Wikipedia:Reference desk/Science: Difference between revisions
→August 20: hey! I wasn't finished with that! |
|||
| Line 7: | Line 7: | ||
[[Category:Wikipedia reference desk|Science]] |
[[Category:Wikipedia reference desk|Science]] |
||
</noinclude> |
</noinclude> |
||
== Low pressure and high temperatures == |
|||
I've been checking the weather report lately since the weather has been pretty hot this summer over here, and I've noticed that the heat waves were well-corellated with times of low atmospheric pressure. For example see [http://meteociel.fr/previsions/54701/zagreb___centar.htm] [http://meteociel.fr/tendances/54701/zagreb___centar.htm]. That seems to be the wrong way around to me, usually we get low pressure with storms, not 24-hour heat. What could cause this? <!-- Template:Unsigned IP --><small class="autosigned">— Preceding [[Wikipedia:Signatures|unsigned]] comment added by [[Special:Contributions/78.0.231.47|78.0.231.47]] ([[User talk:78.0.231.47#top|talk]]) 04:25, 18 August 2017 (UTC)</small> <!--Autosigned by SineBot--> |
|||
:[[Cold front]]) and [[warm front]] have nice tab that will help you understand what's going on. wind direction and strength are also important in that matter. |
|||
:As evidenced in those articles, the pressure in itself is not as important as pressure variation [[User:Gem fr|Gem fr]] ([[User talk:Gem fr|talk]]) 07:40, 18 August 2017 (UTC) |
|||
:Looking at the temp and pressure diagrams at the first link, I don't see any correlation at all. The 2nd link does seem to show pressure peaking mid-morning each day, and of course temps peak mid-afternoon. Not sure what would cause the pressure to behave like that. [[User:StuRat|StuRat]] ([[User talk:StuRat|talk]]) 07:37, 18 August 2017 (UTC) |
|||
::Temperature at high altitude lags behind surface temperature, so you generally get the coldest troposphere in mid-morning. Low temperature means high density, means more weight in that column of air, means more pressure at the surface. So it makes sense. Of course the effect isn't very large. There must be air moving in or out of the column, which happens by high-altitude wind changing with the time of day. [[User:PiusImpavidus|PiusImpavidus]] ([[User talk:PiusImpavidus|talk]]) 10:39, 19 August 2017 (UTC) |
|||
:When the temperature is high, the density of the air is lower. The column of air above you weighs less, so the air pressure drops. If the pressure gets lower than in surrounding areas, the air will rise, clouds will form and the temperature will drop (during the day). But a lot depends on regional effects. When the pressure distribution is such that the wind comes from the continent, the air is dry, which mean less clouds and higher temperatures in summer. [[User:PiusImpavidus|PiusImpavidus]] ([[User talk:PiusImpavidus|talk]]) 10:04, 18 August 2017 (UTC) |
|||
::oh dear. your are basically saying that higher temperature lower pressure. How wrong. |
|||
::[[Ideal gas law]] P =(n/V)RT do not perfectly apply to air, but it does quite well, meaning, when temperature rises, pressure rises, too (opposite of what you said). The higher temperature air do not rise because of lower pressure, it rises because, for a given pressure, if T rises, then density (n/V) must goes down, hence [[Archimedes' principle]] applies and the parcel of air must go up. All that without any pressure effect. [[User:Gem fr|Gem fr]] ([[User talk:Gem fr|talk]]) 12:34, 18 August 2017 (UTC) |
|||
:::I know the ideal gas law. When the air heats up, it expands. The air isn't locked up in a bottle, it's free to expand and flows away horizontally at the [[tropopause]], driven by a slight increase in pressure up there. As the density drops with rising temperature, the pressure difference between the surface and the tropopause (caused by gravity acting on the column of air) drops, so the surface pressure drops too. That's the pressure we measure. The reduced pressure at the surface causes horizontal inflow, continuing as a vertical flow from the surface to the tropopause. That's how Archimedes' principle drives convection. [[User:PiusImpavidus|PiusImpavidus]] ([[User talk:PiusImpavidus|talk]]) 10:39, 19 August 2017 (UTC) |
|||
::::Very complicated way to explain than an isobar process will bring a drop in pressure...which it will not, by definition. |
|||
::::I guess you'll explain likewise that vapor bubbles in a column of boiling water will reduce pressure at the bottom of the column? they won't, either. Actually they would slightly increase the pressure, because slightly rising the whole water surface height (hence pressure, since it depends on height) |
|||
::::Try again [[User:Gem fr|Gem fr]] ([[User talk:Gem fr|talk]]) 16:54, 22 August 2017 (UTC) |
|||
:::::Vapour bubbles will push the surface up, but won't change the pressure at the bottom. You may turn part of the water into vapour, but the weight stays the same. Pressure is the weight of the water+vapour bubbles divided by surface area (+ pressure at the top surface), so boiling the water doesn't change the pressure at the bottom, until the water actually boils away. And all of this is about the average pressure at the bottom, as locally the pressure will change. Where it's heated, the pressure at the bottom drops, where it's not heated, pressure increases, just like in the atmosphere. If heating is uniform over the bottom area, you just get turbulence with random pressure variations. |
|||
:::::In short, the average pressure doesn't change, but there are local changes. Where heating is going on, the pressure at low altitude decreases and at high altitude increases, where there's cooling, pressure at low altitude increases and at high altitude decreases. Just as the world-wide average air pressure is constant, but pressure can drop locally on a hot day. [[User:PiusImpavidus|PiusImpavidus]] ([[User talk:PiusImpavidus|talk]]) 13:50, 23 August 2017 (UTC) |
|||
:Here's a better picture, [http://freeimage.host/image/hjSYS the last 7 days]. The temperature is red, air pressure is dotted gray. You can see how after 14th August temperature is still rising day-to-day, but air pressure has levelled off. I don't think it has much to do with fronts, the first day of that period there was a cold front, but there were no storms/rain on any other day. Even better, check out the relationship from the middle of this graph to the right [http://freeimage.host/image/hjIgJ]. (78.0.231.47) <!-- Template:Unsigned IP --><small class="autosigned">— Preceding [[Wikipedia:Signatures|unsigned]] comment added by [[Special:Contributions/93.139.110.217|93.139.110.217]] ([[User talk:93.139.110.217#top|talk]]) 04:21, 19 August 2017 (UTC)</small> <!--Autosigned by SineBot--> |
|||
::You didn't look at [[cold front]], did you? Or did you find it lacking in some way? [[User:Gem fr|Gem fr]] ([[User talk:Gem fr|talk]]) 16:57, 22 August 2017 (UTC) |
|||
:::The cold front article states that after the front passes, the temperature steadily decreases and the pressure steadily increases. There are three such patterns, each lasting 2-3 days, on the 30-day picture here, but what interests me are the longer periods after the last two, where pressure decreases gradually while temperature increases, and some days the pressure is actually lowest right when the temprature peaks. (78.0.231.47) [[Special:Contributions/78.0.246.130|78.0.246.130]] ([[User talk:78.0.246.130|talk]]) 18:32, 23 August 2017 (UTC) |
|||
= August 20 = |
= August 20 = |
||
Revision as of 03:57, 24 August 2017
of the Wikipedia reference desk.
Main page: Help searching Wikipedia
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Wikipedia:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
Low pressure and high temperatures
I've been checking the weather report lately since the weather has been pretty hot this summer over here, and I've noticed that the heat waves were well-corellated with times of low atmospheric pressure. For example see [1] [2]. That seems to be the wrong way around to me, usually we get low pressure with storms, not 24-hour heat. What could cause this? — Preceding unsigned comment added by 78.0.231.47 (talk) 04:25, 18 August 2017 (UTC)
- Cold front) and warm front have nice tab that will help you understand what's going on. wind direction and strength are also important in that matter.
- As evidenced in those articles, the pressure in itself is not as important as pressure variation Gem fr (talk) 07:40, 18 August 2017 (UTC)
- Looking at the temp and pressure diagrams at the first link, I don't see any correlation at all. The 2nd link does seem to show pressure peaking mid-morning each day, and of course temps peak mid-afternoon. Not sure what would cause the pressure to behave like that. StuRat (talk) 07:37, 18 August 2017 (UTC)
- Temperature at high altitude lags behind surface temperature, so you generally get the coldest troposphere in mid-morning. Low temperature means high density, means more weight in that column of air, means more pressure at the surface. So it makes sense. Of course the effect isn't very large. There must be air moving in or out of the column, which happens by high-altitude wind changing with the time of day. PiusImpavidus (talk) 10:39, 19 August 2017 (UTC)
- When the temperature is high, the density of the air is lower. The column of air above you weighs less, so the air pressure drops. If the pressure gets lower than in surrounding areas, the air will rise, clouds will form and the temperature will drop (during the day). But a lot depends on regional effects. When the pressure distribution is such that the wind comes from the continent, the air is dry, which mean less clouds and higher temperatures in summer. PiusImpavidus (talk) 10:04, 18 August 2017 (UTC)
- oh dear. your are basically saying that higher temperature lower pressure. How wrong.
- Ideal gas law P =(n/V)RT do not perfectly apply to air, but it does quite well, meaning, when temperature rises, pressure rises, too (opposite of what you said). The higher temperature air do not rise because of lower pressure, it rises because, for a given pressure, if T rises, then density (n/V) must goes down, hence Archimedes' principle applies and the parcel of air must go up. All that without any pressure effect. Gem fr (talk) 12:34, 18 August 2017 (UTC)
- I know the ideal gas law. When the air heats up, it expands. The air isn't locked up in a bottle, it's free to expand and flows away horizontally at the tropopause, driven by a slight increase in pressure up there. As the density drops with rising temperature, the pressure difference between the surface and the tropopause (caused by gravity acting on the column of air) drops, so the surface pressure drops too. That's the pressure we measure. The reduced pressure at the surface causes horizontal inflow, continuing as a vertical flow from the surface to the tropopause. That's how Archimedes' principle drives convection. PiusImpavidus (talk) 10:39, 19 August 2017 (UTC)
- Very complicated way to explain than an isobar process will bring a drop in pressure...which it will not, by definition.
- I guess you'll explain likewise that vapor bubbles in a column of boiling water will reduce pressure at the bottom of the column? they won't, either. Actually they would slightly increase the pressure, because slightly rising the whole water surface height (hence pressure, since it depends on height)
- Try again Gem fr (talk) 16:54, 22 August 2017 (UTC)
- Vapour bubbles will push the surface up, but won't change the pressure at the bottom. You may turn part of the water into vapour, but the weight stays the same. Pressure is the weight of the water+vapour bubbles divided by surface area (+ pressure at the top surface), so boiling the water doesn't change the pressure at the bottom, until the water actually boils away. And all of this is about the average pressure at the bottom, as locally the pressure will change. Where it's heated, the pressure at the bottom drops, where it's not heated, pressure increases, just like in the atmosphere. If heating is uniform over the bottom area, you just get turbulence with random pressure variations.
- In short, the average pressure doesn't change, but there are local changes. Where heating is going on, the pressure at low altitude decreases and at high altitude increases, where there's cooling, pressure at low altitude increases and at high altitude decreases. Just as the world-wide average air pressure is constant, but pressure can drop locally on a hot day. PiusImpavidus (talk) 13:50, 23 August 2017 (UTC)
- I know the ideal gas law. When the air heats up, it expands. The air isn't locked up in a bottle, it's free to expand and flows away horizontally at the tropopause, driven by a slight increase in pressure up there. As the density drops with rising temperature, the pressure difference between the surface and the tropopause (caused by gravity acting on the column of air) drops, so the surface pressure drops too. That's the pressure we measure. The reduced pressure at the surface causes horizontal inflow, continuing as a vertical flow from the surface to the tropopause. That's how Archimedes' principle drives convection. PiusImpavidus (talk) 10:39, 19 August 2017 (UTC)
- Here's a better picture, the last 7 days. The temperature is red, air pressure is dotted gray. You can see how after 14th August temperature is still rising day-to-day, but air pressure has levelled off. I don't think it has much to do with fronts, the first day of that period there was a cold front, but there were no storms/rain on any other day. Even better, check out the relationship from the middle of this graph to the right [3]. (78.0.231.47) — Preceding unsigned comment added by 93.139.110.217 (talk) 04:21, 19 August 2017 (UTC)
- You didn't look at cold front, did you? Or did you find it lacking in some way? Gem fr (talk) 16:57, 22 August 2017 (UTC)
- The cold front article states that after the front passes, the temperature steadily decreases and the pressure steadily increases. There are three such patterns, each lasting 2-3 days, on the 30-day picture here, but what interests me are the longer periods after the last two, where pressure decreases gradually while temperature increases, and some days the pressure is actually lowest right when the temprature peaks. (78.0.231.47) 78.0.246.130 (talk) 18:32, 23 August 2017 (UTC)
- You didn't look at cold front, did you? Or did you find it lacking in some way? Gem fr (talk) 16:57, 22 August 2017 (UTC)
August 20
Umbra speed on Earth's surface
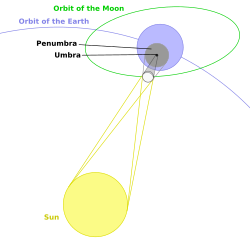
During a solar eclipse, why does the speed of the moon's shadow on the surface of the Earth vary significantly?
- 2410 mph: Western Oregon
- 1747 mph: central Nebraska
- 1462 mph: Western Kentucky
- 1502 mph: near Charleston SC
—2606:A000:4C0C:E200:A90E:D475:2878:2440 (talk) 01:28, 20 August 2017 (UTC)
- What is your source for those numbers? ←Baseball Bugs What's up, Doc? carrots→ 02:49, 20 August 2017 (UTC)
- I assume the time of day should be a factor. Think of a shadow cast on a globe -- if the Sun is just rising, a small movement of a shadow across it will go a long distance on the ground. Likewise the latitude should have an effect. Also, the rate at which the ground moves due to the rotation of the Earth will be different by latitude. The Moon's orbital speed should not vary by much, so the shadow itself (ignoring the terrain and curvature of the Earth in its way) should not change speed much. Wnt (talk) 02:51, 20 August 2017 (UTC)
- Source:
- "How fast is the shadow moving across the US during the eclipse?". eclipse2017.org. Retrieved 20 August 2017. 2606:A000:4C0C:E200:A90E:D475:2878:2440 (talk) 02:55, 20 August 2017 (UTC)
- I don't think time of day should matter. Essentially, the shadow itself doesn't move (relatively speaking) -- the Earth rotates within the umbra; the time as determined by the position of the sun in the sky (not "clock time") is the same for all observers (for same eclipse phase). 2606:A000:4C0C:E200:A90E:D475:2878:2440 (talk) 03:04, 20 August 2017 (UTC)
- To excerpt from that source "Because of the geometry of the Earth’s shape, the shadow will travel faster across its surface and the ends of the eclipse path, and slowest right in the middle." (It should say "at the ends". Must be a typo.) Bus stop (talk) 03:07, 20 August 2017 (UTC)
- I guess the relative speed of the moon (~one orbit per month) does matter. Looking at the top image from that source, the moon's shadow always remains in the "center" of the Earth's surface, since the Sun's direction (vector) must be normal to the surface, with the moon intersecting the vector. 2606:A000:4C0C:E200:A90E:D475:2878:2440 (talk) 03:29, 20 August 2017 (UTC)
- I can't see the image you refer to, but (speaking as an ex-astronomer) I think you must have misinterpreted it, if not the entire physical situation. During a solar eclipse, the angle to the Earth's surface of the direction to the eclipsed sun can vary from 0° (if the Sun & Moon happen to be at the at the Zenith, which would actually be a rare occurance) to 90° (if they're on the horizon, which happens not infrequently). {The poster formerly known as 87.81.230.195} 94.12.90.255 (talk) 05:52, 20 August 2017 (UTC)
- I can see now that the alignment need not be a normal vector to the Earth; but, I reiterate my argument that the reason the shadow moves is due to the rotation of the Earth, and the extraordinary change in the velocity of the shadow is curious. -OP:2606:A000:4C0C:E200:5560:5BBD:5A94:28E2 (talk) 08:00, 20 August 2017 (UTC)
- Umbras start at sunrise and end at sunset. If it goes over the pole before hitting the Earth it can go the "wrong way" (sunset to sunrise). The shadow cannot only move because of the rotation of the Earth, if that were the case there'd be no way to get off the Earth and there'd be a total solar eclipse somewhere on Earth 24/7, even at Full Moon. Sagittarian Milky Way (talk) 09:13, 20 August 2017 (UTC)
- There always is an umbra; however, it only reaches the Earth during an eclipse. 2606:A000:4C0C:E200:EC30:98E9:F083:2A5E (talk) 17:52, 20 August 2017 (UTC)
- The rotation of the Earth contributes to the movement of the umbra over the Earth's surface, but is by no means the dominant contributor. Also important are the movement of the Earth due to its revolution round the Sun, the movement of the Moon due to its revolution around the Earth – both of which alter the relative alignment of the syzygy (astronomy) – and the sphericality of the Earth which means the umbra moves faster towards the Earth's limbs (from the Moon's point of view) limbs due to the umbra's increasingly oblique angle to the Earth's surface. Consider setting up a rough 3D model with balls and a flashlight so that you can demonstrate these factors to yourself, rather than trying to visualise them from descriptions and 2D diagrams. {The poster formerly known as 87.81.230.195} 90.204.183.114 (talk) 19:50, 20 August 2017 (UTC)
- Umbras start at sunrise and end at sunset. If it goes over the pole before hitting the Earth it can go the "wrong way" (sunset to sunrise). The shadow cannot only move because of the rotation of the Earth, if that were the case there'd be no way to get off the Earth and there'd be a total solar eclipse somewhere on Earth 24/7, even at Full Moon. Sagittarian Milky Way (talk) 09:13, 20 August 2017 (UTC)
- I can see now that the alignment need not be a normal vector to the Earth; but, I reiterate my argument that the reason the shadow moves is due to the rotation of the Earth, and the extraordinary change in the velocity of the shadow is curious. -OP:2606:A000:4C0C:E200:5560:5BBD:5A94:28E2 (talk) 08:00, 20 August 2017 (UTC)
- I can't see the image you refer to, but (speaking as an ex-astronomer) I think you must have misinterpreted it, if not the entire physical situation. During a solar eclipse, the angle to the Earth's surface of the direction to the eclipsed sun can vary from 0° (if the Sun & Moon happen to be at the at the Zenith, which would actually be a rare occurance) to 90° (if they're on the horizon, which happens not infrequently). {The poster formerly known as 87.81.230.195} 94.12.90.255 (talk) 05:52, 20 August 2017 (UTC)
- I guess the relative speed of the moon (~one orbit per month) does matter. Looking at the top image from that source, the moon's shadow always remains in the "center" of the Earth's surface, since the Sun's direction (vector) must be normal to the surface, with the moon intersecting the vector. 2606:A000:4C0C:E200:A90E:D475:2878:2440 (talk) 03:29, 20 August 2017 (UTC)
- To excerpt from that source "Because of the geometry of the Earth’s shape, the shadow will travel faster across its surface and the ends of the eclipse path, and slowest right in the middle." (It should say "at the ends". Must be a typo.) Bus stop (talk) 03:07, 20 August 2017 (UTC)
- I don't think time of day should matter. Essentially, the shadow itself doesn't move (relatively speaking) -- the Earth rotates within the umbra; the time as determined by the position of the sun in the sky (not "clock time") is the same for all observers (for same eclipse phase). 2606:A000:4C0C:E200:A90E:D475:2878:2440 (talk) 03:04, 20 August 2017 (UTC)
- Viewed from an observer at Polaris at this time of year, the Earth is a blue dot (formerly white...) and according to Orbit of the Moon the Moon goes around it counterclockwise. The Earth is going around the Sun counterclockwise, or if you want to be parochial about it, the Sun goes around the Earth counterclockwise also, in a non-inertial frame of course; but either way this is so slow we can almost ignore it. And since the Moon is able to extract angular momentum from the spinning Earth, it's a good guess the Earth is also spinning counterclockwise, if your scope can make out those details. So the chief competitors in this race are the Moon, moving an average 1.022 km/s = 2290 mph, and the surface of the Earth, moving 24000 mi/24 = 1000 mph at the equator, times the cosine of your latitude. (The North Pole at 90 N, of course, does not move when rotating). So the Moon's speed is definitely faster than the land's speed, and they're both going counterclockwise, so it's going to catch up. So the East Coast, racing toward sunset, will not outrun the approaching eclipse... unless, that is, it actually reaches sunset first. But when the Moon's shadow is nearing that side of the Earth, then it is extending further and further back along the side at a faster and faster rate, so it is entitled to a last burst of speed near the end of the eclipse (as near its beginning) to cover a large amount of twilit territory. Wnt (talk) 00:51, 21 August 2017 (UTC)
Somewhat related question
Is it purely coincidental that, as seen from Earth, the diameter of the Sun and Moon are the same? — 2606:A000:4C0C:E200:5560:5BBD:5A94:28E2 (talk) 06:09, 20 August 2017 (UTC)
- Yep, funny, huh? However, as the Moon is moving away from the Earth, long in the future, this will no longer be the case, and total solar eclipses will no longer occur. --47.138.161.183 (talk) 06:49, 20 August 2017 (UTC)
- Actually, we already see that, when the moon is closer to apogee and we see an annular eclipse. If the moon drifts farther away, all eclipses will become of the annular variety. ←Baseball Bugs What's up, Doc? carrots→ 00:52, 21 August 2017 (UTC)
- Hmmmm....
The Moon is thought to have moved from 5 Earth radii away to the present 60. The orbital speed is proportional to the square root of this radius. So I'm thinking when it was something like 60/(2.3)^2 = 11 Earth radii away, the orbital speed should have been slow enough that the equator could match its speed. And of course the umbra back then would have been much larger. Does that mean there was a time early on when eclipses could last for hours, or you could even have a double eclipse where the Sun could peek past the Moon, then return behind it? Dang that doesn't seem to make sense - the Earth's surface isn't in orbit - yet I rechecked, Earth's rotation is 1040 km/h, orbit of the Moon is 2290 km/h. Is the orbital speed formula approximation that wrong?? Wnt (talk) 01:54, 21 August 2017 (UTC)- The Precambrian day was less than 24 hours. Did you about for that? What did you mean by the last 2 sentences? Sagittarian Milky Way (talk) 02:02, 21 August 2017 (UTC)
- Wnt, I think you have confused tangential and angular speed. 78.0.199.76 (talk) 03:11, 21 August 2017 (UTC)
- No, I was just being stupid. The orbital speed is proportional to the inverse square root of radius, i.e. something a billion light-years out has an orbital speed of basically zero, while closer orbits are faster. So the Moon doesn't slow down to current rotational speed at the equator until it is 6 times further out, if it ever gets there, at which point the rotational speed has also decreased, and there are no total eclipses anyway. This also reflects that the rotational speed would have to be very much higher than lunar orbital speed to reach orbital velocity near the surface. Now, I still don't actually know that the faster rotation of the early Earth was never faster than the faster orbital speed of the early Moon, and I can't rule it out logically; I'd need formulas for how both have chanced over time. Wnt (talk) 03:33, 21 August 2017 (UTC)
- Wnt, I think you have confused tangential and angular speed. 78.0.199.76 (talk) 03:11, 21 August 2017 (UTC)
- The Precambrian day was less than 24 hours. Did you about for that? What did you mean by the last 2 sentences? Sagittarian Milky Way (talk) 02:02, 21 August 2017 (UTC)
- Hmmmm....
- Actually, we already see that, when the moon is closer to apogee and we see an annular eclipse. If the moon drifts farther away, all eclipses will become of the annular variety. ←Baseball Bugs What's up, Doc? carrots→ 00:52, 21 August 2017 (UTC)
Runway construction on estuary examples
Are there any similar examples to the Haneda Runway D and bridge project ( https://www.jstage.jst.go.jp/article/jgssp/2/2/2_ESD-KL-4/_pdf) which involves the construction of both a runway and bridge strong enough to carry A380s on an estuary? 90.194.48.37 (talk) 10:58, 20 August 2017 (UTC)
- As detailed in Thames Estuary Airport, proposals to build a large new airport serving London somewhere in the Thames estuary have been around for over half a century, but so far none have been implemented. {The poster formerly known as 87.81.230.195} 90.204.183.114 (talk) 19:37, 20 August 2017 (UTC)
LED bulbs and resistors
Do LED bulbs need a resistor to work properly? — Preceding unsigned comment added by 123.201.133.189 (talk) 14:29, 20 August 2017 (UTC)
- Yes, they're in there to limit the current.
Unless you use a very weak source like a penlight battery which has enough internal resistance anyway.Some batteries can have very low resistance so unlike the article I wouldn't leave out the resistance even with 3V coin cells. See LED where it talks a little bit about this in the section on considerations for use. Dmcq (talk) 15:18, 20 August 2017 (UTC)- LED bulbs need something that limits the current, but that something does not have to be a resistor. Unless the product is really cheap, it is far more common to use a tiny Switched-mode power supply because the batteries last a lot longer. --Guy Macon (talk) 17:33, 20 August 2017 (UTC)
- There are two aspects to this: controlled current or controlled voltage. Unlike an incandescent lamp, LEDs have a strongly non-linear relation between current and their junction voltage. So a simple resistor isn't a great solution. It's important to control (or at least, safely limit) both aspects.
- For decades, LEDs were low-powered dim indicators, not powerful illuminators. So their power consumption was low and they were fed from a broadly constant voltage supply. So the simple series resistor was adequate. Also the LED forward voltage (typically 1.7V for a red) was low (compared to the supply), so the resistor was essential. This had both advantages and a disadvantage, also it's somewhat unclear whether the resistor is there to control voltage or current. Because the supply voltage was so much higher than the LED forward voltage, there was a large voltage dropped across the resistor making the circuit relatively insensitive, i.e. the LED current changes little if the supply voltage changed (a good thing). The other advantage of the resistor is that it's cheap and simple, the disadvantage is that the power is mostly dissipated uselessly in the resistor, not the LED.
- It's necessary to control both current and voltage - or at least, to not exceed limits on either, by controlling one of them. For these early LEDs, controlling the current was enough, and was easy.
- Then the 1990s changed everything - LEDs became powerful sources of illumination. Also their forward voltages increased, to maybe 3.6V. We were now trying to make powerful LED lighting, also using LEDs as torches, from low battery voltages (maybe just a single cell at 1.2V) and trying to cope with battery voltages that changed as the cells discharged.
- The series resistor was no longer an adequate control mechanism. As the supply voltage approaches the LED voltage, the circuit no longer regulates so well. But the supply voltage was also reducing at this time, both because the junction voltage was increasing and approaching battery voltages, and because powerful LEDs (especially with batteries) could no longer afford to waste power across the ballast resistor, as they had before.
- Constant current supplies are now popular for lighting, because they are easy to control and they're also usable with varying numbers of LED chips in a series circuit. However they need a supply voltage that's close to the LED junction voltages (or the difference ends up as waste somewhere), so they might require an embedded switch-mode buck converter (a downward voltage converter) to efficiently match these.
- Constant voltage supplies are also used, mostly when driven by batteries. These are boost converters, which can raise the voltage, thus allowing batteries to be used as their voltage falls under discharge. However voltage regulation is difficult, as the LEDs are sensitive to their voltage, and a small excess voltage can cause such a great over-current that the LED overheats and is destroyed. Andy Dingley (talk) 18:39, 20 August 2017 (UTC)
- LED bulbs need something that limits the current, but that something does not have to be a resistor. Unless the product is really cheap, it is far more common to use a tiny Switched-mode power supply because the batteries last a lot longer. --Guy Macon (talk) 17:33, 20 August 2017 (UTC)
-
2 watts, 12 to 16 LEDs feed by LED switching mode driver from 12 V AC or DC >90% efficiency
-
Internal AC adapter driving a series circuit of LEDs >80% efficiency
-
Full insulated single device passive filament LED driver >90% efficiency
--Hans Haase (有问题吗) 10:27, 22 August 2017 (UTC)
Saving wild animals like in Operation_Breakthrough
This question leads to a broader question: is it a good idea to save animals from natural threats? Shouldn't the evolution just do its work? That is, I wonder why save the whales at all in this situation, shouldn't we better let thoughtless stranded whales die?--Hofhof (talk) 16:07, 20 August 2017 (UTC)
- "Is it a good idea...?" and "Shouldn't" both implicitly rest upon some value system. Maybe you don't want to "interfere", but surely other people do think there is good to be done by saving a beached whale. Could be something about how humans are pretty much 100% responsible for endangering wales, and maybe some people think we owe them a little help when we can. Nobody can tell you what you think is right or wrong, but we can give you references that discuss what experts in biology and ethics have said about it.
- For whales specifically, see this report [4] on whale welfare and ethics, this [5] position piece on beached cetaceans from the WDC, including decision making.
- For general background, See Whale_conservation, Marine_mammals_and_sonar, Value theory, bioethics, Conservation_(ethic). SemanticMantis (talk) 16:37, 20 August 2017 (UTC)
- From [6] it appears that this might be a matter of mission creep -- NOAA apparently more often rescues whales tangled in manmade objects, and I would assume the implication there is that they don't expect the sea to be full of crap for the next 10 to 20 whale generations to make it a useful thing to select against. Whether that's because the crap gets cleaned up or the whales die out before then is yours to decide... Of course, the literal answer to your question is that it's a great idea: a million dollars in suckers' money waiting to be spent, and in the process you make yourself a star "real life hero" in two weeks of paid work. Wnt (talk) 00:36, 21 August 2017 (UTC)
- Another possible argument for saving a stranded animal is if it's species is endangered. Of course, it could also be argued that it has defective genes, and should be eliminated for the good of the species. But this must be weighed against the reduction in genetic diversity that will occur if that individual dies. StuRat (talk) 00:54, 21 August 2017 (UTC)
- Laymen typically highly overestimate the effects of selection pressure in driving evolution. Typically, most deaths are entirely accidental in regards to any specific gene. Rather, even if only .01% of deaths are differentially caused by a gene per generation (that is, a surplus of only 1/10,000 deaths) it is enough to drive evolution in geologic time, even though very few excess deaths can be attributed to that gene.
- Consider the extinction of the non-avian dinosaurs. There was no specific gene that the Chicxulub Meteor selected for that day. Likewise, very few people who die who have blue eyes die because they have blue eyes. Also, people who die in old age have already reproduced. Selection pressure doesn't apply at that point, hence the plethora of diseases of old age.
- Finally, there's the example Stephen Jay Gould gave of a single dog which killed over 500 kiwis in 1988, about half the local population. There was no single gene these birds shared that the massacre survivors didn't.
- This is Gould's hypothesis that much of evolution is driven by radical contingency (i.e., blind luck), not gene selection. See Gould's Wonderful Life. If most whale strandings are due to blind misfortune, attempting to save the whales is unlikely to contribute to their long-term extinction.
- There are, of course, counter-examples. Should world civilization fall, the bloodlines of type I diabetics, hemophiliacs, and women whose hip structure allows only Caesarian births, for example, would quickly plummet to almost negligent numbers. That is because we address the effects of these genetic issues without eliminating the genes themselves from our own population. μηδείς (talk) 16:24, 21 August 2017 (UTC)
- A small nitpick. In the Māori language, the plural of kiwi is kiwi. One kiwi, 200 kiwi. One Māori (person), 20,000 Māori. One waka, 30 waka. By convention, this is carried over into New Zealand English when using Māori words. There are many New Zealanders who uncaringly add the 's', but the preference is to follow the Māori style. Akld guy (talk) 20:29, 21 August 2017 (UTC)
- I bet the Chicxulub Meteor selected pretty heavily for some hibernation/estivation variants, and blue eyes could be the difference between life and death under the Nazis. But in order to be effective, selection has to be sustained - the meteor of course could wipe out whole species, but if it left a few copies of a given allele within a species, that allele might eventually return to its original equilibrium. Despite all these things, evidence of selective sweeps is not hard to find. Even if a given gene only accounts for 1/10000 deaths ... there are 30,000 of them. One gene usually matters little, but some gene usually matters. Wnt (talk) 19:52, 21 August 2017 (UTC)
- The birds are normally called kiwis (pl) in American English, this is the term Gould uses repeatedly in Bully for Brontosaurus recounting the story I referred to, and I am sticking to it. You can call moose "elk"; rabbits "coneys"; and opossums "possums" as much as you like.
- As for selection for estivation or hibernation as a means of surviving K/T level events, either life at the time would have had to be small due past such events, or have become small afterwards to survive regular future such events. Neither was the case, so this was one mass culling, not the evolution of small size by (repeated) natural selection. Dinosaurs did not evolve to become extinct and mammals did not evolve to stay small to avoid future mass extinctions.
- Each of you did actually understand my argument and nitpicking for points is not my cup of cricket. μηδείς (talk) 00:39, 22 August 2017 (UTC)
- Don't see any evidence, Akld guy was "nitpicking for points", not sure Wnt was doing so either. Most logical conclusion would be that the person who assumes nitpicks are for points probably believes so because they aren't being quite honest in what their cup of cricket is hence adding non existent motivations just because it's what they normally do. Incidentally, in modern contexts it's not very common to call opossums as possums in NZ. Mostly of course we don't talk about opossums much since they don't really concern us, but possums do. Our article Opossum suggests it's an American thing. There is some history of the possum being called opossum in NZ, including in legislation [7] [8] but from my experience in modern contexts very few Kiwis actually do that. (I don't think I've actually read or heard any example in the years I've been here except for those I read when researching this. I.E. whenever someone has mentioned opossum they're been talking about opossums although as said, this isn't really that common in NZ. But I have read and heard about possums. A lot. And although it isn't something I have that much experience with, a Google Scholar search supports my view that this applies even to scientific papers in modern contexts. Most references to opossum relating to New Zealand since 2000 seem to be titles of older citations. The modern papers themselves use possum. This source suggests it was probably about in the late 1970s and the 1980s [9] that the scientific usage caught up with the vernacular usage.) Nil Einne (talk) 08:52, 22 August 2017 (UTC)
- Basically TLDR, Nil, but both those editors understood my point exactly, and I said they could call opossums "possums" if they liked. All that matters is they were making points irrelevant to the actual science. Mass extinction does not equal evolution, although it obviously opens up niches that other animals can evolve to fill--but mass extinction and evolution by natural selection are separate concepts. As for PC nomenclature, they are still starfish and jellyfish no matter how much the mermen complain. μηδείς (talk) 16:36, 22 August 2017 (UTC)
- I have no idea what relevance moose, elk, rabbits, coneys, and possums had to my little piece about the plural of kiwi. If you assume in bad faith that I was point scoring instead of educating you and other readers, that says more about you than me. Accept in good faith that someone may simply be trying to teach you something. Akld guy (talk) 21:45, 22 August 2017 (UTC)
- I really could care less, Akld, about the politically correct neologisms of a nation half the size of NYC. The Gould standard is gold enough for me. Go file a complaint at WP:ANENGVAR. μηδείς (talk) 03:33, 23 August 2017 (UTC)
- I have no idea what relevance moose, elk, rabbits, coneys, and possums had to my little piece about the plural of kiwi. If you assume in bad faith that I was point scoring instead of educating you and other readers, that says more about you than me. Accept in good faith that someone may simply be trying to teach you something. Akld guy (talk) 21:45, 22 August 2017 (UTC)
- Basically TLDR, Nil, but both those editors understood my point exactly, and I said they could call opossums "possums" if they liked. All that matters is they were making points irrelevant to the actual science. Mass extinction does not equal evolution, although it obviously opens up niches that other animals can evolve to fill--but mass extinction and evolution by natural selection are separate concepts. As for PC nomenclature, they are still starfish and jellyfish no matter how much the mermen complain. μηδείς (talk) 16:36, 22 August 2017 (UTC)
- Don't see any evidence, Akld guy was "nitpicking for points", not sure Wnt was doing so either. Most logical conclusion would be that the person who assumes nitpicks are for points probably believes so because they aren't being quite honest in what their cup of cricket is hence adding non existent motivations just because it's what they normally do. Incidentally, in modern contexts it's not very common to call opossums as possums in NZ. Mostly of course we don't talk about opossums much since they don't really concern us, but possums do. Our article Opossum suggests it's an American thing. There is some history of the possum being called opossum in NZ, including in legislation [7] [8] but from my experience in modern contexts very few Kiwis actually do that. (I don't think I've actually read or heard any example in the years I've been here except for those I read when researching this. I.E. whenever someone has mentioned opossum they're been talking about opossums although as said, this isn't really that common in NZ. But I have read and heard about possums. A lot. And although it isn't something I have that much experience with, a Google Scholar search supports my view that this applies even to scientific papers in modern contexts. Most references to opossum relating to New Zealand since 2000 seem to be titles of older citations. The modern papers themselves use possum. This source suggests it was probably about in the late 1970s and the 1980s [9] that the scientific usage caught up with the vernacular usage.) Nil Einne (talk) 08:52, 22 August 2017 (UTC)
August 21
The Octaeteris and the Cycles of Venus
How aware were the ancients that the Octaeteris coincides with cycles of Venus ? (I am asking this because these two numbers, eight and thirteen, appear conjoined in at least two instances in this ancient monument, dating from the first two centuries BC, and I want to avoid anachronistic interpretations). — 79.118.174.82 (talk) 10:30, 21 August 2017 (UTC)
- Which ancients? There are thousands of cultural groups around the world. --Jayron32 10:35, 21 August 2017 (UTC)
- Europeans (such as the ancient Greeks, for instance), and the civilizations (directly or indirectly) known to them in ancient times (i.e., Egyptians and Babylonians, but not Mayans). — 79.118.174.82 (talk) 12:11, 21 August 2017 (UTC)
- If you don't find a clue in Egyptian astronomy, Babylonian astronomy or Babylonian astrology, we can only try guess
- however, be aware that 8-13 is also part of the Fibonacci sequence, with ubiquitous presence in nature, so you WILL find it pretty everywhere whether people meant it or not. And hence in numerology
- Gem fr (talk) 16:37, 22 August 2017 (UTC)
- Plus you can cannot even know it's 13 Venus years if you think the Earth is the center of the Solar System. Aristarchus the Ancient Greek thought the Sun was the center but that didn't catch on in the West till a few centuries ago. The more relevant numbers for Earthbound observers are 5 and 8 (5 Venus apparition pairs per 8 years) and 1.6 years (AKA 8/5ths years, 1 and 3/5ths years). (5 is coincidentally a Fibonacci number) The octaresis also sucks. It's wrong by a whole tenth lunation after only 2 cycles (16 years) and the Metonic cycle's only wrong by a tenth lunation in ~a millennium (dozens of cycles)) so that's a much better accuracy to length ratio. Sagittarian Milky Way (talk) 17:38, 22 August 2017 (UTC)
- No, the ancients knew how long the planetary years were even in their geocentric model because they could still track the patterns in the repeating motions of the planets. That is, they could calculate the time periods for the patterns they saw, and could arrive at the numbers even if their explanations were crap. See Deferent and epicycle for a rough overview. Pre-modern astronomers had strikingly accurate calculations of the movements of objects. They didn't know what they were actually watching, but they could describe and predict it just fine. --Jayron32 19:25, 22 August 2017 (UTC)
- Okay you're right, inferior planets do seem to go around their main epicycle once per real year in inertial space (this is simplified from small epicycles on main epicycles and maybe more layers right?) but from the observer their years are ~1/3rd and ~8/5ths years or the model wouldn't work. And I wonder if 8/13ths years would be more important to geocentrists than 8/5ths years when the center of the epicycle is glued to the Earth-Sun line like a rod. When it comes down to astrology (which was what astronomy mostly was at the time and paid the astronomologers' bills) who cares what sign Venus is in and what stars it conjuncts as seen from the center of it's epicycle, what sign is Venus in and what stars it conjuncts from Earth? And what's it's angular distance from the Sun as seen from Earth so we can see if it's conjunct or semisextile or semisquare and makes good or bad sex juju? And when's a good time to see Venus in the morning (early bird)/evening (everyone else) so I can contribute to planet location records so the theorists can add epicycles to improve the ephemerides? (which all repeat once every ~8/5ths or 8 years). Sagittarian Milky Way (talk) 21:49, 22 August 2017 (UTC)
- No, the ancients knew how long the planetary years were even in their geocentric model because they could still track the patterns in the repeating motions of the planets. That is, they could calculate the time periods for the patterns they saw, and could arrive at the numbers even if their explanations were crap. See Deferent and epicycle for a rough overview. Pre-modern astronomers had strikingly accurate calculations of the movements of objects. They didn't know what they were actually watching, but they could describe and predict it just fine. --Jayron32 19:25, 22 August 2017 (UTC)
- Plus you can cannot even know it's 13 Venus years if you think the Earth is the center of the Solar System. Aristarchus the Ancient Greek thought the Sun was the center but that didn't catch on in the West till a few centuries ago. The more relevant numbers for Earthbound observers are 5 and 8 (5 Venus apparition pairs per 8 years) and 1.6 years (AKA 8/5ths years, 1 and 3/5ths years). (5 is coincidentally a Fibonacci number) The octaresis also sucks. It's wrong by a whole tenth lunation after only 2 cycles (16 years) and the Metonic cycle's only wrong by a tenth lunation in ~a millennium (dozens of cycles)) so that's a much better accuracy to length ratio. Sagittarian Milky Way (talk) 17:38, 22 August 2017 (UTC)
- Europeans (such as the ancient Greeks, for instance), and the civilizations (directly or indirectly) known to them in ancient times (i.e., Egyptians and Babylonians, but not Mayans). — 79.118.174.82 (talk) 12:11, 21 August 2017 (UTC)
- Thanks, guys ! Problem solved ! Quoting from Ptolemy's Tetrabiblos: “Venus, taking in charge the third age, that of youth, for the next eight years, corresponding in number to her own period...” — 86.125.198.169 (talk) 23:27, 23 August 2017 (UTC)
LED on mains power how is it possible
In this video some guy solders what looks like a ~1v DC Light Emitting Diode onto an AC mains power socket and somehow it works. Can someone explain to me how this is possible? I thought AC travels in both directions so shouldn't the LED not light up? How come it doesn't get hot and / or explode by being overpowered by 239v? — Preceding unsigned comment added by 49.49.98.175 (talk) 11:08, 21 August 2017 (UTC)
- Devices called rectifiers are used to convert AC to DC. Any simple diode can work as a rectifier (with some extra components, IIRC. I am not an electrician, but I did take a few electronics classes many decades ago, and some of it is coming back to me) including a light emitting diode. I suspect the LED acts as its own rectifier in some way for this circuit. --Jayron32 12:14, 21 August 2017 (UTC)
- Oh, and it doesn't get overpowered because he hooks up a ballast resistor. You can clearly see him doing this. --Jayron32 12:20, 21 August 2017 (UTC)
- LEDs are diodes, so they only pass current in one direction. When they do that, they light up. When reversed, they don't conduct. However they're not very good diodes, so they have only a low reverse breakdown voltage. LEDs are available where two chips are placed in one package, connected in reverse parallel and usually of two different colours. They were used as a two-wire connected two colour indicator, or as a simple polarity indicator.
- There are two problems here. He solves the excess forward voltage and current issue by using a resistor (see further up this page too). But he leaves it with a high reverse voltage, so the LED is likely to fail fairly soon. Andy Dingley (talk) 13:21, 21 August 2017 (UTC)
- Looking at a few more of this guy's videos, he's past idiot and into dangerous idiot. Many of the things here are not just pointless, but so pointlessly hazardous that they will try to kill you - in a way that isn't even educational or amusing.
- If you want some real electrical knowledge from YouTube, try Big Clive instead. Andy Dingley (talk) 16:01, 21 August 2017 (UTC)
- Agreed. BC has a bit of an obsession with pyrotechnics, but at least he *warns* you when he is doing something that is not 100% sensible. Gandalf61 (talk) 16:32, 21 August 2017 (UTC)
- Also he uses the finest of safety equipment, such as the Explosion Containment Pie Dish Andy Dingley (talk) 16:42, 21 August 2017 (UTC)
- Interesting, but my favorite is still David L. Jones at [EEVblog]. --Guy Macon (talk) 18:19, 21 August 2017 (UTC)
- Also he uses the finest of safety equipment, such as the Explosion Containment Pie Dish Andy Dingley (talk) 16:42, 21 August 2017 (UTC)
- Agreed. BC has a bit of an obsession with pyrotechnics, but at least he *warns* you when he is doing something that is not 100% sensible. Gandalf61 (talk) 16:32, 21 August 2017 (UTC)
- Nobody has done minimum calculations for efficiency or life span of this circuit. There are BiLEDs or double core LEDs avail, containing two "anti parallel" green/red or yellow/red LEDs chips in a single LED device case. For a red LED, the one in the video looks little "orange". The second LED is a reverse to the first one. Most LEDs allow a max reverse voltage of five volts. As even blue LEDs have the highest voltage drop in operation mode from 3 to 5 volts, the dual LEDs protect each other form reverse voltage. But nobody has seen if his multiple socket is plugged to the wall. There's also no flicker or interference from camera to the red LED visible which makes me being in doubt if the LED is powered with AC. --Hans Haase (有问题吗) 09:52, 22 August 2017 (UTC)
- He's using LEDs from a bag labelled "Red". In the bulk shots you can see the internal frame for a single LED. Andy Dingley (talk) 10:28, 22 August 2017 (UTC)
Does Wikipedia have an article on the territorial behavior of various animals, including humans?
^ 140.254.70.33 (talk) 13:03, 21 August 2017 (UTC)
- Territory (animal) and Territoriality (nonverbal communication). Rojomoke (talk) 13:51, 21 August 2017 (UTC)
Is it possible to have a total solar eclipse on a Full Moon instead of a New Moon?
Is it possible to have a total solar eclipse on a Full Moon instead of a New Moon? 96.66.16.169 (talk) 16:21, 21 August 2017 (UTC)
- No. You get lunar eclipses around the time of a full moon, solar eclipses around a new moon. The ordering has to be either S/E/M or S/M/E for each, and accurately aligned. Andy Dingley (talk) 16:27, 21 August 2017 (UTC)
- Technically no, because a full moon occurs when the moon is on the far side of the earth from the sun, rather than between the earth and sun. But a full moon does occur half a month before and after an eclipse, and there could be back-to-back solar and lunar eclipses 15 days appart if conditions are right. μηδείς (talk) 16:28, 21 August 2017 (UTC)
- [ec] -In other words, in order to be in the moon's shadow, the sun must be directly behind it, which means the moon's dark side is facing you. 2606:A000:4C0C:E200:F9E3:2F35:FA19:8314 (talk) 16:33, 21 August 2017 (UTC)
- You certainly can have one if you're on the Moon! ;) But to Earthly eyes that's a lunar eclipse. Wnt (talk) 19:39, 21 August 2017 (UTC)
- No. By definition, the moon would be on the exact wrong side of Earth during a full moon.
- The only way it could happen is if some other massive object showed up to block the sun. At that point, arguing over whether or not that "technically" still counted as an eclipse would probably be the least of our worries. ApLundell (talk) 14:07, 22 August 2017 (UTC)
- Astronomers don't have to wait for a total eclipse to study the solar corona. They can place a circular mask at the end of their telescopes. A calendar year can have a maximum of seven eclipses (four of the sun and three of the moon if I remember rightly) or a minimum of two (both solar). Eclipses happen when the orbits of the earth and the moon intersect (which happens every six months). So for the maximum seven the orbits would intersect at the beginning of January, in June and at the end of December. 82.14.24.95 (talk) 16:02, 22 August 2017 (UTC)
Would a solar eclipse be visible from the moon?
Another eclipse question: I was reading Total eclipses on the Moon, and wondered if the reverse could occur. Would a solar eclipse (as seen from earth) be visible from the moon? I.e. if you were standing on the moon, looking at the earth, would you see the moon's shadow passing across (umbra and penumbra), or would it be too small to see with the naked eye? Optimist on the run (talk) 19:20, 21 August 2017 (UTC)
- The moon's shadow on the earth would be the same size as the moon looks from earth, about a half a degree. Seems like it would be highly visible if you were on the moon looking at the earth. It would look like a small dark spot moving across the earth. 69.243.146.7 (talk) 19:26, 21 August 2017 (UTC)
- You are wrong: moon's shadow on the Earth is very small - about 250 km. From the the Moon the angular size of this shadow will be about 2 angular minutes. In another words it will be barely visible. Ruslik_Zero 20:40, 21 August 2017 (UTC)
- Binoculars would definitely help, and it would be more visible if it (or rather the lit area surrounding it) was hitting a highly reflective surface, like clouds or ice. StuRat (talk) 22:02, 21 August 2017 (UTC)
Approximately what is considered a deep partial solar eclipse?
What's considered a shallow one? Are there any other terms like this? (moderate?) Presumably this would be in eclipse magnitude or obscuration. (width and area covered) Sagittarian Milky Way (talk) 23:42, 21 August 2017 (UTC)
- I can find no formal definition of the term, which means that deep means "a lot" and shallow means "only a little" without any formal % as the dividing line between the two; it's probably like the differences between "some" "a few" "several" "a lot" "many", etc. These are imprecise relative terms and are not defined strictly. At least, that's how I interpret the absence of evidence on this one; if it had a formal definition, we'd find it. If it lacks a formal definition, we're stuck to falling back to how language works, which is as fuzzy as always. --Jayron32 19:20, 22 August 2017 (UTC)
Solar eclipse: why did the sun still look round in direct images?

In my camera-phone photos of today's solar eclipse, taken where it was not total but peaked above 75%, the sun still appeared round or slightly elliptical in the direct image. Only a reflection, which I'm guessing came off the edge of the lens (since it persisted when I removed the clear plastic case), made the crescent shape of a partially-eclipsed sun visible. Why does this happen? NeonMerlin 23:52, 21 August 2017 (UTC)
- I saw something similar today, but with no camera involved (just using a lens pointing the image at paper). My guess is that the direct image was badly out of focus, since any image sufficiently out of focus will look like that. The reflected image just worked out to be perfectly in focus. StuRat (talk) 23:58, 21 August 2017 (UTC)
- Here in the Northeast the sun appeared crescent both in my pinhole camera and when directly visible through heavy cloud cover. My dad's camera made an hourglass shape, until I asked him how he made the pinhole, which was with a blunt paperclip that tore the foil rather than puncturing it. When he made a new, round pinhole the image appeared quite clearly crescent-shaped. My sister is not too far from Vermont, I'm planning on seeing totality there in 2024. μηδείς (talk) 00:48, 22 August 2017 (UTC)
- A pinhole lens has the feature that everything is always in focus, so that would explain why you wouldn't get a big blob. The downside, of course, is that the image is quite dim. Normally this is a showstopper, but with something as bright as the Sun, some dimming is a good thing. StuRat (talk) 01:09, 22 August 2017 (UTC)
- Here in Virginia, cellphone-carrying people with whom I was talking typically had one of two images: a vaguely round really bright figure through thin or no clouds, or a crescent-shaped figure through not-as-thin clouds. I was left suspecting that cellphones simply can't capture the solar figure properly unless it's dimmed by clouds or something else. Nyttend (talk) 02:06, 22 August 2017 (UTC)
- A pinhole lens has the feature that everything is always in focus, so that would explain why you wouldn't get a big blob. The downside, of course, is that the image is quite dim. Normally this is a showstopper, but with something as bright as the Sun, some dimming is a good thing. StuRat (talk) 01:09, 22 August 2017 (UTC)
- I had a looooooong discussion about this yesterday before the eclipse. A cell phone (and any cheap auto-focus camera) is designed to focus on objects that are designed to focus on objects that are between 6 inches and 100 feet away. This depends greatly on the exact camera and the type of auto focus. So, lets assume yours is a very nice autofocus and it can focus on objects that are 100 miles away, not 100 feet. The sun is almost 100 million miles away. As a result, your camera cannot focus on it and you get a blurry bright dot. That isn't all. The brightness of the sun is so much that it floods the photo sensors and bleeds into nearby sensors, causing more fuzziness. If you take the photo through eclipse viewing glasses, you will reduce the light by about 99%. But, it will still be greatly out of focus. Even during totality, when you can look directly at the sun, your phone cannot focus on an object that far away. So, instead of a pretty ring, you get a fuzzy circle. The fix is to use manual focus. Not all phones have manual focus. Even then, you need to be able to focus on something very far away. An alternative that I saw was to use two cameras. Each had eclipse filters to block 99% of the light. Each got an out-of-focus photo from a slightly different angle (they were about 4 feet apart). Then, the image was fed into a computer that used the two photos to reconstruct an in-focus photo. It looked acceptable, but nothing near the quality of a photo from a filtered telescope. 209.149.113.5 (talk) 11:52, 22 August 2017 (UTC)
- The difference between 100 feet and 93 million miles is irrelevant, as focus is not at all linear, and both those would fall under the infinite focus position. For example, this camera considers anything beyond 7 feet to be infinite focus: [10]. There are cameras which can distinguish longer focal distances than that, but eventually they all go to "∞". StuRat (talk) 16:49, 23 August 2017 (UTC)
- I think the scale seems like an important factor here. I mean, in the photo above the reflection is very clearly a small crescent. That implies to me that the real image is of the same size, buried in the middle of that immense white blob. I'm tempted to think that if the reflection is in focus, the original image is also in focus, but I don't know that - depends on how that camera produced it, especially since it might even have auto-focused the reflection because it provides a better contrast... Wnt (talk) 13:28, 22 August 2017 (UTC)
August 22
Male autism-spectrum correlations with homosexuality, bisexuality, fetishes and paraphilias?
In males, are autism-spectrum disorders statistically correlated with homosexuality, bisexuality or any fetishes or paraphilias? The one study I found on the subject included only 17 males, and the extent to which it oversampled females leads me to question the representativeness of those males. NeonMerlin 00:54, 22 August 2017 (UTC)
- the article you link should have a link to references, which should include known articles about the same subject. As it seems to me highly politically UNcorrect to try to link ASD to any LGBT behavior, I suspect such study would be rare and statistically unsignificant
- Gem fr (talk) 16:15, 22 August 2017 (UTC)
- I think it is a useful topic for study, because some authors think of autism as an "extreme male" phenotype, [11] and so looking at GLBT variations seems like a no-brainer. I've never given that idea much credence (the article I cite doesn't seem to either) but hey, it's biology, and nothing is impossible in biology. That said, the only obvious relevant hit on PubMed for autism homosexuality is this 50-boy study that finds nothing much of note. I actually found three references with autism transsexual, but they amount to anecdotes, something from Med Hypotheses, and this brief report, which takes the idea somewhat seriously and is the closest I have to an answer. But I'll leave it to someone else to pull this off Sci-Hub and decide if there is any meat there. Wnt (talk) 18:52, 22 August 2017 (UTC)
- "extreme male"? Now THAT is a concept! especially when applied to fully functional FEMALE people, by people writing in a Psychology review when we now know that ASD is NOT about psychology (as people though sometime ago)! University job includes producing ideas, most of them utter crap. I already know that Most Published Research Findings Are False and Most Clinical Research Is Not Useful in medicine, i suspect it is even worse in psychology. But that was just my "small" 2 cents Gem fr (talk) 13:16, 23 August 2017 (UTC)
- I think it is a useful topic for study, because some authors think of autism as an "extreme male" phenotype, [11] and so looking at GLBT variations seems like a no-brainer. I've never given that idea much credence (the article I cite doesn't seem to either) but hey, it's biology, and nothing is impossible in biology. That said, the only obvious relevant hit on PubMed for autism homosexuality is this 50-boy study that finds nothing much of note. I actually found three references with autism transsexual, but they amount to anecdotes, something from Med Hypotheses, and this brief report, which takes the idea somewhat seriously and is the closest I have to an answer. But I'll leave it to someone else to pull this off Sci-Hub and decide if there is any meat there. Wnt (talk) 18:52, 22 August 2017 (UTC)
Sunspots
Using premodern equipment, how in the world does one observe sunspots? Even when I was looking through clouds and through a telescope made specifically for eclipse-viewing, the Sun appeared during today's solar eclipse to be a perfect, unblemished shape, aside from the rather big missing chunk of course. I can't imagine how the ancient Chinese, c. 800 BC, or Greeks c. 300 BC (see Solar observation#Early observations, could see sunspots. Nyttend (talk) 02:10, 22 August 2017 (UTC)
- It is fairly easy with premodern equipment to use a pinhole camera to get a projected image of the Sun, on which sunspots are quite readily visible. Double sharp (talk) 02:33, 22 August 2017 (UTC)
- So what, you just take a sheep of papyrus and poke a hole in it, and you're done? Nyttend (talk) 02:36, 22 August 2017 (UTC)
- No poking of sheep is allowed here ! StuRat (talk) 03:09, 22 August 2017 (UTC)
- Poking a piece of parchment would be the most basic approach, I suppose. (Lot's of school children observe an eclipse with two pieces of paper, I don't imagine parchment would be any different.)
- But much more advanced pinhole cameras have been in use for a long time. The article Pinhole camera is a bit thin, but it does cover the history. The article Camera obscura also covers the history a bit.
- It strikes me as an interesting factoid that cameras were invented thousands of years before film. ApLundell (talk) 14:01, 22 August 2017 (UTC)
- The word camera's etymology runs from Greek kamara "vaulted chamber" --> Latin camera "vaulted room" --> English 1708 "vaulted building" --> early 18c. as a short form of Modern Latin camera obscura "dark chamber" (a black box with a lens or pinhole that could project images of external objects) --> c. 1840 "picture-taking device". Blooteuth (talk) 14:36, 22 August 2017 (UTC)
- Note that sunspots vary dramatically in size and temperature/brightness, and hence visibility. StuRat (talk) 03:10, 22 August 2017 (UTC)
- Any sunspots during the eclipse weren't big as sunspot go and were probably covered by the Moon during the time when there was a rather big missing chunk. The butterfly diagram shows that we're in one of the lowest latitude parts of the sunspot cycle and the Sun's poles are only 7 degrees from Earth's orbit's poles so that couldn't really help bring them to the north or south side of the disc either. Sagittarian Milky Way (talk) 04:23, 22 August 2017 (UTC)
- It would be nice if someone could update the articles with information about where the observations took place. I mean, the Sun has the great advantage of being really bright, so you can project it a long way, but at largest size it is still best viewed in a dark setting. This implies that it would really help to have a monumental roofed building, some manner of cathedral where a pinhole aperture made accidentally or deliberately at one window can project an image into a vast darkened space. (I suppose the right cave can do it but you have to postulate a lot of luck) I imagine the Chinese and Greeks had many such buildings but don't know if those observers were known to use them. Wnt (talk) 05:14, 22 August 2017 (UTC)
- See also helioscope.--Shantavira|feed me 06:49, 22 August 2017 (UTC)
- The name and the fact that Galileo and a contemporary worked on it would imply reliance on lenses, which makes that a much higher level of technology than that available to the earlier workers. Wnt (talk) 13:31, 22 August 2017 (UTC)
- Right now, there is just one visible sunspot - a chain on the sun's equator called AR2671. It's possible that you weren't looking in the right place (sunspots are not that big compared to the solar disc, and it's easy to overlook them). That said I find larger sunspots are visible even without magnification, just by looking through eclipse glasses - and if you're very lucky, you can see them in the setting sun. Smurrayinchester 07:59, 22 August 2017 (UTC)
- This article does not go back as far as the notations above, but does go back to the early modern period and discusses some fairly simple methods for observing sunspots. Kepler created a simple camera obscura by poking a pinhole in the roof of his house, for example. --Jayron32 12:07, 22 August 2017 (UTC)
- Note that natural pinholes are quite common. For example, a tree's leaves on a calm day can form many pinholes between the leaves. I often find accidental pinhole lens images. StuRat (talk) 14:39, 22 August 2017 (UTC)
- On rare occasions a sunspot (or sunspot group) can be big enought to be seen directly with the eye when the Sun is dimmed by mist or thin cloud. Over the last 50 years I've read in various astronomy textbooks that ancient Chinese astronomers recorded a number of such observations, although our article on Chinese astronomy doesn't mention them. To give a couple of references:
- George O. Abell's Exploration of the Universe; Third edition; Holt, Rinehart & Winston 1975 (in Section 2.1 Earliest Astronomers, on page 11) states, "The Chinese also kept rather accurate records of comets, meteors and fallen meteorites from 700 B.C. Records were made of sunspots visible to the naked eye . . . ."
- Patrick Moore's The Guinness Book of Astronomy; Fifth Edition; Guinness Publishing 1995 (in The Solar System ∗ The Sun, on page 6) states, "Naked-eye spots had been previously [to J. Fabricius in 1610–11] recorded, but had not been explained; one given in a Chinese record of 28BC is described as 'a black vapour as large as a coin' and there is a Chinese record of an 'obscuration' in the Sun, which may well have been a spot, as early as 800BC."
- {The poster formerly known as 87.81.230.195} 94.12.81.193 (talk) 15:03, 22 August 2017 (UTC)
- What ! Can't see sunspots... reason is below to the tune of La donna è mobile:
- Sun spots are not here this year,
- Less so than yester year.
- Small spots are sometimes seen
- Often with H-alpha screen.
- Sun spots are not here this year,
- Next verse:
- Bigger ones are for future years
- So get in lots of beers
- Get yours before their gone
- Come now try one.
- Bigger ones are for future years
- See: Real-time solar activity for what I mean.Aspro (talk) 18:32, 22 August 2017 (UTC)
- And here's another and perhaps better ref as to why the ancients saw spots before their eyes but you can't. Sun's Current Solar Activity Cycle Is Weakest in a Century Aspro (talk) 19:01, 22 August 2017 (UTC)
- I highly doubt any "pinhole camera" construction without a lens can help with sky or sun observation. There is no need for any of that to see big sunspots since the sun is big enough to observe with sharp eyes and we have frequent moments to look straight at our central star - in the morning and evening, when the weather conditions are right - without any danger of harming the Retina. Besides that some planets where also known surprisingly early but also there have always been humans with exeptional sharp eyes. --Kharon (talk) 22:57, 22 August 2017 (UTC)
- Ganymede, Uranus, some asteroids and possibly Neptune are visible to good eyesight but their sighting wasn't known to science till 1610, 1789, 1800s, and 1846 respectively. (also a very small amount of galaxies: discovered by telescope, some people can see them) Sagittarian Milky Way (talk) 00:03, 23 August 2017 (UTC)
- Kharon is right about some people having exceptionally sharp eyesight. Anecdata: an acquaintance of mine with comparatively little interest in astronomy once revealed that he could routinely see Jupiter's four Galilean moons with his naked eyes. (They are in themselves bright enough to be visible, having magnitudes ranging from 4.6 to 5.6 at opposition, but are normally lost in the greater glare of Jupiter.) Until a short time before our conversation, he hadn't realised that this was exceptional, so had never mentioned it. Doubtless in earlier history some people had similarly seen previously unknown astronomical phenomena without realising their significance, and so had not recorded the fact. {The poster formerly known as 87.81.230.195} 94.12.81.193 (talk) 16:35, 23 August 2017 (UTC)
- But Kharon is wrong about pinholes being worthless for observing sunspots.
- It's a common enough student activity to track sunspots with a pinhole camera.(Example [12]). Ideally you'd want about a meter or more between the pinhole and the viewing surface. This will make the image pretty dim, but with a hood of some kind (or just sticking your head in the cardboard box you're using) your eyes dark-adapt and you can see surprising detail. ApLundell (talk) 17:09, 23 August 2017 (UTC)
- Ganymede, Uranus, some asteroids and possibly Neptune are visible to good eyesight but their sighting wasn't known to science till 1610, 1789, 1800s, and 1846 respectively. (also a very small amount of galaxies: discovered by telescope, some people can see them) Sagittarian Milky Way (talk) 00:03, 23 August 2017 (UTC)
- I highly doubt any "pinhole camera" construction without a lens can help with sky or sun observation. There is no need for any of that to see big sunspots since the sun is big enough to observe with sharp eyes and we have frequent moments to look straight at our central star - in the morning and evening, when the weather conditions are right - without any danger of harming the Retina. Besides that some planets where also known surprisingly early but also there have always been humans with exeptional sharp eyes. --Kharon (talk) 22:57, 22 August 2017 (UTC)
Gluconeogenesis
The gluconeogenesis article says "The process is highly endergonic until it is coupled to the hydrolysis of ATP or GTP, effectively making the process exergonic." What does this mean in layman terms? At what point in a zero carb diet does gluconeogenesis become "coupled to the hydrolysis of ATP or GTP" — Preceding unsigned comment added by 187.1.51.122 (talk) 13:59, 22 August 2017 (UTC)
- Endergonic means energy costly, exergonic means producing energy. An analogous statement to the first part of your question would be that producing gasoline costs a lot of energy (obtaining, transporting, and refining oil) until you burn it in the presence of oxygen. μηδείς (talk) 16:25, 22 August 2017 (UTC)
- it means gluconeogenesis consume energy, that the energy for this is brought in the process by ATP (or GTP), but that not all the energy of ATP is transformed into glucose, the process waste some of it as heat (as is usual for process)
- It also means that gluconeogenesis IS coupled to the hydrolysis of ATP or GTP from its very start, it wouldn't happen at all otherwise
- Gem fr (talk) 17:54, 22 August 2017 (UTC)
- It's a little more complicated than that. The thing to bear in mind is that gluconeogenesis is a complicated multi-step process. The change in Gibbs free energy at each step depends on the concentration of all reagents - Le Chatelier's principle is very much in play. Force one reaction with a large energy change, and the next step will have no choice but to go forward ... once a large enough concentration of metabolite builds up. Look at [13] for a sense of the "energy landscape" for glycolysis, which is a similar situation; then see this comparison for some numbers for gluconeogenesis. But the cell can't deal with massive concentrations of some metabolites. So there is a lot of specific engineering and "horse trading" involved, which has been optimized by unfathomable time scales of microbial evolution. But the bottom line is that just a few steps that split ATP are used to drive many others that are neutral or even unfavorable in terms of free energy. Wnt (talk) 19:11, 22 August 2017 (UTC)
- Fair enough, but the OP asked for a translation in "layman terms" of what is written in the article, so i am pretty sure that adding "It's a little more complicated than that" didn't help (even though I can only hat tip) Gem fr (talk) 12:51, 23 August 2017 (UTC)
- From a general overview, it makes sense for gluconeogenesis to consume energy. As noted at nutrient, both protein and carbohydrate provide about 4 kcal/gram to the body in usable energy. Therefore, you gain nothing from an energy point of view by converting protein to carbs; and since you aren't using that protein as an energy source, you're basically burning 4 kcal/gram just to make that glucose. Couple that with energy losses expected due to inefficiencies (a general physics concept is that SOME energy is always lost in any transfer, per second law of thermodynamics, etc) and you can see where the process must be endergonic. --Jayron32 13:07, 23 August 2017 (UTC)
- Fair enough, but the OP asked for a translation in "layman terms" of what is written in the article, so i am pretty sure that adding "It's a little more complicated than that" didn't help (even though I can only hat tip) Gem fr (talk) 12:51, 23 August 2017 (UTC)
- It's a little more complicated than that. The thing to bear in mind is that gluconeogenesis is a complicated multi-step process. The change in Gibbs free energy at each step depends on the concentration of all reagents - Le Chatelier's principle is very much in play. Force one reaction with a large energy change, and the next step will have no choice but to go forward ... once a large enough concentration of metabolite builds up. Look at [13] for a sense of the "energy landscape" for glycolysis, which is a similar situation; then see this comparison for some numbers for gluconeogenesis. But the cell can't deal with massive concentrations of some metabolites. So there is a lot of specific engineering and "horse trading" involved, which has been optimized by unfathomable time scales of microbial evolution. But the bottom line is that just a few steps that split ATP are used to drive many others that are neutral or even unfavorable in terms of free energy. Wnt (talk) 19:11, 22 August 2017 (UTC)
Dissociation constant, Ph of water 'n' stuff
The formula is amenable to two different interpretations,
Kw=[OH-][H+]
= 10-14 x (1000/17.01) x (1000/1.01)
= 5.82 e -10
or
Kw=[OH-][H3O+]
= 10-14 x (1000/17.01) x (1000/19.02)
= 3.1 e -11
neither correspond to the given values. This would seem to indicate that my textbook is wrong saying that the value of [H2O] being "ignored" is 55.5 = (1000 g/L / 18.015 g/mol).
All this leaves me slightly puzzled.
All the best: Rich Farmbrough, 14:52, 22 August 2017 (UTC).
- Why are you getting 17.01? And water's molecular weight is 18.015 g/mol, not 18.5. Yes, I had a chemistry professor that disputed the textbook's usage of H3O+, so, H3O+ and OH- do not make H2O, only with H+ and OH-. 12.130.157.65 (talk) 15:12, 22 August 2017 (UTC).
- 18.5 was a typo. 17.01 as indicated below by DMacks. All the best: Rich Farmbrough, 16:08, 22 August 2017 (UTC).
- 18.5 was a typo. 17.01 as indicated below by DMacks. All the best: Rich Farmbrough, 16:08, 22 August 2017 (UTC).
- It might help if you attach units to the numbers. For example, I recognize "17.01", "1.01", and "19.02" as the molar masses of the respective ions, but not what "1000" represents in each case (or why 1000 is in each ionic component but a separate powers-of-ten multiplier that is not part of the literal formula is also present). Are you conflating different substances' "liters" or "grams" or "moles" somewhere? DMacks (talk) 15:18, 22 August 2017 (UTC)
- I don't know because the formula is not clear to me. [] "indicates molar concentration". The figure for water is calculated as shown (55.5) and is predicated on "almost all" of the substance being H2O - I use the same method for the other three species. The ratio of the other two components to the whole is 10e-7 - there are two such factors, hence 10E-14. My text book says "The value of ..Kw at 25 C is 10-14." which indicates that either the molarity is not part of the equation, or is being subsumed in the constant factor that differentiates Kw, by ignoring the almost-constant denominator.
- All the best: Rich Farmbrough, 16:06, 22 August 2017 (UTC).
- do you understand molar concentration, to begin with? It would help you, i think.
Please note that it is a dimensionless quantity, and so is any product or ratio of several of them, as Dissociation constant is. That's why we can use a "log" operation on it.(my bad, i stand corrected below). And that's why it is SO important to NEVER, EVER, use raw number without attached unit (for instance, don't use 1.01, use 1.01g/mol, etc.), as DMacks too politely tried to tell you. If you don't you may add up liters with kg, and lose all meaning without seeing it. I suspect this is precisely what just happened to you. Gem fr (talk) 17:30, 22 August 2017 (UTC)- 55.5 M is not dimensionless it is mol/L as is clear from the above.
- We would expect the dissociation constant to have the same dimensionality, as it is the product of two concentrations divided by another, and indeed our article confirms this "Kd, which has the dimensions of concentration…" although it later seems to get confused between molality and molarity.
- Incidentally it is the cologarithm we (may) take to get Ph, and in that case we have the concentration of H+ ions (or equivalent) over the concentration of H2O, which is trivially dimensionless.
- It may be worth re-framing my questions:
- When the text book says:
- Kw=[OH-][H+]
- instead of :
- what is the actual change in value and dimension between K and Kw?
- Kw=[OH-][H+]
- Given that we can treat the ions in water in two different ways, either OH- and H+ or OH- and H3O+, does this choice affect the value of K or Kw. If so, what does this represent? If not, why not?
- When the text book says:
- All the best: Rich Farmbrough, 18:49, 22 August 2017 (UTC).
- Rich: to start out with, there's no H2O+ -- you want H2O there. But to answer your question, [H2O] = 55 mol/l, so the dimensions of the constant of the other side change accordingly. Kw is mol^2/l^2 and Keq is mol/l. Wnt (talk) 16:54, 23 August 2017 (UTC)
- do you understand molar concentration, to begin with? It would help you, i think.
- To start out with, bear in mind that Kw is equal to the product of two concentrations; therefore it has units of (mol/L)^2. This is relevant if someone asks you to do this in the gas phase and your concentrations turn into partial pressures in atm; then you need to bear in mind that the ideal gas constant R = 0.082 L atm / mol K --> then you can multiply your mol/L by (RT)^2, where T is the temperature of the gas, and get something in atm^2 that you can use to figure out the relevant partial pressures ... or at least, could if the dissociation constant didn't depend on temperature and phase a lot - as you see in self-ionization of water the values for steam and supercritical water are ridiculously high since the charges aren't stabilized the same way as in polar aggregates. But you couldn't compare them at all without having units on your equilibrium constants to guide you!
- Now, it should be clear that the Keq for [H+][OH-]/[H2O] will be reduced by a factor of 55.5 M (by which I mean 55.5 mol/l; honestly, I despise the italic M notation). And so the units of that will be in mol/l only. You get that number by noting that 1 g H2O ~= 1 cm^3 H2O and 1 mol H2O = 18.01 g H2O and 1 cm^3 (cubic centimeter) = 1 ml = 1/1000 L. You can multiply anything in chemistry by the ratio of two things that are equal, because that is always 1, so putting these together like dominoes you get (1 g H2O / 1 ml H2O) * ( 1 mol H2O / 18.01 g H2O) * (1000 ml / 1 L ). By cancelling out all the units on both top and bottom and multiplying or dividing numbers as given, that's a net result of 1 mol H2O * 1000 / 18.01 L = 55.5 mol/L.
- As for your other numbers, I'm not sure where they come from. At room temperature Kw is around 10-14 (mol/L)^2, i.e. the pKw = 14 + 2 log L - 2 log mol. But nobody else on Earth but me would try to keep track of units once they take a log; they say oogledyboo and the units go away and they just try to remember not to take a log of anything but mol/l in the same context. Wnt (talk) 19:42, 22 August 2017 (UTC)
- Thanks! I think you can say "the units go away" in a fairly legitimate manner, if everything is normalised, or you are otherwise Very Careful™. You answered Q1, and also made me feel that my questions weren't somehow stupid, which is the vibe I was starting to get from the other answers. So thanks again.
- All the best: Rich Farmbrough, 19:59, 22 August 2017 (UTC).
- (Actually my initial question was stupid, because it didn't explain itself enough.) All the best: Rich Farmbrough, 20:01, 22 August 2017 (UTC).
- I think you should read self-ionization of water. Please, remember that the basic quantity is activity, which is dimensionless by definition. Only in case when concentrations are low it is proportional to molality. Ruslik_Zero 20:04, 22 August 2017 (UTC)
- It is indeed true that the equation is really for activity. That said, introductory students and biochemists alike rarely are in a position to figure out the activity rather than the simple concentration. As explained at Thermodynamic activity, it is still debatable whether the activity of H+ ion is even a measurable quantity! To be sure, pH is a very deep rabbit hole and the more you look into it the more confusing it gets. I don't pretend to have understood it the last time I went there. But to the basic student it is all very simple. ;) In any case, for equilibria it is convenient to use activity coefficients, which are dimensionless and typically close to 1, which are multiplied by the concentrations. Thus, the equilibrium constants retain the same units as if true concentrations are used - so the activities are, for most practical purposes, empirical corrections used to make the formula match observed behavior.
- Rich: so far as units are concerned, there are different schools of thought on the matter. There are some who love natural units and would like to do everything dimensionless. Personally I hate dropping units and like having the check as I go along. The key thing with units is that as long as you watch your math, they'll tell you if you're doing the wrong calculation. You rarely get an answer in the right units if you used the wrong formula. Wnt (talk) 22:59, 22 August 2017 (UTC)
- The thing I always dislike, pedagogically, about thermodynamic activity (which you get at) is that it is both literally true and yet it is fully tautological in practice. By that I mean that a) Strictly speaking, equilibrium constant calculations are only absolutely true for ratios of activities and b) The only way to find activities is to know the equilibrium constant first; that is to "back calculate" from a known EQ constant. Since I can't actually use activity to calculate K, it's a pretty useless concept, even if true. --Jayron32 11:15, 23 August 2017 (UTC)
- I don't feel comfortable saying that - the article describes a lot of different things that the activity effects, like melting point depression and vapor pressure if I recall correctly. The main thing that hinders me in applying the concept comfortably is that I could use a better mental picture of how you inactivate a substance - I would think in some cases there are dimers or even little crystals formed or something? But I don't actually know. Wnt (talk) 16:58, 23 August 2017 (UTC)
- The origin of the activity concept lies in a specific parameterization of the chemical potential of component i: (n - concentration). The second term corresponds to some additional entropy and . In equilibrium the thermodynamic potential should be the same before and after the reaction. So, one can obtain the equilibrium conditions. Ruslik_Zero 19:24, 23 August 2017 (UTC)
- That math seems informative - I didn't realize the concentration-dependent term would tend to zero at low temperature (even if that isn't generally achievable). But I still don't understand how the extra entropy would come about. I mean, one of the examples given is sodium chloride - I know the sodium ion doesn't vibrate into some weird conformation where it can't react, right? Wnt (talk) 20:57, 23 August 2017 (UTC)
- The origin of the activity concept lies in a specific parameterization of the chemical potential of component i: (n - concentration). The second term corresponds to some additional entropy and . In equilibrium the thermodynamic potential should be the same before and after the reaction. So, one can obtain the equilibrium conditions. Ruslik_Zero 19:24, 23 August 2017 (UTC)
- I don't feel comfortable saying that - the article describes a lot of different things that the activity effects, like melting point depression and vapor pressure if I recall correctly. The main thing that hinders me in applying the concept comfortably is that I could use a better mental picture of how you inactivate a substance - I would think in some cases there are dimers or even little crystals formed or something? But I don't actually know. Wnt (talk) 16:58, 23 August 2017 (UTC)
- The thing I always dislike, pedagogically, about thermodynamic activity (which you get at) is that it is both literally true and yet it is fully tautological in practice. By that I mean that a) Strictly speaking, equilibrium constant calculations are only absolutely true for ratios of activities and b) The only way to find activities is to know the equilibrium constant first; that is to "back calculate" from a known EQ constant. Since I can't actually use activity to calculate K, it's a pretty useless concept, even if true. --Jayron32 11:15, 23 August 2017 (UTC)
- I think you should read self-ionization of water. Please, remember that the basic quantity is activity, which is dimensionless by definition. Only in case when concentrations are low it is proportional to molality. Ruslik_Zero 20:04, 22 August 2017 (UTC)
- (Actually my initial question was stupid, because it didn't explain itself enough.) All the best: Rich Farmbrough, 20:01, 22 August 2017 (UTC).
STDs that spread despite use of condoms
What STDs can still be caught even if people use condoms correctly and systematically for vaginal/anal, but not for oral sex? Assuming a non-monogamous person, with regular sexual contact (including kissing) with other promiscuous people.--Hofhof (talk) 16:27, 22 August 2017 (UTC)
- You're question need clarification for me: are you talking about the effect of condom on sexually transmitted infection, or about STI that also transmit NON sexually, through kisses and oral contact?
- Gem fr (talk) 17:08, 22 August 2017 (UTC)
- Oral sex counts as sex.B8-tome (talk) 18:38, 22 August 2017 (UTC)
- You can get papilomavirus infection, syphilis, gonorrhea and possibly chlamydia by oral route (in both directions). In rare cases you can get HIV. Ruslik_Zero 18:33, 22 August 2017 (UTC)
- Herpes (HSV-1, or oral herpes and HSV-2, or genital herpes) and HPV. You can also catch gonorrhea in the throat, or transmit it to someone else’s genitalia. — Preceding unsigned comment added by B8-tome (talk • contribs) 18:35, 22 August 2017 (UTC)
- It's important to note as well that people can still use condoms correctly and the condom can have a fault in it. Nothing is perfect. So the answer to "What STDs can people still caught even if they use condoms correctly and systematically" is every STD. Assuming "can" means "it could happen at all to at least one person in the entire history of humanity" The world is a big enough place that even low percentage events occur in large absolute numbers. This source from the CDC is probably a good place to start your research, and it leads to additional sources. --Jayron32 19:37, 22 August 2017 (UTC)
- You are technically right. I should have asked which STDs are common in the scenario above, without disregarding low risks. Hofhof (talk) 20:07, 22 August 2017 (UTC)
- The only perfect STD barrier is sexual abstinence, which is a form of isolation. If you isolate yourself, then you will not catch a pathogen. Even if you do catch a pathogen, the likelihood of falling ill really depends on the health of your immune system. If you are immunocompromised, then you will be susceptible to all sorts of diseases. If you are relatively healthy, then you will be unaware that you carry the pathogen. 140.254.70.33 (talk) 22:18, 22 August 2017 (UTC)
- That's a dangerously broad statement. To begin with, the "healthiness" of the immune system can only be tested by putting it to the test, which makes this a tautology -- if you shake off a virus, you had a healthy immune system! It would be different if you could stick your finger in a machine and get back a message that thanks to eating your vegetables you're AIDS-proof, but that won't happen. (There are folks with mutations like CCR5 that make them resistant to HIV, but they are actually more vulnerable to other pathogens for the same reason) The other thing is that the virulence of pathogens varies - there are some, like HIV, that a person can be exposed to a dozen times and get lucky every time, and others like pneumonic plague where exposure pretty much guarantees a gruesome death without artificial treatment. Wnt (talk) 23:05, 22 August 2017 (UTC)
- One's strong immune system can also contribute to organ damage and death when an infection occurs, so I agree that "if you have a strong immune system" is a misleading overgeneralization. —PaleoNeonate – 06:03, 23 August 2017 (UTC)
- It's also simply wrong. The effectiveness of "sexual abstinence", like all other forms of protection from pregnancy or sexually transmitted disease, is evaluated in terms of both theoretical effectiveness and use effectiveness. While the theoretical effectiveness of sexual abstinence may be zero, the use effectiveness isn't. So, for example, the theoretical effectiveness of male condoms is about 97%. Use effectiveness is about 86%. As an estimate of the use effectiveness of "abstinence," about 60% of people who took virginity pledges in high school were sexually active in their college years. This is probably the biggest "gap" of any method between theoretical and use effectiveness. When you recommend sexual abstinence, you recommend a method with a 60% failure rate. - Nunh-huh 05:19, 23 August 2017 (UTC)
- ... though I would expect that more than 99% of individuals would know, looking back at the past, whether or not they have practised abstinence consistently ... Dbfirs 06:23, 23 August 2017 (UTC)
- That's a dangerously broad statement. To begin with, the "healthiness" of the immune system can only be tested by putting it to the test, which makes this a tautology -- if you shake off a virus, you had a healthy immune system! It would be different if you could stick your finger in a machine and get back a message that thanks to eating your vegetables you're AIDS-proof, but that won't happen. (There are folks with mutations like CCR5 that make them resistant to HIV, but they are actually more vulnerable to other pathogens for the same reason) The other thing is that the virulence of pathogens varies - there are some, like HIV, that a person can be exposed to a dozen times and get lucky every time, and others like pneumonic plague where exposure pretty much guarantees a gruesome death without artificial treatment. Wnt (talk) 23:05, 22 August 2017 (UTC)
- But then you need to define common and low risk. Depending on the community you're in and other factors, your risk of being infected by HIV is fairly low even if you regularly engage in unprotected vaginal sex in non-monogamous relationships. It's still generally considered a bad idea though considering the consequences are quite high and the risk isn't so low that you can assume it isn't going to happen. Nil Einne (talk) 08:50, 23 August 2017 (UTC)
- The only perfect STD barrier is sexual abstinence, which is a form of isolation. If you isolate yourself, then you will not catch a pathogen. Even if you do catch a pathogen, the likelihood of falling ill really depends on the health of your immune system. If you are immunocompromised, then you will be susceptible to all sorts of diseases. If you are relatively healthy, then you will be unaware that you carry the pathogen. 140.254.70.33 (talk) 22:18, 22 August 2017 (UTC)
- Exactly. I don't know the quality of the sources but these say the risk of HIV transmission from unprotected insertive oral sex [14] [15] [16] may be 0.04% per act between serodiscordant people, which is equal to the "estimated risk for insertive anal sex between men, with use of a condom, with a partner whose HIV status is unknown". (Although I would imagine this isn't perfect use for the condom.) Nil Einne (talk) 08:50, 23 August 2017 (UTC)
- You are technically right. I should have asked which STDs are common in the scenario above, without disregarding low risks. Hofhof (talk) 20:07, 22 August 2017 (UTC)
- Also note that condoms won't prevent the spread of pubic lice. StuRat (talk) 20:04, 23 August 2017 (UTC)
- I'm curious how a person who practices abstinence can get an STD. Some of the above advice is suggesting that abstinence is a dangerous practice. Akld guy (talk) 01:37, 24 August 2017 (UTC)
- Some STDs can also be spread by non-sexual contact, like some forms of herpes from kissing. StuRat (talk) 02:04, 24 August 2017 (UTC)
- Herpes spread in that way is a form of virus, not an STD. Genital herpes spread by sexual intercourse is indeed an STD. So again, I ask. Akld guy (talk) 03:10, 24 August 2017 (UTC)
- Some STDs can also be spread by non-sexual contact, like some forms of herpes from kissing. StuRat (talk) 02:04, 24 August 2017 (UTC)
What causes red/green coloration in eclipse images?
Here [17] are a few images of the recent eclipse. The first is taken through a telescope with a cell phone camera. Note how the sun/sky border is limned with green tones, while the sun/moon border has red. I also saw the same pattern of colors using different imaging methods. I could see it with the naked eye looking at natural camera obscura e.g. through leaf shadow like the second image in the album. The third photo is of a projection by binoculars on to a white paper, red/green was also visible in live viewing. N.B. you can't quite see the colors in the leaf shadow photo but I could see it as it happened, and several nearby watchers said they could see the same color pattern.
Q: What causes this red/green coloration on the different boundaries? Perhaps some sort of diffraction? Since I see it with three fairly different methods, it seems unlikely to be a property of any given camera or lens.
Thanks! SemanticMantis (talk) 16:39, 22 August 2017 (UTC)
- red appears for the very same reason the sun look red at sunset or sunrise
- I cannot explain the green, someone else may help
- Gem fr (talk) 17:16, 22 August 2017 (UTC)
- I believe you are seeing various forms of chromatic aberration, which is fundamentally an optical artifact that is aggravated by the very high light/dark contrast ratio that is a characteristic of this type of photograph of a solar eclipse - whether it is an image of a projection, or a direct view of the sun.
- To make matters worse, complicated engineering systems - carefully-designed complications of optical glass lenses, and carefully-designed software and hardware inside the camera - may attempt to correct for chromatic aberration. Those corrections only work up to a point - but for the extreme case of a direct solar image or a high-contrast projection of the sun - these corrections might actually aggravate the artifact.
- First, the lenses - there are achromats, apochromats, super-apochromats, multi-element super-apochromats,
superheterodynes, ... I sure wish this book wasn't so darned expensive! - Next, the algorithms - there are radial correctors, chromatic gradient detectors, desaturation filters, nonlinear edge estimators, ... heck, it's 2017... somebody's probably trained a recurrent convolutional neural network to detect-and-correct chromatic aberration.
- The core cause of the visual artifact we call chromatic aberration is optical refraction occurring at the exact same part of the image as optical diffraction - places where a hard edge in the image corresponds to an extreme, high-contrast change in the illumination.
- Here's an open-source algorithm - Fix-CA - that is commonly distributed with the GIMP. It's pretty simple, and it works in RGB-colorspace, so it should be "easy" for an ordinary programmer to follow.
- Here's a much more famous and complex/sophisticated algorithm - Radial, part of PanoTools. Here's a blog documenting how it works, and here's another one. The algorithm is harder to read, because it's split across the tools library, but the meat of it is in correct.c.
- Those tools are meant to allow a pro-user to fix up an artifact in a digital image after they've captured it. A modern-vintage camera probably implements a built-in, probably-proprietary, possibly-similar algorithm to fix up the image before you ever see it.
- See if you can spot where and why such an algorithm could fail - and imagine what a proprietary camera algorithm might do differently - and imagine how that might fail too! The great bane of digital image processing algorithms is that in some corner-case conditions, instead of solving a visual artifact problem, the algorithms can make the problem worse!
- Nimur (talk) 19:34, 22 August 2017 (UTC)
- Couldn't this be caused in the same way as the Green flash?B8-tome (talk) 20:22, 22 August 2017 (UTC)
- Thanks @Nimur:. That's helpful but two things I'm still not clear on: 1) I can accept that my neighbor's binoculars and my friends' telescope may have the same pattern of aberration, but why would they consistently show red on one the sun/moon border while showing green on the sun/sky boundary? 2) I suppose the same aberration can occur in my own naked eye?
- Put another way, if I accept this to be chromatic aberration in the telescope, the binoculars, and my naked eye, I'm still left with the question of what is different between the sun/moon boundary and the sun/sky boundary. I have yet to see any photo that shows both sides with the same color aberration, or with a reversed color scheme. If you search google images for /eclipse through telescope/ (e.g. [18]) or /eclipse projection/ (e.g. [19]), you will see many photos with the same pattern that I describe, and I feel like there should be a good reason why none of the putative aberrations have the same color on both sides, or a reversed pattern, etc. SemanticMantis (talk) 21:02, 22 August 2017 (UTC)
- Well, don't those two sides have very different things on them? On one side you are seeing light that comes from the center of the Sun and grazes the edge of the Moon; on the other side you are seeing light that comes from the edge of the Sun's globe. Looie496 (talk) 21:41, 22 August 2017 (UTC)
- The root cause of red/green coloration of images is dispersion which is the change in all optical materials of their refractive index with the frequency (color) of light. Dispersion causes a glass prism to spread a beam of white light into spectral colors that Newton observed in 1666, gives rise to the Rainbow, and causes a single glass lens to have too much chromatic aberration for camera use, though wearers of spectacles have to get used to it. Compound lenses of greater complexity and cost have been designed to achieve compromises between various optical aberrations (spherical, coma, and chromatic) but a perfectly achromatic or even Apochromatic lens will not be found in a cell phone. Incidentally, the human eye has only a single lens that presumably exhibits all the optical aberrations which must thereafter be corrected by retinal/neural processes. These processes are not normally trained to work with extreme contrasts and may be saturating during SM's eclipse observations. Blooteuth (talk) 22:09, 22 August 2017 (UTC)
- At a more concrete level, the reddish tinge of the inner edge probably comes from bending of light by the Earth's atmosphere, the same process that causes the sky to be red at sunrise and sunset. The greenish tinge of the outer edge probably results from the fact that the light has to pass through a long stretch of the Sun's atmosphere, which filters out reddish hues more than greenish hues (this second part is speculation). Looie496 (talk) 14:25, 23 August 2017 (UTC)
Physical mechanism of leafhopper song
This article has a pretty good description of how leafhopper communicate and the purpose of their vibrations. But how do they make the vibration? Is it by moving their wings back and forth, or using their legs to strike drum-like tymbals or something else? http://www.wired.com/2014/09/lustful-leafhoppers-locate-good-vibrations/ --Captain Breakfast (talk) 18:40, 22 August 2017 (UTC)
- I'm also curious if these sounds are easily detectable by human ears, or if they are only heard by other leafhoppers.--Captain Breakfast (talk) 18:46, 22 August 2017 (UTC)
- Wikipedia has a brief overview article on Leafhoppers, unfortunately it doesn't say anything about the song. Leafhopper is a pretty large family of insects, which is part of the cicada superfamily, and THAT article describes how they produce their song. --Jayron32 19:14, 22 August 2017 (UTC)
- As described in our Article Tymbal. Generally its easy to generate vibrations. If you use one of your fingernails to bend another fingernail and then let it flip by gliding off the edge you can make a loud sound yourself like if you had an exoskelet. Do the fingernail flip right next to your ear for a try to notice how loud it actually is. If you could do that very rapidly and you had a resonating shell on top, you could make similar impressive loud sounds too. --Kharon (talk) 23:15, 22 August 2017 (UTC)
- I think you are looking for stridulation and Cicada#Song
- Gem fr (talk) 12:29, 23 August 2017 (UTC)
The Multiplication of Food Grains
Let's say I have one of each seeds of typical wheat, rice, barley, maize, millet ... and many other common food grains. They are all properly planted in well-prepared fields under favorable climate. They are all successfully harvested.
How many grains can I get from each plant?
How many grains can I get if I plant one seed of typical wheat, rice, barley, maize, or millet ... -- Toytoy (talk) 21:27, 22 August 2017 (UTC)
- There are many varieties of each of these grains. The number of grain could easily be double between varieties of one species, and it should be noted that not all grain is fertile seed, such as in two-row barley.Of 19 (talk) 22:14, 22 August 2017 (UTC)
- One seed of maize (sweetcorn or field corn) typically produces one or two ears of harvested corn if it sprouts and grows to maturity without suffering from insects, drought, blight, etc. (Maize intended to be harvested pre-maturely for "baby corn" may have more ears and thus more immature harvested kernels). [20]. Each ear has 500 to 1000 kernels, with 800 being typical. [21]. That could imply 500 to 2000 for each healthy stalk. In the Genesis 26:12 in the Bible, a farmer harvested 100 fold, so he might get 100 seeds harvested from each seed planted. In Mattheh 13:8 seed sown into "good ground" produced a yield of 30 to 100 fold. Not sure what grain the Biblical farmers planted. Edison (talk) 22:16, 22 August 2017 (UTC)
- [double edit conflict; I wrote this before Of 19] Two further considerations: (1) are they sheltered from birds, and (2) do you have a second maize plant nearby? We tried growing a few wheat and oat plants in our vegetable garden (just to see what would happen) when I was growing up, and they all produced good grain, but between the fewness of the plants and the hunger of nearby birds, the "entire" yield was eaten before it ripened. Most of your grains are self-pollinating, but it looks like maize isn't, so exactly one maize plant will produce nothing. Nyttend (talk) 22:21, 22 August 2017 (UTC)
- One seed of maize (sweetcorn or field corn) typically produces one or two ears of harvested corn if it sprouts and grows to maturity without suffering from insects, drought, blight, etc. (Maize intended to be harvested pre-maturely for "baby corn" may have more ears and thus more immature harvested kernels). [20]. Each ear has 500 to 1000 kernels, with 800 being typical. [21]. That could imply 500 to 2000 for each healthy stalk. In the Genesis 26:12 in the Bible, a farmer harvested 100 fold, so he might get 100 seeds harvested from each seed planted. In Mattheh 13:8 seed sown into "good ground" produced a yield of 30 to 100 fold. Not sure what grain the Biblical farmers planted. Edison (talk) 22:16, 22 August 2017 (UTC)
- Certainly they must be sheltered from birds and bugs. If I were on Mars without potatoes, I need to grow something for my breakfast cereals and Saturday night beers. I need to have grains that multiply A LOT!!!! -- Toytoy (talk) 03:55, 23 August 2017 (UTC)
- Corn is monoecious; the tassel holding the male organs, the ear holding the female organs. The two organs however may not emerge at the same time so for a small garden staggering planting by a week or two will help with pollination. Also Self-incompatibility in plants can be an issue for about half of flowering plants but I'm not sure for these grains. Of 19 (talk) 22:53, 22 August 2017 (UTC)
- It's not clear whether the OP is literally planting just one seed of each item. In any case, you're right that maize can self-pollinate (or be induced to do so) but any significant time gap between emergence of the male parts (tassel) and emergence of the female parts (silk) can result in little or no yield. ←Baseball Bugs What's up, Doc? carrots→ 08:00, 23 August 2017 (UTC)
- It is well known among gardeners that maize has to be planted in substantial patches (say 2 meters across at minimum) in order to pollinate reliably. If you just plant one or two plants, you probably won't get any ears. Looie496 (talk) 14:19, 23 August 2017 (UTC)
- So the question remains, how many maize seeds does the OP intend to plant? ←Baseball Bugs What's up, Doc? carrots→ 17:08, 23 August 2017 (UTC)
- It is well known among gardeners that maize has to be planted in substantial patches (say 2 meters across at minimum) in order to pollinate reliably. If you just plant one or two plants, you probably won't get any ears. Looie496 (talk) 14:19, 23 August 2017 (UTC)
- It's not clear whether the OP is literally planting just one seed of each item. In any case, you're right that maize can self-pollinate (or be induced to do so) but any significant time gap between emergence of the male parts (tassel) and emergence of the female parts (silk) can result in little or no yield. ←Baseball Bugs What's up, Doc? carrots→ 08:00, 23 August 2017 (UTC)
- Corn is monoecious; the tassel holding the male organs, the ear holding the female organs. The two organs however may not emerge at the same time so for a small garden staggering planting by a week or two will help with pollination. Also Self-incompatibility in plants can be an issue for about half of flowering plants but I'm not sure for these grains. Of 19 (talk) 22:53, 22 August 2017 (UTC)
- To answer this, i would just divide the maximum yield by the standard weight of seeds needed
- wheat for instance can yield more than 1O t for a seed dose of 125 kg, meaning you can expect ~100 grains par seed with good but not exceptional earth condition (i guess not all seed contribute to yield, some die in the process)
- I leave it to you to find both maximum yield and standard seed dose for different plants using your favorite search tool.
- Gem fr (talk) 12:14, 23 August 2017 (UTC)
Biological maladaptions of living too far away from homeland
When light-skinned people migrate to Africa near the equator, their skin is probably going to be overwhelmed by the sun and get skin cancer. Likewise, when dark-skinned people migrate to the polar region, they may develop rickets. African sickle cell heterozygotes are protected from malaria. Are there others? With the advent of fast pace movement from one continent to another, how can humans adapt to the environment? If a population of light-skinned settlers move to the equator, will their descendants develop dark skin? 140.254.70.33 (talk) 22:56, 22 August 2017 (UTC)
- Unless the cancer or rickets reduces a group's ability to have otherwise healthy children who will also go on to breed at a rate similar to the rest of the local population there is no pressure on the group (forgetting silly race based violence) to lose their seemingly negative traits. So with modern medicine, the settler's offspring will continue to have light coloured skin.
- Humans can adapt to these challenges with clothing and sun-screen and vitamin fortified foods. In the distant future we may be able to edit our DNA to change our skin colour or disease resistance, or anything really so long as the change doesn't kill us. Of 19 (talk) 23:13, 22 August 2017 (UTC)
- "How can humans adapt to the environment?"
- We don't have to. In general, we adapt the natural environment to us. There is some cold and heat adaptations and high-altitude adaptation, that tunes our body to the environment though.--B8-tome (talk) 23:31, 22 August 2017 (UTC)
- Genetic studies have shown that skin color does adapt over the course of thousands of years. Dark skin is the base state that evolved in our African ancestors, but in populations that move far from the equator, skin color gradually lightens over generations. There are also other interesting features relating to this. For example, people who live in Africa experience minimal changes in the length of the light and dark parts of the day, even at the extreme north and south ends of the continent, but people who live on other continents experience much larger changes. It would be interesting to know what effect this has had on the genes that underlie biological rhythms, particularly those involved in sleep. Looie496 (talk) 14:15, 23 August 2017 (UTC)
- As for other "geographic" traits:
- There are high altitude adaptations, such as being short with large lungs, more red blood cells, etc. I suspect they would be fine at sea level, though those adapted to live at sea level would not survive at high altitudes.
- There are adaptations to extreme cold, such as having a short, pudgy body. Handling extreme heat might be difficult for such people. Conversely, a tall, thin body is best in hot climates, but could be deadly in the cold. See ectomorph and endomorph. StuRat (talk) 20:31, 23 August 2017 (UTC)
- Lactose-tolerance has developed in areas where dairy farming is most practical. I can't see this being a problem to those who move away, though. Lactose-intolerance, on the other hand, could be a problem for those moving into a dairy farming area, at least before we knew how to deal with it (lactose-free milk, etc.). StuRat (talk) 20:14, 23 August 2017 (UTC)
August 23
Before a storm, do electrons turn from - to + or + to - ?
}}}} — Preceding unsigned comment added by Lgklange (talk • contribs) 00:23, 23 August 2017 (UTC)
- Electrons are always negative.Dja1979 (talk) 04:00, 23 August 2017 (UTC)
- Dja is right, but the flow can be up or down, with hugely different effects, see lightning. μηδείς (talk) 04:45, 23 August 2017 (UTC)
- I'm guessing that what you meant to ask is if positive or negative ions form before a storm. The answer is both. That is, a roughly equal number of positive and negative ions form, and are physically separated. Lightning occurs when the charge equalizes by the electrons moving from the negatively charged area back to the positive. StuRat (talk) 20:19, 23 August 2017 (UTC)
Name for optical illusion?
Hello, how is this optical illusion called? Obviously some kind of tunnel-y 3D effect :), but I am looking for a more formal descriptive name for this Commons image. Bonus question: which Commons category in Commons:Category:Optical illusions should this image be placed in? GermanJoe (talk) 10:55, 23 August 2017 (UTC)
- Is this a Fraser spiral illusion? --Jayron32 10:57, 23 August 2017 (UTC)
- No. The Fraser spiral illusion only appears to be a spiral. The illustration shown here really is a spiral. I'm not convinced it is an "illusion" of any sort.--Shantavira|feed me 11:20, 23 August 2017 (UTC)
- This seems simply an Interference (wave propagation)-picture with no special name, as far as i am aware. Sorry if i hurt the artist who generated it by revealing his rather simple technology. The possible Interference-patterns are almost endless and only a few typical patterns have a name, usually in connection to their meaning in/for technology and science, like the better known Moiré pattern due to its broad appearence in display and image technology today. This is also the base of Interferometry which is very important and often used in science and technology, like for example as Astronomical interferometer. --Kharon (talk) 11:49, 23 August 2017 (UTC)
- No. The Fraser spiral illusion only appears to be a spiral. The illustration shown here really is a spiral. I'm not convinced it is an "illusion" of any sort.--Shantavira|feed me 11:20, 23 August 2017 (UTC)
- If you are referring to the fact that the pattern seems to vibrate, that's basically a type of grid illusion often generated by interference patterns. If you're referring to the fact that it gives a sense of three-dimensionality, that isn't considered an optical illusion, just an aspect of human depth perception. Looie496 (talk) 14:06, 23 August 2017 (UTC)
- Well, it's the cover to a book on optical illusions.
- We have articles on grid illusion and Illusory motion, which both seem to be present in this image. But I don't know that this exact image has a name. ApLundell (talk) 14:23, 23 August 2017 (UTC)
- The illusory motion could be creating a secondary Kinetic depth effect; that is the fact that the image appears to vibrate slightly may also add to the sense of depth in it. --Jayron32 14:52, 23 August 2017 (UTC)
- Hah! That article looks like it's trying to cram the whole spinning dancer article into an image caption. ApLundell (talk) 16:56, 23 August 2017 (UTC)
- Not anymore. Good thing we have a freely-editable encyclopedia. --Jayron32 18:21, 23 August 2017 (UTC)
- Hah! That article looks like it's trying to cram the whole spinning dancer article into an image caption. ApLundell (talk) 16:56, 23 August 2017 (UTC)
- The illusory motion could be creating a secondary Kinetic depth effect; that is the fact that the image appears to vibrate slightly may also add to the sense of depth in it. --Jayron32 14:52, 23 August 2017 (UTC)
What does it mean to have a mental age of 8 or 12?
There is that Kennedy girl, Rosemarie, who has a below average IQ. The Wiki article says she has the intelligence level of an 8- year-old to 12-year-old child. What does that mean? When I was 8, I could do multiplication and division and write essays. When I was 12, I could do the order of operations and basic algebra. Though, even when I was 3 or 4, my workbooks indicated that I practiced addition. At all stages of my life, I like toys, especially stuffed animals. 140.254.70.33 (talk) 14:06, 23 August 2017 (UTC)
- Yes, but you are one person, so you are statistically insignificant. Your singular experience means nothing at all, and cannot be used to draw conclusions about humanity as a whole. However, if you take all people (or a representative sample of all people) and from that sample, develop an average set of capabilities, you have a benchmark by which to compare people against known expectations. When statements like the one you note above are made, they are being made looking at an average across all humanity, not against YOU. You, and your experiences, don't matter to such a comparison. --Jayron32 14:46, 23 August 2017 (UTC)
- It basically means that her score on a standard IQ test would be near the mean for children in the 8-12 year old range. Looie496 (talk) 15:14, 23 August 2017 (UTC)
- Mental age[22] --AboutFace 22 (talk) 15:31, 23 August 2017 (UTC)
- When a person is older than its mental age, see Mental Retardation. --Hans Haase (有问题吗) 18:46, 23 August 2017 (UTC)
- That would be substantially older than their mental age. StuRat (talk) 20:21, 23 August 2017 (UTC)
Design and build construction contracts
Is it generally the case that with design and build construction contracts, the clients are more hands off and a lot of the risk and accountability is transferred to the suppliers? Compared to framework contracts where the client is very much in control and breaks contracts down into small work packages. 82.132.235.85 (talk) 16:35, 23 August 2017 (UTC)




![{\displaystyle K={[OH^{-}][H^{+}] \over [H_{2}O^{+}]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/1b9a11a4e90d45ad4eec70b98e873b340db1b8b1)

