Ebola: Difference between revisions
m →Prevention: Rewording to clarify sentence/correct grammar. |
|||
| Line 117: | Line 117: | ||
[[Universal precautions]], barrier nursing, and adquate sterilization is used to prevent the spread of the disease.<ref>{{harvnb|CDC|WHO|1998|p=11}}</ref> |
[[Universal precautions]], barrier nursing, and adquate sterilization is used to prevent the spread of the disease.<ref>{{harvnb|CDC|WHO|1998|p=11}}</ref> |
||
Vaccines have been produced for both Ebola<ref name="Jones2005">{{Cite doi|10.1038/nm1258}}</ref> and Marburg<ref name="Hevey1998">{{Cite doi|10.1006/viro.1998.9367}}</ref> that were 99% effective in protecting a group of monkeys from the disease. These vaccines are based on either a [[Recombinant DNA|recombinant]] [[Vesicular stomatitis virus]] or a recombinant [[Adenoviridae|Adenovirus]]<ref name="Sullivan2003">{{Cite doi|10.1038/nature01876}}</ref> carrying the Ebola spike protein on its surface. A recent vaccine trial conducted by the Vaccine Research Center (VRC) of the [[National Institutes of Health]] (NIH) in Bethesda, MD successfully demonstrated an immune response to the virus in humans.<ref>{{cite press release|title=Ebola/Marburg Vaccine Development|publisher=National Institute of Allergy and Infectious Diseases|date=2008-09-15|url=http://www.niaid.nih.gov/vrc/scientificupdates_ebola.htm}}</ref> The biggest problem with the vaccine is that, unless the patient is given it near the onset of the virus (1–4 days after the symptoms begin), there will be too much damage to the human body to repair, e.g., ruptured arteries and capillaries, vomiting, and other symptoms that may still cause enough harm to kill or seriously traumatize the patient. Another downside is that Ebola is a RNA virus, meaning that it can mutate into a different form. Nevertheless, such vaccines may play a crucial role by allowing health care workers and primary responders to safely help patients.<ref>{{citation|last=Oplinger|first=Anne A.|date=2003-11-18|title=NIAID Ebola vaccine enters human trial|url=http://news.bio-medicine.org/medicine-news-2/NIAID-Ebola-vaccine-enters-human-trial-4881-3/|publisher=Bio-Medicine}}</ref> |
Vaccines have been produced for both Ebola<ref name="Jones2005">{{Cite doi|10.1038/nm1258}}</ref> and Marburg<ref name="Hevey1998">{{Cite doi|10.1006/viro.1998.9367}}</ref> that were 99% effective in protecting a group of monkeys from the disease. These vaccines are based on either a [[Recombinant DNA|recombinant]] [[Vesicular stomatitis virus]] or a recombinant [[Adenoviridae|Adenovirus]]<ref name="Sullivan2003">{{Cite doi|10.1038/nature01876}}</ref> carrying the Ebola spike protein on its surface. A recent vaccine trial conducted by the Vaccine Research Center (VRC) of the [[National Institutes of Health]] (NIH) in Bethesda, MD successfully demonstrated an immune response to the virus in humans.<ref>{{cite press release|title=Ebola/Marburg Vaccine Development|publisher=National Institute of Allergy and Infectious Diseases|date=2008-09-15|url=http://www.niaid.nih.gov/vrc/scientificupdates_ebola.htm}}</ref> The biggest problem with the vaccine is that, unless the patient is given it near the onset of the virus (1–4 days after the symptoms begin), there will be too much damage to the human body to repair, e.g., ruptured arteries and capillaries, vomiting, and other symptoms that may still cause enough harm to kill or seriously traumatize the patient. Another downside is that Ebola is a RNA virus, meaning that it can mutate into a different form. Nevertheless, such vaccines may play a crucial role by allowing health care workers and primary responders to safely help patients.<ref>{{citation|last=Oplinger|first=Anne A.|date=2003-11-18|title=NIAID Ebola vaccine enters human trial|url=http://news.bio-medicine.org/medicine-news-2/NIAID-Ebola-vaccine-enters-human-trial-4881-3/|publisher=Bio-Medicine}}</ref> Clinical trails are currently underway to determine the safety and immunogenicity of candidate DNA vaccines for Ebola and Marburg.<ref>{{citation|title=Ebola/Marburg Vaccine Development|url=http://www3.niaid.nih.gov/topics/ebolaMarburg/default.htm|date=2009-02-19|publisher=National Institute of Allergy and Infectious Diseases (NIAID)}}</ref> |
||
===Symptoms=== |
===Symptoms=== |
||
Revision as of 00:36, 11 June 2009
| Ebola | |
|---|---|

| |
| An electron micrograph of an Ebola virus | |
| Virus classification | |
| Group: | Group V ((−)ssRNA)
|
| Order: | |
| Family: | |
| Genus: | Ebolavirus
|
| Type species | |
| Zaïre virus | |
| Species | |
|
Ivory Coast ebolavirus | |
| Ebola | |
|---|---|
| Specialty | Infectious diseases |
Ebola is the common term for a group of viruses belonging to genus Ebolavirus (EBOV), which is a part of the family Filoviridae, and for the disease that they cause, Ebola hemorrhagic fever. The virus is named after the Ebola River, where the first recognized outbreak of Ebola hemorrhagic fever occurred. The viruses are characterized by long filaments, and have a shape similar to that of the Marburg virus, also in the family Filoviridae, and possessing similar disease symptoms.
There are a number of species within the ebolavirus genus, which in turn have a number of specific strains or serotypes. The Zaïre virus is the type species, which is also the first discovered and the most lethal. Ebola is transmitted primarily through bodily fluids and to a limited extent through skin and mucous membrane contact. Also, through contact with dead bodies. The virus interferes with the endothelial cells lining the interior surface of blood vessels and platelet cells. As the blood vessel walls become damaged and the platelets are unable to coagulate, patients succumb to hypovolemic shock.
Ebola first emerged in 1976 in Zaire. It remained largely obscure until 1989 with the outbreak in Reston, Virginia.
Etymology
The virus is named after the Ebola River Valley in the Democratic Republic of the Congo (formerly Zaïre), which is near the site of the first recognized outbreak in 1976, in a mission hospital run by Flemish nuns.[1]
Classification
The genera Marburgvirus and Ebolavirus was originally classified as the species of the now nonexistent Filovirus genus. In March 1998, the Vertebrate Virus Subcommittee proposed to the International Committee on Taxonomy of Viruses (ICTV) to change the Filovirus genus to Filovirus family with two specific generas: Ebola-like viruses and Marburg-like viruses. This proposal was implemented in Washington, D.C. as of April 2001 and in Paris as of July 2002. In 2000, another proposal was made in Washington, D.C. to change the "-like viruses" to "-virus" (e.g. Ebolavirus, Marburgvirus).[2]
- Zaïre virus (ZEBOV)
- The Zaïre virus, formerly named Zaïre Ebola Virus, has the highest case-fatality rate, up to 90% in some epidemics, with an average case fatality rate of approximately 83% over 27 years. The case-fatality rates were 88% in 1976, 59% in 1994, 81% in 1995, 73% in 1996, 80% in 2001-2002, and 90% in 2003. There have been more outbreaks of Zaïre ebolavirus than any other strain. The first outbreak took place on 26 August 1976 in Yambuku. Mabalo Lokela, a 44-year-old schoolteacher, became the first recorded case. The symptoms resembled malaria, and subsequent patients received quinine. The initial transmission was believed to be due to reuse of the needle for Lokela's injection without sterilization. Subsequent transmission was also due to lack of barrier nursing and the traditional burial preparation method, which involves washing and gastrointestinal tract cleansing.[3]
- Sudan ebolavirus (SEBOV)
- The virus was the second species of Ebola emerging simultaneous with the Zaïre virus. It was believed to have originated amongst cotton factory workers in Nzara, Sudan, with the first case reported as a worker exposed to a potential natural reservoir. Scientists tested all animals and insects in response to this; however, none tested positive for the virus. The carrier is still unknown. The lack of barrier nursing facilitated the spread of the disease. The most recent outbreak occurred in May 2004. 20 confirmed cases of it were reported in Yambio County, Sudan, with five deaths resulting. The average fatality rates for were 54% in 1976, 68% in 1979, and 53% in 2000 and 2001.
- Reston ebolavirus (REBOV)
- The virus is classified as species of Ebola. It was discovered during an outbreak of Simian hemorrhagic fever virus (SHFV) in crab-eating macaques from Hazleton Laboratories (now Covance) in 1989. Since the initial outbreak in Reston, it has emerged in the Philippines, Siena Italy, Texas,[4] and recently among pigs in the Philippines.[5] Despite its status as a Level-4 organism, it is non-pathogenic to humans however hazardous to monkeys.[6]
- Ivory Coast ebolavirus (CIEBOV)
- The virus was first discovered among chimpanzees of the Tai Forest in Côte d'Ivoire, Africa. On 1 November 1994, the corpses of two chimpanzees were found in the forest. Necropsies showed blood within the heart to be liquid and brown; no obvious marks were seen on the organs; and one necropsy displayed lungs filled with liquid blood. Studies of tissues taken from the chimps showed results similar to human cases during the 1976 Ebola outbreaks in Zaïre and Sudan. Later in 1994, more dead chimpanzees were discovered, with many testing positive to Ebola using molecular techniques. The source of contamination was believed to be the meat of infected Western Red Colobus monkeys, upon which the chimpanzees preyed. One of the scientists performing the necropsies on the infected chimpanzees contracted Ebola. She developed symptoms similar to those of dengue fever approximately a week after the necropsy, and was transported to Switzerland for treatment. She was discharged from hospital after two weeks and had fully recovered six weeks after the infection.[7]
- Bundibugyo ebolavirus
- On November 24 2007, the Uganda Ministry of Health confirmed an outbreak of Ebola in the Bundibugyo District. After confirmation of samples tested by the United States National Reference Laboratories and the CDC, the World Health Organization confirmed the presence of the new species. On 20 February 2008, the Uganda Ministry officially announced the end of the epidemic in Bundibugyo with the last infected person discharged on 8 January 2008.[8] Ugandan officials confirmed a total of 149 cases of this new Ebola species, with 37 deaths attributed to the strain (24.83%).[9]
Virology
Structure
Electron micrographs of members of genus Ebolavirus show them to have the characteristic thread-like structure of a filovirus.[10] EBOV VP30 is around 288 amino acids long.[11] The virions are tubular in general form but variable in overall shape and may appear as the classic shepherd's crook or eyebolt, as a U or a 6, or coiled, circular, or branched. However, laboratory purification techniques, such as centrifugation, may contribute to some of these.[12] Virions are generally 80 nm in diameter with a lipid bilayer anchoring the glycoprotien which projects 7 to 10 nm long spikes from its surface.[13] They are of variable length, typically around 800 nm, but may be up to 1000 nm long. In the center of the virion is a structure called nucleocapsid, which is formed by the helically-wound viral genomic RNA complexed with the proteins NP, VP35, VP30, and L.[14] It has a diameter of 80 nm and contains a central channel of 20–30 nm in diameter. Virally-encoded glycoprotein (GP) spikes 10 nm long and 10 nm apart are present on the outer viral envelope of the virion, which is derived from the host cell membrane. Between envelope and nucleocapsid, in the so-called matrix space, the viral proteins VP40 and VP24 are located.[citation needed]
Genome
Each virion contains one minor molecule of linear, single-stranded, negative-sense RNA, totaling 18,959 to 18,961 nucleotides in length. The 3′ terminus is not polyadenylated and the 5′ end is not capped. It was found that 472 nucleotides from the 3' end and 731 nucleotides from the 5' end are sufficient for replication.[15] It codes for seven structural proteins and one non-structural protein. The gene order is 3′ - leader - NP - VP35 - VP40 - GP/sGP - VP30 - VP24 - L - trailer - 5′; with the leader and trailer being non-transcribed regions, which carry important signals to control transcription, replication, and packaging of the viral genomes into new virions. The genomic material by itself is not infectious, because viral proteins, among them the RNA-dependent RNA polymerase, are necessary to transcribe the viral genome into mRNAs, as well as for replication of the viral genome.
Life cycle
Viruses do not grow through cell division, because they are acellular; instead, they use the machinery and metabolism of a host cell to produce multiple copies of themselves, and they assemble in the cell.[14][16]
- The virus attaches to host receptors through the GP (glycoprotein) surface peplomer and is endocytosed into vesicles in the host cell.
- Fusion of virus membrane with the vesicle membrane occurs; nucleocapsid is released into the cytoplasm.
- The encapsidated, negative-sense genomic ssRNA is used as a template for the synthesis ( 3' - 5') of polyadenylated, monocistronic mRNAs.
- Translation of the mRNA into viral proteins occurs using the host cell's machinery.
- Post-translational processing of viral proteins occurs. GP0 (glycoprotein precursor) is cleaved to GP1 and GP2, which are heavily glycosylated. These two molecules assemble, first into heterodimers, and then into trimers to give the surface peplomers. SGP (secreted glycoprotein) precursor is cleaved to SGP and delta peptide, both of which are released from the cell.
- As viral protein levels rise, a switch occurs from translation to replication. Using the negative-sense genomic RNA as a template, a complementary +ssRNA is synthesized; this is then used as a template for the synthesis of new genomic (-)ssRNA, which is rapidly encapsidated.
- The newly-formed nucleocapsides and envelope proteins associate at the host cell's plasma membrane; budding occurs, and the virions are released.
Natural reservoirs
Despite numerous studies, the wildlife reservoir of Ebolavirus has not been identified. Between 1976 and 1998, from 30,000 mammals, birds, reptiles, amphibians, and arthropods sampled from outbreak regions, no Ebolavirus was detected[17] apart from some genetic material found in six rodents (Mus setulosus and Praomys species) and a shrew (Sylvisorex ollula) collected from the Central African Republic in 1998.[18] Ebolavirus was detected in the carcasses of gorillas, chimpanzees, and duikers during outbreaks in 2001 and 2003 (the carcasses were the source of the initial human infections), but the high mortality from infection in these species precludes them from acting as reservoirs.[17] As of late 2005, three species of fruit bat have been identified as carrying the virus but not showing disease symptoms, and they are now believed to be the natural host species, or reservoir, of the virus.[19]
Plants, arthropods, and birds have also been considered as reservoirs; however, bats are considered the most likely candidate.[20] Bats were known to reside in the cotton factory in which the index cases for the 1976 and 1979 outbreaks were employed, and they have also been implicated in Marburg infections in 1975 and 1980.[17] Of 24 plant species and 19 vertebrate species experimentally inoculated with Ebolavirus, only bats became infected.[21] The absence of clinical signs in these bats is characteristic of a reservoir species. In 2002-03, a survey of 1,030 animals from Gabon and the Republic of the Congo including 679 bats found Ebolavirus RNA in 13 fruit bats (Hypsignathus monstrosus, Epomops franquetti and Myonycteris torquata).[22] Bats are also known to be the reservoirs for a number of related viruses including Nipah virus, Hendra virus and Lyssaviruses.
Pathogenesis
Ebolavirus interferes mainly with the endothelial cells lining the interior surface of blood vessels and platelet cells. As the vessel walls become damaged from infection and the platelet cells are unable to coagulate, patients quickly lose blood and succumb to shock. Filoviruses replicate well in a wide range of organs and cell types such as hepatocytes, epithelial cells, fibroblasts, fibroblastic reticular cells, and adrenal cortical cells.[23]
Epidemiology
Prevalence
Outbreaks of Ebola have mainly been restricted to Africa,[24] from which many of the outbreaks consume the population before it can effectively spread.[citation needed]Lack of roads also helps to contain the outbreak.
Confirmed cases of Ebola Hemorrhagic Fever (EHF) has occured in the Democratic Republic of the Congo, Gabon, Sudan, the Ivory Coast, Uganda, and the Republic of the Congo.[25]
Reston ebolavirus (EBO-R), believed to potentially be either another subtype of Ebola or another filovirus, has spread to numerous areas via air transport of infected monkeys. It has emerged in: Reston, Virginia; Alice, Texas; the Philippines; and Italy.
On 11 December 2008, pigs from farms slightly north of Manila, Philippines tested positive for the virus. [26]
Transmission
The virus has been confirmed to be transmitted through body fluids, however, transmission through oral exposure and through conjunctiva exposure is possible.[27] Transmission through the oral and conjunctiva route has been confirmed in non-human primates.[28] Filoviruses are not naturally transmitted by aerosol, they are highly infectious as breathable 0.8-1.2 micron dropllets in laboratory conditions. Because of this potential route of infection, these viruses have been classified as Category A biological weapons.[29][30]
The chance that a mutation would allow ebola to transmitt by airborn means are unlikely.[31]
Although airborne transmission between monkeys has been demonstrated by an accidental outbreak in a laboratory located in Virginia, USA, there is very limited evidence for human-to-human airborne transmission in any reported epidemics. Nurse Mayinga might represent the only possible case. The means by which she contracted the virus remains uncertain.
The infection of human cases with Ebolavirus has been documented through the handling of infected chimpanzees, gorillas, and forest antelopes — both dead and alive — as was documented in Côte d'Ivoire, the Republic of Congo, and Gabon. The transmission of the Ebola Reston strain through the handling of cynomolgus monkeys has also been reported.[32] Ebola Reston has been found in pigs in Luzon.
So far, all epidemics of Ebola have occurred in sub-optimal hospital conditions, where practices of basic hygiene and sanitation are often either luxuries or unknown to caretakers and where disposable needles and autoclaves are unavailable or too expensive. In modern hospitals with disposable needles and knowledge of basic hygiene and barrier nursing techniques, Ebola has never spread on such a large scale.
In the early stages, Ebola may not be highly contagious. Contact with someone in early stages may not even transmit the disease. As the illness progresses, bodily fluids from diarrhea, vomiting, and bleeding represent an extreme biohazard. Due to lack of proper equipment and hygienic practices, large-scale epidemics occur mostly in poor, isolated areas without modern hospitals or well-educated medical staff. Many areas where the infectious reservoir exists have just these characteristics. In such environments, all that can be done is to immediately cease all needle-sharing or use without adequate sterilization procedures, to isolate patients, and to observe strict barrier nursing procedures with the use of a medical rated disposable face mask, gloves, goggles, and a gown at all times. This should be strictly enforced for all medical personnel and visitors.
Ebola is unlikely to develop into a pandemic, or world-wide infection, due to its difficulty in spreading by airborne transmission and the period of time that the virus can use a living and contagious victim to spread compared to other infectious diseases. In isolated settings such as a quarantined hospital or a remote village, most victims are infected shortly after the first case of infection is present. In addition, the quick onset of symptoms from the time the disease becomes contagious in an individual makes it easy to identify sick individuals and limits an individual's ability to spread the disease by traveling. Because bodies of the deceased are still infectious, some doctors had to take measures to make sure that the disposal of dead bodies were conducted in a safe manner despite any local traditional burial rituals.[33]
Medical aspects
Prevention
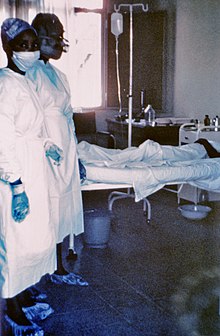
Universal precautions, barrier nursing, and adquate sterilization is used to prevent the spread of the disease.[34]
Vaccines have been produced for both Ebola[35] and Marburg[36] that were 99% effective in protecting a group of monkeys from the disease. These vaccines are based on either a recombinant Vesicular stomatitis virus or a recombinant Adenovirus[37] carrying the Ebola spike protein on its surface. A recent vaccine trial conducted by the Vaccine Research Center (VRC) of the National Institutes of Health (NIH) in Bethesda, MD successfully demonstrated an immune response to the virus in humans.[38] The biggest problem with the vaccine is that, unless the patient is given it near the onset of the virus (1–4 days after the symptoms begin), there will be too much damage to the human body to repair, e.g., ruptured arteries and capillaries, vomiting, and other symptoms that may still cause enough harm to kill or seriously traumatize the patient. Another downside is that Ebola is a RNA virus, meaning that it can mutate into a different form. Nevertheless, such vaccines may play a crucial role by allowing health care workers and primary responders to safely help patients.[39] Clinical trails are currently underway to determine the safety and immunogenicity of candidate DNA vaccines for Ebola and Marburg.[40]
Symptoms
Symptoms are varied and often appear suddenly. Initial symptoms include high fever (at least 38.8°C; 101.8°F), severe headache, muscle, joint, or abdominal pain, severe weakness, exhaustion, sore throat, nausea, dizziness, internal and external bleeding.[41] Before an outbreak is suspected, these early symptoms are easily mistaken for malaria, typhoid fever, dysentery, influenza, or various bacterial infections, which are all far more common and reliably less fatal.
Ebola may progress to cause more serious symptoms, such as diarrhea, dark or bloody feces, vomiting blood, red eyes due to distension and hemorrhage of sclerotic arterioles, petechia, maculopapular rash, and purpura. Other, secondary symptoms include hypotension (low blood pressure), hypovolemia, tachycardia, organ damage (especially the kidneys, spleen, and liver) as a result of disseminated systemic necrosis, and proteinuria. The interior bleeding is caused by a reaction between the virus and the platelets that produces a chemical that will cut cell-size holes into the capillary walls.
On occasion, internal and external hemorrhage from orifices, such as the nose and mouth, may also occur, as well as from incompletely-healed injuries such as needle-puncture sites. Ebola virus can affect the levels of white blood cells and platelets, disrupting clotting. More than 50% of patients will develop some degree of hemorrhaging. Eventually, Ebola will turn the host's organs to a mush-like substance.
Diagnosis
Methods of diagnosis of Ebola include testing saliva and urine samples. Ebola is diagnosed with an Enzyme-Linked ImmunoSorbent Assay (ELISA) test. This diagnosis method has produced potentially ambiguous results during non-outbreak situations. Following Reston, and in an effort to evaluate the original test, Dr. Karl Johnson of the CDC tested San Blas Indians from Central America, who have no history of Ebola infection, and observed a 2% positive result. Other researchers later tested sera from Native Americans in Alaska and found a similar percentage of positive results. To combat the false positives, a more complex test based on the ELISA system was developed by Tom Kzaisek at USAMRIID, which was later improved with Immunofluorescent antibody analysis (IFA). It was however not used during the serosurvey following Reston.[42] These treatments are not commerically available.[32]
Prognosis
The incubation period can range from 2 to 21 days but is generally 5–10 days.[43]
Ebola hemorrhagic fever is potentially lethal and encompasses a range of symptoms including fever, vomiting, diarrhea, generalized pain or malaise, and sometimes internal and external bleeding. The span of time from onset of symptoms to death is usually between 2 and 21 days. By the second week of infection, patients will either defervesce (the fever will lessen) or undergo systemic multi-organ failure. Mortality rates are typically high, with the human case-fatality rate ranging from 50–89%, depending on the species or viral strain.[44] The cause of death is usually due to hypovolemic shock or organ failure.[45]
Treatment

There is no standard treatment for Ebola hemorrhagic fever. Treatment is primarily supportive and includes minimizing invasive procedures, balancing electrolytes, and, since patients are frequently dehydrated, replacing lost coagulation factors to help stop bleeding, maintaining oxygen and blood levels, and treating any complicating infections. Convalescent plasma (factors from those that have survived Ebola infection) shows promise as a treatment for the disease. Ribavirin is ineffective. Interferon is also thought to be ineffective. In monkeys, administration of an inhibitor of coagulation (rNAPc2) has shown some benefit, protecting 33% of infected animals from a usually 100% (for monkeys) lethal infection (however, this inoculation does not work on humans). In early 2006, scientists at USAMRIID announced a 75% recovery rate after infecting four rhesus monkeys with Ebolavirus and administering Morpholino antisense drugs.[46] Development of improved Morpholino antisense conjugated with cell penetrating peptides is ongoing.[47]
History
Emergence
Ebolavirus first emerged in 1976 in outbreaks of Ebola hemorrhagic fever in Zaire and Sudan.[48] The strain of Ebola that broke out in Zaire has one of the highest case fatality rates of any human pathogenic virus, roughly 90%.[49] The strain that broke out later in Sudan has a case fatality rate of around 50%.[49] The virus is believed to be transmitted to humans via contact with an infected animal host. The virus is then transmitted to other people that come into contact with blood and bodily fluids of the infected person, and by human contact with contaminated medical equipment such as needles. Both of these infectious mechanisms will occur in clinical (nosocomial) and non-clinical situations. Due to the high fatality rate, the rapidity of demise, and the often remote areas where infections occur,plus poor hygiene in the area, the potential for widespread epidemic outbreaks is considered low.
Proceedings of an International Colloquium on Ebola Virus Infection and Other Haemorrhagic Fevers held in Antwerp, Belgium on December 6 through December 8 in 1977.[50]
Public attention
While investigating an outbreak of Simian hemorrhagic fever (SHFV) in November 1989, an electron microscopist from USAMRIID discovered filoviruses similar in appearance to Ebola in tissue samples taken from Crab-eating Macaque imported from the Philippines to Hazleton Laboratories Reston, Virginia.[51] Due to the lethality of the suspected and previously obscure virus, the investigation quickly attracted attention.[citation needed]
Blood samples were taken from 178 animal handlers during the incident.[52] Of those, six animal handlers eventually seroconverted. When the handlers failed to become ill, the CDC concluded that the virus had a very low pathogenicity to humans.[53]
Both the Philippines and the United States had no previous cases of infection, and upon further isolation it was concluded to be another species of Ebola or a new filovirus of Asian origin, and named Reston ebolavirus (REBOV) after the location of the incident.
Recent cases
In 1992, members of Japan's Aum Shinrikyo cult considered using Ebola as a terror weapon. Their leader, Shoko Asahara, led about forty members to Zaire under the guise of offering medical aid to Ebola victims in a presumed attempt to acquire a virus sample.[54] Because of the virus's high morbidity, it can be a potential agent for biological warfare.[55]
Given the lethal nature of Ebola, and, since no approved vaccine or treatment is available, it is classified as a biosafety level 4 agent, as well as a Category A bioterrorism agent by the Centers for Disease Control and Prevention. It has the potential to be weaponized for use in biological warfare.[56] The effectiveness as a biological weapon is compromised by its rapid lethality as patients quickly die off before they are capable of effectively spreading the contagion.[citation needed]
The attention gathered from the outbreak in Reston prompted an increase in public interest, leading to the publication of numerous fictional works.
The BBC reports that in a study that frequent outbreaks of ebola may have resulted in the deaths of 5,000 gorillas.[57]
As of August 30, 2007, 103 people (100 adults and three children) were infected by a suspected hemorrhagic fever outbreak in the village of Kampungu, Democratic Republic of the Congo. The outbreak started after the funerals of two village chiefs, and 217 people in four villages fell ill. The World Health Organization sent a team to take blood samples for analysis and confirmed that many of the cases are the result of Ebolavirus.[58][59] The Congo's last major Ebola epidemic killed 245 people in 1995 in Kikwit, about 200 miles from the source of the August 2007 outbreak.[60]
On November 30, 2007, the Uganda Ministry of Health confirmed an outbreak of Ebola in the Bundibugyo District. After confirmation of samples tested by the United States National Reference Laboratories and the Centers for Disease Control, the World Health Organization confirmed the presence of a new species of Ebolavirus.[61] The epidemic came to an official end on February 202008. While it lasted, 149 cases of this new strain were reported, and 37 of those led to deaths.
An International Symposium to explore the environment and filovirus, cell system and filovirus interaction, and filovirus treatment and prevention is to be held at Centre Culturel Français, Libreville, Gabon during March 2008.[62] The virus appeared in southern Western Kasai province on November 27, 2008,[63] and blood and stool samples were sent to laboratories in Gabon and South Africa for identification.
A mysterious disease that has killed eleven and infected twenty-one people in southern Democratic Republic of Congo has been identified as the Ebola virus.[64] Doctors Without Borders reports 11 deaths as of Monday 29 December 2008 in the Western Kasai province of the Democratic Republic of Congo. It is said that a further 24 cases are being treated. In January 2009, Angola closed down part their border with DRC to prevent the spread of ebola.[65]
On March 12, 2009, an unidentified 45-year-old female scientist from Germany accidentally pricked her finger with a needle used to inject Ebola into lab mice. She was given an experimental vaccine never before used on humans. Since the peak period for an outbreak during the 21-day Ebola incubation period has passed as of April 2, 2009, she has been declared healthy and safe. It remains unclear whether or not she was ever actually infected with the virus.[66]
References
Notes
- ^ Bardi, Jason Socrates (2002). "Death Called a River". Scripps Research Institute. 2 (1). Retrieved 2006-12-08.
- ^ Büchen-Osmond, Cornelia (2006-04-25). "ICTVdB Virus Description - 01.025.0.02. Ebolavirus". International Committee on Taxonomy of Viruses. Retrieved 2009-06-02.
- ^ Isaacson, Margaretha. "Two Belgian nurses died of Ebola".
{{cite journal}}: Cite journal requires|journal=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Special Pathogens Branch CDC (2008-01-14). "Known Cases and Outbreaks of Ebola Hemorrhagic Fever". Center for Disease Control and Prevention. Retrieved 2008-08-02.
- ^ "Pig-to-Human Ebola Case Suspected in Philippines". New York Times. 2009-01-24. Retrieved 2009-01-26.
- ^ McCormick & Fisher-Hoch 1999, p. 300
- ^ Waterman, Tara (1999), Ebola Cote D'Ivoire Outbreaks, Stanford University, retrieved 2009-05-30
- ^ "End of Ebola outbreak in Uganda" (Press release). World Health Organization. 2008-02-20.
- ^ Cocks, Tim (2007-12-11). "Uganda confirms 113 suspected Ebola cases". Reuters. Retrieved 2008-02-25.
- ^ Klenk & Feldmann 2004, p. 2
- ^ Klenk & Feldmann 2004, p. 13
- ^ Klenk & Feldmann 2004, pp. 33–35
- ^ Klenk & Feldmann 2004, pp. 28
- ^ a b Biomarker Database, Ebola virus, Korea National Institute of Health, retrieved 2009-05-31
- ^ Klenk & Feldmann 2004, p. 9
- ^ ViralZone: Ebola-like viruses, Swiss Institute of Bioinformatics, retrieved 2009-05-31
- ^ a b c Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/j.micinf.2005.04.006, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/j.micinf.2005.04.006instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/S1286-4579(99)00242-7, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/S1286-4579(99)00242-7instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 17940947, please use {{cite journal}} with
|pmid=17940947instead. - ^ "Fruit bats may carry Ebola virus". BBC News. 2005-12-11. Retrieved 2008-02-25.
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 8969248, please use {{cite journal}} with
|pmid=8969248instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1038/438575a, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1038/438575ainstead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1128/JVI.77.18.9733-9737.2003, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1128/JVI.77.18.9733-9737.2003instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 15078595, please use {{cite journal}} with
|pmid=15078595instead. - ^ Questions and Answers about Ebola Hemorrhagic Fever, Centers for Disease Control and Prevention, 2009-03-25, retrieved 2009-05-31
- ^ Gale, Jason (2008-12-11). "Pig Ebola May Lead Scientists to 'Elusive Reservoir' of Virus". New York City: Bloomberg L.P. Retrieved 2008-12-22.
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 8551825, please use {{cite journal}} with
|pmid=8551825instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 8712894, please use {{cite journal}} with
|pmid=8712894instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 15588056, please use {{cite journal}} with
|pmid=15588056instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 7547435, please use {{cite journal}} with
|pmid=7547435instead. - ^ Russell, Brett, What is the probability of a dangerous strain of Ebola mutating and becoming airborne?, Self, retrieved 2009-05-31
- ^ a b Ebola and Marburg haemorrhagic fever - Factsheet, European Centre for Disease Prevention and Control, 2008-07-10, retrieved 2009-05-31
{{citation}}: Check date values in:|year=/|date=mismatch (help) - ^ Harden, Blaine (2001-02-18). "Dr. Matthew's Passion". New York Times Magazine. Retrieved 2008-02-25.
- ^ CDC & WHO 1998, p. 11
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1038/nm1258, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1038/nm1258instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1006/viro.1998.9367, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1006/viro.1998.9367instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1038/nature01876, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1038/nature01876instead. - ^ "Ebola/Marburg Vaccine Development" (Press release). National Institute of Allergy and Infectious Diseases. 2008-09-15.
- ^ Oplinger, Anne A. (2003-11-18), NIAID Ebola vaccine enters human trial, Bio-Medicine
- ^ Ebola/Marburg Vaccine Development, National Institute of Allergy and Infectious Diseases (NIAID), 2009-02-19
- ^ World Health Organization (2007-09), Ebola haemorrhagic fever, retrieved 2009-05-02
{{citation}}: Check date values in:|date=(help) - ^ McCormick & Fisher-Hoch 1999, pp. 302–303
- ^ Francesconi, Paolo (2003-10-09), "Ebola Hemorrhagic Fever Transmission and Risk Factors of Contacts, Uganda", Emerging Infectious Diseases, vol. 9, no. 11, Centers for Disease Control and Prevention, retrieved 2009-05-30
{{citation}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 15752448, please use {{cite journal}} with
|pmid=15752448instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/j.biocel.2005.02.018, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/j.biocel.2005.02.018instead. - ^ Linden, Caree Vander (2006-01-13), Gene-Specific Ebola Therapies Protect Nonhuman Primates from Lethal Disease (New Release), U.S. Army Medical Research Institute of Infectious Diseases, retrieved 2009-05-31
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1128/AAC.00936-08, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1128/AAC.00936-08instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 7787519, please use {{cite journal}} with
|pmid=7787519instead. - ^ a b King, John W (April 2, 2008). "Ebola Virus". eMedicine. WebMd. Retrieved 2008-10-06.
- ^ Pattyn 1978, p. 3
- ^ McCormick & Fisher-Hoch 1999, pp. 277–279
- ^ Waterman, Tara (1999), Ebola Reston Outbreaks, Stanford University, retrieved 2008-08-02
- ^ McCormick & Fisher-Hoch 1999, pp. 298–299
- ^ Monterey Institute for International Studies (2001), Chronology of Aum Shinrikyo's CBW Activities (PDF), James Martin Center for Nonproliferation Studies
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 15207310, please use {{cite journal}} with
|pmid=15207310instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1001/jama.287.18.2391, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1001/jama.287.18.2391instead. - ^ Ebola 'kills over 5,000 gorillas', BBC, 2006-12-08, retrieved 2009-05-31
- ^ "Ebola Outbreak Confirmed in Congo". NewScientist.com. 2007-09-11. Retrieved 2008-02-25.
- ^ Ebola outbreak in Congo, CDC news, 2007-09-12, retrieved 2009-05-31
- ^ "Mystery DR Congo fever kills 100". BBC News. 2007-08-31. Retrieved 2008-02-25.
- ^ "Uganda: Deadly Ebola Outbreak Confirmed - UN". UN News Service. 2007-11-30. Retrieved 2008-02-25.
- ^ The IV International Symposium on Filoviruses, l'Institut de recherche pour le développement (IRD), retrieved 2009-0-31
{{citation}}: Check date values in:|accessdate=(help) - ^ World Health Organization (2008-12-27), RD Congo : Fièvre hémorragique à virus Ebola au Kasaï Occidental, Rapport de situation No 1 des 26 & 27 décembre 2008 (in French), Relief Web, retrieved 2009-06-02
- ^ Ebola epidemic kills nine in central DR Congo: report, Agence France-Presse, 2008-12-25, retrieved 2009-05-30
- ^ Ebola alert shuts Angolan border, BBC, 2009-01-06, retrieved 2009-05-31
- ^ Eddyn, Melissan (2009-03-27), Scientist Injects Self With Ebola, Associated Press, retrieved 2009-05-02
Bibliography
- Centers for Disease Control and Prevention and World Health Organization (1998), Infection Control for Viral Haemorrhagic Fevers in the African Health Care Setting (PDF), Atlanta, Georgia, USA: Centers for Disease Control and Prevention, retrieved 2009-05-31
- Klenk, Hans-Dieter; Feldmann, Heinz (2004-03-10), Ebola and Marburg viruses: molecular and cellular biology, Taylor & Francis, ISBN 978-0954523237, retrieved 2009-05-16
- McCormick, Joseph; Fisher-Hoch, Susan (1999) [1996], Level 4: Virus Hunters of the CDC, Horvitz, Leslie Alan ("Updated edition" 3rd ed.), Barnes & Noble, ISBN 9780760712085, retrieved 2008-08-02
{{citation}}: Unknown parameter|origmonth=ignored (help) - Pattyn, S.R. (1978), Ebola Virus Haemorrhagic Fever, Elsevier / North-Holland Biomedical Press 335 Jan van Galenstraat, P.O. Box 211 Amsterdam, The Netherlands: Elsevier / North-Holland Biomedical Press, ISBN 0-444-80060-3, retrieved 2009-05-31
{{citation}}: CS1 maint: location (link)
