Boltzmann and Bose-Einstein respectively, but (1) doesn't seem to correspond to anything very physical. --Reuben 19:45, 28 September 2007 (UTC)
- Effectively yes (for type 1) - I would put it this way - that the energy is quantised and comes in chunks of energy q only. It does give a fairly good model of blackbody radiation. (does this distribution have a mathematical name - also I'd like to know more about getting a continuous version of this - ie not quantised ..)
- For type 2 : Bose einstein statistics looks promising - I found this page http://hyperphysics.phy-astr.gsu.edu/hbase/quantum/disbex.html#c1 that seems to show that I'm using the same method to calculate states in 2 as is used in bose-einstein - so that's good - however - I get probability of 'atom' having energy E is proportional to (k-E)N-2 - ie the shape of the graph depends on the number of molecules (as is easily tested with small numbers of molecules without a derivation) - the bose-einstein equation doesn't do that - so I'd like to ask 3. why? and 4. Is there a distribution function (something I can look up) that does take into account the numbers of molecules..87.102.83.163 19:56, 28 September 2007 (UTC)
- Are you saying that in (1), you mean not only the atoms but also the quanta of energy to be labeled and distinguishable? In that case, 2(a) and 2(b) are Maxwell-Boltzmann and Bose-Einstein respectively, but (1) doesn't seem to correspond to anything very physical. --Reuben 19:45, 28 September 2007 (UTC)
- The difference between 1 and 2 is in the case given above- for the state (3,0,0) there is one way to obtain the state in 1, but for the state (1,1,1) there are 6 (3!) ways to obtain the state in 1. So in 1 each state is not equally likely.87.102.83.163 19:37, 28 September 2007 (UTC)
- It seems reasonable to begin an accounting of the quanta of action that take place in ergonomic work or exercises. The number of quanta won't be exact, they are so small. But it won't be less than, say, 10^10 quanta for lifting a curling iron, and it won't be more than 10^40. Progressive estimates can narrow the number a great deal. SyntheticET (talk) 04:03, 22 December 2008 (UTC)
Enkephalin and enkephaline
Enkephalin has its own article in Wikipedia, enkephaline is redirected to endorphin. Is this really correct?? Lova Falk 13:18, 27 September 2007 (UTC)
- Enkephalin is an endorphin, it looks like enkephaline is a french spelling of enkaphalin, but seems to refer to a family of enkephalins? so maybe should redirect to enkephalin, though the current redirect is not really wrong - just not specific enough.83.100.254.236 13:22, 27 September 2007 (UTC)
- I don't think enkephaline is actaully an english word...83.100.254.236 13:24, 27 September 2007 (UTC)
- I found enkephaline in an english book (Kolb & Whishaw: Fundamentals of Human Neuropsychology).
- Basically you're saying:
- enkephalin = enkephaline
- there are different kinds of endorphins, and enkephalin is one of them. (This is how I interpret your writing an endorphin.)
- Are you sure this is correct? Lova Falk 13:31, 27 September 2007 (UTC)
- The consensus at the AFD that made enkephaline a redirect was that it is a mis/alt-spelling of enkephalin. Since enkephalin was a redirect then, enkephaline was redirected straight to endorphin. Thus I have just changed enkephaline to redirect to enkephalin. I hope this is correct. btw, enkephalin is strictly speaking two endorphins, met- and leu-. Algebraist 16:02, 27 September 2007 (UTC)
- The issue of the extra e is solved, but I'm still confused about enkephalin being two endorphins. Why does neither the article on enkephalin nor the article on endorphin mention this? Why does the article Opioid peptide name three opioid peptides produced by the body and not mention that enkephalin is an endorphin?? I'm sorry for being so stubborn, but it just doesn't make sense to me that enkephalin is an endorphin. Lova Falk 16:56, 27 September 2007 (UTC)
- I expanded the section Opioid peptides produced by the body. Goodman & Gilman's The Pharmacological Basis of Therapeutics is sorta the bible for these kinds of things and it uses "endogenous opioid peptides" as the general term for which there are three well-characterized families, enkephalins, endorphins and dynorphins. Since these three families of opioid peptides are produced by homologous genes, things can get a bit confused, particularly since the met-enkephalin peptide sequence is coded for by both the enkephalin gene and the endorphin gene (also known as the POMC gene) and the leu-enkephalin peptide sequence is coded for by both the enkephalin gene and the dynorphin gene. The peptides were found and named first and only later were the genes sequenced, so the nomenclature is a bit of a mess. --JWSchmidt 03:16, 28 September 2007 (UTC)
- Thank you! I added your comment to Enkephalin. Lova Falk 15:05, 28 September 2007 (UTC)
- I expanded the section Opioid peptides produced by the body. Goodman & Gilman's The Pharmacological Basis of Therapeutics is sorta the bible for these kinds of things and it uses "endogenous opioid peptides" as the general term for which there are three well-characterized families, enkephalins, endorphins and dynorphins. Since these three families of opioid peptides are produced by homologous genes, things can get a bit confused, particularly since the met-enkephalin peptide sequence is coded for by both the enkephalin gene and the endorphin gene (also known as the POMC gene) and the leu-enkephalin peptide sequence is coded for by both the enkephalin gene and the dynorphin gene. The peptides were found and named first and only later were the genes sequenced, so the nomenclature is a bit of a mess. --JWSchmidt 03:16, 28 September 2007 (UTC)
- The issue of the extra e is solved, but I'm still confused about enkephalin being two endorphins. Why does neither the article on enkephalin nor the article on endorphin mention this? Why does the article Opioid peptide name three opioid peptides produced by the body and not mention that enkephalin is an endorphin?? I'm sorry for being so stubborn, but it just doesn't make sense to me that enkephalin is an endorphin. Lova Falk 16:56, 27 September 2007 (UTC)
- The consensus at the AFD that made enkephaline a redirect was that it is a mis/alt-spelling of enkephalin. Since enkephalin was a redirect then, enkephaline was redirected straight to endorphin. Thus I have just changed enkephaline to redirect to enkephalin. I hope this is correct. btw, enkephalin is strictly speaking two endorphins, met- and leu-. Algebraist 16:02, 27 September 2007 (UTC)
decline of the human body
I am a 24 year old male who is very athletic. I can run really fast, jump high, and I am very flexibile. I play a lot of sports, especially basketball. I wanted to know at what age does the body start to slow down? How many more years do I have left until I won't be able to run fast or jump high and overall start to loose my abilities? —Preceding unsigned comment added by 68.223.217.95 (talk) 15:51, 27 September 2007 (UTC)
- One way of finding an answer to your question is to check for each ability at what ages professional athletes are at their peak. For instance, gymnasts (for flexibility) usually reach their peak performance at a much younger age than runners do. Lova Falk 17:04, 27 September 2007 (UTC)
- I'm sure its different for everybody. Brett Favre is pushing 38 and he still dominates the NFL. Tiki Barber was only like 31 when he retired. —Preceding unsigned comment added by 128.101.53.147 (talk) 18:24, 27 September 2007 (UTC)
- Of course it's also individual, and injuries can complicate matters. But you can calculate averages. Lova Falk 18:44, 27 September 2007 (UTC)
- Also, keep in mind that as you age, your speed goes down, but your endurance goes up. This is why, for example, top sprinters tend to be in their 20s, but top endurance runners tend to be in their 30s or 40s. --Carnildo 23:30, 27 September 2007 (UTC)
- In many important physiological aspects, it is all downhill after age 19. Intellectually, it has been said that mathematicians make their best contributions by age 35. Then there is the truism "Age and guile beat youth and talent, every time." Edison 04:29, 28 September 2007 (UTC)
- I'm sure its different for everybody. Brett Favre is pushing 38 and he still dominates the NFL. Tiki Barber was only like 31 when he retired. —Preceding unsigned comment added by 128.101.53.147 (talk) 18:24, 27 September 2007 (UTC)
- In other words, it's gradual. Your abilities will very slowly diminish. On the other hand, your experience will grow and that experience will help you make better use of the physical abilities you have. So that will compensate for a while. I'd say that on average the effect of declining physical abilities will start to have a bigger effect than the experience in one's forties. But that also depends on what kind of activity we're talking about, more physical, like running or rowing, or more skill-based, like hiking or sailing. Also, it depends on how much you wear out your body. Such as with sports. Physical exercise (eg taking the stairs instead of the lift) is good, but sports tend to put too much ('unnatural') stress on specific body parts (think tennis-elbow and football-knee). Especially if you stick to just one sport and/or do it more fanatically. If you want to stay fit as long as possible, then forget about sports and get your exercise from ordinary daily activities, preferably as varied as possible. If that's not enough exercise for you, then maybe do several sports and don't try to excel at them. In other words, remain active, but don't overdo it either. DirkvdM 06:57, 28 September 2007 (UTC)
For the record, it's extremely arguable whether or not Brett Favre currently "dominates" the NFL, but it's nonetheless impressive that he's still playing and winning games at his age. 38.112.225.84 13:57, 28 September 2007 (UTC)
chemistry/food-grade
I am looking for information on what compound 1036 is.
207.41.37.140 15:54, 27 September 2007 (UTC)Rachel
- 3-methyl-6-(ethoxycarbonylbutoxy)-2-oxo-1,2-dihydroquinoxaline Someguy1221 18:38, 27 September 2007 (UTC)
Positronium energy levels and instability
Why is the "reduced mass" used for energy level calculations in positronium but not in hydrogen. What quantum mechanical forces lead to instability of positronium.68.226.119.129 18:07, 27 September 2007 (UTC)
- 1. It should be reduced mass for the hydrogen atom too, but the electron mass and reduced mass are only different by about 0.05%, so the distinction is usually ignored as negligible. 2. In the ground state, the electron and positron wave functions overlap quite considerably. They annihilate. See positronium for decay channels. --Reuben 18:14, 27 September 2007 (UTC)
- Ummm, no. The reduced mass is and hence the reduced mass of the electron in positronium is half the electron's normal mass. 136.152.153.30 21:26, 28 September 2007 (UTC)
- You're talking about positronium. I was explaining why the distinction is not necessary for the hydrogen atom. --Reuben 21:43, 28 September 2007 (UTC)
- Ummm, no. The reduced mass is and hence the reduced mass of the electron in positronium is half the electron's normal mass. 136.152.153.30 21:26, 28 September 2007 (UTC)
Proper temperature for deep freeze?
My deep freeze has a digital display showing the current temp inside. How cold should I set it? I don't want to set it unnecessarily cold and waste electricity. --24.249.108.133 18:15, 27 September 2007 (UTC)
- Deep freeze = standard home freezer which is possibly attached to the refrigerator? Dismas|(talk) 19:07, 27 September 2007 (UTC)
- Freezers are conventionally set at 0F/-18C. Colder probably makes things last longer but uses more energy, and "longer" is asymptotic anyway -- things last a long, long time at 0F.
- FYI: In American usage, "deep freeze" usually refers to a separate freezer unit, often chucked off in the garage or basement because they're not accessed all that often. These units are frequently horizontally-oriented with the door opening upwards so that the spill they least amount of cold air when the top (door) is opened. Baskets within allow easy access into the freezer to get at things down by the bottom.
- Interesting that there isn't an entry on these, eh? BTW, my deep freeze has a fridge-like vertical orientation. --24.249.108.133 21:58, 27 September 2007 (UTC)
- In English/English, the kind that you put in the garage are 'chest freezers' - but the term 'deep freezer' refers to how cold they are kept rather than how physically deep they are. I'm surprised that -18C is right - I thought it was much warmer than that. -7C or so. The only requirement is for everything inside to be frozen and there aren't many foods that are still liquid below that. (Although concentrated brine solutions won't freeze until -21C. The amount of heat leaking into your freezer (and therefore the amount of electricity you'll need to run the thing) is proportional to the square of the difference in temperature between the air and the freezer. If your room temperature is (say) 20C and you keep your freezer at -18C then you'll be using 382 'units' of electricity - at (say) -8C, it's only 282...that's about half as much! So running your freezer colder than it needs to be is not cheap! SteveBaker 22:54, 27 September 2007 (UTC)
- Even in American English, technically speaking, I think you're correct about "chest freezer" and "deep freeze", but colloquially, I think it's more common to refer to the freezer-only unit, whether upright or horizontal, as the "deep freeze".
- When attached to the refrigerator it is often referred to as the freezer compartment and its temperature is usually
notowner adjustable. - hydnjo talk 11:37, 28 September 2007 (UTC) Oops, strike "not". - hydnjo talk 21:17, 28 September 2007 (UTC)
- When attached to the refrigerator it is often referred to as the freezer compartment and its temperature is usually
- The temperature of the freezer compartment is always adjustable, it's just that the temperature of the refrigerator compartment may track along with it (or vice-versa, depending on your point of view). But in most American kitchen 'friges (with a separate door for the freezer compartment), some provision is made to adjust the temperature of the compartments separately. Low-end friges let you manually set an air door (damper) that varies the relative amount of cooling between the two compartments. Higher-end fridges have separate thermostats that probably automatically affect an air door; I haven't taken mine apart yet. ;)
- I know that -18C is often the recommended storage temperature for ice cream. Whether it makes a significant different to the texture or taste I don't know. Also I don't think the only requirement is that everything is frozen. You ideally want most things to be a few degrees below their freezing temperature since otherwise they may thaw too quickly. I'm not saying -8C doesn't achieve this simply pointing out everything being frozen isn't usually sufficient. Also I would expect the actual temperature inside the freezer is allowed to vary by at least 1-2 degrees. The freezer turns on when it's above the high threshold and turns off when below the low threshold (similar to the way the fridge part works). Nil Einne 14:52, 28 September 2007 (UTC)
- My experience is that small changes in temperature have big effects on the texture of ice cream, especially the more-watery varieties.
- Lower temperatures also help to stave off the effects of freezer burn. 136.152.153.30 21:33, 28 September 2007 (UTC)
- A few points on why a lower temp than freezing may be needed. One is that many frozen foods contain fat or oils, which substantially lowers their freezing temp. Another is that adding a large room temperature object (say the carcass of a turkey after a meal) can melt things around it. Every time a frost-freeze cycle occurs the food degrades further, so you want to avoid this, if at all possible. StuRat 22:49, 28 September 2007 (UTC)
- I don't think that's true. Fat and oils don't dissolve in water - they forms an emulsion, which is really just teeny-tiny bubbles of oil floating in the water - and that doesn't change the freezing points of either substance. AFAIK, the lowest temperatures you can get are with salt. In fact, the one thing that lends some credance to the -18C figure is that it is zero Fahrenheit. The Fahrenheit temperature scale was designed such that 0F was the coldest temperature that could be obtained in a laboratory at the time - using salt and water (100F was thought to be human body temperature at the time). But Mr Fahrenheit wasn't much of an experimenter and he got both ends off the scale off by a few degrees and actually, you can get water down a few degrees below 0F with a really concentrated salt solution. SteveBaker 01:17, 29 September 2007 (UTC)
- I didn't say oils mixed with water. I'm quite clear on oils and waters forming an emulsion, as I mentioned just recently here. I said that some "foods contain fat or oils, which substantially lowers their freezing temp". That means that to be frozen solid, the oils must be frozen, too, which have a much lower freezing temp than water. (I suppose you can leave the oils in liquid form, but that leaves them free to migrate, which can cause the food quality to diminish.) StuRat 02:06, 29 September 2007 (UTC)
- I did find the proper 'official' temperature for freezing foods. In the UK (at least) frozen foods are labelled with their storage life against the temperature of the freezer using a 'star rating' - so the amount of time you can keep food depends on the star rating of your freezer:
- One Star = -6°C
- Two Stars = -12°C
- Three Stars = -18°C
- Four Stars = -18°C - but with a 'fast freeze' setting down to -26C
- So you could save a LOT of energy by adjusting your freezer down to -6C and pretending you only have a one-star freezer - but you'd have to be aware that you are reducing the amount of time that your food will remain in good shape. Bacteria can't grow at -6C - so that's not the issue...it's purely the texture, flavor and such that suffers at warmer settings over long periods. SteveBaker 01:33, 29 September 2007 (UTC)
- Wouldn't that be setting the temp "up" to -6°C ? StuRat 01:54, 29 September 2007 (UTC)
neurophysiology
im looking for a few pointers how to write a 3000-5000 word essay on the reason why even though you are running after a bus for example,you can still read the number on the bus with reference to the nervous system and responses Saradennehy 19:48, 27 September 2007 (UTC)
- The tell them three times rule of thumb may apply. Introduction -- Tell them what your are going to tell them. Body -- Tell them. Conclusion -- Tell them what you told them. Lazyquasar 23:33, 27 September 2007 (UTC)
- Oh, hey, you're looking for a specific reflex. I'm not going to tell you what it is, because I think you're doing homework. However, there is a link to it at Eye movement. Let us know if you run into trouble! --Mdwyer 01:28, 28 September 2007 (UTC)
- If I'm not mistaken, Steven Rose uses that very example in his book The 21st century brain explaining how we can identify a big red patch moving accross our field of vision as a bus and act instinctively upon that realisation (such as jumping out of the way). This might touch on your subject (is it vision and body coordination?). It's a classic that should be in your college library. 80.200.155.140 00:13, 30 September 2007 (UTC)
Examples of Buffer Solutions
Can you tell me some examples of buffer solutions that work in human body? I need to know them to prepare an assignment.What is their biological imprtance and how does they function? Please, share your knowledge. thanks a lot. —Preceding unsigned comment added by 201.220.222.140 (talk) 20:22, 27 September 2007 (UTC)
Well, the human body uses several buffer systems (not solutions). The two main systems are the bicarbonate/carbonate ionic system, and the phosphate buffer system. I don't see a good overview here on Wikipedia, though you could look at the specifics here. Some explanation can be found at this site. - Nunh-huh 20:41, 27 September 2007 (UTC)
- "Oh yeah, a buffer. The family had a lot of buffers!" - hydnjo talk 03:21, 28 September 2007 (UTC)
Physiological Effects of Niacin
I am doing an article on niacin and am trying to simplify a paragraph found at http://www.feinberg.northwestern.edu/nutrition/factsheets/vitamin-b3.html . Could someone explain it to me?
Niacin is an essential vitamin that supports energy metabolism and reactions involving biosynthesis and degradation as part of the pyridine nucleotide coenzymes, nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP). The levels of oxidized and reduced forms of these coenzymes establish the redox potential in cells that regulates metabolic activities involving mitochondrial electron transport and numerous enzyme reactions.
Thanks!
Felix 22:23, 27 September 2007 (UTC)
Niacin is a vitamin required to produce NAD and NADP, which govern the redox state of a cell, and thereby regulate its metabolism. - Nunh-huh 22:52, 27 September 2007 (UTC)
- Basically, Niacin is required to make NADP and NAD. (Niacin makes up parts of the structures of NADP and NAD.) NADP and NAD are two molecules that help in making stuff that the body needs (biosynthesis). They do this by helping in redox reactions (redox is a type of reaction that transfers electrons from one molecule to another).
- The paragraph also talks about NADP and NAD being coenzymes. Coenzymes are molecules that work together with enzymes (proteins that help speed up biological reactions) to complete the reactions. Hope this helps.
128.163.224.222 20:33, 30 September 2007 (UTC)
insect brain
Other than size, what is the difference betweem human and insect brains? Clem 23:47, 27 September 2007 (UTC)
- Our insect article discusses the nervous system a bit. An insect barely has anything worth calling a "brain": what passes for a brain is sixed fused ganglia in the head. The insect nervous system is much more "decentralized" than the vertebrate nervous system, with ganglia in various body segments controlling local funcitons. - Nunh-huh 00:25, 28 September 2007 (UTC)
- It also weighs much less. --Sean 03:24, 28 September 2007 (UTC)
- Which is a logical result from it being smaller. Duh! :) Keep in mind that a larger body requires a larger brain, just to control the body. As for the mental functions, I was almost tempted to point out that the neocortex gives mammals the ability to learn. But bees can tell each other where a nice and nectary flower field can be found, and that is a form of learning. DirkvdM 07:08, 28 September 2007 (UTC)
- "The developing Drosophila melanogaster brain, like the vertebrate brain, contains many compartments that give rise to multiple, anatomically distinct processing centers...." Compartmentalization of visual centers in the Drosophila brain requires Slit and Robo proteins provides a description of the major fly brain regions involved in vision. You can compare these to the major visual centers of the human brain. Exoskeletons impose restrictions on the evolution of sensory organs and brains, but insect brains are as "highly" evolved as human brains. --JWSchmidt 14:25, 28 September 2007 (UTC)
- Do you mean to say that the basic functions are the same, but insects just have less of them? Concerning my remark about learning - the example I gave is short-term memory. And I now wonder if that is essentially different from long-term memory or that the latter is just a matter of 'more of the same', therefore spanning a longer time period. However, I doubt that insect brains will be essentially the same as human brains because the two are so different in so many ways. I suppose what matters here is if nerves as we know them evolved before the insect and mammal branches split off from each other. DirkvdM 18:09, 28 September 2007 (UTC)
- Invertebrates and vertebrates have been found to use many proteins (example: CREB) in similar ways for making changes in neurons that are required for the storage of long term memories. However, many hundreds of millions of years have passed since there was a common ancestor for insects and vertebrates, so there are very different patterns in how insect and human brains are laid out. A Beetle is very different from a Liebherr T 282B, but are they "essentially" the same? --JWSchmidt 20:29, 28 September 2007 (UTC)
- Do you mean to say that the basic functions are the same, but insects just have less of them? Concerning my remark about learning - the example I gave is short-term memory. And I now wonder if that is essentially different from long-term memory or that the latter is just a matter of 'more of the same', therefore spanning a longer time period. However, I doubt that insect brains will be essentially the same as human brains because the two are so different in so many ways. I suppose what matters here is if nerves as we know them evolved before the insect and mammal branches split off from each other. DirkvdM 18:09, 28 September 2007 (UTC)
September 28
Subway menu
What does BMT stand for? —Preceding unsigned comment added by 84.64.122.81 (talk) 03:50, 28 September 2007 (UTC)
- "Biggest, Meatiest, Tastiest", with a nod towards "Brooklyn-Manhattan Transit." --Mdwyer 04:07, 28 September 2007 (UTC)
- Back when the names of the sandwiches were taken from subway lines, it did stand for Brooklyn Manhattan Transit. They moved away from the naming scheme but that one stuck. At least that's what the Subway manager I spoke to told me when I asked this years ago. Dismas|(talk) 15:47, 28 September 2007 (UTC)
- Subway's own website plays coy:
- "Italian B.M.T.® An old-world favorite. Sliced Genoa salami, pepperoni and ham and your choice of fresh vegetables and condiments served on freshly baked bread. Some say BMT stands for biggest, meatiest, tastiest. We wouldn’t disagree."
- I've also seen "Bigger, meatier, tastier" on in-store posters, though not recently. TenOfAllTrades(talk) 16:00, 28 September 2007 (UTC)
I've been reading the Transverse Doppler effect article, and I just don't get what it's saying. If the redshift factor is , then does that mean that the factor is less than one and an object moving laterally appears redshifted to the observer? 18.238.6.77 04:06, 28 September 2007 (UTC)
- Have you noticed that there is an article on the relativistic Doppler effect, and it even has colourful figures and animations. Looking a bit at these will show you that, yes, an object moving laterally gets redhsifted a bit, too. See relativistic beaming for something even stranger. 138.232.67.15 12:17, 28 September 2007 (UTC)
Fibreglass for oven insulation
This probably isn't the best place to ask but what the heck... After my stupid cat brought in and lost one (or more) mice in my house, it evidentally took up nest in the oven. Today when I turned it on (mostly use the elements), the heat and/or air movement because of the heat caused the outside to stink terribly (inside it smelled fine). After finally working out how to take the damn thing apart I decided the fibreglass insulation which had some holes in some places had probably been contaminated and it was unlikely I could get the smell off so I removed it. I now of course have to replace the insulation if I don't want to waste a lot of electricity.
Does anyone know if you need special high temperature fibreglass or most stuff will be fine (obviously wool or something is a bad idea). I have some left over from a water heater job but which looks more or less the same but of course that only needs to work at 100 C whereas this will in theory get close to 250C in some cases.
N.B. The often is fairly old so if worse comes to worse it can be replaced. Hopefully I can put I back together okay
Nil Einne 15:09, 28 September 2007 (UTC)
- YIKES! Are you sure that's fibreglass and not some kind of asbestos wool? The latter is not stuff you'd want to be messing with! Asbestos fibre looks almost identical to fibreglass - it's much more temperature-resistant and if your oven is old then it may well have been made before the stuff was banned. Asbestos is a nasty health hazard. I don't know if that's what you have here (obviously) - but then, neither do you - so for sure wear a mask and (even if it's only fibreglass), you should certainly wear gloves! SteveBaker 15:33, 28 September 2007 (UTC)

- Asbestos thermal insulation really doesn't look much like the fiberglass used in ovens, but your suggestion of caution is still welcomed. Having said that, I'll make three observations and a suggestion:
- The insulation may not be just for efficiency. It may be for safety, as in keeping the exterior of the oven at a low-enough temperature so as not to cause thermal burns and light the kitchen cabinets, floors, etc. on fire. Don't omit it!
- For a self-cleaning oven that uses pyrolytic decomposition, you need insulation that will go a lot higher than 250C, more like 500-560C.
- I personally wouldn't trust "improvised" insulation so I'd go to my nearest appliance parts dealer and try to buy replacement appropriate insulation board which you could then cut to shape.
- Asbestos thermal insulation really doesn't look much like the fiberglass used in ovens, but your suggestion of caution is still welcomed. Having said that, I'll make three observations and a suggestion:
- I'm pretty sure it's not asbestos because amongst other things asbestos has been more or less completely banned in NZ since 1987 AFAIK, altho the regulations were fairly strict before then anyway (since the early 80s or so I think). And the oven is old but I don't think it's that old. Also as Atlant said (although I've no experience in recognising asbestos) I don't think it looks like fibreglass. It's not a Self-cleaning oven BTW, it's not that fancy although it did seem to be quite well insulated (from experience of how well it kept the heat when off). Nil Einne 16:55, 28 September 2007 (UTC)
- You're probably right - you said the oven was old...but not how old...and I felt it better to be "safe, quickly" than to do a whole lot of research. Anyway, I strongly disagree about asbestos and fibreglass looking different - check out the two photos from our asbestos and fibreglass articles respectively. I couldn't tell the difference. SteveBaker 18:16, 28 September 2007 (UTC)
- Perhaps in their "raw" form, but as a final product, I still think they're pretty unmistakable. For example, I've never seen pink asbestos ;-), and even the more-common fiberglass "yellow" color is pretty unique to fiberglass. Asbestos and rock wool tended to be used in their natural colors.
Insect identification
Can anyone identify this... thing? It was seen in Vermont. It's about 3-4" long and about 3/4" in diameter. Sorry for the crappy cell phone pics... [6] [7] [8] [9] Thanks, Dismas|(talk) 16:57, 28 September 2007 (UTC)
- I'm no bugologist, but it looks like a Sphinx Moth caterpillar, perhaps a Eumorpha pandorus. Was he by any chance munching on a grape vine? There are some nice pics of them here. Some Eumorpha do an absurdly good job of tricking birds into thinking they're snakes: [10], [11] (!). --Sean 18:09, 28 September 2007 (UTC)
- Kind of close but I don't recall the one I saw having that horn on the head. Oh, and a "bugologist" would be an entomologist. Dismas|(talk) 22:21, 28 September 2007 (UTC)
- Kind of close, but an entomologist studies insects, which are a subset or superset of bugs depending on your vantage point. There are many bugs which aren't insects and true bugs are studied by hemipterologists. --Rajah 01:33, 29 September 2007 (UTC)
- Kind of close but I don't recall the one I saw having that horn on the head. Oh, and a "bugologist" would be an entomologist. Dismas|(talk) 22:21, 28 September 2007 (UTC)
preproprotein
Can you please add a definition for preproprotein and examples? thanks208.146.45.110 17:46, 28 September 2007 (UTC)
- Wikipedia has a fairly long article on insulin, but does not do a very good job of explaining the preproinsulin molecule. Most peptide hormones and neuropeptides are derived from preproproteins that use a signal peptide to interact with signal recognition particles and gain entry into the endoplasmic reticulum. After the signal peptide is cleaved from the preproproteins, the prohormone must be further processed to produce a functioning signal molecule that can bind to its receptor. Proopiomelanocortin is derived from preproopiomelanocortin. The articles on oxytocin and cholecystokinin also indicate that they are produced from a larger preproproteins. --JWSchmidt 19:55, 28 September 2007 (UTC)
- Proprotein is also currently without an article. The Transhumanist 20:31, 28 September 2007 (UTC)
- I just added redirects for proprotein and preproprotein to protein precursor. I also described prepropeptides there. The pre- prefix should be elaborated upon at either signal peptide or protein targeting, in my opinion. --David Iberri (talk) 01:25, 29 September 2007 (UTC)
Hunger and grumpiness
Howdy, does anyone know why huger leads to being grumpy, irritable, or otherwise tetchy? I'm curious about both the biochemical cause (is there some hormone release?) or evolutionary (ultimate) cause would be greatly appreciated. Thanks, --TeaDrinker 20:31, 28 September 2007 (UTC)
- I’ll go out on a limb and hypothesize that it may be caused by very mild hypoglycemia (low blood sugar) due to not eating for an unusual stretch of time. Although the article points out that blood plasma glucose levels are usually maintained at about 70-140 mg/dL throughout the day in healthy humans, if a person breaks from their routine (skips a meal for instance) the unaccustomed fasting could cause the blood sugar level to dip. --S.dedalus 23:51, 28 September 2007 (UTC)
Geos
Hi. The article, Geo (landscape) states that the term is only used in Shetland and Orkney Islands but Im quite sure that the term can also be used to refer to similar features in England. For example clefts formed when chalk caves on the coast collapse in. An example being on Flamborough head, Yorkshire. Could someone help me out here? Tbo 157(talk) (review) 22:03, 28 September 2007 (UTC)
September 29
Venusian atmospheric pressure
The earth and venus have aprroximately the same size, but venus has about 90 times the atmospheric pressure of earth, why's that? —Preceding unsigned comment added by 88.203.105.128 (talk) 00:14, 29 September 2007 (UTC)
- There is 90 times as much gas in the atmosphere - which makes it push down 90 times harder. Venus' gravity is a little less than Earths - but that's made up for by the fact of much the atmosphere being almost entirely CO2 - and CO2 is heavier than Oxygen and Nitrogen. SteveBaker 00:40, 29 September 2007 (UTC)
Best place to start...Atmosphere of Venus. It has a history section, bits that suggest that it may have been more earth-like in the past. ny156uk 00:41, 29 September 2007 (UTC)
using electrical appliances in Israel
Please help me. What kind of plug or adaptor do O ineed on appliances to use in Israel? And does Israel use 240 or 120v?… Bonnie Ralph bonnie.ralph@btinternet.com —Preceding unsigned comment added by 86.142.73.24 (talk) 07:33, 29 September 2007 (UTC)
- Looks like you need a "European" adapter, as Israeli sockets take type H or type C plugs. See our article domestic AC power plugs and sockets. Hopefully someone else here will have hands-on experience and can tell you more.--Shantavira|feed me 07:52, 29 September 2007 (UTC)
Penny battery - another wild and sovereign funded idea
The penny is no longer made of solid copper but of zinc encased in a copper foil. Would the savings in material cost of recyclable copper and zinc oxide justify the energy that could be produced (for powering personal electronic devices, etc.) by using a redesigned coin with one side made of zinc at 90% total coin thickness and the other side made of copper at 10% total coin thickness so the end user could stack the coins between pieces of blotter paper saturated baking soda solution? Clem 10:27, 29 September 2007 (UTC)
- The biggest and best savings would be to stop making the damned things.
Your information is outdated, there is now less than 2.5% copper in a penny - the rest is zinc.The trouble with making batteries out of them is that it's a lot of hassle to mess around with blotting paper and baking soda - and the resulting battery wouldn't fit in anything that takes regular batteries. But worst of all - your pennies would have to be zinc on one side and copper on the other. If the mint believed that people would accept such a coin, they'd make them zinc on both sides - 100% zinc - and save more money. Since a penny costs a lot more than a penny to make - that's an important thing! But the best reform of all would be to simply stop making them. SteveBaker 16:00, 29 September 2007 (UTC)- (Actually - you might be right about the 10% copper thing - I was assuming you were talking about US 1c coins - you probably mean a UK 1p coin or something.) SteveBaker 16:04, 29 September 2007 (UTC)
- The OP is presumably not referring to the British one penny coin, which is of copper-plated steel. Algebraist 19:23, 29 September 2007 (UTC)
- Yes, I am referring to the US penny. At 1.5 volts per coin I could stack 9 (8 blotter inserts) and get 12 volts. ...Just thought of something. You can cause a piece of steel wool to ignite by using it to short a 9 volt battery. So if you fell out of a plane and landed safely near some salt water and had some pieces of cloth to use as a blotter substitute and had a pocket full of the new, lets call them energy pennies, and had a piece of steel wool then you could make a fire and stay warm until a spotter plane saw the smoke - all because the government invested in energy pennies. Cool. Clem 01:17, 30 September 2007 (UTC)
- And if you had some ham, you could have ham and eggs, if you had some eggs. --Sean 15:47, 30 September 2007 (UTC)
- Yes, I am referring to the US penny. At 1.5 volts per coin I could stack 9 (8 blotter inserts) and get 12 volts. ...Just thought of something. You can cause a piece of steel wool to ignite by using it to short a 9 volt battery. So if you fell out of a plane and landed safely near some salt water and had some pieces of cloth to use as a blotter substitute and had a pocket full of the new, lets call them energy pennies, and had a piece of steel wool then you could make a fire and stay warm until a spotter plane saw the smoke - all because the government invested in energy pennies. Cool. Clem 01:17, 30 September 2007 (UTC)
- (outdent):::::A pocket full U.S. one cent coins in the possession of a McGyver quality artificer on a desert island could be made into a battery of respectable voltage and of very modest current output. By using a flat rock to grind away the copper coating from the edges and one face, a stack of these separated by paper or thin pasteboard soaked in a conductive solution (brine, etc) could be assembled to produce the required voltage (say 12 volts to power a small radio transmitter whose battery is dead, lost, or unsalvageable). But the output current would be very small due to the small area of a cent. Early 19th century batteries used zinc and copper, but the pieces were typically many times the area of a U.S. cent, like several square inches. Several penny voltaic piles could be paralled to increase the current output. I built such a penny battery, after reading this question, using 100 grit sandpaper as a surrogate for a flat stone, to expose the zinc on 2 pennies, leaving the copper on the head side (obverse). With a piece of paper soaked in lemon juice between them, I saw an open circuit voltage of about 0.4 volts and a short circuit current of about 25 microamperes (this was an old crappy analog multimeter, so the numbers are approximate). I checked the current draw of a small instrument powered by 2 AAA batteries, and it was 2 milliamperes, so it would have taken about 8 of these units to get the voltage and about 80 in parallel to get the current to run even a low power instrument that doesn't light up, move, or transmit messages. Various alternatives for such Galvanic cells might just use aluminum (such as from a boat or plane) as one electrode, covered by cardboard or paper soaked in a conductive liquid, with a number of intact pennies covering the top of the cardboard, to increase the area, then a piece of aluminum on that followed by the cardboard, pennies, etc to get both the current and voltage needed. Sand could be used to remove any paint, etc., from the aluminum. Edison 20:11, 30 September 2007 (UTC)
Has somebody access to this article on Earthdoc?
Hi, I hope this is the right place to ask (please forgive me if it isn't): Can someone with access to Earthdoc (meaning someone who is member of the European Association of Geoscientists & Engineers) please send me an article (PDF 2,13MB)? The text Geophysical Investigation of the Nasca Lines by A. Weller, K. Hartsch, S. Rosas, G. Reppchen (2006) seems to published nowhere else. Concerning its content, the website says: "The content on this site is provided without charge as a service to members, to be used for information and educational purposes only." So, I would welcome a EAGE member willing to inform and educate me in this respect :-) Yours, --Jonas kork 12:28, 29 September 2007 (UTC)
Buffers
What shifts in the following reactions may occur in the event of addition of a) H+ ions and b) OH- ions.
NaHCO3 ↔ Na+ (+) HCO3-
H2O ↔ H+ (+) OH-
H+ (+) HCO3- ↔ H2CO3 —Preceding unsigned comment added by 89.242.33.223 (talk) 12:44, 29 September 2007 (UTC)
- Maybe look at Le Chatelier's principle. Someguy1221 17:27, 29 September 2007 (UTC)
Human eye frames per second
Just as video cameras have a certain number of frames per second that they can record, does our eye have a certain number of frames per second that we can process? I'm assuming this must be true because if we see something going very fast we see it as a blur. If it is true, than about how many do we see per second? Would this number change throughout our life? One of the reasons I find this so interesting is because if our FPS is lower, we can comprehend less things per second, and therefore our perception of time might seem to go faster, and vice versa. Imaninjapiratetalk to me 14:47, 29 September 2007 (UTC)
- This question seems to get asked every few months. See for example Wikipedia:Reference desk/Archives/Science/2007 January 16 and persistence of vision.--Shantavira|feed me 14:56, 29 September 2007 (UTC)
- Certainly check the archives - we've discussed this to death in the past. The short answer is "No, the human eye doesn't have a 'frame rate'". It just doesn't work like that. Using our peripheral vision (which is most sensitive to time-varying information) we can see things flickering at maybe between 30 to 100 Hz - it depends a lot on the individual and on the ambient lighting conditions. I know that in an otherwise utterly dark room I can reliably see flicker at 72Hz and I can reliably NOT see it at 76Hz - but other people have different limits a few don't see flicker at 30Hz and I met an unfortunate chap who can't watch TV or operate a normal computer because he can see flicker directly in front of him at over 100Hz! That indicates some limits on minimum 'response time' - but that's not at all the same thing as 'frame rate'. SteveBaker 15:37, 29 September 2007 (UTC)
- Does anyone else see flicker after the source is removed? It's sort of like a dynamic version of staring at a yellow and green picture and then switching to a white background and seeing blue and red. If I turn off a tube TV in a dark room, I will see a flickering image that is square and TV sized that persists in my main field of vision for quite a while longer. It's not a definable image, just a flickering rectangle. Chemical persistence doesn't seem to explain this. --DHeyward 08:37, 1 October 2007 (UTC)
- I can only see flicker while it's present, but I haven't actively tested my "persistence of flicker". BTW, I can see flicker of 70Hz, but not 75Hz in peripheral. —Preceding unsigned comment added by 203.22.236.14 (talk) 09:25, 1 October 2007 (UTC)
- Does anyone else see flicker after the source is removed? It's sort of like a dynamic version of staring at a yellow and green picture and then switching to a white background and seeing blue and red. If I turn off a tube TV in a dark room, I will see a flickering image that is square and TV sized that persists in my main field of vision for quite a while longer. It's not a definable image, just a flickering rectangle. Chemical persistence doesn't seem to explain this. --DHeyward 08:37, 1 October 2007 (UTC)
- I think it's very possible that you still "see" flicker after it's gone away. Our brains are good at cancelling out unchanging distractions. So you stop 'hearing' the 'shhhhhh' noise of the fan of your computer and only notice that it was ever there when you turn it off. For a moment, the silence seems somehow more silent than a mere absence of sound - it's almost like there was 'anti-sound' (bear with me here!). There are also those 'illusions' where you stare for 30 seconds directly at a US or UK flag that's painted in cyan, black and orange instead of red, white and blue - then you look at a blank page and see the flag mysteriously floating there in it's normal red/white/blue colours...those work the same way. Your eyes get bored with looking at orange and cyan and 'cancel them out' - so when you look at a white sheet - you see the complements of those colours ("anti-colours") for a few seconds until your eyes adapt to the change. I think it's possible you could be doing the same thing with the flicker - except that when the flicker goes away you are seeing 'anti-flicker'. Dunno - but it seems plausible. SteveBaker 14:33, 1 October 2007 (UTC)
Renaming proteins after discovery of function(s)
Does anyone else feel that scientific things and processes should be named according to what they do? I note that people refer to such names as unimaginative but as a student, I find them so much easier to remember. I hold the highest respect for scientists who name their discoveries after their function etc, instead of slapping their name or something on it. I know that a protein's function isn't always known at the time of discovery but is it unfeasible to rename proteins as their functions are established? Sonic hedgehog is probably characterised well enough to rename it, right? Am I the only one that gets fustrated? I envision some organisation like IUPAC, renaming proteins for the common good... IUPAC did it with chemicals, and we all survived. This isn't just a rant; ideally someone would say "yes, we all agree and <<acronym>> is already making progress in doing exactly as you describe". It's probably just me... —Preceding unsigned comment added by 201.220.222.140 (talk) 20:22, 29 September 2007 (UTC)
- I suspect that at some point most proteins will exist within a standardization system such as the one for enzymes. There are some relatively young efforts to categorize all proteins (see List of types of proteins, Gene Ontology) that should eventually lead to ways to develop standardized names....hopefully names that will reflect function. In the case of enzymes, it is often possible to identify a single active site that defines the function of the enzyme. However, in the case of many other proteins there are multiple binding sites on the protein and it is often not a trivial matter to define "the" function of the protein. For some types of non-enzymatic proteins, groups of scientists sometimes get together and propose a standard nomenclature, but so far it is not unusual for the proposed standard nomenclature to be ignored by other scientists. It might be educators who have to step in and push for sensible names rather than wait for working scientists to do it. (external links: a wiki thesaurus for gene and protein names, Gene and protein nomenclature in public databases). --JWSchmidt 17:26, 29 September 2007 (UTC)
- As a student, you are frustrated by this, but as a reasearcher, you will soon find the opposite source of frustration: After some lengthy discussion, some phenomenon has been finally named somehow but for some reasons you want to study the discussion that ed to this naming and locate the research papers involved in it. What do you use as search term if you look for old articles from a time when a name was not yet established and everybody just wrote "that strange phenomenon that occurs in X, Y and Z, and hes been described by, among others A, C, D, and F? Then, you'll be happy if the name that the first researcher proposed simply stuck. But you might be pleased to hear that recently on a conference of geneticists, a resolution was passed urging scientists to stop using too flamboyant gene names, because --so they argues-- that may be funny with fruit flies but becomes embarassing with the equivalent human genes. No doctor would want to have to say: "I am so sorry, but your baby seems to suffer from a sonic hedgehog mutation." Simon A. 18:06, 29 September 2007 (UTC)
- The problem with this suggestion is that proteins do not have a single function. For example, what should one call Adrenocorticotropic hormone? It can act through a number of different receptors to influence many different biological processes. As it happens, it was named after the process that was first discovered. In addition, protein families to provide information about their phylogenetic relationship. Consider the 800+ olfactory receptors in mice. Each one will bind a number of different odorants with higher or lower affinity. Currently a numerical nomenclature is used based on chromosome cluster (and hence phylogeny) and order. Even if we knew all the odorants that even one receptor can bind (and we don't) exactly what would we call it? This is one example, but the principle hold true for pretty much all proteins. At very best all we could do is name than after one of the functions we happen to know they have. Rockpocket 04:32, 30 September 2007 (UTC)
Nitrogenous polysaccharide
What elements, other than nitrogen, make up the nitrogenous polysaccharide capsule of prokaryotik cells? Thanks alot 88.110.203.63 16:31, 29 September 2007 (UTC)
- see polysaccharide -- Flyguy649 talk contribs 16:36, 29 September 2007 (UTC)
- Thanks alot, thats just great, i got mixed up with amino acids, and started thinking there may be an 'r' (variable) group, wich can contain sulphur as well, but im just dumb, your not :) thanks again, 88.110.203.63 16:48, 29 September 2007 (UTC)
(me again, wow i just read your user page flyguy, and read you are studying amino acids, and i dont think its geeky at all) —Preceding unsigned comment added by 88.110.203.63 (talk) 16:50, 29 September 2007 (UTC)
Pupil size
Is pupil size a genetic trait? I know some people whose pupils are like pinheads in sunlight and other people whose pupils are like planets at night time. Are there any advantages/disadvantages to having smaller/larger than average pupils? --Candy-Panda 16:49, 29 September 2007 (UTC)
- First of all, the pupil size of a single healthy individual has a wide range (about a factor of 4 in diameter, or 4 stops in photography jargon). Therefore it doesn't make sense to talk about "pupil size" itself as a genetic trait, but it does make sense to ask about the heritability of minimum or maximum pupil size. This study found that maximum pupil size after mydriasis has a heritability of up to 80%, so it has a strong genetic component. Here's an important quote:
- This study only examined final pupil size, and so it cannot be certain whether the strong genetic influence is on actual maximum pupil size or on the response to mydriatics. Bertler and Smith's previous twin study found a high MZ [monozygotic] correlation for initial pupil size and no difference in the rate of dilatation between MZ and DZ [dizygotic] twins, suggesting that the genes determine mydriasis not in response to drug or dose, but determine the maximal possible size of the pupil.
- I couldn't find an analogous study for the minimum size of the pupil.
- The advantages of small and large pupils are exactly the same as those of large and small f-numbers in a camera, with the additional consideration that if you're in bright sunlight too often and your pupils aren't small enough, the ultraviolet does long-term damage to your eyes. —Keenan Pepper 18:06, 29 September 2007 (UTC)
STOP YOUR BODY'S MELANIN PRODUCTION
is there something that can stop your body from producing melanin in hair —Preceding unsigned comment added by 81.99.212.22 (talk) 18:34, 29 September 2007 (UTC)
- The short answer is no. -- Flyguy649 talk contribs 20:26, 29 September 2007 (UTC)
- With all respect, no matter how many times you ask the same question, albeit phrased slightly differently, you are going to get the same answer. There is no safe way one can change one's hair colour permanently. Rockpocket 04:17, 30 September 2007 (UTC)
- Come now. Surely it's well known that aging or stress can stop melanin production in hair. —Tamfang 08:08, 30 September 2007 (UTC)
- Aging and stress are not safe, though. And I think it would be nice if someone produced a post regarding the reason why the original poster would like to know that, which seems to be to become a white person. It is not healthy for one to wish to become a white person, since this is impossible. I believe that, if the original poster wants to become a white person, they ought to look for a psychologist. A.Z. 08:16, 30 September 2007 (UTC)
- I don't see anything about safety in the OP, do you? If I asked "is there something that can cause skin to become red and itchy" would you say "No" because poison oak is unhealthy? Tsk. —Tamfang 16:09, 30 September 2007 (UTC)
- The poster didn't make it explicit that they want something safe. No, I wouldn't say no to that question about the red and itchy skin. A.Z. 19:25, 30 September 2007 (UTC)
- ?Stress, Tamfang are you sure about that. I know it's a popular myth that stress turns your hair grey or white but is there any scientific evidence? I have serious doubts that stress can affect your hair colour although this idea has been perpetuated in many films. As the colour is dictated by the growth of the hair in the follicle it is likely to take many weeks to change the colour of a sizeable strand. I will accept that nutritional stress may cause a change in hair colouration , as seen in pellagra or beri-beri.Richard Avery 13:17, 30 September 2007 (UTC)
- Yes, we all know that any change in your metabolism won't be visible in your hair until it has time to grow out, nobody here implied otherwise. —Tamfang 16:09, 30 September 2007 (UTC)
- Ok, for the sake of hypotheticals, it may be possible using genetic tricks. This is not possible currently in humans. Disrupting the melanin biosynthetic pathway throughout the body would likely not be good for you, since many other molecules your body needs, such as some neurotransmitters, are produced by the same (or related) pathways. Also, melanin is really important in the eye to prevent excess light reaching the retina. Albinos have poor vision in part because of a lack of melanin. And remember that melanin is important for the protection from UV damage in the skin. Back to the question. The trick is to block the creation of melanin only in hair; this could be done using a virus that would deliver DNA coding for small interfering RNA sequences that would disrupt the production of melanin synthesis enzyme or enzymes. If such DNA was under the control of a hair-specific promoter, it would only disrupt melanin production in hair follicle cells. I don't think it's likely that you'd be able to "hit" all the cells in all the hair follicles, so you'd still likely end up with some coloured hair. Also, even if all hair melanin production were stopped immediately, that would only affect new hair; existing hair would still be coloured until it grew out. You'd need to bleach it to remove the colour in older hair. -- Flyguy649 talk contribs 15:44, 30 September 2007 (UTC)
- There seems to be an ever-increasing amount of money being spent by cosmetics companies in an attempt to find ways to alter melanin production and storage. Such efforts are of interest to the US government because there are important health implications of research aimed at providing tools that could be used to change to melanin production and storage. Here is a recent article that mentions some existing melanin research directions: Regulation of Skin Pigmentation via Modification of Tyrosinase Function. --JWSchmidt 17:21, 30 September 2007 (UTC)
- Funny, I want to darken my skin. I live in Australia and of Irish ancestory, so every summer it takes a couple of months to tan sufficiently to avoid quick sunburn. I envy a darker skinned Sri Lanken person I work with who has never suffered sunburn. Wouldn't it be nice to be able to adjust one's skin colour to the environment in which they live. --203.22.236.14 09:19, 1 October 2007 (UTC)
Swappin' spit reagents
Frequently in journal articles, I read about experiments being performed with reagents sent as gifts from other researchers. I was wondering what limitations there are in this practise? If I buy mice, I'm allowed to breed them for my own use but not allowed to send them to other researchers. What about expression vector plasmids for example? Are some unpatented? Perhaps the research community would benefit from some sort of network/website through which scientists could swap reagents more easily. I suppose some big companies might oppose this... --Seans Potato Business 18:53, 29 September 2007 (UTC)
- As a condition of publishing in peer reviewed journals, one usually has to agree to make one's reagents available for other scientists. There are limits to this, obviously, when the materials are very expensive or rare or commercially available for purchase. That said, some scientists will resolutely ignore any requests for materials (not that I'm bitter or anything...) but most will make an effort to provide materials at no cost when asked. Since many novel materials may have commercial, value most institutes, universities and companies will make your sign a material transfer agreement, which can limit your use of the gift. The most common limit is that you will not pass the material on to anyone else and that your relinquish the rights to any commercial application you may stumble upon. MTAs have to go through the legal and/or technology transfer department of your institution, so an online swap-meet would probably not work.
- When you purchase plasmids from companies, you usually agree to a limited license of use, which restricts you from copying the plasmid and giving it to others for free. Like in the computer software industry, this is the way producers ensure they recoup profit from their work. Rockpocket 04:08, 30 September 2007 (UTC)
- "...And the clueless newb was enlightened" :) Do you say that I can't take my plasmid and copy it so that I never have to buy more of it or just that I can't copy and give to someone else? --Seans Potato Business 17:55, 30 September 2007 (UTC)
- You can amplify plasmids as much as you want, they would not be of much use otherwise. This right could probably be restricted for commercial applications, but I doubt that this happens a lot. As long as you don't make money out of selling the plasmid directly you are by all means on the safe side. Cacycle 19:10, 30 September 2007 (UTC)
- Andrew Ryan would disagree. -Wooty [Woot?] [Spam! Spam! Wonderful spam!] 08:16, 1 October 2007 (UTC)
- You can amplify plasmids as much as you want, they would not be of much use otherwise. This right could probably be restricted for commercial applications, but I doubt that this happens a lot. As long as you don't make money out of selling the plasmid directly you are by all means on the safe side. Cacycle 19:10, 30 September 2007 (UTC)
- "...And the clueless newb was enlightened" :) Do you say that I can't take my plasmid and copy it so that I never have to buy more of it or just that I can't copy and give to someone else? --Seans Potato Business 17:55, 30 September 2007 (UTC)
How to choose best undergraduate school for best mcat scores
Hello, I am going to attend undergraduate university next year. I searched for statistics of students who take the MCAT from certain undergraduate schools to compare undergraduate programs, but could not find any results. Is there a site/listing of the best programs that prepare students to take the MCAT exam?
Thanks, Robert —Preceding unsigned comment added by 71.56.231.40 (talk) 20:27, 29 September 2007 (UTC)
- Honestly, I'm a little dubious about the idea of picking your undergrad location based on average standardized testing scores of graduates. I think as a metric that is likely to be highly unreliable, and will have little guarantee of giving you, the individual, high MCAT scores. --24.147.86.187 21:35, 29 September 2007 (UTC)
- If your goal is to do well on the MCAT, start experiencing what you need to know now. Go to a hospital and volunteer (especially in the emergency room). Classes and studying are necessary, but they don't replace experience. -- kainaw™ 00:19, 30 September 2007 (UTC)
- Pick a university where you think you will be happy. If you are happy, it will be easier to put you best efforts into your studies. ike9898 17:29, 30 September 2007 (UTC)
Resistor capacitance
Any estimates for the capacitance of a metal film resistor with a body length of 6mm? —Preceding unsigned comment added by 88.110.150.148 (talk) 21:32, 29 September 2007 (UTC)
- Is there anything written on it? --Mdwyer 23:05, 29 September 2007 (UTC)
- For a specific inidivual resistor, you should measure the capacitance. If you are designing for production, you should use the manufacturer's spec sheet. Resistors are typically manufactured for a specific tolerance in resistance, so the tolerance in capacitance will typically be much larger. Your circuit design should therefore accommodate the entire range of possible capacitance values from the manufacturer's spec sheet. -Arch dude 23:44, 29 September 2007 (UTC)
130.15.208.183 08:32, 30 September 2007 (UTC)
Contact Lenses and Frothy Solution
I have begun to notice that whenever I apply solution to my contact lenses, (for example after removing them from the case, just before I would put them in my eyes) the solution frothes up and many small bubbles form. Does this indicate some sort of reaction taking place, or that the lenses are very dirty?
The good sign is I haven't gone blind, even if this does indicate something, since I still put them in. But, I was just wondering, since it only started happening recently...
Thanks, Eclipse45 22:01, 29 September 2007 (UTC)
They, I mean we, don't do medical advice here. The reason is obvious when you think about it. Sorry. --Milkbreath 22:50, 29 September 2007 (UTC)
- Most contact lens solutions have a consumer help telephone number on the label. You should immediately call this number for an answer to your question. If you are not completely satisfied by the information you get over the phone, you should immediately contact your eye-care professional. Do this now. Do not delay. -Arch dude 23:33, 29 September 2007 (UTC)
- (In response to the comment of Milkbreath) The way I read it, the question is about chemistry, not health. --71.175.68.224 02:31, 30 September 2007 (UTC)
- It's borderline; that's why it's still here. She mentions going blind from a chemical reaction on her contact lenses, however facetiously. That's medical in addition to chemical. Suppose someone here tells her not to worry about it, and she actually does go blind from it. Don't say it can't happen; you're not an eye doctor, and neither am I. I'm tempted to remove it even now, but her intent clearly was not to ask a medical question, and, to judge by her user page, she's at least relatively sane. --Milkbreath 03:19, 30 September 2007 (UTC)
- Contact lens mentions cleaning and disinfecting fluids. Then look up Hydrogen peroxide.Polypipe Wrangler 02:52, 30 September 2007 (UTC)
Ok, first of all, DID I say I was a she? Where did you get that from? And second of all, you can notice I said the good thing was I DIDN'T GO BLIND, AS IN A JOKE, that nothing did happen to me even when I still put the contacts on afterwards, after noticing the froth.
And yes, for the record I asked about the chemistry involved. Maybe the 2nd part of the last sentence was unclear, but, the rest I think is fine. Learn to read. And yes, I know it says not to ask medical advice.
Thanks for the whole fiasco. If I was blind, could I see the screen to type that question? Think about it. 130.15.208.183 08:32, 30 September 2007 (UTC)
- We are supposed to assume good faith in Wikipedia. I did. And all caps is considered shouting. Don't shout. I haven't. Flinging insults invites a flame war. We don't do that here (though I, personally, think they're tons of fun). We have to be nice, dammit. So. I had a fifty-fifty chance with the sex. Why should there be a problem with a gender-neutral "she", anyway? As for the going blind, you haven't gone blind, yet. But that isn't the issue. I don't know how to put it any clearer than I have. There was a medical aspect to your question. You expressed concern for your safety. If the froth had appeared on your car instead of on something you put in your eyes, that would be different. Blindness is no joking matter, by the way. We couldn't tell whether you were joking or whether you had any sense at all because we don't know you, but we're learning fast. --Milkbreath 14:28, 30 September 2007 (UTC)
- The chemical reaction is the decomposition of hydrogen peroxide into water and oxygen:
- 2 H2O2 -> 2 H2O + O2
- I believe this reaction occurs quite slowly spontaneously, but quite quickly in the presence of the catalyst catalase, contained in protein deposits on your contact lenses.
- Incidentally, blind people can use computers, via screen readers and other technologies. StuRat 11:46, 30 September 2007 (UTC)
- Indeed, Jack Vance (for example) has cranked out a handful of novels since he lost his sight. —Tamfang 16:14, 30 September 2007 (UTC)
Ok, so then, as you put it milkbreath, there does seem to be a medical aspect to my question (i swear i never thought of it like that when I typed it), but just the same, i wanted to know about the chemical aspect, which others have answered. as for insults, no I didn't want to cause anything, nor am i particularly nuts. I just got a bit angry to see that instead of answers I got a bunch of 'don't seek medical advice' - which in retrospect follows with wikipedia policy if you saw my question in a medical light. in any case, i'm sorry for anything that did happen: heat of the moment thing? but i did get an answer so i'm still content. 130.15.208.183 17:49, 30 September 2007 (UTC)
- Sorry for the slow response; I missed your reply. No hard feelings. I know only too well how easy it is to get, or make someone, exasperated in this medium. My rule is "Shit is a dish that is best served cold", if you'll pardon my French. That applies literally, too, come to think of it. --Milkbreath 15:40, 1 October 2007 (UTC)
September 30
Hydrocloride vs Dihydrochloride
Whats the different between dihydrochloride and hydrochloride? I was looking at betahistine and found both versions, the HCL was used in a study and I found the dihydrochloride version online. Are they much different? —Preceding unsigned comment added by 76.167.139.80 (talk) 02:22, 30 September 2007 (UTC)
- No - it dissociates anyway. Icek 03:08, 30 September 2007 (UTC)
See betahistine The hydrochloride has formula C8H12N2 HCl, the dihydrochloride has formula C8H12N2 2HCl A dihydrochloride can exist because it has two basic sites - the amine and the nitrogen in the benzene ring pyridine - they are different salts of betahistine87.102.11.118 12:22, 30 September 2007 (UTC)
solar wind hydro and geothermal energies
how can i get the questions where does the resource come from how its obtained for usage what are its uses what are some of the advantages of it and what are some of the disadvantages of it —Preceding unsigned comment added by 67.60.91.27 (talk) 03:15, 30 September 2007 (UTC)
- Solar power, hydroelectric power, geothermal power. When you're looking for general information, you can just type the term of interest into the search bar on the left of this window. Please come back if you have questions not answered in the articles. Someguy1221 03:30, 30 September 2007 (UTC)
Diagnostic and Statistical Manual
I have read the artical on the DSM but am not clear: is the DSM considered "evidence-based" and, if so, how is the research to establish this conducted? —Preceding unsigned comment added by 209.161.212.229 (talk) 04:42, 30 September 2007 (UTC)
- It depends on what you mean by "evidence-based". A DSM practitioner would probably say that the descriptive approach which leads to a DSM classification is "evidence-based" in that it is based on clinical or analytical experience and is often culled from a larger body of work in psychology. However a skeptic of the DSM, of which there are many, would likely argue that it was not "evidence-based" as the categorizations are not established by rigorous empirical standards, and the differentiations between categories are meant to be simply recognized by an experienced professional rather than being testable in a standard empirical sense. That is my understanding of it, anyway, but I am not a huge expert in such things. --24.147.86.187 10:50, 30 September 2007 (UTC)
Life!...
When will MAN 'give up' religion, and start relying on FACT!? Just been watching too much news. Dave 64.230.233.222 05:23, 30 September 2007 (UTC)
- Because humans need something to believe in. If "MAN" gives up religion then he would need to believe in something else.
- It's easy to believe in religion because it's requirements are so low. Compare that to quantum physics where you literally need to spend years studying before you can even do the basic problems. 220.239.107.201 06:26, 30 September 2007 (UTC)
- Perfect answer, I think. Reminds me of when I asked a certain person, "What would happen if it were PROVED/PROVEN that God didn`t/doesn`t exist?" This person mentioned , and I`m paraphrasing, something that Voltaire once said, "If God died, 'man' would just invent another God."!!! Dave 64.230.233.222 07:35, 30 September 2007 (UTC)
- Believing and relying on facts isn't exclusive. – b_jonas 13:24, 30 September 2007 (UTC)
- To attempt a proper answer to this question: The fact is that religion appears to have sprung up independently in many (if not all) civilisations suggests that at some time in the past it served an important function. In an era before modern science, perhaps it produced answers to difficult questions and allowed important men to make themselves more important by claiming to be speakers for and interpreters of the greatest possible power in the universe. Nowadays we're able to use the tools of mathematics, astronomy, geology, paleontology, cosmology, physics, chemistry and biology that humanity has worked so hard over centuries to perfect - and over just the last 50 or so years have we produced more straight, clear-cut answers to the difficult questions of life than any of the previous millenia. We don't have to guess anymore - we finally know. The first black hole was found just three years ago - over the last decade, the results of quantum theory are so well understood that we use them to make mundane things like MP3 players. We've only been able to do detailed DNA analysis for a dozen years - but now we have the human genome laid out in detail before us. Those of us who are alive today are the first in all of human history to be able to deal with these questions of "how did we get here" without the need for ancient pseudo-science and myth - we don't have to guess how the universe was formed - we have proof. We don't have to wonder which animals descended from which - we can see it clearly in the DNA. I think it'll take a few more generations for the consequences to fully play out - but I honestly believe that we have no further need for religion - and the terrible wars and apalling deeds that are done in religion's name are things we are definitely better off without. SteveBaker 19:52, 30 September 2007 (UTC)
- Another thing that IMHO religion often provided was a resonably simplistic sense of right and wrong and rules to live by guided by other people who help you understand the 'rules'. It also provided a sense of justice and a reason to live by these rules. Note that even nowadays, some religious people opposed to atheism say that without religion/God, people don't have to be 'good'. It is perhaps easier for people to be willing to do good when they believe those that don't will ultimately be punished. Nil Einne 03:29, 1 October 2007 (UTC)
- Given the Crusades and recent Islamic extremist terrorism, I reckon that (organised) religion is also good at excusing or encouraging rather immoral behaviour. --203.22.236.14 04:45, 1 October 2007 (UTC)
- Organised religion has a lot to answer for in terms of the troubles of the world - but small-scale religion is no better (Branch Davidians, Jonestown, Heaven's Gate, etc). SteveBaker 19:44, 1 October 2007 (UTC)
- Given the Crusades and recent Islamic extremist terrorism, I reckon that (organised) religion is also good at excusing or encouraging rather immoral behaviour. --203.22.236.14 04:45, 1 October 2007 (UTC)
SUCH ANSWERS SHOWS ME THAT YOU GUYS DONT HAVE A LIFE WELL IF GOD DIDNOT EXSIST WHO CREATED THE WORLD? WHO CREATED THE UNIVERSE? WHO CREATED HUMANS,ANIMALS,AND LIVING THINGS? CAN YOU ANSWER THESE QUESTIONS? I AM COMPLETLY POSTIVE THAT GOD EXSIST AND HE IS THE ONLY ONE WHO GOT ANSWERS TO THESE QUESTIONS AND SOON WE WILL SEE —Preceding unsigned comment added by 212.71.37.107 (talk) 14:50, 30 September 2007 (UTC)
- To 64.230.233.222: you might be interested in the book Consilience: The Unity of Knowledge by E. O. Wilson. Wilson mentions the idea that given the realities of human nature, it might be useful to create new religions that incorporate scientific facts.
To 212.71.37.107: Since this is the science desk I'll provide a reply that is found in many scientific publications. If there is a God of the type you mention, then where did that God come from? Most scientists are not satisfied by taking a mystery, putting a name on it and pretending that you are done. --JWSchmidt 15:12, 30 September 2007 (UTC)
- I`m gonna read that book JWS. Thank you. I have no reply for 212. Dave 64.230.233.222 15:30, 30 September 2007 (UTC)
- 212.71.37.107, if you reject the hypothesis that the answer to WHO could be "nobody, no more than someone makes water run off a boulder," there's not much we can say to you. But I'm curious as to what makes you think THAT YOU GUYS DONT HAVE A LIFE. If you think only losers ponder such questions, rather than accepting what someone told them in childhood and never seriously considering them again, I reckon that tells us something about you and I wonder why you're even looking at the Science Desk. (But please note that I'm only making a guess here about what you think, based on the limited evidence available; the Voices haven't spoken up yet on this subject.) —Tamfang 16:22, 30 September 2007 (UTC)
Smart Question JWSchmidt .... but that is why we call him god we humans are not supposed to prove god presence or not god created us to worship him and if you look deep you will find the one true religion in the world that will help us find our way through life why was there prophets remember something god is going to judje us cause god created us look deep and you will find the truth as we know from physics ....everything is there for a reason and i beleive god created these reasons
- I have an excellent life without any sign of belief in any god or gods - it's very possible to have a full and satisfying life without religion. No gods were required to make the universe - the Big Bang Theory has the creation of the universe explained down to a fairly exacting degree. Therefore there is no "WHO" in "who created the universe"...and even if there was, that would only push the question back to "How did your god/gods get created?" - adding another step doesn't in any way help you find the answer. Humans, animals and living things come about through evolution from a single random coincidence that caused the first self-replicating molecule to appear (probably in the primordial earth - but perhaps elsewhere if panspermia turns out to be true). Can I answer these questions? Yes, easily - anyone who goes around with their eyes wide open can find these answers. Since you have absolutely no evidence or proof of any kind that your idea of god exists - your belief is merely an act of faith (as I recall your religion teaches you). Blind faith is a risky business - without evidence, how do you know that you are worshipping the correct god. I'm pretty sure the devil worshippers will also tell their supporters to have faith. Anyway, if god is the only one who can answer these questions - and I can certainly answer these questions then I am your god and "SOON YOU WILL SEE" - because I just told you. The only question I find tough to answer is WHY THE HECK YOU CAN"T FIND YOUR CAPS LOCK KEY. SteveBaker 16:44, 30 September 2007 (UTC)
To begin with , we are just discussing so it would be nice to respect each other. i want to ask you a question did u read about old times , and the real prophets that once was present and if you read about them , i suppose you heard about their miracles . what makes you think that god is made or created , then how on earth is he called god. let me go back to miracles of the prophets that was once there and the fact that alot of people beleived in them because they found the truth. another thing how do you explain that everything that consider life science and everything was mentioned either directly or indirect in the holy books of god if you think that someone wrote them then how do expect him to find out about all this so early ,,(was he a genius!!!)
- Actually, if you believe in god (or gods) I honestly don't respect you. It's hard to respect someone who bases his or her entire life on something that's exactly as believable to me as Santa Claus or the Tooth Fairy...respect has to be earned and claiming that everything about science is in this one skinny 2,000 year old book does nothing to help you here. Did I read about the old times? Sure - I've read the bible from cover to cover - I have a passing knowledge of world history. The miracles the bible describes would indeed be fairly impressive if they were true - but there is really no evidence that they ever happened. Someone wrote it down in a book - and the book has been repeatedly retranslated from a source language that's confusing as all hell because the written form contains no vowels. How do we know it's true? It could just as easily be a work of fiction. Since it contradicts itself in many, MANY places - it certainly can't all be true.
- You say that all of the answers are there. OK - let's try a nice simple test. Let's look at the creation of life itself. What does the bible say in answer to the question "Which came first - animals or people?" - do me a favor. Look it up in your bible and post the answer here - tell me simply and clearly: "Animals came before people" or "People came before animals". It's right there in Genesis - I'd like you to tell everyone here what it says - post your answer here. I want to know what your holy book - on whose teachings you base your entire life - tells you about this very basic question.
- SteveBaker 21:00, 30 September 2007 (UTC)
- They way "I" understand God and the various religions is that they were invented to give people guidance. Perhaps even to encourage people to behave. Offer them an incredible reward, Heaven, if they behaved and of course, the most hedious of punishments if they didn`t. Almost sounds sane, until you really think about it though. I very much tend to agree along the same lines as SteveBaker, only problem is, how does one change all that brainwashing that has been around for so long? One cannot stop believers from believing. And to force them to not believe, if possible, would surely be unethical/immoral, to say the least. I too, have a very difficult time showing respect towards a person who follows the BOOK err, religiously. I think I`d better stop else this could turn ugly. Thanks for all your thoughts. Dave 64.230.233.222 21:48, 30 September 2007 (UTC)
- Reluctantly, I'm increasingly agreeing with Richard Dawkins - atheists have to take off the kid gloves and stop treating religion and the religious with respect. This is not a popular point of view in the middle of Bible-belt America - but that's where I happen to live. The people who believe in this pile of nonsense don't deserve my respect. I now feel very confident about calling them idiots - mocking their beliefs - disbelieving their sincerity because not a one of them is consistent about their beliefs. I intend to continue pointing out the ridiculousness of the crap they are espousing. It's easy to focus on this way of thinking - try my recipe: Whenever they say "God", think "Tooth Fairy" and whenever they say "Heaven" think "Santa's grotto". After all, if you are an atheist, those terms are equally believable - and switching them around enables us to rid ourselves of thousands of years of brainwashing. It's very hard not to giggle when a priest in his silly costume starts telling us (in all seriousness) that if we believe in the Tooth Fairy, we'll end up spending eternity in Santa's grotto...but that's exactly as believable to me as what he's actually saying. SteveBaker 03:12, 1 October 2007 (UTC)
- They way "I" understand God and the various religions is that they were invented to give people guidance. Perhaps even to encourage people to behave. Offer them an incredible reward, Heaven, if they behaved and of course, the most hedious of punishments if they didn`t. Almost sounds sane, until you really think about it though. I very much tend to agree along the same lines as SteveBaker, only problem is, how does one change all that brainwashing that has been around for so long? One cannot stop believers from believing. And to force them to not believe, if possible, would surely be unethical/immoral, to say the least. I too, have a very difficult time showing respect towards a person who follows the BOOK err, religiously. I think I`d better stop else this could turn ugly. Thanks for all your thoughts. Dave 64.230.233.222 21:48, 30 September 2007 (UTC)
- SteveBaker 21:00, 30 September 2007 (UTC)
- Very few, if any, people entirely rely on 'fact' alone; almost everyone has things that they think or do which are not supported by facts. If you look at populations where few people practise religion, you do not tend to see the majority of the population building their lives on facts; rather, these people practise feng shui, read their horoscopes, mutter phrases to magpies, repeat 'please please please' under their breath after bowling and before the ball hits the pins. They buy lottery tickets, they believe that they have paranormal abilities, or that someone else who wants their money does, they see ghosts, they see UFOs, they press the button at the pelican crossing again when the lights take a long time to change, they think that their loved ones are looking down on them, or have gone on to a better place, or are waiting for them, or have come back to guard them. For whatever reason, most people do not really want to live lives based on fact alone; they get something out of religion and supernatural beliefs that they do not get elsewhere. If you do not, that's fine, but please do not assume that people believe these things just because they are ignorant or stupid, or that they would be happier without them. Oh, and if it's just brainwashing, why so many converts to so many religions and beliefs? 79.65.119.193 22:53, 30 September 2007 (UTC)
- Oh, and if it's just brainwashing, why so many converts to so many religions and beliefs? People do "irrational" things because it brings meaning to their lives. Before you jump on that let me tell you that:
- People do hobbyX not because hobbyX is rational but because hobbyX bring meaning to their lives. hobbyX can be {swiming, jogging, horseridding, mountain climbinb, sking, dancing}. Now no one suggest that hobbyX is rational or that hobbyX is TRUE or that if anyone insults hobbyX that they should have their head chopped off. No one gets angry if you suggest that hobbyX is a social construct or if you choose to join hobbyX and hobbyY at the same time.
- Compare that with religionX. People do religionX not because religionX is rational but because religionX bring meaning to their lives. People gets very angry if you suggest that religionX is NOT TRUE or if you suggest that religionX is a social construct. And people get angry if you choose to join religionX and religionY at the same time.
- Religions are just like hobbies, except that it is a philosophical hobby that insists on it's own validity and rejects any other philosophies that suggests that itself is FALSE.202.168.50.40 23:26, 30 September 2007 (UTC)
- I understand why many people 'need' religion. They would feel that life was unimportant, for some reason, without it. I just feel that any sort of unsubstantiated belief system is a little silly, that`s all. Humans are so knowledgeable now. Way back when, when just about everything was mysterious, even the wind was a 'god', then I can see how people needed religion. It helped 'explain' such mysteries. The fact that almost everything is fairly easily explainable now makes me wonder how ANYONE can still 'believe'. Dang, I`ve said too much already. I really didn`t want to start anything here. Sorry if I offended anyone. Dave 64.230.233.222 23:52, 30 September 2007 (UTC)
- Religions are just like hobbies, except that it is a philosophical hobby that insists on it's own validity and rejects any other philosophies that suggests that itself is FALSE.202.168.50.40 23:26, 30 September 2007 (UTC)
- Exactly! The point of religion isn't really to explain things that science explains, it's because it gives people something they cannot get anywhere else. If you see why people need religion, why talk about changing 'brainwashing'? Most people need religion or supernatural beliefs, and it is not to explain the things which are explained. People who read their horoscopes (and many people really, truly believe them, and some even become quite aggressive when you suggest horoscopes are silly) do not do so because they require an explanation for the movements of the planets. People who pray to a god don't generally do so because they want an explanation for why wind blows. With very few exceptions (if even those) people do not want to live only with verified facts. "I understand why many people 'need' religion. They would feel that life was unimportant, for some reason, without it." Does this mean that you feel life is important? Do you have any way of backing that up; is it supported by facts? Does believing it matter to you? :) 79.65.119.193 00:20, 1 October 2007 (UTC)
- Very good questions 79.65.119.193. If I`m forced to think about it long enough, I would have to conclude that life is more or less meaningless. It`s neat though. It`s quite a curiosity. But heck, everyone dies. How cruel is THAT? Life can still be important though. Just skip a couple of car or house payments to quickly find out just HOW important a life is, especially to a banker. As for astrology, sheesh! That`s not even worth a comment. I am utterly amazed at all the crap people can be made to believe in. My original question merely wanted to get some sort of 'blanket' answer as to why, or how people can still believe, given all the knowledge at hand. Wouldn`t you agree that religion has caused more harm than good? Just think about all the wars based on it. Thanks for listening. CHEERS! Dave 64.230.233.222 00:50, 1 October 2007 (UTC)
- :) I think you're starting to feel what I'm trying to show. Logically, when you think about it, you can see there is nothing to support the belief that life matters. But, it feels better to believe that life matters. If someone offered to prove to you that life didn't matter, would you welcome it? Equally, if thinking logically, people know that there is nothing supporting the belief that any gods exist. Nonetheless, it feels better (to them) to believe that a god, or gods, exist, so they do. Thinking logically, people know that pressing the button again at the pelican crossing doesn't speed up the change, but they won't welcome you explaining this to them when they do it; it feels good to do it and to believe, at some level, that you have affected the process. No, I wouldn't agree that religion has done more harm than good, any more than I believe that politics has done more harm than good. I have never seen a war that was fought because religion existed; religion is an excuse and a marker, it is useful in propaganda and in countering it, but it is not the cause of the wars. Since religion and politics seem to be pretty much hardwired into humans and their interactions, it's sadly not really possible to do a control study :P Who knows what the human race would be like without them, but they wouldn't be the human race. 79.65.119.193 01:04, 1 October 2007 (UTC)
- You have quite a way with words 79. Your points are well taken. Just a quick guess at your last comment: Without religion and politics, I think we`d still be savages. I think humans NEED rules and regulations to live in a civilized sort of way. Thanks for your fine points. I know Wikipedia is important. I love this place. lol Dave 64.230.233.222 01:21, 1 October 2007 (UTC)
- I love this place too. Happy thinking :) 79.65.119.193 07:02, 1 October 2007 (UTC)
first , you don't deserve to be respected if you dont respect others whatever their belief is. next the bible is not my book. and if this makes you feel better the bible was changed with by people over time and that's why you cant beleive in it , next do you actually beleive in the bigbang theory if you do so then who created what's before bigbang or was it a coincedince that earth was formed and we were created . no reason just like that , that is impossible —Preceding unsigned comment added by 212.71.37.73 (talk) 08:20, 1 October 2007 (UTC)
- To address your points one by one: Respect is indeed a bi-directional thing. It's not just that withhold my respect from people with crazy beliefs - I also don't give a damn whether they respect me or not. Truly - why would I care about the opinions of someone who runs his/her life on a rock-solid belief in the tooth-fairy? That person is a nut-job and I no more care how they feel about me than how they feel about the tooth-fairy. I would be more upset if someone with opinions I can understand (if not agree with) didn't respect me...but those are people I can respect.
- So - I can well understand why you wouldn't believe in the things written in the bible - that's a good thing! But without that - what do you know about this "god"? How do you know what she/he/it demands of you? So you are just making up your entire personal cosmology as you go along without any evidence whatever? Don't you think that's a dangerous viewpoint? If you are going to go around just believing in anything on a whim without evidence, what's to stop you suddenly believing that your god wants you to blow up tall buildings with airplanes...or poisoning the Kool-Aid and waiting for the aliens to come and resurrect you? Those who believe in random things without proof or reason are dangerous, scary people!
- I do believe that there is strong evidence for the big bang theory - cosmic background radiation measurements pretty much clinched that debate. But this continual demand for "What created the ultimate thing" - then when I tell you, you demand the thing that created the ultimate thing, then the thing that created that. It might make you happier to believe that some god or other created the big bang - but that raises an even nastier question: "How did the god get there?". Most religious people simply say that their god has always been there because god is outside of time - and they are happy with that answer. But why is that a better answer than "The singularity that was the source of the big bang was always there and is 'outside' of time". I don't see how a complex thing like a god (with thoughts and powers and strong opinions and all of these abilities) is a more probable starting point than a simple 'dot' of infinite density.
- Describing the formation of the earth as a 'coincidence' is missing the point that science is making. Science says that the universe is VAST - the number of planets that have formed is hugely greater than the number of grains of sand on a beach. The formation of the earth was no coincidence - it seems that there are billions and billions of planets out there, they are formed from simple physical processes that are exceedingly common in our galaxy. Perhaps the only oddity is that this particular planet has all of this complex life on it (although we don't really know whether that's an oddity or not - maybe all planets have complex life on them but we just haven't found them yet). But even if we are alone in the universe, that's not a 'coincidence'. Beings that are capable of asking the question "How did I get here" can only arise on those planets which have exactly the right conditions - so it is inevitable that we live on a planet like this one that is exactly suited to us. If only one planet in the entire universe happens to have those exact conditions - then it is certain that the beings asking the question must be living on it. SteveBaker 14:17, 1 October 2007 (UTC)
- Anyone (especially atheists like me) who wants to understand religion, its evolutionary fitness, and its appeal to adherents must watch the PBS Frontline expisode "The Mormons" (available online). This documentary was extremely enlightening to me even though I had already read some books on the subject. Just watch how the young woman's eyes light-up when she describes the mormon concept of marriage in the section "The Family" in part 2 and you will understand how religion is a natural outgrowth (exploitation?) of human evolutionary desires. -- Diletante 15:54, 1 October 2007 (UTC)
how to test caffeine content??
please suggest a possible experiment(home test) to find caffeine content in common drinks an coffee and tea -sumit —Preceding unsigned comment added by 59.94.140.159 (talk) 06:47, 30 September 2007 (UTC)
- Try searching the internet for the two keywords Daphnia and caffeine. You might be able to use the heart rate of Daphnia as a bioassay for the concentration of caffeine. --JWSchmidt 14:57, 30 September 2007 (UTC)
- This question came up a few months ago - we concluded that there was no easy test that could be done in the home. But using Daphnia could maybe work with appropriate calibration of the method against samples with a known content. SteveBaker 15:21, 30 September 2007 (UTC)
- Here's that thread, easily found by googling 'spider web cafeine site:http://en.wikipedia.org/wiki/Wikipedia:Reference_desk/Archives'. I rather liked my suggestion of using a spider. :) DirkvdM 18:24, 30 September 2007 (UTC)
thanks sir, but i had asked this qn on 30 or 29 sep not a month ago--59.94.146.227 12:59, 1 October 2007 (UTC)
- Yeah - we know that - it's just that it's a surprisingly common question and it's easier to point you to the replies that were given last time than to answer it all over again. SteveBaker 13:38, 1 October 2007 (UTC)
FIRE FIGHTING ROBOT
My question regarding the above topic is
I wanted to know how does the software part has to be explored as on case of fire fighting robot which is basically dependent on the temperature sensors used(LM315). Wanted to know how the KEIL software come into play with the following project. my query was how is the programming going to be used in the above project fire fighting robot. Miki619 09:38, 30 September 2007 (UTC)
- OK - back up a bit here. Which fire-fighting robot are you talking about? Do you have link to it? Your question is a bit confusing - so let's take this slowly. The robot uses some temperature sensing mechanism - using LM313 chips maybe - and you want to know what the software does with the data that comes out of those chips? This doesn't sound like a 'real' project. Is it some school project with a home-made robot or something? If so, and if I were programming it for a competition, and assuming the robot has just one temperature sensor pointing forwards, I'd write something like this:
- Make the the software turn the robot slowly around in a full circle taking temperature readings all the while and noting the highest reading and the direction we were pointing in when that reading was taken.
- Rotate back to the direction where we got the highest reading (so, hopefully we're pointing towards the hottest part of the fire).
- Then, I'd look at the actual temperature we detected in that direction and say:
- If the temperature is lower than a certain threshold, drive forwards for a few seconds.
- Else, if the temperature is high enough that we believe the fire is immediately in front of the robot we squirt water/foam/CO2 at the fire until the temperature drops below the pre-programmed threshold - or some short time limit is reached (10 seconds maybe).
- Then, go back to step (1) and look for a new direction to drive in.
- Hopefully, this would cause the robot to drive towards the hottest part of the fire that it can see - put it out - then go looking for the next most hot part - put that out and so on. At the end, when there are no more heat sources hot enough to be fires, the robot will drive towards the next hottest thing it can see - which is probably the sun - which would probably cause it to triumphantly leave the building. Of course in the real world, things would be vastly, insanely, more complicated than that - but for some kind of school robotics class, that would do the job quite nicely.
- But we have no way to know whether the robot you are thinking of is at all like that - there are other ways to program the thing. Maybe it has cameras - maybe it has a map of the room - maybe it has temperature sensors pointing in all directions, an infra-red camera. Maybe it has to ration it's water/CO2/foam supplies, maybe it has to distinguish between different kinds of fire so it knows whether it's safe to use water or not. Maybe it has to climb staircases - to put out fires up high on a rooftop or down low on the floor. Maybe it has to be able to smash down doors to get into a room - or avoid fallen debris. Perhaps it has to check that the floor is still strong enough to support it. Maybe it has to give a higher priority to saving lives than to putting out the fire.
- You really didn't give us enough information! SteveBaker 15:17, 30 September 2007 (UTC)
Possible amnesia question.
Is there a certain type of amnesia to where a person only remembers things for as long as they stay conscious? So basically, after they fall asleep, they forget everything they learned when they wake up.--SeizureDog 10:12, 30 September 2007 (UTC)
- You don't have to fall asleep to forget with Anterograde amnesia though - it's a failure of the mechanism that transfers data from short term to long term memory - so memories fade after 20 to 30 minutes because short-term memory can't hold them any longer and long term memory can't kick in. I can't imagine a mechanism that would cause you to forget things only when you fell asleep - so I think the answer to the question is "No". The opposite can happen though - if someone is deprived of REM sleep for too many nights, they become forgetful. SteveBaker 14:54, 30 September 2007 (UTC)
- 50 First Dates is, I assume, the source of this musing? 79.65.119.193 22:22, 30 September 2007 (UTC)
- Could be. The real-world cases are a lot sadder than that. There was a story on the radio about a guy who has this terrible condition - every time his wife steps into the room he greets her like he's been separated from her for years - since the time he suffered the injury that caused the disease. He says that he's "waking up for the very first time" - and the poor woman has to explain to him what the problem is and that this happens all the time to him - dozens of times per day. 50 First Dates sugar-coats it to hell and back! SteveBaker 13:36, 1 October 2007 (UTC)
- At least he can hide his own Easter eggs. --Sean 18:12, 1 October 2007 (UTC)
- Could be. The real-world cases are a lot sadder than that. There was a story on the radio about a guy who has this terrible condition - every time his wife steps into the room he greets her like he's been separated from her for years - since the time he suffered the injury that caused the disease. He says that he's "waking up for the very first time" - and the poor woman has to explain to him what the problem is and that this happens all the time to him - dozens of times per day. 50 First Dates sugar-coats it to hell and back! SteveBaker 13:36, 1 October 2007 (UTC)
Amiodarone
Many sources refer to the chemists Tondeur and Binon as the original discoverers of amiodarone, working for the Belgian company Labaz. Despite this, I could not find a reference to their original publications even in the 1985 Cordarone FDA approval documents[12]. Anyone? (Reply on my talkpage please, or on Talk:Amiodarone.) JFW | T@lk 10:16, 30 September 2007 (UTC)
- It's allright - PubMed worked. How odd that Google does not index all pubmed abstracts?? JFW | T@lk 10:28, 30 September 2007 (UTC)
Mitochondria
How would you count the number of mitochondria within a liver cell? Using a series of images from a transmission electon microscope, or could a image from a scanning microscope be used,( or does a scanner produce an image of the exterior of the cell?) 88.110.203.63 11:11, 30 September 2007 (UTC)
- If you search PubMed here you might be able to find the full text of articles that describe how to use either transmission electron microscopy or confocal microscopy to estimate the number of mitochondria in cells. --JWSchmidt 14:46, 30 September 2007 (UTC)
(Carbon) Emissions Data
I am looking for comprehensive, standardised, reliable emissions data please —Preceding unsigned comment added by Canuinti (talk • contribs) 13:58, 30 September 2007 (UTC)
- Emissions from what? SteveBaker 14:48, 30 September 2007 (UTC)
- And where and over what period. I assume you mean antrhopogenic emissions of CO2, probably in connection with global warming. Is that correct? Googling 'co2 emissions' gives loads of results on that, so you'd have to narrow it down a bit. If you mean worldwide, then the IPCC might be a reliable source for that, such as the fourth assessment report]. Alas, they're bloody pdf's and I don't know how to search through those. DirkvdM 18:36, 30 September 2007 (UTC)
DNA
can DNA be manupilated to add or remove certain characteristics ? can this possibly be true ? i mean as a good mutation!! —Preceding unsigned comment added by 212.71.37.108 (talk) 14:53, 30 September 2007 (UTC)
- Try reading Genetic engineering. --JWSchmidt 15:54, 30 September 2007 (UTC)
- Yes, and we're getting better at it all the time. It does bring up many moral issues, however, like stem-cell research. StuRat 22:32, 30 September 2007 (UTC)
Calculus needed?
Hello. I am just a high school student. I am considering studying thermodynamics as well as the methods used to date geological strata and the age of the Earth. I want to know about these things so I can evaluate claims in the creationism vs. evolution debate. Is it necessary to know calculus to have an adequate grasp of these subjects? Thanks. 68.77.186.32 16:23, 30 September 2007 (UTC)
- I linked a couple of the keywords in your question.
- You might find some useful info in http://www.talkorigins.org/origins/faqs-youngearth.html
- As a layman I can think of a couple of points where differential equations are relevant, but they seem to be simple ones requiring only the basics of calculus – or you could use a "cookbook" approach, i.e. plug in numbers to a formula without needing to understand the formula. —Tamfang 16:33, 30 September 2007 (UTC)
- I would say it depends on how deeply you want to go into these subjects. Calculus turns up in many, many areas of science when they are studied at a deep level. However, I think you could get a good overall understanding of both these subjects with only basic math. ike9898 17:25, 30 September 2007 (UTC)
- Common sense and simple arithmetic should suffice, butif during your research you run into the need for higher math, please come back and ask for help. Someone here may be able to either help you with the math or direct you to a simpler way to handle the particular problem. You will need higher math if you proceed to University-level thermodynamics, of course. -Arch dude 17:30, 30 September 2007 (UTC)
- If you're doing 'real' thermodynamics using numbers and energies and things, rather than just vaguely talking about the rules of thermodynamics, then you definitely need calculus; there's a lot of integrating and differentiating. So it depends how much thermodynamics you want to understand. I'd recommend you study calculus if you get the chance, if you're interested in science at all; it isn't as hard as cartoons would have you believe, and it lets you learn at lot of other things. 79.65.119.193 22:21, 30 September 2007 (UTC)
- I'd say you would need to use logarithms for calculating ages based on decay of radioactive isotopes. That's the most complex math that comes to mind. StuRat 22:28, 30 September 2007 (UTC)
- I would also like to point out that calculus is very useful for the lifetime of thinking you have ahead of you, even if you only have a qualitative ("calculus for poets") understanding of it. You should definitely learn it if you're curious about the world around you. --Sean 18:16, 1 October 2007 (UTC)
- Slightly off-topic, but regarding thermodynamics (specifically entropy and the Second Law) and creationism vs evolution, and claims about entropy and disorder from order being a natural consequence of systems (and therefore, supposedly, of evolution being "false") I was reminded of the following point (thanks to M.C. Hawking):
"Creationists always try to use the second law, to disprove evolution, but their theory has a flaw. The second law is quite precise about where it applies, only in a closed system must the entropy count rise. The earth's not a closed system; it's powered by the sun, so **** the damn creationists, Doomsday get my gun!"
- Hope this helps in your evaluations :) -Wooty [Woot?] [Spam! Spam! Wonderful spam!] 23:25, 1 October 2007 (UTC)
Graphics of a X-box 360 to graphics of a ps3
Can I get a website that shows a comparison of the graphics for the x-box 360 and ps3. It would be nice if it would be a same game comparison. Thank you in advance for your help. (Wookiemaster 17:35, 30 September 2007 (UTC))
- It's a really complicated trade-off. I can't reveal what I know because I've signed non-disclosure agreements (I'm an Xbox360/PS-3 game designer - and my job is the graphics). I would say this though. There isn't that much to choose between them. The horrific cost of designing video games these days means that most (if not all) games that come out on both platforms will have pretty much the same graphic design for both of them. The result is the games looks pretty much identical - no matter whether one is better than the other or not - it's a compromise between what the two machines can do. If you want to choose which one to buy - look only at whether the games you want are available on the console you want and ignore the millions of fanboi sites because not a single one of them know all of the facts (because to find them out you'd be under NDA and unable to write about them) - and for sure none of them are unbiassed. SteveBaker 21:50, 30 September 2007 (UTC)
Search for "xbox ps3 graphics comparison" like this http://ww.google.co.uk/search?hl=en&q=xbox+ps3+graphics+comparison&meta= and as the above says - mostly the stills look exactly the same - though sometimes the dynamic range or brightness differs...87.102.19.191 21:56, 30 September 2007 (UTC)
ionosing gas
i would like to know about the best ionising gas121.246.251.238 17:43, 30 September 2007 (UTC)
- Best for what? Best at what? --Mdwyer 17:55, 30 September 2007 (UTC)
- A gas with low ionisation energy is good - such as Xenon,
or maybe sulphur hexafluoride.. Radon is a good ionising gas as it is radioactive and a such self ionises.87.102.19.191 21:58, 30 September 2007 (UTC)
Physics Phenomena i don't understand
1) If a plane with two jet engines has the left one fail, the plane will deccelerate, and rotate left, is this correct?
2) Why does your reflection run at you twice your speed
3) i understand circular motion, so why do the planets move ellipcically?
4) if i shot a bullet out of a gun pointed in the opposite direction to my motion in a car that is travelling say 50 m/s and the bullet also travels at this speed what wil happen, does the bullet just stay in the gun?
5) I'm sitting here trying to figure out this damn questions, its not homework i promise, in fact i've changed the numbers. ok so lets say there are 40 chlorine particles per 10^7 air particle (made that up), and your lungs together can take 1 litre of air, atmospheric pressure is 1.0x10^5 Pa, how many chlorine molecules would you breath in?
78.146.4.0 18:10, 30 September 2007 (UTC)
- 1) Thankfully, all planes have some form of yaw control, usually a rudder. 2) If you are running towards a mirror that is facing you, from the perspective of someone standing next to the mirror, you are both running towards eachother at the same speed. Therefore, the distance between you two is closing at twice whatever that speed is, so from your perspective your reflection is approaching you twice as fast as you are running (assuming you're not running near the speed of light). 3) A circular orbit is merely the perfect orbital situation, where an orbiting object has the exact right velocity and altitude that gravity cannot pull it any closer, or let it go any further away. As you can imagine, in the chaos that was our early solar system, the chance of a planet forming in such a perfect situation was about nil. You might want to check out orbit. 4) No, the bullet exits the gun at 50m/s with respect to the gun. Anyone standing on the road sees the bullet plop out of the gun and fall harmlessly to the ground with no horizontal motion. According to the laws of special relativity, there is no preferred frame of reference. I am sitting still in my chair typing at my computer, but i'm on a spinning globe, which is hurtling around the sun, which is hurtling around the milky way galaxy, which is hurtling in some direction I don't know with respect to nearby galaxies. So what makes the road any better a frame from which to measure velocities than any other frame i can think of? Any inertial reference frame (a frame with constant velocity) is a valid one from which to draw physical conclusions. So as long as your car is moving with constant velocity, you can say that, from the car's perspective, the bullet will leave the gun with the same velocity that it would if the car were stopped. This is actually a better reference frame to use than the road because with the road you have to deal with a bullet from a moving gun, which is more complex. 5) One liter of gas at that pressure contains about 0.045 moles of particles. Someguy1221 18:35, 30 September 2007 (UTC)
- 1) If a plane with two jet engines has the left one fail, the plane will deccelerate, and rotate left, is this correct? -- Well, it would - but such aircraft are designed to still be flyable under those circumstances - so generally one has to throttle back the one remaining engine and apply rudder to keep it flying straight. In some kinds of aircraft you get other ikky problems due to the flow of the exhaust gasses over the tail plane being asymmetrical so that when you climb or dive, the plane wants to roll slowly to one side or the other. But again, the remaining controls are designed to be sufficient to keep the plane flyable.
- 2) Why does your reflection run at you twice your speed - you run towards the mirror as speed X, your reflection runs towards the mirror at speed X. Relative to you, you are stationary and the mirror is moving towards you at X - so your reflection is moving towards you at X+X = 2X.
- 3) i understand circular motion, so why do the planets move ellipcically? - To move in a circular orbit, you'd have to be moving with a velocity that's exactly at right angles to the line between you and the star/sun. If your initial velocity is pointing at some other angle (inwards towards the star, for example) then you are going to start moving closer to it instead of staying at the exact same distance. Because you are moving closer, you start going faster and faster - but this increases the amount of centrifugal force - which eventually pushes you away from the star. So now you are moving further away from it, you will gradually slow down and then move back towards it again. If the speeds are just right, you remain in a stable - but elliptical - orbit forever. Another way to look at it is that all orbits are 'elliptical' but a circle is just a special case of an ellipse.
- 4) if i shot a bullet out of a gun pointed in the opposite direction to my motion in a car that is travelling say 50 m/s and the bullet also travels at this speed what wil happen, does the bullet just stay in the gun? -- We have to be very careful about what you mean by the speed of the bullet. If you mean that if you were standing still (in a vacuum) and you fired the gun then the bullet would come out of the barrel at 50 ms-1 - then when you fire it from the car (still in a vacuum), the speed imparted to the bullet by the propellant in the barrel will be added to the speed of the car - so someone beside the road would say that the bullet was travelling at 0 ms-1 - the bullet would come out of the gun and fall to the ground. In reality, things will be much more complicated because of air resistance. The pressure of the air in the barrel of the gun will be lower because the speed of the car is pulling air out of the barrel creating a partial vacuum. So the pressure of the explosive propellant behind the bullet minus the air pressure in front of the bullet will be more than it would be if you were firing the gun in still air. In the case of a real gun, the difference would be almost negligable - but your gun is an AMAZINGLY slow one - so it might not be so negligable. Certainly, the bullet would come out of the barrel - but nowhere near as fast as it 'should'.
- 5) I'm sitting here trying to figure out this damn questions, its not homework i promise, in fact i've changed the numbers. ok so lets say there are 40 chlorine particles per 10^7 air particle (made that up), and your lungs together can take 1 litre of air, atmospheric pressure is 1.0x10^5 Pa, how many chlorine molecules would you breath in? -- Well, I'm not doing this one for you - check out Gas laws to figure out the number of molecules in the air from the pressure and volume - divide by ten to the seven and multiply by 40 - but it's made very messy by the fact that air is a mixture of lots of gasses. SteveBaker 21:32, 30 September 2007 (UTC)
- for 1), see ETOPS. -Arch dude 02:33, 1 October 2007 (UTC)
- THANKS guys! for question 5, can i use pV=nrT, but i do not have a value of temperature, what do i do? 89.240.40.152 17:08, 1 October 2007 (UTC)
- When unspecified, you normally use either STP (273K) or room temperature (293K). Since you're talking about breathing, I'd imagine it would be the latter. Someguy1221 17:24, 1 October 2007 (UTC)
- Perhaps a more practical example than the bullet one, since gas pressures and high muzzle velocities would not play a role, and you could titrate the amount of force used, would be to shoot an arrow backwards from a car. It's interesting (and counterintuitive to many) that the bullet or arrow would not hang suspended in the air at all, but would fall at the same rate as if you just dropped it by hand. --Sean 18:23, 1 October 2007 (UTC)
- People are not good at those kinds of thought experiment. We used to ask job candidates: "If you are driving along in a car at constant speed with a ball in your hand. There is a bucket beside the road. You need to drop the ball into the bucket. Should you drop the ball (A) just before you reach the bucket, (B) exactly as you pass it, or a (C) little while after you pass it?" - an amazing number of people answer "as you pass it" or "after you pass it" - the latter group usually tell you "because of air resistance" and the middle group often say "assuming we can neglect air resistance" (which is a shame because they are closer to having the right answer if we take air resistance into account). It's very sad. SteveBaker 19:27, 1 October 2007 (UTC)
- Hmm. Are you sure the question was stated the same way you posed it here? If so, it's difficult to comprehend how the incorrect answers could be appealing even in an intuitive sense. Even if you were sitting in a stationary chair with a stationary bucket in a total vacuum, the ball is still going to take time T > 0 to reach the bucket, so (C) doesn't even seem to make sense. My guess: there were other considerations (such as time pressure) you haven't mentioned here. dr.ef.tymac 21:45, 1 October 2007 (UTC)
- Still dr., talk about a simple problem. I`m guessing your candidates are college graduates? Sheesh! That is VERY sad. Dave 64.230.233.209 21:54, 1 October 2007 (UTC)
- Yep - college grads. Their thinking goes like this: When I stick my hand out of the window of the car - there is a really strong wind blowing. Balls aren't very heavy so they're going to be blown backwards really fast. So I need to let go of the ball after we've passed the bucket so that the ball gets blown backwards into the bucket. Yeah - I'm well aware of all of the fatal flaws in that logic - but that's what they say. I'm not putting them under time pressure - but then they never seem to take time to come to their conclusions - they always think it's so "obvious". In fact, I prefer that they take time and think - because I wasn't in the business of needing people who could come to a snap decision - I wanted people who'd take the time to get the right answer. The nastier question we used was "If you are in a boat on small 10' deep pond with a large, heavy, iron anvil on board - if you chuck the anvil over the side, will the water level in the pond go up, down or stay the same?" It's not a trick question. SteveBaker 22:54, 1 October 2007 (UTC)
- I know you`re not asking me, per se, but I believe the water level would drop some. Briefly: With the anvil in the boat, being so much more dense than water, it would displace its whole 'mass worth' of water. Once completely submerged, it will only displace its equivalent volume of water. This would be much less water than it would displace while in the boat. Of course, the water would be at its lowest level while the anvil was in the air, before submerging. Do I get the job? lol Dave 64.230.233.209 01:15, 2 October 2007 (UTC)
- Yep - college grads. Their thinking goes like this: When I stick my hand out of the window of the car - there is a really strong wind blowing. Balls aren't very heavy so they're going to be blown backwards really fast. So I need to let go of the ball after we've passed the bucket so that the ball gets blown backwards into the bucket. Yeah - I'm well aware of all of the fatal flaws in that logic - but that's what they say. I'm not putting them under time pressure - but then they never seem to take time to come to their conclusions - they always think it's so "obvious". In fact, I prefer that they take time and think - because I wasn't in the business of needing people who could come to a snap decision - I wanted people who'd take the time to get the right answer. The nastier question we used was "If you are in a boat on small 10' deep pond with a large, heavy, iron anvil on board - if you chuck the anvil over the side, will the water level in the pond go up, down or stay the same?" It's not a trick question. SteveBaker 22:54, 1 October 2007 (UTC)
- Still dr., talk about a simple problem. I`m guessing your candidates are college graduates? Sheesh! That is VERY sad. Dave 64.230.233.209 21:54, 1 October 2007 (UTC)
- Hmm. Are you sure the question was stated the same way you posed it here? If so, it's difficult to comprehend how the incorrect answers could be appealing even in an intuitive sense. Even if you were sitting in a stationary chair with a stationary bucket in a total vacuum, the ball is still going to take time T > 0 to reach the bucket, so (C) doesn't even seem to make sense. My guess: there were other considerations (such as time pressure) you haven't mentioned here. dr.ef.tymac 21:45, 1 October 2007 (UTC)
Perfect sharpness?
Is it possible to achieve a nearly perfect sharp blade by making it as much thin as possible (about 1 micron or something like that, assuming that the blade is of very firm material), so one would be able to cut up a considerable parts of nearly every material even by a slight strike? --85.132.14.38 18:43, 30 September 2007 (UTC)
- See Monomolecular wire and Monofilament (science fiction) for some idea. Basically this is science fiction. Exxolon 20:54, 30 September 2007 (UTC)
- some diamond knives can do close to this (can cut apart a cell) read "The Hot Zone" —Preceding unsigned comment added by 12.217.195.89 (talk) 00:00, 1 October 2007 (UTC)
- Considering we can move separate atoms these days (can someone provide a link?) it may be becoming more science than fiction. However, when you use such a blade, it will become blunt again, so it's a one-off thing. Unless it's extremely tough material, like diamond. But then I suppose one cannot make diamond with the method I cannot find a link to. So maybe more fiction than science after all. DirkvdM 08:15, 1 October 2007 (UTC)
- Hold on, what about this? Cover a knife in some insulating material, then use a laser (how thin can that be?) to cut out a strip right at the edge. Then put it in a galvanising bath and a perfectly sharp edge will form. Of course, this will still suffer the problem that it will go 'blunt' very fast. It might help if this was done in several stages, ever narrower, so the edge will be wedge-shaped. DirkvdM 08:23, 1 October 2007 (UTC)
- I always wondered whether you could do this by electro-plating ultra-thin layers of progressively harder metals onto some kind of substate - ending with the hardest metal you can find - then adding more layers of progressively softer metals. Remove the substrate and you have a sandwich of a spectacularly thin layer of hard metal encased in softer metals on both sides. Sharpen it so the hard metal is the cutting edge of the V-cross-section blade. Now, as you use the knife, the softer metals will wear away faster than the hard one - leaving a perpetually ultra-sharp knife that maintains it's own sharpness as you use it. I imagine there must be a terrible problem with this idea because nobody seems to be doing it already - but if we're postulating nanotech solutions - that's the way to go. SteveBaker 15:41, 1 October 2007 (UTC)
- That sounds a bit like the Dayak method of machete-making taken several steps further. See the last paragraph of the Dayak people#Society section. I wrote that. :) (how does one make a proud emoticon?) DirkvdM 18:57, 1 October 2007 (UTC)
What is the whitest substance ?
I recently read an article on research to create the whitest substance using nanotechnology, but I can't find it anymore. I looked at wikipedia hoping to find a definition of what being "whitest" means, and a list of very white natural substances (such as the foam of a breaking ocean wave). Any information on these subjects ? Pcarbonn 20:09, 30 September 2007 (UTC)
- Well, I suppose 'whitest' would mean 'reflecting all of the visible wavelengths of light equally' - but a mirror does that pretty well and it's not what we'd call 'white'. So I suppose it also has to be a diffuse reflector. Most tests that are done where a perfectly white diffuse reflector is required (eg in calibrating a camera or something) use very pure calcium carbonate blocks - but I guess it's not perfectly white or the question would never have arisen. SteveBaker 21:09, 30 September 2007 (UTC)
- Are you sure it was a substance and not a light? There have been many advances towards producing pure white LEDs using nanotechnology. -- kainaw™ 21:45, 30 September 2007 (UTC)
- Titanium dioxide seems to be claimed to be the whitest, but I recall hearing that there are coatings used on the fibres of white clothes which absorb UV light and re-emit it in the visible spectrum to make the clothes look whiter (or at least brighter). —Preceding unsigned comment added by 203.22.236.14 (talk) 04:17, 1 October 2007 (UTC)
- UV dyes are mixed in with your detergents for exactly that reason. Look at your washing detergent under black-light for a fun time. --Mdwyer 05:07, 1 October 2007 (UTC)
- Thanks for the answer. I guess 'white' is a mix of reflection and scattering, and nanostructures can contribute to building a good diffuse reflector. The article I mentioned was not about LED. Pcarbonn 07:16, 1 October 2007 (UTC)
- There was an article a while back on an extremely white beetle. http://news.bbc.co.uk/2/hi/science/nature/6272485.stm 71.226.56.79 02:01, 2 October 2007 (UTC)
Calories in alcohol
Does alcohol have any calrories? If so, how many (per ounce or what have you)? Please do not respond by telling me how many calories alcoholic drinks have; I'm only interested in how many calories are found in ethyl alcohol itself.--The Fat Man Who Never Came Back 20:19, 30 September 2007 (UTC)
- 7 calories per gram. More than portein and carbohydrates, less than lipids. I imagine that that number is just an average though, and that specific types of alcohols will yhave different energy densities based on their chemical structure. --YbborTalk 20:47, 30 September 2007 (UTC)
- The question was about ethyl alcohol, which is the only alcohol used in beverages, so the "specific types of alcohols" bit is irrelevant. --Anonymous, 21:23 UTC, September 30, 2007.
- It is however true that ethyl alcohol/ethanol is the only one that really matters when it comes to alcoholic beverage. While other alcohols are consumed like sugar alcohol & propylene glycol, this is usually only im minor quantities. Also, as anon said, fat man did specify ethanol in particular near the end of his question Nil Einne 03:08, 1 October 2007 (UTC)
- Perfect, thanks. Now how many grams are in an a shot of alcohol... maybe an ounce or so? Not that I'm going around doing everclear shots... I'm just curious.--The Fat Man Who Never Came Back 21:14, 30 September 2007 (UTC)
- Well, a US fluid ounce is about 29.57 ml, and ethyl alcohol has a density of 0.789 g/ml, so one US fluid ounce of pure ethyl alcohol would be 23.33 g or about 160 calories worth. --Anonymous, 21:23 UTC, September 30, 2007.
- I suspected as much.... more calories than bread, less than butter. Thanks, folks.--The Fat Man Who Never Came Back 21:47, 30 September 2007 (UTC)
- Also bear in mind that most alcoholic drinks will also contain calories in the form of carbs and some will also contain calories from protein and fats (such as those with milk in them). StuRat 22:07, 30 September 2007 (UTC)
big bang theory
although there are many explanations given as to how earth came about from the "big bang" theory, i wanted to no how the material and atoms actually got there in the first place to create this big bang? how was this material invented from nothing?
thanks liam —Preceding unsigned comment added by 86.4.197.35 (talk) 22:23, 30 September 2007 (UTC)
- I don't think anyone really knows the answer to that. —Preceding unsigned comment added by 12.217.195.89 (talk) 23:57, 30 September 2007 (UTC)
- Timeline of the Big Bang#The very early universe lists links to some proposals. It is important to note that the big bang theory is actually entirely meant to describe what happened right after the universe started existing. For the actual moment of the big bang itself, or what, if anything, came before it, there is no accepted theory. Someguy1221 00:05, 1 October 2007 (UTC)
- The big bang supposes that an infinitely small 'dot' of infinite density is the start of it all. In a sense, that is nothing - it's zero sized - but space itself is also scrunched up to zero size, also time, information - everything that ever was or ever will be is in this infinitesimal dot. Like the singularity at the center of a black hole perhaps. Since no information can pass through a singularity - it would be impossible to know what (if anything) created the singularity. One popular suggestion it that it arises from a 'big crunch' of some previous universe. But we can never know. It has also been suggested that time itself would be crunched up in the singularity - so that time would be kick-started up by the same process. In that sense, the singularity would have created time too. Without a 'before' - there can be no thing that 'created' it because the thing that created it would have to have been around before...no before - no creator.
- There is a serious problem with asking the question "What was the ultimate cause of everything?" - and when you get the answer 'X' (a space-time singularity in this case) - going on to ask what caused 'X'? When someone provides the answer 'Y' - you're just going to ask "What caused 'Y'?" - if there is some kind of ultimate answer - then that something pretty much has to have no cause - to have been around for infinite time or to have created time. A zero sized, infinitely dense dot that's crunched up time too - through which no information can pass...that's a pretty definite "ultimate" beginning.
- The big bang theory is based on the FLRW solution to general relativity, which has a singularity at the beginning, but physicists do not give this mathematical singularity a physical interpretation. It's just a sign that the theory doesn't work there. Big bang cosmology is about the evolution of the universe, not how it began. The name "big bang" is very unfortunate. It was, as you might know, originally invented as an insult (by Fred Hoyle). Imagine the confusion if the theory of natural selection were known as the tornado-in-a-junkyard theory, after his (senseless) criticism of it. -- BenRG 11:00, 1 October 2007 (UTC)
- Stephen Hawkins writings indicate that he is talking about a literal singularity with scrunched up time. It's explained reasonably well in 'A brief history of time' - although you need to read his published papers to get a proper mathematical grasp of the idea...of course he could be wrong, there isn't any actual evidence until a fair amount of time after the actual start of the universe. SteveBaker 13:25, 1 October 2007 (UTC)
- Ok, I`m going out on a limb here. This might very possibly be complete rubbish, but this idea came to me not very long ago while trying to explain, to my own satisfaction, if nothing else, to attempt an explaination to how 'our' Universe began. Here goes...please don`t laugh TOO hard!
While reading-up on the possibility(ies) of there being multiple universes, I thought, "What happens when a black hole is 'created' in another 'neighbouring' universe? Where does the matter go?" I`ve coined the term 'white-burster'. This white-burster is what 'could be' the beginning of OUR universe, as we know it. Of course, I coined to term to make reference to its connection with the 'other side' of a black hole. I know, this is totally unfalsefiable, so therefore not science, but I think it 'could' work. One of course, would have to presume that that 'other' universe, if it exists, would be many magnitudes bigger than the one we presently know. Carl Sagan's most famous, I think, quote obviously comes to mind right now: "Extraordinary claims require extraordinary evidence." Of which of course I have none. Sorry if this just wasted everyone`s time. But, if I may just add: Despite being impossible, I think, to prove true, I think it may be just as impossible to prove untrue, which is, of course, nearly assinine as an argument. I`m very afraid to click on "Save page". lol CHEERS! Dave 64.230.233.209 14:29, 1 October 2007 (UTC)
- Ok, I`m going out on a limb here. This might very possibly be complete rubbish, but this idea came to me not very long ago while trying to explain, to my own satisfaction, if nothing else, to attempt an explaination to how 'our' Universe began. Here goes...please don`t laugh TOO hard!
- Stephen Hawkins writings indicate that he is talking about a literal singularity with scrunched up time. It's explained reasonably well in 'A brief history of time' - although you need to read his published papers to get a proper mathematical grasp of the idea...of course he could be wrong, there isn't any actual evidence until a fair amount of time after the actual start of the universe. SteveBaker 13:25, 1 October 2007 (UTC)
- See White hole and White holes in fiction - it's hard to come up with something we don't already have an article about! SteveBaker 15:34, 1 October 2007 (UTC)
- Simply amazing! I swear I hadn`t heard of any such thing before. Only reason being, I refused to call 'my' white-bursters 'holes'. Maybe I could get the name changed to bursters. I DID however read Hawking`s Black Holes and Baby Universes, but I did not think of each Black Hole being a White Hole someplace else. "MY" white-burster was coined to 'explain' OUR Universe, not other baby ones. So, a little bit of a twist there. Perhaps WE are one of those huge universe`s babies! Thanks for finding that for me. QI! Dave 64.230.233.209 15:57, 1 October 2007 (UTC)
- For the record though - I don't think the material that falls into a black hole goes anywhere - it stays right where it is - in the middle of the black hole. That's why these things get gradually bigger. As more material falls in and is compressed down into the singularity, the mass of the hole increases - an the event horizon gradually increases in diameter. If the material somehow fell through and popped out of someplace else (either in our universe - or in another) then the black hole would presumably be unable to grow - and therefore no black hole would ever be any bigger than the star that formed it. Since we know there are 'supermassive' black holes (like the one in the center of our very own Milky Way) - there must be some way for them to grow. Hence, no white holes of the kind you are thinking of. SteveBaker 19:15, 1 October 2007 (UTC)
- Yes, I know that Steve. I didn`t get 'into it' as deeply as I could have, just for clarity and conciseness sake. I`m thinknig that maybe there`s some sort of theoretical limit to just how massive a black hole can get, or maybe even a finite age for a huge black hole, then BOOM!...it 'disappears' from huge universe, to create a baby. Maybe once it gets to a certain theoretical limit, the 'dimensions' can no longer hold onto it, and it disappears, creating said 'new' universe. I know, still totally unfalsifiable. This will be my last post right here. Thanks all. Dave 64.230.233.209 20:47, 1 October 2007 (UTC)
- For the record though - I don't think the material that falls into a black hole goes anywhere - it stays right where it is - in the middle of the black hole. That's why these things get gradually bigger. As more material falls in and is compressed down into the singularity, the mass of the hole increases - an the event horizon gradually increases in diameter. If the material somehow fell through and popped out of someplace else (either in our universe - or in another) then the black hole would presumably be unable to grow - and therefore no black hole would ever be any bigger than the star that formed it. Since we know there are 'supermassive' black holes (like the one in the center of our very own Milky Way) - there must be some way for them to grow. Hence, no white holes of the kind you are thinking of. SteveBaker 19:15, 1 October 2007 (UTC)
What is meg
Look at this picture. http://www.theoildrum.com/files/Slide7_0.png
At the bottom half, it says "O2/N2 (per meg)", can anyone explain what meg is? Thank you. 202.168.50.40 23:18, 30 September 2007 (UTC)
- this site www.eol.ucar.edu/~stephens/talks/stephens_occc0508.ppt claims it is mol/m^2/year —Preceding unsigned comment added by 12.217.195.89 (talk) 23:50, 30 September 2007 (UTC)
- I would think megaliter as a volumetric measure of atmosphere.
However, I'm not sure where you got that from, but it looks fishy all around. I wouldn't source it or anything.
Mrdeath5493 03:50, 1 October 2007 (UTC)
- I would think megaliter as a volumetric measure of atmosphere.
- Well "per mille" (also "per mil") means thousandths (i.e. 5 per mille = 0.005) in the same way "percent" means hundredths (5% = 0.05). Maybe "per meg" is meant to convey millionths or something like that but its terminology I've never seen before. I dislike 12.217...'s answer because O2/N2 (i.e. a ratio of concentrations) should be a number and hence dimensionless. 76.240.228.205 03:58, 1 October 2007 (UTC)
- If you look at the original graph the O2/N2 (per meg) axis has a numeric scale going from 0 down to -500 - so it can't be anything like a percent/per mil/per million thing because those numbers can't be negative. Also the top graph does everything in ppm (parts per million) and if per-meg were a per-million thing then surely that would have been a 'ppm' scale too. At least 12.217.195.89's suggestion of mol/m^2/year would be a negative number - as CO2 builds up, oxygen declines and the ratio of oxygen to nitrogen would decrease. But a rate of decrease of a few hundred moles per meter cubed per year would have us all gasping for breath a long time ago!
- This article [13] has a definition of 'per meg' that says:
- per meg = ((O2/N2)/(O2/N2)ref - 1} x 106 - in other words the ratio of present-day oxygen levels to the oxygen level at some reference date - messed around until it's some kind of reasonable number scale that looks good on a graph.
- This graph [14] in that same article appears to be presenting the same data as the bottom half of the graph that our OP posted - except that there is a 'delta' symbol in front of the "02/N2 per meg" axis - indicating that this is the rate of change of oxygen nitrogen as expressed in this peculiar 'per meg' notation. Both graphs are attributed to 'R. Keeling' - I suspect they are intended to represent the same underlying data - but the version our OP found has been 'messed about with' by people who didn't know what they were doing. I wouldn't pay too much attention to a graph that spells 'dioxide' with a 'y'. Without that delta symbol (and especially without telling us what reference date is being talked about), the numbers don't make much sense. Urgh. SteveBaker 13:21, 1 October 2007 (UTC)
tick + blade of grass
why is it recommended that when you submit a tick to a lab for analysis you put it in a plastic bag with a blade of grass?
- Here is a guess: The grass is a plant, taking in carbon dioxide and giving off Oxygen. That way the tick can continue to breathe and it will stay alive and retain all the diseases so they can be detected. John 03:57, 1 October 2007 (UTC)
- Is it even possible to get a tick off you without killing it? I thought that the things have to be dead before you can safely twist them out of your skin? --Kurt Shaped Box 09:14, 1 October 2007 (UTC)
- No, you're supposed to twist them off alive with tweezers (preferably ones specially designed for the purpose). If you kill them first (how?), they're more likely to spit some possibly infected fluids back into you as they die. —Ilmari Karonen (talk) 09:58, 1 October 2007 (UTC)
- Whenever I've had a tick, I've doused it with isopropyl alcohol (or vodka) until it stops moving, *then* twisted it out with tweezers. A doctor once told me to do that! I had no idea! I have also burned them (and the surrounding area) away with a cigarette when nothing alcoholic was available. I was under the impression that if you tried to remove the tick whilst still alive, then the head would 'lock', snap off and stay firmly attached to your flesh (ticks being tenacious little buggers) and cause an abscess. It's probably lucky that I haven't had a lot of ticks! F-ck me!
- How do the 'tick tweezers' work, as a matter of interest, then? At the end of the day, it's a smallish bug - how can you apply enough pressure to the thing to get a grip on it without squashing it flat? --Kurt Shaped Box 13:30, 1 October 2007 (UTC)
- When "in their shells", They are extremely hard, almost like an unpopped popcorn kernel. 86.153.44.115 20:28, 1 October 2007 (UTC)
- Cool. How are they 'constructed'? Any idea? --Kurt Shaped Box 22:46, 1 October 2007 (UTC)
- When "in their shells", They are extremely hard, almost like an unpopped popcorn kernel. 86.153.44.115 20:28, 1 October 2007 (UTC)
- I have also heard the “burn or douse in pesticide first, then remove” advise about leeches. In both cases it was ostensibly to prevent bits of the mouth parts from remaining in the wound. I saw both bits of advice in first aid books. Not sure what is the right answer though. --S.dedalus 00:22, 2 October 2007 (UTC)
- Thinking about it, I suppose the advice given might depend on what sort of diseases the local parasites are likely to be carrying - whether the risk of an infection from leaving the head/mouth stuck in your flesh is worse than the risk of the dying critter vomiting god-knows-what into your bloodstream. --Kurt Shaped Box —Preceding signed but undated comment was added at 00:27, 2 October 2007 (UTC)
- That’s a pretty picture. :) --S.dedalus 00:51, 2 October 2007 (UTC)
- Thinking about it, I suppose the advice given might depend on what sort of diseases the local parasites are likely to be carrying - whether the risk of an infection from leaving the head/mouth stuck in your flesh is worse than the risk of the dying critter vomiting god-knows-what into your bloodstream. --Kurt Shaped Box —Preceding signed but undated comment was added at 00:27, 2 October 2007 (UTC)
DEET
what is the metabolic pathway (if there is any) that makes DEET effective at preventing insects from attacking me?
- From DEET:
DEET is believed to work by blocking insect receptors (notably those which detect carbon dioxide and lactic acid) which are used to locate hosts. DEET effectively "blinds" the insect's senses so that the biting/feeding instinct is not triggered by humans or animals which produce these chemicals.
Someguy1221 00:10, 1 October 2007 (UTC)
Deserts
Hi
I've had a look at deserts, but surprisingly it's a little scarce on this topic. My question is: what's the biodiversity really like in deserts in general? I want to say that there are few animals but more insects/microorganism/plants than you'd expect. Would I be right? Comments appreciated. Aaadddaaammm 23:54, 30 September 2007 (UTC)
- Sounds about right. Even though there are more than you'd expect, there are fewer than in most other land types (arctic tundra may be an exception). StuRat 01:49, 1 October 2007 (UTC)
- Comment: an insect is an animal Nil Einne 02:57, 1 October 2007 (UTC)
- I wonder if he appreciated that one. :) DirkvdM 09:11, 1 October 2007 (UTC)
- Thanks for your input, guys! Helpful comments (yes, even Nil Einne's - I'll have to specify vertebrates). Aaadddaaammm 23:06, 1 October 2007 (UTC)
October 1
Lightning as source of electricity
Is it possible to harvest the electricity from lightning strikes? If it is, then we can put an end to burning fossil fuel for electricity. Oidia (talk) 04:41, 1 October 2007 (UTC)
- Electricity doesn't store all that well, sadly. So, if you're intending to use it all up at once -- say, launching your Delorean through time or jump-starting your monster, the I suppose lightning would work. It practice, humans just don't have a way to convert and store that much energy in that little time. Besides, you've also got to be in the right place at the right time to collect it! --Mdwyer 05:04, 1 October 2007 (UTC)
- Electricity can be used for the electrolysis of water, which will produce Hydrogen, which is handy stuff to store and use energy (see Hydrogen economy). However, as Mdwyer pointed out, lightning comes in extreme bolts and it is hard to predict where it will strike, so you'd have to 'harness' it somehow. The latter can be solved by using a good lightning conductor. But then you'd have to convert all that energy into hydrogen in a split second (or could it be delayed somehow?) and for that you'd need a pretty big installation, I suppose (how big?), so that would have to be stationary, so it had better be in a place where there is a lot of lightning to be economically viable.
- Btw, suppose we could somehow (by my method or otherwise) harvest all the energy in electricity. How much would that amount to? Would it be enough to fulfill all our energy needs if it were all 'harvested'? I heard on QI the other day that worldwide, there are about 200 lightning strikes per second. Stephen Fry did not say if this included minor strikes, if there are such things. The lightning article says an average lightning bolt has an energy of 500 MJ. Times 200 per second is 100 GW. Energy consumption says we use up 15 TW (2004, so probably 20 TW now). So even if we could harvest all lightning energy in the world (including in very remote places) at 100% efficiency, that would only contribute 0.5 % of our energy demand. This surprises me. I thought it would be much more. Did I make a mistake somewhere? DirkvdM 08:32, 1 October 2007 (UTC)
- Apparently the idea of using lightning as an energy source is not quite as flaky as it sounds. There is a detailed discussion here and an overview article here. A company called Alternative Energy Holdings, Inc.] produced a press release last year claiming it had developed a prototype "lightning farm", but the company's website does not give an update, so practical results may not have lived up to expectations. Gandalf61 09:40, 1 October 2007 (UTC)
- The real problem is that any plant that took advantage of a lightning strike - capturing and storing the energy and pushing it out onto the electrical grid - would be quite large and expensive. But how many times a year would it get struck? Even in places where the most storms are found, a handful of times at most, I'd guess. That's hardly going to be worthwhile as an investment because it's sitting idle 360 days of the year. I'm also a little skeptical about that figure of 500MJ - sure, that's the total energy of the lightning bolt - but isn't most of that consumed in ionising the air to allow the strike to reach the ground - and producing all of that light and sound energy. I always assumed that only relatively little of that actually hit the ground. SteveBaker 12:55, 1 October 2007 (UTC)
- Isn't it a question of ease of collection / cost really? Surely alternatives such as solar power or wind farming would be much more economical? It does however sound like a very macho way of harvesting electricty. "We shall gather it from The Gods themselves - plucked from the heavens in my lightning-dirigible!" Don your flying goggles and scarves. Lanfear's Bane 14:06, 1 October 2007 (UTC)
- I believe some places, like radio towers on top of mountains, are struck by lightning quite often, perhaps many times a month. I would expect the key would be harvesting the electrical potential before it becomes an unmanageable lightning bolt. This could perhaps be done using thin wires hanging from weather balloons, launched from mountaintops. These would pose an obvious hazard to navigation, however, so any area where this was done would have to be off-limits to airplanes. StuRat 15:03, 1 October 2007 (UTC)
Hall of mirrors
Just curious, but has anyone ever tested the hall of mirrors in Versailles for mercury vapor? —Preceding unsigned comment added by KeeganB (talk • contribs) 15:12, 1 October 2007 (UTC)
- Why? We know that mercury is used in the manufacture of mirrors. Do you think there is a problem because of the age of the mirrors - or because there are so many of them? If it's the former, I doubt that the mirrors are the original ones - when I visited there a couple of years ago, they certainly didn't have the tarnished yellowish look of very old mirrors. If it's the latter, then there are lots of places in the world with many more mirrors than that. SteveBaker 15:29, 1 October 2007 (UTC)
- I haven't looked at a lot of old mirrors to be familiar with the tarnished look Steve has in mind, but I would have thought that a "yellowish look" in an old mirror was due to the use of a cheaper reflecting metal that was yellowish to begin with. (Why would the reflecting metal tarnish if it's covered with glass?) But I was in the Hall of Mirrors last week, and what I noticed was that a lot of the mirrors have a gray or cloudy appearance. I didn't take too close a look (the place was chock-full with other tourists), but I certainly assumed that they were the originals, and this coloring was introduced during manufacture. --Anonymous, 16:26 UTC, October 1, 2007.
- (The reflecting metal tarnishes because only the front face of the metal is covered with glass - the back is not. SteveBaker 19:07, 1 October 2007 (UTC)
- I haven't looked at a lot of old mirrors to be familiar with the tarnished look Steve has in mind, but I would have thought that a "yellowish look" in an old mirror was due to the use of a cheaper reflecting metal that was yellowish to begin with. (Why would the reflecting metal tarnish if it's covered with glass?) But I was in the Hall of Mirrors last week, and what I noticed was that a lot of the mirrors have a gray or cloudy appearance. I didn't take too close a look (the place was chock-full with other tourists), but I certainly assumed that they were the originals, and this coloring was introduced during manufacture. --Anonymous, 16:26 UTC, October 1, 2007.
Don't get your panties in a wad, Stevie. I was just wondering if the possibility of dangerous levels of mercury vapor in the hall has ever been considered.
Penis question
Is there any way of decreasing the time it takes for my penis to beome hard again after ejaculating? Thanks. —Preceding unsigned comment added by 90.240.244.96 (talk) 16:32, 1 October 2007 (UTC)
- Well viagra is a popular 'hardening' drug, similar to starch but for people... ny156uk 16:35, 1 October 2007 (UTC)
- First off, slow down and don't stress over it. I don't know that you are but I do know that stress is the anti-viagra. Slow down, and be intimate following ejaculation; touch, rub, fondle, do whatever turns you and your partner on. Based on my original research I'd say the erection is 80% in your mind. It comes when you are aroused, assuming you do not have a medical condition. Also it takes longer the older you get. I could keep going here but I'm not Dr. Ruth. Man It's So Loud In Here 17:20, 1 October 2007 (UTC)
Iridescence
Why does iridescence occur only in very thin surfaces such as soap bubbles and not in, e.g. 5 mm-thick glass? Goodgerster 18:04, 1 October 2007 (UTC)
- If you have a soap bubble that's one micron thick, then 2.5 wavelengths of 400 nm blue light can fit within it, and 1.4 wavelengths of 700 nm red light can fit within it. If you have two wavelengths that are close together, like 500 nm green and 501 nm green, then the number of wavelengths will be very close (2 versus 1.996). The point is, the accumulated phase difference changes slowly with wavelength.
- In contrast, if you have a 5 mm thick piece of glass, then 10,000 wavelengths of 500 nm green light fit inside, but 9980 wavelengths of 501 nm light fit inside. The difference is more than one whole wavelength, so the phase is very sensitive to the wavelength and in practice that just means it's unpredictable. The thick film has totally different effects on very close wavelengths, so they appear to average out over the whole spectrum (because your eye isn't sensitive to such tiny changes in wavelength).
- In other words, a thin film modulates the spectrum by a slowly varying function (for example, stopping blue and passing red), and a thick film modulates it by a rapidly varying function that's impossible to perceive. —Keenan Pepper 18:24, 1 October 2007 (UTC)
What are the isomers for C7H16 (isomeric heptanes)?
What are the isomers for C7H16 (isomeric heptanes)? —Preceding unsigned comment added by 69.179.253.76 (talk) 19:03, 1 October 2007 (UTC)
- The isomers of C7H16 are the different structures that have that formula.
 Please do your own homework.
Please do your own homework.- Welcome to the Wikipedia Reference Desk. Your question appears to be a homework question. I apologize if this is a misinterpretation, but it is our aim here not to do people's homework for them, but to merely aid them in doing it themselves. Letting someone else do your homework does not help you learn nearly as much as doing it yourself. Please attempt to solve the problem or answer the question yourself first. If you need help with a specific part of your homework, feel free to tell us where you are stuck and ask for help. If you need help grasping the concept of a problem, by all means let us know. DMacks 19:35, 1 October 2007 (UTC)
- Heptane may be of use. Algebraist 19:38, 1 October 2007 (UTC)
C7H16 is an alkane because it fits the CnH(nx2)+2 definition. So all you have to do is figure out how many structures you can create with 7 carbon atoms. Remember to look out for structures that look different on paper but bond can be rotated to give the same structure. Don't forget a linear molecule, and make sure that no C has more than 4 carbons attached to it! Good luck, have fun - I used to love these kind of questions! Aaadddaaammm 23:04, 1 October 2007 (UTC)
"God initiated the Big Bang and then burned and died in the flames of his own creation..."
Would this statement be any less wrong than any of the other current theories about what happened just before and just after the Big Bang? --Kurt Shaped Box 19:18, 1 October 2007 (UTC)
- Well, most theories of God suggest he is all-powerful and omni-present, do they not. So He should not be able to be killed, surely? I much prefer the simpler and more logical explanation: God doesn't exist. --80.229.152.246 19:45, 1 October 2007 (UTC)
- There is currently no scientific theory describing anything "before" the Big Bang, so this is purely a philosophical question. The general idea behind objection to your theory is that the only reason to include God in the picture is that no one likes the idea of something coming from nothing. Unfortunately, you still have that problem because you start the story with God already existing; anything you can say about His provenance, one could also say about the universe's provenance, so it really doesn't buy you anything. The only reason it sounds nicer to do an end run with "God created Himself" than "the universe created itself" is that people who believe in the supernatural are sometimes willing to accept lower evidentiary standards than those who do not. --Sean 20:20, 1 October 2007 (UTC)
- Speaking about something "before" the Big Bang is absurd, since current theories consider that time was created with the Big Bang. And I'd say your statement is somewhat more wrong than the other one, since yours includes God, who is completely unnecessary for the consistence of the theory. The addition of God only adds complication, making the theory, therefore, more improbable. --Taraborn 20:31, 1 October 2007 (UTC)
If time was created by the big bang, where did all the matter that is the universe come from? And if you think it came from God, what created God? —Preceding unsigned comment added by 128.101.53.147 (talk) 20:43, 1 October 2007 (UTC)
- I believe the comforting part of the Judeo-Christian God is that he doesn't require a creator of his own. Someguy1221 20:49, 1 October 2007 (UTC)
- How convenient! Dave 64.230.233.209 21:02, 1 October 2007 (UTC)
- That's not comforting. That's infuriatingly absurd. Beekone 21:03, 1 October 2007 (UTC)
- Did you miss the hint of sarcasm in there? Dave 64.230.233.209 21:09, 1 October 2007 (UTC)
- It would be impossible to prove it either true or false - because no information can pass through a singularity. This makes it 'unfalsifiable' - which is a huge red flag for any theory you want science to take seriously.
- It would also be impossible to disprove the statement "The tooth-fairy did it" or "Kurt the Almighty caused the initial singularity to be pooped out by his 'seagull of eternal light'" - or "A pink and purple striped hamster named Norbert created the initial singularity by running too fast on his 'hamster wheel of ultimate destiny'"...there are an infinite number of such possible statements and all of them are impossible to either prove or disprove. Why would we believe one over the other? The probability that the Christian God did it is no greater than that a Hindu god or a Roman god or a Greek god or "The Devil" or the Tooth Fairy or Norbert the ethereal hamster did it.
- Worse still, all of those kinds of statement just force you to ask things like "What created Norbert?"...and when someone randomly (and without proof) says "Norbert was created by a small yellow teacup", we simply MUST ask "What created the teacup?" and when we find out who/what created THAT, we simply have another unanswerable riddle to solve. None of these complicated (and unfounded) claims are ever "final" - none has any measurable reason for being more true than the infinity of other possible creation stories. It's possible to suppose that "Norbert created the universe - and Norbert lives 'outside of time'" (whatever that means) - thereby making moot the question of what created Norbert...but why not simply say that the singularity is 'outside of time' - that's a much simpler answer.
- We are typically left with two options in the case of unfalsifiable claims: Occams' Razor - which may be roughly stated as: "The simplest explanation is usually the best" and Carl Sagan's: "Extraordinary claims require extraordinary evidence" - since we have none of the latter - we'd best rule out extraordinary claims and stick with the Occam - ruling out all but the simplest explanation - which is that no weird god-like thing did the job and a simple physical explanation for the beginning of the universe is much more likely to be true.
- Right now, I think the best is the one that Stephen Hawkins seems to promote - that time itself came out of the big bang, so there was no "before". Without a "before", nothing can have created the big bang - it is the first thing. To ask "what came before the beginning of time" is about as meaningful as asking what number comes after infinity. Mathematically, he could certainly be correct. But not everyone agrees with that point of view.
- Another plausible suggestion is that the universe ends with a 'big crunch' as gravity pulls everything back into one gigantic black hole...the singularity of which then explodes to form the big bang of the 'next' universe. This is simple - but it's starting to look like our universe doesn't have enough dark energy/matter/whatever in it to halt the expansion - so no convenient 'big crunch' will ever happen. However, the 'previous' universes could all have had more matter/energy in them and ended in a crunch - with ours being the first to last forever.
- We'll never know though.
Mix dogs
Aside from the obvious physical difficulties, is it possible to get puppies from dogs of very different sizes? Are there examples of puppies from say a great dane and a chiwawa? If it doesn't work what other characteristic would it need for them to be different species? 80.200.230.7 21:49, 1 October 2007 (UTC)
- Yes - all dogs can interbreed between breeds. SteveBaker 23:05, 1 October 2007 (UTC)
- Dogs are in fact all of one species, and they can therefore breed and produce fertile offspring whether they are poodles or Mexican rat terriers. In fact, the subspecies of dogs, Canis lupus familiaris falls into the same species as wolves, so dogs and wolves can actually interbreed. - Nunh-huh 23:09, 1 October 2007 (UTC)
You might find reading Poodle hybrid interesting (though as far as I know poodles can be of various sizes) ---- Xil...sist! 23:16, 1 October 2007 (UTC)
- (I think this user is talking more about the obvious physical differences.) Probably only if the chiwawa is the male. . . --S.dedalus 00:10, 2 October 2007 (UTC)
There can be a problem of, well, "mechanics" when two dogs of very different sizes attempt to mate. But, I once saw a video of a small dog trying to mate with a rather annoyed-looking lioness, so anything is possible. StuRat 00:12, 2 October 2007 (UTC)
- I've often wondered what the offspring of a chihuahua and a Great Dane would look like. -- JackofOz 01:05, 2 October 2007 (UTC)
Is this a new question?
If there is so many breeds of dogs, why aint there more breeds of humans? Is this a new question? —Preceding unsigned comment added by 88.109.239.189 (talk) 00:14, 2 October 2007 (UTC)
Locust flying speed
What is it? page does not say —Preceding unsigned comment added by 88.109.239.189 (talk) 23:38, 1 October 2007 (UTC)
- ~3.5 m/s or about 7.8 mph. [15]. Someguy1221 23:48, 1 October 2007 (UTC)
- Note, however, that the flight speed of an individual locust is much higher than the rate of progression of a locust swarm. Each individual locust flies and then stops, and each flight is at some angle with respect to the average vector of the swarm. for example, assume that on average an individual locust spends 10% of its time flying and flies on average at an angle of 45 degrees to the average direction of the swarm. Then, the rate of progression of the swarm would be 0.1/root 2 of the flight speed of the average locust. -Arch dude 01:59, 2 October 2007 (UTC)
Weighty of charge
I think about charge and current. what is weight of charge and current plese? —Preceding unsigned comment added by 88.109.239.189 (talk) 00:17, 2 October 2007 (UTC)
Another tick question I just thought of...
Is it possible for a tick to transmit HIV from one human to another? --Kurt Shaped Box 00:28, 2 October 2007 (UTC)
- theoretically yes, practically no. Each individual tick (or mosquito or other arthropod vector) almost never attacks more than one human. Ticks in particular usually attack an animal host and then a human host. The chances of a particular human being attacked by a tick that had previously attacked a HIV-infected human are vanishingly small. A flea is theoretically more likely, but I have not seen any reports in the literature. -Arch dude 01:42, 2 October 2007 (UTC)
- But leeches are another matter. Our article states that blood borne illnesses can be spread through a leech biting an infected person. --S.dedalus 01:45, 2 October 2007 (UTC)
Does water expand when heated?
If a glass of water is heated from 20 Celsius to 80 celsius, does the water expand and become less concentrated? Acceptable 01:28, 2 October 2007 (UTC)
- Yes the water will expand a very small amount and become less dense, if that is what you mean by concentrated. Sifaka talk 02:05, 2 October 2007 (UTC)


