Bortezomib
 | |
 | |
| Clinical data | |
|---|---|
| License data |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | n/a |
| Protein binding | 83% |
| Metabolism | Hepatic, CYP extensively involved |
| Elimination half-life | 9 to 15 hours |
| Excretion | ? |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.125.601 |
| Chemical and physical data | |
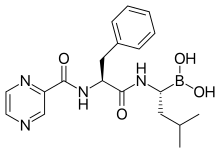
| Formula | C19H25BN4O4 |
| Molar mass | 384.237 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| (verify) | |
Bortezomib (INN, originally codenamed PS-341; marketed as Velcade by Millennium Pharmaceuticals) is the first therapeutic proteasome inhibitor to be tested in humans. It is approved in the U.S. for treating relapsed multiple myeloma[1] and mantle cell lymphoma. In multiple myeloma, complete clinical responses have been obtained in patients with otherwise refractory or rapidly advancing disease.
History
Bortezomib was originally synthesized in 1995 (MG-341) at a company called Myogenics, which soon changed its name to ProScript. After promising preclinical results, the drug (PS-341) was tested in a small Phase I clinical trial on patients with multiple myeloma cancer. ProScript ran out of money and was bought by Leukosite in May 1999. Leukosite in turn was bought by Millennium Pharmaceuticals in October 1999. At this point in time, the project had low priority amongst other projects at the company. This changed significantly when one of the first volunteers to receive the drug in the clinical trial achieved a complete response and was still alive four years later. At the time this was a remarkable result. Later clinical experimentation indicates the possibility of a complete response in 15% of patients in a similar condition, when treated with bortezomib. In May 2003, seven years after the initial synthesis, bortezomib (Velcade) was approved in the United States by the Food and Drug Administration (FDA) for use in multiple myeloma, based on the results from the SUMMIT Phase II trial.[2]
Pharmacology

Structure
The drug is a tripeptide and can be written as Pyz-Phe-boroLeu, which stands for pyrazinoic acid, phenylalanine and Leucine with boronic acid instead of a carboxylic acid. Peptides are written N-terminus to C-terminus, but as in vitro peptide synthesis proceeds C-terminus to N-terminus, peptide drugs are illustrated C to N, as in this case.
Mechanism
The boron atom in bortezomib binds the catalytic site of the 26S proteasome[3] with high affinity and specificity. In normal cells, the proteasome regulates protein expression and function by degradation of ubiquitinylated proteins, and also cleanses the cell of abnormal or misfolded proteins. Clinical and preclinical data support a role in maintaining the immortal phenotype of myeloma cells, and cell-culture and xenograft data support a similar function in solid tumor cancers. While multiple mechanisms are likely to be involved, proteasome inhibition may prevent degradation of pro-apoptotic factors, permitting activation of programmed cell death in neoplastic cells dependent upon suppression of pro-apoptotic pathways.
Pharmacodynamics
Bortezomib is rapidly cleared following intravenous administration.[4] Peak concentrations are reached at about 30 minutes. Drug levels can no longer be measured after an hour. Pharmacodynamics are measured by measuring proteasome inhibition in peripheral blood mononuclear cells. The much greater sensitivity of myeloma cell lines and mantle cell lines to proteasome inhibition compared with normal peripheral blood mononuclear cells and most other cancer cell lines is poorly understood.
Costs
UK
NICE recommended against Velcade in Oct 2006 due to its cost.[5]
The company proposed a cost reduction for multiple myeloma,[6] and this was taken up in the UK.[7]
Adverse effects
Bortezomib is associated with peripheral neuropathy in 30% of patients; occasionally, it can be painful. This can be worse in patients with pre-existing neuropathy. In addition, myelosuppression causing neutropenia and thrombocytopenia can also occur and be dose-limiting. However, these side effects are usually mild relative to bone marrow transplantation and other treatment options for patients with advanced disease. Bortezomib is associated with a high rate of shingles.[8]
Gastro-intestinal (GI) effects and asthenia are the most common adverse events.[9]
Drug interactions
Green tea extract Epigallocatechin gallate(EGCG), which had been expected to have a synergistic effect, was found by Encouse B. Golden, et al. to reduce the effectiveness of bortezomib.[10][11][12][13]
Therapeutic Efficacy
Two open-label, phase III trials established the efficacy of bortezomib 1.3mg/m2(with or without dexamethasone) administered by intravenous bolus on days 1,4,8, and 11 of a 21-day cycle for a maximum of eight cycles in heavily pretreated patients with relapsed/refractory multiple myeloma.[14]. Another trial demonstrated the superiority of bortezomib 1.3mg/m2 over a high-dose dexamethasone regimen.[14]
Further improvement of anticancer potency
Laboratory studies and clinical trials are investigating whether it might be possible to further increase the anticancer potency of bortezomib by combining it with novel types of other pharmacologic agents. For example, clinical trials have indicated that the addition of thalidomide, lenalidomide, inhibitors of vascular endothelial growth factor (VEGF), or arsenic trioxide might be beneficial.[15][16] In laboratory studies, it was found that bortezomib killed multiple myeloma cells more efficiently when combined, for example, with histone deacetylase inhibitors,[17] thapsigargin,[18] or celecoxib.[19] However, the therapeutic efficacy of any of these latter combinations has not yet been confirmed in cancer patients.
References
- ^ Takimoto CH, Calvo E. "Principles of Oncologic Pharmacotherapy" in Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ (Eds) Cancer Management: A Multidisciplinary Approach. 11 ed. 2008.
- ^ Adams J, Kauffman M (2004). "Development of the Proteasome Inhibitor Velcade (Bortezomib)". Cancer Invest. 22 (2): 304–11. doi:10.1081/CNV-120030218. PMID 15199612.
- ^ Bonvini P, Zorzi E, Basso G, Rosolen A (2007). "Bortezomib-mediated 26S proteasome inhibition causes cell-cycle arrest and induces apoptosis in CD-30+ anaplastic large cell lymphoma". Leukemia. 21 (4): 838–42. doi:10.1038/sj.leu.2404528. PMID 17268529.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Voorhees PM, Dees EC, O'Neil B, Orlowski RZ (2003). "The proteasome as a target for cancer therapy". Clin Cancer Res. 9 (17): 6316–25. PMID 14695130.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "NHS watchdog rejects cancer drug". BBC News UK. 20 October 2006. Retrieved 2009-08-14.
- ^ "Summary of VELCADE Response Scheme" (PDF). Retrieved 2009-08-14.
- ^ "More Velcade-Style Risk-Sharing In The UK?". Euro Pharma Today. 21 January 2009. Retrieved 2009-08-14.
- ^ Oakervee HE, Popat R, Curry N; et al. (2005). "PAD combination therapy (PS-341/bortezomib, doxorubicin and dexamethasone) for previously untreated patients with multiple myeloma". Br J Haematol. 129 (6): 755–62. doi:10.1111/j.1365-2141.2005.05519.x. PMID 15953001.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Highlights Of Prescribing Information
- ^ "Cancer drug benefits could be negated by healthy tea treatment". Belfast Telegraph. 3 February 2009. Retrieved 2009-08-14.
- ^ http://www.news-medical.net/?id=45529 "Green tea may counteract anticancer effects of cancer therapy, bortezomib (Velcade)"
- ^ http://www.ecancermedicalscience.com/news-insider-news.asp?itemId=414
- ^ Golden EB; et al. (2009). "Green tea polyphenols block the anticancer effects of bortezomib and other boronic acid-based proteasome inhibitors". Blood. 113 (23): 5927–37. doi:10.1182/blood-2008-07-171389. PMID 19190249.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ a b Curran M, McKeage K.[1].Drugs 2009;69(7):859-888.doi: 10.2165/00003495-200969070-00006.
- ^ Anargyrou K; et al. (2008). "Novel anti-myeloma agents and angiogenesis". Leuk Lymphoma. 49 (4): 677–689. doi:10.1080/10428190701861686. PMID 18398734.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Richardson PG; et al. (2005). "Novel biological therapies for the treatment of multiple myeloma". Best Pract Res Clin Haematol. 18 (4): 619–634. doi:10.1016/j.beha.2005.01.010. PMID 16026741.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Nawrocki ST; et al. (2006). "Aggresome disruption: a novel strategy to enhance bortezomib-induced apoptosis in pancreatic cancer cells". Cancer Res. 66 (7): 3773–3781. doi:10.1158/0008-5472.CAN-05-2961. PMID 16585204.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Nawrocki ST; et al. (2005). "Bortezomib sensitizes pancreatic cancer cells to endoplasmic reticulum stress-mediated apoptosis". Cancer Res. 65 (24): 11658–11666. doi:10.1158/0008-5472.CAN-05-2370. PMID 16357177.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Kardosh A; et al. (2008). "Aggravated endoplasmic reticulum stress as a basis for enhanced glioblastoma cell killing by bortezomib in combination with celecoxib or its non-coxib analogue, 2,5-dimethyl-celecoxib". Cancer Res. 68 (3): 843–851. doi:10.1158/0008-5472.CAN-07-5555. PMID 18245486.
{{cite journal}}: Explicit use of et al. in:|author=(help)
External links
- Myeloma patients campaigning for access to a life prolonging cancer drug
- Millennium Pharmaceuticals website on Velcade
- Multiple Myeloma Research Foundation article on Velcade
- International Myeloma Foundation article on Velcade
- U.S. Food and Drugs Administration on Velcade
- Dedicated website for European audience
- Presentation at 2006 ASCO of the PINNACLE Study on MCL by Dr. Goy, with video/slides
