Statin: Difference between revisions
merge dupe refs, fixes volume number error |
→Drug interactions: deleted uncited practice with potentially dangerous medical effects |
||
| Line 66: | Line 66: | ||
Combining any statin with a [[fibrate]] or niacin, another category of lipid-lowering drugs, increases the risks for [[rhabdomyolysis]] to almost 6.0 per 10,000 person-years.<ref name="Graham2004"/> Most physicians have now abandoned routine monitoring of liver enzymes and [[creatine kinase]], although they still consider this prudent in those on high-dose statins or in those on statin/fibrate combinations, and mandatory in the case of muscle cramps or of deterioration in [[renal function]]. |
Combining any statin with a [[fibrate]] or niacin, another category of lipid-lowering drugs, increases the risks for [[rhabdomyolysis]] to almost 6.0 per 10,000 person-years.<ref name="Graham2004"/> Most physicians have now abandoned routine monitoring of liver enzymes and [[creatine kinase]], although they still consider this prudent in those on high-dose statins or in those on statin/fibrate combinations, and mandatory in the case of muscle cramps or of deterioration in [[renal function]]. |
||
Consumption of [[grapefruit]] or [[grapefruit juice]] inhibits the metabolism of statins. [[Bitter orange]]s may have a similar effect.<ref>[http://www.mayoclinic.com/health/food-and-nutrition/AN00413 Mayo clinic: article on interference between grapefruit and medication]</ref> Furanocoumarins in grapefruit juice (i.e. [[bergamottin]] and [[dihydroxybergamottin]]) inhibit the [[Cytochrome P450 oxidase|cytochrome P450]] enzyme [[CYP3A4]], which is involved in the metabolism of most statins (however it is a major inhibitor of only lovastatin, simvastatin and to a lesser degree atorvastatin) and some other medications<ref name="Kane2000">{{cite journal |author=Kane GC, Lipsky JJ |title=Drug-grapefruit juice interactions |journal=Mayo Clin. Proc. |volume=75 |issue=9 |pages=933–42 |year=2000 |pmid=10994829 |doi=10.4065/75.9.933}}</ref> (it had been thought that flavonoids (i.e. [[naringin]]) were responsible). This increases the levels of the statin, increasing the risk of dose-related adverse effects (including [[myopathy]]/[[rhabdomyolysis]]) |
Consumption of [[grapefruit]] or [[grapefruit juice]] inhibits the metabolism of statins. [[Bitter orange]]s may have a similar effect.<ref>[http://www.mayoclinic.com/health/food-and-nutrition/AN00413 Mayo clinic: article on interference between grapefruit and medication]</ref> Furanocoumarins in grapefruit juice (i.e. [[bergamottin]] and [[dihydroxybergamottin]]) inhibit the [[Cytochrome P450 oxidase|cytochrome P450]] enzyme [[CYP3A4]], which is involved in the metabolism of most statins (however it is a major inhibitor of only lovastatin, simvastatin and to a lesser degree atorvastatin) and some other medications<ref name="Kane2000">{{cite journal |author=Kane GC, Lipsky JJ |title=Drug-grapefruit juice interactions |journal=Mayo Clin. Proc. |volume=75 |issue=9 |pages=933–42 |year=2000 |pmid=10994829 |doi=10.4065/75.9.933}}</ref> (it had been thought that flavonoids (i.e. [[naringin]]) were responsible). This increases the levels of the statin, increasing the risk of dose-related adverse effects (including [[myopathy]]/[[rhabdomyolysis]]). |
||
==Mechanism of action== |
==Mechanism of action== |
||
Revision as of 09:16, 20 December 2011
| Statin | |
|---|---|
| Drug class | |
 Lovastatin, a compound isolated from Aspergillus terreus, was the first statin to be marketed. | |
| Class identifiers | |
| Use | High cholesterol |
| ATC code | C10AA |
| Biological target | HMG-CoA reductase |
| Clinical data | |
| Drugs.com | hmg-coa-reductase-inhibitors |
| External links | |
| MeSH | D019161 |
| Legal status | |
| In Wikidata | |
Statins (or HMG-CoA reductase inhibitors) are a class of drugs used to lower cholesterol levels by inhibiting the enzyme HMG-CoA reductase, which plays a central role in the production of cholesterol in the liver. Increased cholesterol levels have been associated with cardiovascular diseases,[1] and statins are therefore used in the prevention of these diseases. Evidence has found that they are most effective in those with cardiovascular disease (secondary prevention), with questionable benefit in those without previous CVD but with elevated cholesterol levels.[2][3] Statins have rare but severe adverse effects, particularly muscle damage, and some doctors believe they are overprescribed.
The best-selling of the statins is atorvastatin, marketed as Lipitor and manufactured by Pfizer. By 2003 it had become the best-selling pharmaceutical in history,[4] with Pfizer reporting sales of US$12.4 billion in 2008.[5] As of 2010, a number of statins are on the market: atorvastatin (Lipitor and Torvast), fluvastatin (Lescol), lovastatin (Mevacor, Altocor, Altoprev), pitavastatin (Livalo, Pitava), pravastatin (Pravachol, Selektine, Lipostat), rosuvastatin (Crestor) and simvastatin (Zocor, Lipex).[6] Several combination preparations of a statin and another agent—such as ezetimibe/simvastatin are available.
Medical uses
Clinical practice guidelines generally recommend that the people have tried "lifestyle modification", including a cholesterol-lowering diet and physical exercise, before statin use is considered; statins or other pharmacologic agents may then be recommended for those who do not meet their lipid-lowering goals through diet and lifestyle approaches.[7][8]
Primary prevention
There is debate over whether or not statins are effective in those with high cholesterol but no other health problems.[9] One review did not find a mortality benefit in those at high-risk but without prior cardiovascular disease.[9] Other reviews concluded that there is a mortality and morbidity benefit[10][11] but there was concerns regarding the quality of the evidence.[3] With respect to quality of life there is limited evidence of improvement when statins are used for primary prevention.[3] No studies as of 2010 show improved clinical outcomes in children with high cholesterol even though statins decrease cholesterol levels.[12]
Secondary prevention
Statins are effective in decreasing mortality in people with preexisting cardiovascular disease. They are also currently advocated for use in patients at high risk of developing heart disease.[2] On average, statins can lower LDL cholesterol by 1.8 mmol/l (70 mg/dl), which translates into a 60% decrease in the number of cardiac events (heart attack, sudden cardiac death) and a 17% reduced risk of stroke after long-term treatment.[13] They have less effect than the fibrates or niacin in reducing triglycerides and raising HDL-cholesterol ("good cholesterol").
Comparative effectiveness
While no direct comparison exists all statins appear equally effective regardless of potency or degree of cholesterol reduction.[11]
A comparison of atorvastatin, pravastatin and simvastatin, based on their effectiveness against placebos, found that, at commonly prescribed doses, there are no statistically significant differences among agents in reducing cardiovascular morbidity and mortality.[14]
Adverse effects
The most common adverse side effects are raised liver enzymes and muscle problems. In randomized clinical trials, reported adverse effects are low; but they are "higher in studies of real world use", and more varied.[15] In randomized trials statins increased the risk of an adverse effect by 39% compared to placebo (odds ratios 1.4); two-thirds of these were myalgia or raised liver enzymes with serious adverse effects similar to placebo.[16] However, reliance on clinical trials can be misleading indications of real-world adverse effects – for example, the statin cerivastatin was withdrawn from the market in 2001 due to cases of rhabdomyolysis (muscle breakdown), although rhabdomyolysis did not occur in a meta-analysis of cerivastatin clinical trials.[15] Other possible adverse effects include cognitive loss, neuropathy, pancreatic and hepatic dysfunction, and sexual dysfunction.[15]
Some patients on statin therapy report myalgias,[17] muscle cramps,[17] or, less frequently, gastrointestinal or other symptoms. Liver enzyme derangements may also occur, typically in about 0.5%,[citation needed] are also seen at similar rates with placebo use and repeated enzyme testing, and generally return to normal either without discontinuance over time or after briefly discontinuing the drug. Multiple other side-effects occur rarely; typically also at similar rates with only placebo in the large statin safety/efficacy trials. Two randomized clinical trials found cognitive issues while two did not; recurrence upon reintroduction suggests that these are causally related to statins in some individuals.[18] A Danish case-control study published in 2002 suggested a relation between long term statin use and increased risk of nerve damage or polyneuropathy[19] but suggested this side effect is "rare, but it does occur";[20] other researchers have pointed to studies of the effectiveness of statins in trials involving 50,000 people which have not shown nerve damage as a significant side effect.[21]
Rare reactions include myositis and myopathy, with the potential for rhabdomyolysis (the pathological breakdown of skeletal muscle) leading to acute renal failure. Coenzyme Q10 (ubiquinone) levels are decreased in statin use;[22] Q10 supplements are sometimes used to treat statin-associated myopathy, though there was no evidence of their effectiveness as of 2007[update].[23] A common variation in the SLCO1B1 gene, which participates in the absorption of statins, has been shown to significantly increase the risk of myopathy.[24]
Graham et al. (2004) reviewed records of over 250,000 patients treated from 1998 to 2001 with the statin drugs atorvastatin, cerivastatin, fluvastatin, lovastatin, pravastatin, and simvastatin.[25] The incidence of rhabdomyolyis was 0.44 per 10,000 patients treated with statins other than cerivastatin. However, the risk was over tenfold greater if cerivastatin was used, or if the standard statins (atorvastatin, fluvastatin, lovastatin, pravastatin, simvastatin) were combined with fibrate (fenofibrate or gemfibrozil) treatment. Cerivastatin was withdrawn by its manufacturer in 2001.
All commonly used statins show somewhat similar results, however the newer statins, characterized by longer pharmacological half-lives and more cellular specificity, have had a better ratio of efficacy to lower adverse effect rates.[citation needed] Some researchers have suggested that hydrophilic statins such as fluvastatin, rosuvastatin, and pravastatin are less toxic than lipophilic statins such as atorvastatin, lovastatin, and simvastatin, but other studies have not found a connection;[26] it is suggested that the risk of myopathy is lowest with pravastatin and fluvastatin probably because they are more hydrophillic and as a result have less muscle penetration.[citation needed] Lovastatin induces the expression of gene atrogin-1, which is believed to be responsible in promoting muscle fiber damage.[26]
Although there have been concerns that statins might increase cancer, several meta-analyses have found no relationship to cancer, the largest of which as of 2006 included nearly 87,000 participants.[27] However, in 2007 a meta-analysis of 23 statin treatment arms with 309,506 person-years of follow-up found that the risk of cancer was significantly associated with lower achieved LDL-C levels; the authors say that this requires further investigation.[28]
Several case-control studies have found that statins reduce cancer incidence,[27] including one[29] which showed that patients taking statins for over 5 years reduced their risk of colorectal cancer by 50%; this effect was not exhibited by fibrates although the trialists warned that the number needed to treat would approximate 5000, making statins unlikely tools for primary prevention.[29]
Drug interactions
Combining any statin with a fibrate or niacin, another category of lipid-lowering drugs, increases the risks for rhabdomyolysis to almost 6.0 per 10,000 person-years.[25] Most physicians have now abandoned routine monitoring of liver enzymes and creatine kinase, although they still consider this prudent in those on high-dose statins or in those on statin/fibrate combinations, and mandatory in the case of muscle cramps or of deterioration in renal function.
Consumption of grapefruit or grapefruit juice inhibits the metabolism of statins. Bitter oranges may have a similar effect.[30] Furanocoumarins in grapefruit juice (i.e. bergamottin and dihydroxybergamottin) inhibit the cytochrome P450 enzyme CYP3A4, which is involved in the metabolism of most statins (however it is a major inhibitor of only lovastatin, simvastatin and to a lesser degree atorvastatin) and some other medications[31] (it had been thought that flavonoids (i.e. naringin) were responsible). This increases the levels of the statin, increasing the risk of dose-related adverse effects (including myopathy/rhabdomyolysis).
Mechanism of action
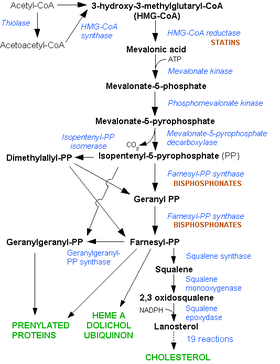
Statins act by competitively inhibiting HMG-CoA reductase, the first committed enzyme of the HMG-CoA reductase pathway. Because statins are similar to HMG-CoA on a molecular level they take the place of HMG-CoA in the enzyme and reduce the rate by which it is able to produce mevalonate, the next molecule in the cascade that eventually produces cholesterol, as well as a number of other compounds. This ultimately reduces cholesterol via several mechanisms.
Inhibiting cholesterol synthesis
By inhibiting HMG-CoA reductase, statins block the pathway for synthesizing cholesterol in the liver. This is significant because most circulating cholesterol comes from internal manufacture rather than the diet. When the liver can no longer produce cholesterol, levels of cholesterol in the blood will fall. Cholesterol synthesis appears to occur mostly at night,[32] so statins with short half-lives are usually taken at night to maximize their effect. Studies have shown greater LDL and total cholesterol reductions in the short-acting simvastatin taken at night rather than the morning,[33][34] but have shown no difference in the long-acting atorvastatin.[35]
Increasing LDL uptake
Liver cells sense the reduced levels of liver cholesterol and seek to compensate by synthesizing LDL receptors to draw cholesterol out of the circulation.[36] This is accomplished via protease enzymes that cleave a protein called "membrane-bound sterol regulatory element binding protein", which migrates to the nucleus and causes increased production of various other proteins and enzymes, including the LDL receptor. The LDL receptor then relocates to the liver cell membrane and binds to passing LDL and VLDL particles (the "bad cholesterol" linked to disease). LDL and VLDL are drawn out of circulation into the liver where the cholesterol is reprocessed into bile salts. These are excreted, and subsequently recycled mostly by an internal bile salt circulation.
Other effects
Statins exhibit action beyond lipid-lowering activity in the prevention of atherosclerosis. The ASTEROID trial showed direct ultrasound evidence of atheroma regression during statin therapy.[37] Researchers hypothesize that statins prevent cardiovascular disease via four proposed mechanisms (all subjects of a large body of biomedical research):[38]
- Improve endothelial function
- Modulate inflammatory responses
- Maintain plaque stability
- Prevent thrombus formation
Statins may even benefit those without high cholesterol. In 2008 the JUPITER study showed fewer stroke, heart attacks, and surgeries even for patients who had no history of high cholesterol or heart disease, but only elevated C-reactive protein levels. There were also 20% fewer deaths (mainly from reduction in cancer deaths) though deaths from cardiovascular causes were not reduced.[39]
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
- ^ The interactive pathway map can be edited at WikiPathways: "Statin_Pathway_WP430".
Pharmacogenomics
A 2004 study showed that patients with one of two common single-nucleotide polymorphisms (SNP) (small genetic variations) in the HMG-CoA reductase gene were less responsive to statins.[40] A 2008 study showed that carriers of the KIF6 genetic mutation were more responsive to statin treatment.[41]
Likewise, a 2008 study demonstrated a link between an increased risk of myopathy at higher doses of statins (40 – 80 mg) and a SNP in SLCO1B1, a gene encoding for the organic anion transporter peptide OADP1B1.[24]
History
In 1971, Akira Endo, a Japanese biochemist working for the drug company Sankyo, began the search for a cholesterol-lowering drug. Research had already shown that cholesterol is mostly manufactured by the body in the liver, using an enzyme known as HMG-CoA reductase.[4] Endo and his team reasoned that certain microorganisms may produce inhibitors of the enzyme to defend themselves against other organisms, as mevalonate is a precursor of many substances required by organisms for the maintenance of their cell wall (ergosterol) or cytoskeleton (isoprenoids).[42] The first agent they identified was mevastatin (ML-236B), a molecule produced by the fungus Penicillium citrinum.
Mevastatin was never marketed, because of its adverse effects of tumors, muscle deterioration, and sometimes death in laboratory dogs. P. Roy Vagelos, chief scientist and later CEO of Merck & Co, was interested, and made several trips to Japan starting in 1975. By 1978, Merck had isolated lovastatin (mevinolin, MK803) from the fungus Aspergillus terreus, first marketed in 1987 as Mevacor.[4]
A link between cholesterol and cardiovascular disease, known as the lipid hypothesis, had already been suggested. Cholesterol is the main constituent of atheroma, the fatty lumps in the wall of arteries that occur in atherosclerosis and, when ruptured, cause the vast majority of heart attacks. Treatment consisted mainly of dietary measures such as a low-fat diet, and poorly tolerated medicines such as clofibrate, cholestyramine and nicotinic acid. Cholesterol researcher Daniel Steinberg writes that while the Coronary Primary Prevention Trial of 1984 demonstrated that cholesterol lowering could significantly reduce the risk of heart attacks and angina, physicians, including cardiologists, remained largely unconvinced.[43]
To market statins effectively, Merck had to convince the public about the dangers of high cholesterol, and doctors that statins were safe and would extend lives. As a result of public campaigns, people became familiar with their cholesterol numbers and the difference between "good" and "bad" cholesterol, and rival pharmaceutical companies began producing their own statins, such as pravastatin (Pravachol), manufactured by Sankyo and Bristol-Myers Squibb. In April 1994, the results of a Merck-sponsored study, the Scandinavian Simvastatin Survival Study or "4S", were announced. Researchers tested simvastatin, later sold by Merck as Zocor, on 4,444 patients with high cholesterol and heart disease. After five years, the study concluded that patients saw a 35-percent reduction in their cholesterol, and their chances of dying of a heart attack were reduced by 42 percent.[4][44] In 1995, Zocor and Mevacor both made Merck over US$1 billion.[4] Endo was awarded the 2006 Japan Prize, and the Lasker-DeBakey Clinical Medical Research Award in 2008.
Available forms
The statins are divided into two groups: fermentation-derived and synthetic. They include, along with brand names, which may vary between countries:
| Statin | Image | Brand name | Derivation | Metabolism[45] |
|---|---|---|---|---|
| Atorvastatin |  |
Lipitor, Torvast | Synthetic | CYP3A4 |
| Cerivastatin |  |
Lipobay, Baycol. (Withdrawn from the market in August, 2001 due to risk of serious Rhabdomyolysis) | Synthetic | various CYP3A isoforms [46] |
| Fluvastatin |  |
Lescol, Lescol XL | Synthetic | CYP2C9 |
| Lovastatin |  |
Mevacor, Altocor, Altoprev | Fermentation-derived. Naturally-occurring compound. Found in oyster mushrooms and red yeast rice. | CYP3A4 |
| Mevastatin |  |
Compactin | Naturally-occurring compound. Found in red yeast rice. | CYP3A4 |
| Pitavastatin |  |
Livalo, Pitava | Synthetic | |
| Pravastatin |  |
Pravachol, Selektine, Lipostat | Fermentation-derived. (A fermentation product of bacterium Nocardia autotrophica). | Non CYP[47] |
| Rosuvastatin |  |
Crestor | Synthetic | CYP2C9 and CYP2C19 |
| Simvastatin |  |
Zocor, Lipex | Fermentation-derived. (Simvastatin is a synthetic derivate of a fermentation product ofAspergillus terreus.) | CYP3A4 |
| Simvastatin+Ezetimibe | Vytorin | Combination therapy | ||
| Lovastatin+Niacin extended-release | Advicor | Combination therapy | ||
| Atorvastatin+Amlodipine Besylate | Caduet | Combination therapy - Cholesterol+Blood Pressure | ||
| Simvastatin+Niacin extended-release | Simcor | Combination therapy |
LDL-lowering potency varies between agents. Cerivastatin is the most potent, (withdrawn from the market in August, 2001 due to risk of serious Rhabdomyolysis) followed by (in order of decreasing potency), rosuvastatin, atorvastatin, simvastatin, lovastatin, pravastatin, and fluvastatin.[48] The relative potency of pitavastatin has not yet been fully established.

Some types of statins are naturally occurring, and can be found in such foods as oyster mushrooms and red yeast rice. Randomized controlled trials found them to be effective, but the quality of the trials was low.[49] Most of the block-buster branded statins will be generic by 2012,including atorvastatin, the largest selling branded drug.
| Statin Equivalent Dosages | ||||||
|---|---|---|---|---|---|---|
| % LDL Reduction (approx.) | Atorvastatin | Fluvastatin | Lovastatin | Pravastatin | Rosuvastatin | Simvastatin |
| 10-20% | -- | 20 mg | 10 mg | 10 mg | -- | 5 mg |
| 20-30% | -- | 40 mg | 20 mg | 20 mg | -- | 10 mg |
| 30-40% | 10 mg | 80 mg | 40 mg | 40 mg | 5 mg | 20 mg |
| 40-45% | 20 mg | -- | 80 mg | 80 mg | 5–10 mg | 40 mg |
| 46-50% | 40 mg | -- | -- | -- | 10–20 mg | 80 mg* |
| 50-55% | 80 mg | -- | -- | -- | 20 mg | -- |
| 56-60% | -- | -- | -- | -- | 40 mg | -- |
| * 80mg dose no longer recommended due to increased risk of rhabdomyolysis | ||||||
| Starting dose | ||||||
| Starting dose | 10–20 mg | 20 mg | 10–20 mg | 40 mg | 10 mg; 5 mg if hypothyroid, >65 yo, Asian | 20 mg |
| If higher LDL reduction goal | 40 mg if >45% | 40 mg if >25% | 20 mg if >20% | -- | 20 mg if LDL >190 mg/dL (4.87 mmol/L) | 40 mg if >45% |
| Optimal timing | Anytime | Evening | With evening meals | Anytime | Anytime | Evening |
Research
Research continues into other areas where statins also appear to have a favorable effect, including dementia,[50] lung cancer,[51]nuclear cataracts,[52] hypertension,[53] prostate cancer [54]
Controversy
Some scientists believe that statins are overused. Their use has expanded into groups with lesser benefit, and lesser evidence of benefit. The lower the risk of cardiovascular events, the lower the ratio is of benefits to costs.[55]
A smaller group of scientists, The International Network of Cholesterol Skeptics, question the lipid hypothesis and argue that elevated cholesterol has not been adequately linked to heart disease. These organizations maintain that statins are not as beneficial or safe as suggested.[56]
References
- ^ Lewington S, Whitlock G, Clarke R; et al. (2007). "Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths". Lancet. 370 (9602): 1829–39. doi:10.1016/S0140-6736(07)61778-4. PMID 18061058.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b National Institute for Health and Clinical Excellence (May 2008, reissued March 2010). "Lipid modification - Cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular disease - Quick reference guide" (PDF). Retrieved 2010-08-25.
{{cite web}}: Check date values in:|date=(help) - ^ a b c Taylor F, Ward K, Moore TH; et al. (2011). Taylor, Fiona (ed.). "Statins for the primary prevention of cardiovascular disease". Cochrane Database Syst Rev (1): CD004816. doi:10.1002/14651858.CD004816.pub4. PMID 21249663.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ a b c d e Simons, John. "The $10 billion pill", Fortune magazine, January 20, 2003.
- ^ "Doing Things Differently", Pfizer 2008 Annual Review, April 23, 2009, p. 15.
- ^ Sweetman, Sean C., ed. (2009). "Cardiovascular drugs". Martindale: the complete drug reference (36th ed.). London: Pharmaceutical Press. pp. 1155–434. ISBN 978-0-85369-840-1.
- ^ National Cholesterol Education Program (2001). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): Executive Summary. Bethesda, MD: National Institutes of Health. National Heart, Lung, and Blood Institute. p. 40. NIH Publication No. 01-3670.
- ^ National Collaborating Centre for Primary Care (2010). NICE clinical guideline 67: Lipid modification (PDF). London: National Institute for Health and Clinical Excellence. p. 38.
- ^ a b Ray; Seshasai, S. R. K.; Erqou, S.; Sever, P.; Jukema, J. W.; Ford, I.; Sattar, N.; et al. (2010). "Statins and All-Cause Mortality in High-Risk Primary Prevention: A Meta-analysis of 11 Randomized Controlled Trials Involving 65 229 Participants". Arch Intern Med. 170 (12): 1024. doi:10.1001/archinternmed.2010.182. PMID 20585067. Retrieved 2010-11-04.
{{cite journal}}: Explicit use of et al. in:|author=(help); More than one of|pages=and|page=specified (help) - ^ Mills, EJ (2011 Feb). "Efficacy and safety of statin treatment for cardiovascular disease: a network meta-analysis of 170,255 patients from 76 randomized trials". QJM : monthly journal of the Association of Physicians. 104 (2): 109–24. doi:10.1093/qjmed/hcq165. PMID 20934984.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Tonelli, M (2011 Nov 8). "Efficacy of statins for primary prevention in people at low cardiovascular risk: a meta-analysis". CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 183 (16): E1189–E1202. PMID 21989464.
{{cite journal}}: Check date values in:|date=(help); Missing pipe in:|journal=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Lebenthal Y, Horvath A, Dziechciarz P, Szajewska H, Shamir R (2010). "Are treatment targets for hypercholesterolemia evidence based? Systematic review and meta-analysis of randomised controlled trials". Arch Dis Child. 95 (9): 673–80. doi:10.1136/adc.2008.157024. PMID 20515970.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Law MR, Wald NJ, Rudnicka AR (2003). "Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis". BMJ. 326 (7404): 1423. doi:10.1136/bmj.326.7404.1423. PMC 162260. PMID 12829554.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Zhou Z, Rahme E, Pilote L (2006). "Are statins created equal? Evidence from randomized trials of pravastatin, simvastatin, and atorvastatin for cardiovascular disease prevention". Am. Heart J. 151 (2): 273–81. doi:10.1016/j.ahj.2005.04.003. PMID 16442888.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Golomb BA, Evans MA (2008). "Statin adverse effects : a review of the literature and evidence for a mitochondrial mechanism". Am J Cardiovasc Drugs. 8 (6): 373–418. doi:10.2165/0129784-200808060-00004. PMC 2849981. PMID 19159124.
- ^ Silva MA, Swanson AC, Gandhi PJ, Tataronis GR (2006). "Statin-related adverse events: a meta-analysis". Clin Ther. 28 (1): 26–35. doi:10.1016/j.clinthera.2006.01.005. PMID 16490577.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Anne Harding (August 28, 2007). "Docs often write off patient side effect concerns". Reuters. Retrieved 2009-10-06.
- ^ Holman JR. (2007). Some Docs in Denial About Statin Side Effects. Doc News.
- ^ Gaist D, Jeppesen U, Andersen M, García Rodríguez LA, Hallas J, Sindrup SH (2002). "Statins and risk of polyneuropathy—a case-control study". Neurology. 58 (9): 1333–1337. PMID 12011277.
{{cite journal}}: Unknown parameter|laydate=ignored (help); Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Sandra G. Boodman, The Washington Post (September 10, 2002). "Study links statins to nerve damage". Pittsburgh Post-Gazette. Retrieved 2009-10-06.
- ^ Julie Appleby and Steve Sternberg (2002-08-18). "Statin side effect rare, but be aware". USA TODAY. Retrieved 2009-10-06.
{{cite news}}: CS1 maint: date and year (link) - ^ Ghirlanda G, Oradei A, Manto A, Lippa S, Uccioli L, Caputo S, Greco A, Littarru G (1993). "Evidence of plasma CoQ10-lowering effect by HMG-CoA reductase inhibitors: a double-blind, placebo-controlled study". J Clin Pharmacol. 33 (3): 226–9. PMID 8463436.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Marcoff L, Thompson PD (2007). "The role of coenzyme Q10 in statin-associated myopathy: a systematic review". J. Am. Coll. Cardiol. 49 (23): 2231–7. doi:10.1016/j.jacc.2007.02.049. PMID 17560286.
- ^ a b The SEARCH Collaborative Group (2008). "SLCO1B1 Variants and Statin-Induced Myopathy - A Genomewide Study". NEJM. 359: 789–799. doi:10.1056/NEJMoa0801936. PMID 18650507.
- ^ a b Graham DJ, Staffa JA, Shatin D; et al. (2004). "Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs". JAMA. 292 (21): 2585–90. doi:10.1001/jama.292.21.2585. PMID 15572716.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ a b Hanai J, Cao P, Tanksale P; et al. (2007). "The muscle-specific ubiquitin ligase atrogin-1/MAFbx mediates statin-induced muscle toxicity". J. Clin. Invest. 117 (12): 3940–51. doi:10.1172/JCI32741. PMC 2066198. PMID 17992259.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ a b Dale KM, Coleman CI, Henyan NN, Kluger J, White CM (2006). "Statins and cancer risk: a meta-analysis". JAMA. 295 (1): 74–80. doi:10.1001/jama.295.1.74. PMID 16391219.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Alsheikh-Ali AA; Maddukuri, PV; Han, H; Karas, RH (2007). "Effect of the Magnitude of Lipid Lowering on Risk of Elevated Liver Enzymes, Rhabdomyolysis, and Cancer: Insights From Large Randomized Statin Trials". Journal of the American College of Cardiology. 50 (5): 409–418. doi:10.1016/j.jacc.2007.02.073. PMID 17662392.
- ^ a b Poynter JN, Gruber SB, Higgins PD; et al. (2005). "Statins and the risk of colorectal cancer". N. Engl. J. Med. 352 (21): 2184–92. doi:10.1056/NEJMoa043792. PMID 15917383.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Mayo clinic: article on interference between grapefruit and medication
- ^ Kane GC, Lipsky JJ (2000). "Drug-grapefruit juice interactions". Mayo Clin. Proc. 75 (9): 933–42. doi:10.4065/75.9.933. PMID 10994829.
- ^ Miettinen TA (1982). "Diurnal variation of cholesterol precursors squalene and methyl sterols in human plasma lipoproteins". Journal of Lipid Research. 23 (3): 466–73. PMID 7200504.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Saito Y; Yoshida S; Nakaya N; Hata Y; Goto Y (1991). "Comparison between morning and evening doses of simvastatin in hyperlipidemic subjects. A double-blind comparative study". Arterioscler Thromb. 11 (4): 816–26. PMID 2065035.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Wallace A; Chinn D; Rubin G (4 October 2003). "Taking simvastatin in the morning compared with in the evening: randomised controlled trial". British Medical Journal. 327 (7418): 788. doi:10.1136/bmj.327.7418.788. PMID 14525878.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cilla DD Jr; Gibson DM; Whitfield LR; Sedman AJ (1996). "Pharmacodynamic effects and pharmacokinetics of atorvastatin after administration to normocholesterolemic subjects in the morning and evening". Journal of Clinical Pharmacology. 36 (7): 604–9. PMID 8844442.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Ma PT, Gil G, Südhof TC, Bilheimer DW, Goldstein JL, Brown MS (1986). "Mevinolin, an inhibitor of cholesterol synthesis, induces mRNA for low density lipoprotein receptor in livers of hamsters and rabbits" (PDF). Proc. Natl. Acad. Sci. U.S.A. 83 (21): 8370–4. Bibcode:1986PNAS...83.8370M. doi:10.1073/pnas.83.21.8370. PMC 386930. PMID 3464957.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Nissen S, Nicholls S, Sipahi I, Libby P, Raichlen J, Ballantyne C, Davignon J, Erbel R, Fruchart J, Tardif J, Schoenhagen P, Crowe T, Cain V, Wolski K, Goormastic M, Tuzcu E (2006). "Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial". JAMA. 295 (13): 1556–65. doi:10.1001/jama.295.13.jpc60002. PMID 16533939.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Furberg CD (19 January 1999). "Natural Statins and Stroke Risk". Circulation. 99 (2): 185–188. PMID 9892578.
- ^ Ridker PM, Danielson E, Fonseca FAH; et al. (2008). "Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein" (PDF). NEJM. 359 (21): 2195–207. doi:10.1056/NEJMoa0807646. PMID 18997196.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Chasman DI, Posada D, Subrahmanyan L, Cook NR, Stanton VP, Ridker PM (2004). "Pharmacogenetic study of statin therapy and cholesterol reduction". JAMA. 291 (23): 2821–7. doi:10.1001/jama.291.23.2821. PMID 15199031.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 18222355, please use {{cite journal}} with
|pmid=18222355instead. - ^ Endo A (1 November 1992). "The discovery and development of HMG-CoA reductase inhibitors" (PDF). J. Lipid Res. 33 (11): 1569–82. PMID 1464741.
- ^ Steinberg, Daniel. The Cholesterol Wars: The Skeptics vs. The Preponderance of Evidence. Academic Press, 2007, pp. 6–9.
- ^ "Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S)". Lancet. 344 (8934): 1383–9. 1994. doi:10.1016/S0140-6736(94)90566-5. PMID 7968073.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Safety of Statins: Focus on Clinical Pharmacokinetics and Drug Interactions. Circulation. 2004:109:III-50-IIIi-57
- ^ Metabolism of cerivastatin by human liver microsomes in vitro. Characterization of primary metabolic pathways and of cytochrome P450 isozymes involved. Drug Metab Dispos. 1997 Mar;25(3):321-31.
- ^ Comparison of Cytochrome P-450-Dependent Metabolism and Drug Interactions of the 3-Hydroxy-3-methylglutaryl-CoA Reductase Inhibitors Lovastatin and Pravastatin in the Liver. DMD February 1, 1999 vol. 27 no. 2 173-179
- ^ Shepherd J, Hunninghake DB, Barter P, McKenney JM, Hutchinson HG (2003). "Guidelines for lowering lipids to reduce coronary artery disease risk: a comparison of rosuvastatin with atorvastatin, pravastatin, and simvastatin for achieving lipid-lowering goals". Am. J. Cardiol. 91 (5A): 11C–17C, discussion 17C–19C. doi:10.1016/S0002-9149(03)00004-3. PMID 12646338.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Liu J, Zhang J, Shi Y, Grimsgaard S, Alraek T, Fønnebø V (2006). "Chinese red yeast rice (Monascus purpureus) for primary hyperlipidemia: a meta-analysis of randomized controlled trials". Chin Med. 1: 4. doi:10.1186/1749-8546-1-4. PMC 1761143. PMID 17302963.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Wolozin, B (July 19, 2007). "Simvastatin is associated with a reduced incidence of dementia and Parkinson's disease". BMC Medicine. 5: 20. doi:10.1186/1741-7015-5-20. PMC 1955446. PMID 17640385.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: unflagged free DOI (link) - ^ Khurana V, Bejjanki HR, Caldito G, Owens MW (2007). "Statins reduce the risk of lung cancer in humans: a large case-control study of US veterans". Chest. 131 (5): 1282–1288. doi:10.1378/chest.06-0931. PMID 17494779.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Klein BE, Klein R, Lee KE, Grady LM (2006). "Statin use and incident nuclear cataract". JAMA. 295 (23): 2752–8. doi:10.1001/jama.295.23.2752. PMID 16788130.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Golomb BA, Dimsdale JE, White HL, Ritchie JB, Criqui MH (2008). "Reduction in blood pressure with statins: results from the UCSD Statin Study, a randomized trial". Arch. Intern. Med. 168 (7): 721–7. doi:10.1001/archinte.168.7.721. PMID 18413554.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Mondul AM, Han M, Humphreys EB, Meinhold CL, Walsh PC, Platz EA (2011). "Association of statin use with pathological tumor characteristics and prostate cancer recurrence after surgery". Journal of Urology. 185 (4): 1268–1273. doi:10.1016/j.juro.2010.11.089. PMID 21334020.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Abramson J, Wright J (2007). "Are lipid-lowering guidelines evidence-based?". Lancet. 369 (9557): 168–9. doi:10.1016/S0140-6736(07)60084-1. PMID 17240267.
- ^ Ravnskov U, Rosch P, Sutter M, Houston M (2006). "Should we lower cholesterol as much as possible?". BMJ. 332 (7553): 1330–2. doi:10.1136/bmj.332.7553.1330. PMC 1473073. PMID 16740566.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
External links
- Statin page at Bandolier, an evidence-based medicine journal (little content after 2004)

