Bendamustine: Difference between revisions
m r2.7.1) (Robot: Adding es:Bendamustina |
No edit summary |
||
| Line 51: | Line 51: | ||
}} |
}} |
||
'''Bendamustine''' ([[International Nonproprietary Name|INN]], trade names '''Ribomustin''' and '''Treanda'''; also known as '''SDX-105''') is a [[nitrogen mustard]] used in the treatment of [[chronic lymphocytic leukemia]]s |
'''Bendamustine''' ([[International Nonproprietary Name|INN]], trade names '''Ribomustin''' and '''Treanda'''; also known as '''SDX-105''') is a [[nitrogen mustard]] used in the treatment of [[chronic lymphocytic leukemia]]s<ref>{{cite journal |author=Kath R, Blumenstengel K, Fricke HJ, Höffken K |title=Bendamustine monotherapy in advanced and refractory chronic lymphocytic leukemia |journal=J. Cancer Res. Clin. Oncol. |volume=127 |issue=1 |pages=48–54 |year=2001 |month=January |pmid=11206271 |doi= 10.1007/s004320000180|url=http://link.springer.de/link/service/journals/00432/bibs/1127001/11270048.htm}}</ref> and [[lymphoma]]s. It belongs to the family of drugs called [[alkylating antineoplastic agent|alkylating agent]]s. It is also being studied for the treatment of [[sarcoma]].<ref>{{cite journal |author=Bagchi S |title=Bendamustine for advanced sarcoma |journal=Lancet Oncol. |volume=8 |issue=8 |pages=674 |year=2007 |month=August |pmid=17726779 |doi= 10.1016/S1470-2045(07)70225-5|url=}}</ref> |
||
==History== |
==History== |
||
| ⚫ | Bendamustine was first synthesized in 1963 by Ozegowski and Krebs in [[East Germany]] (the former German Democratic Republic). Until 1990 it was available only in East Germany. East German investigators found that it was useful for treating [[chronic lymphocytic leukemia]], [[Hodgkin’s disease]], [[non-Hodgkin’s lymphoma]], [[multiple myeloma]] and [[lung cancer]]. |
||
| ⚫ | Bendamustine received its first marketing approval in Germany, where it is marketed under the tradename Ribomustin, by Astellas Pharma GmbH's licensee, Mundipharma International Corporation Limited. It is indicated as a single-agent or in combination with other anti-cancer agents for indolent [[non-Hodgkin's lymphoma]], multiple myeloma, and chronic lymphocytic leukemia. SymBio Pharmaceuticals Ltd holds exclusive rights to develop and market bendamustine HCl in Japan and selected Asia Pacific Rim countries. |
||
| ⚫ | Bendamustine was first synthesized in 1963 by Ozegowski and Krebs in East Germany (the former German Democratic Republic) |
||
In March 2008, [[Cephalon]] received approval from the [[United States]] [[Food and Drug Administration]] to market bendamustine in the US, where it is sold under the tradename Treanda, for treatment of chronic lymphocytic leukemia.<ref>{{cite web |url=http://www.cephalon.com/newsroom/news_reader.aspx?ID=1120688 |title=Cephalon press release - Cephalon Receives FDA Approval for TREANDA, a Novel Chemotherapy for Chronic Lymphocytic Leukemia |accessdate=2008-03-23 |format= |work= }}</ref> |
|||
| ⚫ | Bendamustine received its first marketing approval in Germany, |
||
In |
In October 2008, the FDA granted further approval to market Treanda for the treatment of indolent B-cell non-Hodgkin's lymphoma that has progressed during or within six months of treatment with rituximab or a rituximab-containing regimen. <ref>{{cite web |url=http://www.cephalon.com/media/news-releases/article/video-cephalon-receives-fda-approval-for-treanda-to-treat-patients-with-relapsed-indolent-non-hodgk/ |title=Cephalon press release -Cephalon Receives FDA Approval for TREANDA to Treat Patients with Relapsed Indolent Non-Hodgkin's Lymphoma |accessdate=2008-11-03 |format= |work= }}</ref> |
||
In October 2008, the FDA granted further approval to market Treanda for the treatment of indolent B-cell [[non-Hodgkin's lymphoma]] (NHL) that has progressed during or within six months of treatment with rituximab or a rituximab-containing regimen. <ref>{{cite web |url=http://www.cephalon.com/media/news-releases/article/video-cephalon-receives-fda-approval-for-treanda-to-treat-patients-with-relapsed-indolent-non-hodgk/ |title=Cephalon press release -Cephalon Receives FDA Approval for TREANDA to Treat Patients with Relapsed Indolent Non-Hodgkin's Lymphoma |accessdate=2008-11-03 |format= |work= }}</ref> |
|||
==Pharmacology== |
==Pharmacology== |
||
Betamustine acts as an alkylating agent causing intra-strand and inter-strand cross-links between DNA bases. |
Betamustine is a white, water soluble microcrystalline powder with [[amphoterism|amphoteric]] properties. It acts as an alkylating agent causing intra-strand and inter-strand cross-links between [[DNA]] bases. |
||
After intravenous infusion it is extensively metabolised in the [[liver]] by [[cytochrome |
After intravenous infusion it is extensively metabolised in the [[liver]] by [[cytochrome p450]]. More than 95% of the drug is bound to protein - primarily [[albumin]]. Only free bendamustine is active. Elimination is biphasic with a half-life of 6–10 minutes and a terminal half-life of approximately 30 minutes. It is eliminated primarily through the [[kidney]]s. |
||
== Chemotherapeutic uses == |
== Chemotherapeutic uses == |
||
| Line 76: | Line 75: | ||
==Adverse effects== |
==Adverse effects== |
||
Common adverse reactions are typical for |
Common adverse reactions are typical for the class of [[nitrogen mustard]]s, and include nausea, fatigue, vomiting, diarrhea, fever, constipation, loss of appetite, cough, headache, unintentional weight loss, difficulty breathing, rashes, and [[stomatitis]], as well as immunosuppression, anemia, and [[thrombocytopenia|low platelet counts]]. |
||
==References== |
==References== |
||
Revision as of 00:53, 8 April 2012
 | |
| Clinical data | |
|---|---|
| Trade names | Treanda |
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a608034 |
| License data | |
| Routes of administration | Intravenous infusion |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | NA (intravenous only) |
| Protein binding | 94–96% |
| Metabolism | Hydrolyzed to inactive metabolites. Two minor metabolites (M3 and M4) formed by CYP1A2 |
| Elimination half-life | 40 min (bendamustine), 3 h (M3), 30 min (M4) |
| Excretion | Mostly fecal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.205.789 |
| Chemical and physical data | |
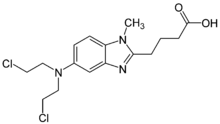
| Formula | C16H21Cl2N3O2 |
| Molar mass | 358.262 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Bendamustine (INN, trade names Ribomustin and Treanda; also known as SDX-105) is a nitrogen mustard used in the treatment of chronic lymphocytic leukemias[1] and lymphomas. It belongs to the family of drugs called alkylating agents. It is also being studied for the treatment of sarcoma.[2]
History
Bendamustine was first synthesized in 1963 by Ozegowski and Krebs in East Germany (the former German Democratic Republic). Until 1990 it was available only in East Germany. East German investigators found that it was useful for treating chronic lymphocytic leukemia, Hodgkin’s disease, non-Hodgkin’s lymphoma, multiple myeloma and lung cancer.
Bendamustine received its first marketing approval in Germany, where it is marketed under the tradename Ribomustin, by Astellas Pharma GmbH's licensee, Mundipharma International Corporation Limited. It is indicated as a single-agent or in combination with other anti-cancer agents for indolent non-Hodgkin's lymphoma, multiple myeloma, and chronic lymphocytic leukemia. SymBio Pharmaceuticals Ltd holds exclusive rights to develop and market bendamustine HCl in Japan and selected Asia Pacific Rim countries.
In March 2008, Cephalon received approval from the United States Food and Drug Administration to market bendamustine in the US, where it is sold under the tradename Treanda, for treatment of chronic lymphocytic leukemia.[3]
In October 2008, the FDA granted further approval to market Treanda for the treatment of indolent B-cell non-Hodgkin's lymphoma that has progressed during or within six months of treatment with rituximab or a rituximab-containing regimen. [4]
Pharmacology
Betamustine is a white, water soluble microcrystalline powder with amphoteric properties. It acts as an alkylating agent causing intra-strand and inter-strand cross-links between DNA bases.
After intravenous infusion it is extensively metabolised in the liver by cytochrome p450. More than 95% of the drug is bound to protein - primarily albumin. Only free bendamustine is active. Elimination is biphasic with a half-life of 6–10 minutes and a terminal half-life of approximately 30 minutes. It is eliminated primarily through the kidneys.
Chemotherapeutic uses
Bendamustine has been used both as sole therapy and in combination with other agents including etoposide, fludarabine, mitoxantrone, methotrexate, prednisone, rituximab, vincristine and 90Y-ibritumomab tiuxetan.
One combination for stage III/IV relapsed or refractory indolent lymphomas and mantle cell lymphoma (MCL), with or without prior rituximab-containing chemoimmunotherapy treatment, is bendamustine with mitoxantrone and rituximab.[5]
Adverse effects
Common adverse reactions are typical for the class of nitrogen mustards, and include nausea, fatigue, vomiting, diarrhea, fever, constipation, loss of appetite, cough, headache, unintentional weight loss, difficulty breathing, rashes, and stomatitis, as well as immunosuppression, anemia, and low platelet counts.
References
- ^ Kath R, Blumenstengel K, Fricke HJ, Höffken K (2001). "Bendamustine monotherapy in advanced and refractory chronic lymphocytic leukemia". J. Cancer Res. Clin. Oncol. 127 (1): 48–54. doi:10.1007/s004320000180. PMID 11206271.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Bagchi S (2007). "Bendamustine for advanced sarcoma". Lancet Oncol. 8 (8): 674. doi:10.1016/S1470-2045(07)70225-5. PMID 17726779.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ "Cephalon press release - Cephalon Receives FDA Approval for TREANDA, a Novel Chemotherapy for Chronic Lymphocytic Leukemia". Retrieved 2008-03-23.
- ^ "Cephalon press release -Cephalon Receives FDA Approval for TREANDA to Treat Patients with Relapsed Indolent Non-Hodgkin's Lymphoma". Retrieved 2008-11-03.
- ^ Weide R, Hess G, Köppler H; et al. (2007). "High anti–lymphoma activity of bendamustine/mitoxantrone/rituximab in rituximab pretreated relapsed or refractory indolent lymphomas and mantle cell lymphomas. A muticenter phase II study of the German Low Grade Lymphoma Study Group (GLSG)". Leuk. Lymphoma. 48 (7): 1299–1306. doi:10.1080/10428190701361828. PMID 17613757.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link)
External links
- Manufacturer's official website intended for US patients
