Hydrogen peroxide
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
dihydrogen dioxide
| |||
| Other names
Dioxidane
Oxidanyl | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.028.878 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2015 (>60% soln.) 2014 (20–60% soln.) 2984 (8–20% soln.) | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| H2O2 | |||
| Molar mass | 34.0147 g/mol | ||
| Appearance | Very light blue color; colorless in solution | ||
| Odor | slightly sharp | ||
| Density | 1.135 g/cm3 (20 °C, 30-percent) 1.450 g/cm3 (20 °C, pure) | ||
| Melting point | −0.43 °C (31.23 °F; 272.72 K) | ||
| Boiling point | 150.2 °C (302.4 °F; 423.3 K) | ||
| Miscible | |||
| Solubility | soluble in ether, alcohol insoluble in petroleum ether | ||
| Acidity (pKa) | 11.75 | ||
Refractive index (nD)
|
1.4061 | ||
| Viscosity | 1.245 cP (20 °C) | ||
| 2.26 D | |||
| Thermochemistry | |||
Heat capacity (C)
|
1.267 J/g K (gas) 2.619 J/g K (liquid) | ||
Std enthalpy of
formation (ΔfH⦵298) |
-187.80 kJ/mol | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
1518 mg/kg | ||
| Related compounds | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Hydrogen peroxide (H
2O
2) is the simplest peroxide (a compound with an oxygen-oxygen single bond). It is also a strong oxidizer. Hydrogen peroxide is a colorless liquid, slightly more viscous than water. In dilute solution, it appears colorless. Due to its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent. The oxidizing capacity of hydrogen peroxide is so strong that it is considered a highly reactive oxygen species. Concentrated hydrogen peroxide, or 'high-test peroxide', is therefore used as a propellant in rocketry.[1]
Organisms naturally produce hydrogen peroxide as a by-product of oxidative metabolism. Consequently, nearly all living things (specifically, all obligate and facultative aerobes) possess enzymes known as catalase peroxidases, which harmlessly and catalytically convert low concentrations of hydrogen peroxide to water and oxygen. Hydrogen peroxide has been shown to play a role in the immune system.
At one time, the most common household use of hydrogen peroxide was to disinfect wounds, but it is now thought to slow healing by affecting tissue growth through several possible factors. Only a very minute concentration of H2O2 can induce healing, and only if not repeatedly applied.[2] Surgical use can lead to gas embolism formation.[3]
Structure and properties

Structure
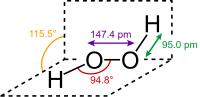
Hydrogen peroxide (H2O2), also known as hydroperoxic acid, is a nonplanar molecule with a (twisted) structure of C2 symmetry. Although chiral (the twist can be left or right-handed), the molecule undergoes rapid racemization, the result of which is that the left and right-handed twist forms cannot be isolated as they can quickly "flip" their handed-ness. The observed anticlinal "skewed" shape is a compromise between two conformers, called syn and anti. If the molecule had the flat shape of the anti conformer, it would minimize steric repulsions. However, if it had the 90° torsion angle of the syn conformer, there would be optimized mixing between the filled p-type orbital of the oxygen (one of the lone pairs) and the LUMO of the vicinal O-H bond. The compromise angle has the lowest energy state.[4] The bond angles can also be affected by hydrogen bonding between molecules. As the molecules in gasses are too far apart for hydrogen bonding, the molecular structure of the gaseous and crystalline forms is different; indeed a wide range of values is seen in crystals containing H
2O
2.
Although the O−O bond is a single bond, the molecule has a relatively high barrier to rotation, of 29.45 kJ/mol; for comparison, the rotational barrier for ethane is 12.5 kJ/mol. The increased barrier is ascribed to repulsion between nonbonding electrons (lone pairs) of the adjacent oxygen atoms.[5]
Comparison with analogues
Analogues of hydrogen peroxide include the chemically identical deuterium peroxide, and hydrogen disulfide (H2S2).[6] Hydrogen disulfide has a boiling point of only 70.7 °C despite having a higher molecular weight. The relatively high boiling point of hydrogen peroxide is the result of hydrogen bonding, which is of minor importance in H2S2.
Physical properties of hydrogen peroxide solutions
In aqueous solutions hydrogen peroxide differs from the pure material. This demonstrates the effects of hydrogen bonding between water and hydrogen peroxide molecules. Hydrogen peroxide and water form a eutectic mixture, exhibiting freezing-point depression. Pure water melts and freezes at approximately 273 K, and pure hydrogen peroxide just 0.4 K below that, but a 50% (by volume) solution melts and freezes at 221 K. The boiling point of the same mixture is less than the average (398 K) of the boiling points of pure water (373 K) and hydrogen peroxide (423 K) at 387 K.[7]
pH of H
2O
2
Pure hydrogen peroxide has a pH of 6.2; thus it is considered to be a weak acid. The pH can be as low as 4.5 when diluted at approximately 60%.[8]
Density
The percentage given here is v/v.
1.0095 g/cm3 for 3% @ 15 °C 1.10 g/cm3 for 27% @ 20 °C 1.13 g/cm3 for 35% @ 20 °C 1.20 g/cm3 for 50% @ 20 °C 1.29 g/cm3 for 70% @ 20 °C 1.33 g/cm3 for 75% @ 20 °C 1.42 g/cm3 for 96% @ 20 °C 1.43 g/cm3 for 98% @ 20 °C 1.450 g/cm3 for 100% @ 20 °C
History
Louis Jacques Thénard first described hydrogen peroxide in 1818. He produced it by treating barium peroxide with nitric acid.[9] An improved version of this process used hydrochloric acid, followed by addition of sulfuric acid to precipitate the barium sulfate byproduct. Thénard's process was used from the end of the 19th century until the middle of the 20th century.[10] Modern production methods are discussed below.
Pure hydrogen peroxide was long believed to be unstable. This was because of failed attempts to separate the hydrogen peroxide from the water, which is present during synthesis. However, this instability was due to traces of impurities (transition metals salts) that catalyze the decomposition of the hydrogen peroxide. One hundred percent pure hydrogen peroxide was first obtained through vacuum distillation by Richard Wolffenstein in 1894.[11] At the end of the 19th century, Petre Melikishvili and his pupil L. Pizarjevski showed that of the many proposed formulas of hydrogen peroxide, the correct one was H−O−O−H.
The use of H
2O
2 sterilization in biological safety cabinets and barrier isolators is a popular alternative to ethylene oxide (EtO) as a safer, more efficient decontamination method. H
2O
2 has long been widely used in the pharmaceutical industry. In aerospace research, H
2O
2 is used to sterilize artificial satellites and space probes.
The U.S. FDA has granted 510(k) clearance to use H
2O
2 in individual medical device manufacturing applications. EtO criteria outlined in ANSI/AAMI/ISO 14937 may be used as a validation guideline. Sanyo was the first manufacturer to use the H
2O
2 process in situ in a cell culture incubator, which is a faster and more efficient cell culture sterilization process.[citation needed]
Manufacture
Formerly, hydrogen peroxide was prepared industrially by hydrolysis of the ammonium peroxydisulfate:
- (NH4)2S2O8 + 2 H2O → H2O2 + 2 (NH4)HSO4
The required ammonium peroxydisulfate was obtained electrolysis of a solution of ammonium bisulfate (NH
4HSO
4) in sulfuric acid.
Today, hydrogen peroxide is manufactured almost exclusively by the anthraquinone process, which was formalized in 1936 and patented in 1939. It involves the autoxidation of a 2-alkyl anthrahydroquinone (or 2-alkyl-9,10-dihydroxyanthracene) to the corresponding 2-alkyl anthraquinone. Major producers commonly use either the 2-ethyl or the 2-amyl derivative. The cyclic reaction depicted below shows the 2-ethyl derivative, where 2-ethyl-9,10-dihydroxyanthracene (C
16H
12(OH)
2) is oxidized to the corresponding 2-ethylanthraquinone (C
16H
12O
2) and hydrogen peroxide. Most commercial processes achieve this by bubbling compressed air through a solution of the derivatized anthracene, whereby the oxygen present in the air reacts with the labile hydrogen atoms (of the hydroxy group), giving hydrogen peroxide and regenerating the anthraquinone. Hydrogen peroxide is then extracted and the anthraquinone derivative is reduced back to the dihydroxy (anthracene) compound using hydrogen gas in the presence of a metal catalyst. The cycle then repeats itself.[12][13]

The simplified overall equation for the process is deceptively simple:[12]
- H
2 + O
2 → H
2O
2
The economics of the process depend heavily on effective recycling of the quinone (which is expensive) and extraction solvents, and of the hydrogenation catalyst.
In 1994, world production of H
2O
2 was around 1.9 million tonnes and grew to 2.2 million in 2006,[14] most of which was at a concentration of 70% or less. In that year bulk 30% H
2O
2 sold for around US $0.54 per kg, equivalent to US $1.50 per kg (US $0.68 per lb) on a "100% basis".[15][16]
New developments
A new high-productivity/high-yield process, based on an optimized distribution of isomers of 2-amyl anthraquinone, has been developed by Solvay. In 2008, this process allowed the construction of a mega-scale single-train plant in Zandvliet (Belgium). The plant has an annual production capacity more than twice that of the world's next-largest single-train plant. An even larger plant was commissioned in 2011 by a joint venture of Solvay and Dow in Map Ta Phut (Thailand). This plant had a projected production capacity of 330,000 tons of hydrogen peroxide per year at 100% concentration.[17] It is likely that this will lead to a reduction in the cost of production due to economies of scale.[18]
A process to produce hydrogen peroxide directly from the elements has been of interest for many years. The problem with the direct synthesis process is that, in terms of thermodynamics, the reaction of hydrogen with oxygen favors production of water. It had been recognized for some time that a finely dispersed catalyst is beneficial in promoting selectivity to hydrogen peroxide, but, while selectivity was improved, it was still not sufficiently high to permit commercial development of the process. However, an apparent breakthrough was made in the early 2000s by researchers at Headwaters Technology. The breakthrough revolves around development of minute (nanometer-size) phase-controlled noble metal crystal particles on carbon support. This advance led, in a joint venture with Evonik Industries, to the construction of a pilot plant in Germany in late 2005. It is claimed that there are reductions in investment cost because the process is simpler and involves less equipment; however, the process is also more corrosive and unproven. This process results in low concentrations of hydrogen peroxide (about 5–10 wt% versus about 40 wt% through the anthraquinone process).[18]
In 2009, another catalyst development was announced by researchers at Cardiff University.[19] This development also relates to direct synthesis, but in this case using gold–palladium nanoparticles. Under normal circumstances, direct synthesis must be carried out in an acid medium to prevent decomposition of the hydrogen peroxide as soon as it is formed. (Hydrogen peroxide in any case tends to decompose on its own, which is why, even after production, it is often necessary to add stabilisers to the commercial product when it is to be transported or stored for long periods.) However the nature of a catalyst can cause this decomposition to accelerate rapidly, and it is claimed that this gold-palladium catalyst reduces the decomposition and, as a consequence, little to no acid is required. The process is in a very early stage of development and currently results in very low concentrations of hydrogen peroxide being formed (less than about 1–2 wt%). Nonetheless, it is envisaged by the inventors that the process will lead to an inexpensive, efficient, and environmentally friendly process.[18][19][20][21]
That same year it was announced that scientists at the University of Liverpool have devised a fuel cell which creates hydrogen peroxide rather than electricity, with no known environmental impact. This would be used as a propellant or for the chemical industry. The project was to terminate at 2004 with conclusions, but none were given.[22]
A novel electrochemical process for the production of alkaline hydrogen peroxide has been developed by Dow. The process employs a monopolar cell to achieve an electrolytic reduction of oxygen in a dilute sodium hydroxide solution.[18]
Availability
Hydrogen peroxide is most commonly available as a solution in water. For consumers, it is usually available from pharmacies at 3 and 6 wt% concentrations. The concentrations are sometimes described in terms of the volume of oxygen gas generated; one milliliter of a 20-volume solution generates twenty milliliters of oxygen gas when completely decomposed. For laboratory use, 30 wt% solutions are most common. Commercial grades from 70% to 98% are also available, but due to the potential of solutions of >68% hydrogen peroxide to be converted entirely to steam and oxygen (with the temperature of the steam increasing as the concentration increases above 68%) these grades are potentially far more hazardous, and require special care in dedicated storage areas. Buyers must typically allow inspection by commercial manufacturers.
Reactions
Decomposition
- 2 H
2O
2 → 2 H
2O + O
2
This process is thermodynamically favorable. It has a ΔHo of −98.2 kJ·mol−1 and a ΔS of 70.5 J·mol−1·K−1. The rate of decomposition is dependent on the temperature (a cool environment slows down decomposition, therefore hydrogen peroxide is often stored in a refrigerator) and on the concentration of the peroxide, as well as the pH and the presence of impurities and stabilizers. Hydrogen peroxide is incompatible with many substances that catalyse its decomposition, including most of the transition metals and their compounds. Common catalysts include manganese dioxide, silver, and platinum.[23] The same reaction is catalysed by the enzyme catalase, found in the liver, whose main function in the body is the removal of toxic byproducts of metabolism and the reduction of oxidative stress. The decomposition occurs more rapidly in alkali, so acid is often added as a stabilizer.
The liberation of oxygen and energy in the decomposition has dangerous side-effects. Spilling high concentrations of hydrogen peroxide on a flammable substance can cause an immediate fire, which is further accelerated by the oxygen released from the decomposing hydrogen peroxide. High test peroxide, or HTP (also called high-strength peroxide) must be stored in a suitable, vented container to prevent the buildup of oxygen gas, which would otherwise lead to the eventual rupture of the container.[24]
In the presence of certain catalysts, such as Fe2+
or Ti3+
, the decomposition may take a different path, with free radicals such as HO· (hydroxyl) and HOO· (hydroperoxyl) being formed. A combination of H
2O
2 and Fe2+
is known as Fenton's reagent.
A common concentration for hydrogen peroxide is 20-volume, which means that, when 1 volume of this solution of hydrogen peroxide is decomposed, it produces 20 volumes of oxygen (STP). A 20-volume concentration of hydrogen peroxide is equivalent to 1.761 mol/dm3 (Molar solution) or about 6.08%(w/v).
Redox reactions
In acidic solutions, H
2O
2 is one of the most powerful oxidizers known—stronger than chlorine, chlorine dioxide, and potassium permanganate. Also, through catalysis, H
2O
2 can be converted into hydroxyl radicals (•OH), which are highly reactive.
| Oxidant/Reduced product | Oxidation potential, V |
|---|---|
| Fluorine/Hydrogen fluoride | 3.0 |
| Ozone/Oxygen | 2.1 |
| Hydrogen peroxide/Water | 1.8 |
| Potassium permanganate/Manganese dioxide | 1.7 |
| Chlorine dioxide/HClO | 1.5 |
| Chlorine/Chloride | 1.4 |
In aqueous solutions, hydrogen peroxide can oxidize or reduce a variety of inorganic ions. When it acts as a reducing agent, oxygen gas is also produced.
In acidic solutions Fe2+
is oxidized to Fe3+
(hydrogen peroxide acting as an oxidizing agent),
and sulfite (SO2−
3) is oxidized to sulfate (SO2−
4). However, potassium permanganate is reduced to Mn2+
by acidic H
2O
2. Under alkaline conditions, however, some of these reactions reverse; for example, Mn2+
is oxidized to Mn4+
(as MnO
2).
Other examples of hydrogen peroxide's action as a reducing agent are reaction with sodium hypochlorite or potassium permanganate, which is a convenient method for preparing oxygen in the laboratory.
- NaOCl + H
2O
2 → O
2 + NaCl + H
2O
- 2 KMnO
4 + 3 H
2O
2 → 2 MnO
2 + 2 KOH + 2 H
2O + 3 O
2
Hydrogen peroxide is frequently used as an oxidizing agent in organic chemistry. One application is for the oxidation of thioethers to sulfoxides.[citation needed] For example, methyl phenyl sulfide can be readily oxidized in high yield to methyl phenyl sulfoxide:[25]
- Ph−S−CH
3 + H
2O
2 → Ph−S(O)−CH
3 + H
2O
Alkaline hydrogen peroxide is used for epoxidation of electron-deficient alkenes such as acrylic acids, and also for oxidation of alkylboranes to alcohols, the second step of hydroboration-oxidation.
Formation of peroxide compounds
Hydrogen peroxide is a weak acid, and it can form hydroperoxide or peroxide salts or derivatives of many metals.
For example, on addition to an aqueous solution of chromic acid (CrO
3) or acidic solutions of dichromate salts, it will form an unstable blue peroxide CrO(O
2)
2. In aqueous solution it rapidly decomposes to form oxygen gas and chromium salts.
It can also produce peroxoanions by reaction with anions; for example, reaction with borax leads to sodium perborate, a bleach used in laundry detergents:
- Na
2B
4O
7 + 4 H
2O
2 + 2 NaOH → 2 Na
2B
2O
4(OH)
4 + H
2O
H
2O
2 converts carboxylic acids (RCOOH) into peroxy acids (RCOOOH), which are themselves used as oxidizing agents. Hydrogen peroxide reacts with acetone to form acetone peroxide, and it interacts with ozone to form hydrogen trioxide, also known as trioxidane. Reaction with urea produces the adduct hydrogen peroxide - urea, used for whitening teeth. An acid-base adduct with triphenylphosphine oxide is a useful "carrier" for H
2O
2 in some reactions.
Alkalinity
Hydrogen peroxide can still form adducts with very strong acids. The superacid HF/SbF
5 forms unstable compounds containing the [H
3O
2]+
ion.
Biological function
Hydrogen peroxide is also one of the two chief chemicals in the defense system of the bombardier beetle, reacting with hydroquinone to discourage predators.
A study published in Nature found that hydrogen peroxide plays a role in the immune system. Scientists found that hydrogen peroxide inside of cells increased after tissues are damaged in zebra fish, which is thought to act as a signal to white blood cells to converge on the site and initiate the healing process. When the genes required to produce hydrogen peroxide were disabled, white blood cells did not accumulate at the site of damage. The experiments were conducted on fish; however, because fish are genetically similar to humans, the same process is speculated to occur in humans. The study in Nature suggested asthma sufferers have higher levels of hydrogen peroxide in their lungs than healthy people, which could explain why asthma sufferers have inappropriate levels of white blood cells in their lungs.[26][27]
Hydrogen peroxide has important roles as a signaling molecule in the regulation of a wide variety of biological processes.[28] The compound is a major factor implicated in the free-radical theory of aging, based on how readily hydrogen peroxide can decompose into a hydroxyl radical and how superoxide radical byproducts of cellular metabolism can react with ambient water to form hydrogen peroxide.[29] These hydroxyl radicals in turn readily react with and damage vital cellular components, especially those of the mitochondria.[30] At least one study has also tried to link hydrogen peroxide production to cancer.[31] These studies have frequently been quoted in fraudulent treatment claims.
The amount of hydrogen peroxide in biological systems can be assayed using a fluorimetric assay.[32]
Uses
Water treatment
Drinking water treatment
Hydrogen peroxide can be used for cleaning well water or other drinking water sources, by removing odors, organic materials that change the water taste, and the removal of H2S and Iron, while reducing trihalomethanes and haloacetic acids. Hydrogen peroxide can be used to increase or decrease the amount of ozone in drinking water.[33]
Wastewater treatment
Hydrogen peroxide is replacing prechlorination as a way to deal with odors entering wastewater treatment plants:[34]
Sulfide oxidation
Hydrogen peroxide has been utilized to minimize hydrogen sulfide (H2S) formation.[35]
The processing of wastewater sludge (or biosolids) can cause the generation of hydrogen sulfide, a poisonous and odoriferous gas. Hydrogen sulfide can also damage equipment and concrete structures.
Sulfides are found throughout the environment as a result of both natural and industrial processes. Most sulfides found in nature were produced biologically (under anaerobic conditions) and occur as free hydrogen sulfide (H2S) – characterized by its rotten egg odor. Biogenic H2S is encountered in sour groundwaters, swamps and marshes, natural gas deposits, and sewage collection/treatment systems. Man-made sources of H2S typically occur as a result of natural materials containing sulfur (e.g., coal, gas and oil) being refined into industrial products. For a variety of reasons – aesthetics (odor control), health (toxicity), ecological (oxygen depletion in receiving waters), and economic (corrosion of equipment and infrastructure) – sulfide laden wastewater must be handled carefully and go through a remediation process before it can be released to the environment. Typical discharge limits for sulfide are < 1 mg/L.
Hydrogen peroxide is a strong oxidizer effective in controlling sulfide and organic-related odors in wastewater collection and treatment systems. It is typically applied to a wastewater system where there is a retention time of 30 minutes to 5 hours before hydrogen sulfide is released. Hydrogen peroxide oxidizes the hydrogen sulfide and promotes bio-oxidation of organic odors.[36]
BOD and COD removal from wastewater
Hydrogen peroxide decomposes to oxygen and water, adding dissolved oxygen to the system, thereby negating some Biochemical Oxygen Demand (BOD). Typical sewage at its first stage has aerobic organisms quickly consuming the oxygen, then dying and decomposing, and anaerobic organisms usually bacteria, set in, creating a toxic environment through their anaerobic digestion. In order to "re-vitalize" the water, various methods of aeration are typically used.
Hydrogen peroxide has been used to reduce the BOD and COD of industrial waste-water for many years. While the cost of removing BOD/COD through chemical oxidation is typically greater than that through physical or biological means, there are nonetheless specific situations which justify its use. These include:
- Pre-digestion of wastewater which contains moderate to high levels of compounds that are toxic, inhibitory, or recalcitrant to biological treatment (e.g., pesticides, plasticizers, resins, coolants, and dyestuffs);
- Pretreatment of high strength / low flow wastewater – where biotreatment may not be practical – prior to discharge to a Publicly Owned Treatment Works (POTW);
- Enhanced separation of entrained organics by flotation and settling processes; and
Supply of supplemental Dissolved Oxygen (DO) when biological treatment systems experience temporary overloads or equipment failure.
As indicated by these examples, hydrogen peroxide can be used as a stand-alone treatment or as an enhancement to existing physical or biological treatment processes, depending on the situation.[37]
Nitrogen oxide (NOx) abatement
Nitrogen oxides are major pollutants in the atmosphere, being a precursor to acid rain, photochemical smog, and ozone accumulation. The oxides are mainly nitric oxide (NO) and nitrogen dioxide (NO2) both of which are corrosive and hazardous to health, typically created from the decomposition of organic materials, assisted by anaerobic organisms, or released during the combustion of fossil fuels.[38]
With the use of catalytic converters on automobiles, the initial regulatory focus of controlling of mobile NOx emissions has reached the point where further restriction has become economically impractical. Consequently, the stationary sources of NOx emissions are now being subjected to more stringent standards in many areas of the U.S. Stationary sources include nitric acid manufacturing plants, manufacturers of nitrated materials such as fertilizer and explosives, and industrial manufacturers (metallurgical processors, glass manufacturers, cement kilns, power generators, etc.) where high processing temperatures are used.[38]
Because of the environmental concerns posed by air pollution, much research time and money have been expended to develop methods for controlling NOx emissions. Several 'NOx scrubbing' processes have been developed, using H2O2 as part of the solution, where the nitrogen oxides are converted to nitrate, nitric acid or nitrogen.[38]
Hydrogen peroxide is also used to eliminate nitrogen oxide development 'at the source', by reacting with HNO as it is formed, and eliminating its decomposition into NO or NO2.[38]
Pollutants removal
Hydrogen peroxide is one of the most versatile, dependable and environmentally compatible oxidizing agents. The relative safety and simplicity of its use as an oxidizing agent has led to the development of a number of applications in refinery wastewater systems:
- Uncatalyzed hydrogen peroxide
The strong oxidizing power of hydrogen peroxide makes it suitable for the destruction of a variety of pollutants. Optimization of conditions using hydrogen peroxide to destroy these pollutants can involve control of pH, temperature and reaction time. No additional additives are required.
- Catalyzed hydrogen peroxide
Pollutants that are more difficult to oxidize require hydrogen peroxide to be activated with catalysts such as iron. Catalyzed oxidation can also be used to destroy easily oxidized pollutants more rapidly.
Under acid pH conditions, the addition of iron salts to a wastewater solution activates hydrogen peroxide to generate free radicals, which can attack a variety of organic compounds. Other metal salts and conditions can apply (e.g. in cyanide destruction, a copper catalyst can be used at a pH of 8.5 – 11.5).[39]
Aeration for fish and plants
- Horticulture
Some horticulturalists and users of hydroponics advocate the use of weak hydrogen peroxide solution in watering solutions. Its spontaneous decomposition releases oxygen that enhances a plant's root development and helps to treat root rot (cellular root death due to lack of oxygen) and a variety of other pests.[40][41][42]
- Fish Aeration
Laboratory tests conducted by fish culturists in recent years have demonstrated that common household hydrogen peroxide can be used safely to provide oxygen for small fish. The hydrogen peroxide releases oxygen by decomposition when it is exposed to catalysts such as manganese dioxide.[43][44]
Bombs
Hydrogen peroxide has been used for creating organic peroxide explosives for improvised explosive devices, including the 7 July 2005 London bombings.[45]
Industrial applications

Bleaching wood pulp
About 50% of the world's production of hydrogen peroxide in 1994 was used for pulp- and paper-bleaching.[14]
Possible alternative to chlorine bleaches
Other bleaching applications are becoming more important as hydrogen peroxide is seen as an environmentally benign alternative to chlorine-based bleaches.[46] However scientific studies have found hydrogen peroxide to be ineffective in certain cases, and generally instruct hospitals, medical institutions, and other locations where public health is monitored, to use chlorine-based bleaches for disinfection.[47]
Mild bleaches in laundry detergents
Other major industrial applications for hydrogen peroxide include the manufacture of sodium percarbonate and sodium perborate, used as mild bleaches in laundry detergents.
Intermediate processes in the chemical industry
It is used in the production of certain organic peroxides, such as dibenzoyl peroxide, used in polymerisations and other chemical processes.
Hydrogen peroxide is also used in the production of epoxides, such as propylene oxide: Reaction with carboxylic acids produces a corresponding peroxy acid. Peracetic acid and meta-chloroperoxybenzoic acid (commonly abbreviated mCPBA) are prepared from acetic acid and meta-chlorobenzoic acid, respectively. The latter is commonly reacted with alkenes to give the corresponding epoxide.
Micro-etching
In the PCB manufacturing process, hydrogen peroxide mixed with sulfuric acid was used as the microetch chemical for copper surface roughening preparation.
Instant steam
A combination of a powdered precious metal-based catalyst, hydrogen peroxide, methanol and water can produce superheated steam in one to two seconds, releasing only CO
2 and high-temperature steam for a variety of purposes.[48]
Bio-decontamination validation
Recently, there has been increased use of vaporized hydrogen peroxide in the validation and bio-decontamination of half-suit and glove-port isolators in pharmaceutical production.
Rapid oxidation for nuclear plant shutdown
Nuclear pressurized water reactors (PWRs) use hydrogen peroxide during the plant shutdown to force the oxidation and dissolution of activated corrosion products deposited on the fuel. The corrosion products are then removed with the cleanup systems before the reactor is disassembled.
Oil and gas exploration fossil analysis
Hydrogen peroxide is also used in the oil and gas exploration industry to oxidize rock matrix in preparation for micro-fossil analysis.
Propylene oxide
A method of producing propylene oxide from hydrogen peroxide has been developed. The process is claimed to be environmentally friendly, since the only significant byproduct is water. Two of these "HPPO" (hydrogen peroxide to propylene oxide) plants came onstream in 2008: One of them located in Belgium is a Solvay, Dow-BASF joint venture, and the other in Korea is an EvonikHeadwaters, SK Chemicals joint venture. A caprolactam application for hydrogen peroxide has been commercialized. Potential routes to phenol and epichlorohydrin utilizing hydrogen peroxide have been postulated.[18]
Therapeutic use
Hydrogen peroxide is generally recognized as safe (GRAS) as an antimicrobial agent, an oxidizing agent and for other purposes by the U.S. FDA.[49] For example, 35% hydrogen peroxide is used to prevent infection transmission in the hospital environment, and hydrogen peroxide vapor is registered with the US EPA as a sporicidal sterilant.
On the other hand many false claims have been made about the therapeutic properties of hydrogen peroxide, some even lethal, as discussed below.[50]
Disinfectant for inanimate objects
Hydrogen peroxide has been used, in sufficient concentrations, to disinfect inanimate objects.[51] As stated above scientific research concluded that chlorine based disinfectants are better used in hospital and other public institutions.[47]
Veterinary practice
Hydrogen peroxide is used as an emetic in veterinary practice.[52][53]
Toothpaste
Hydrogen peroxide mixed with baking soda and salt is used as a toothpaste, but its use was shown to be no more effective than toothpaste.[54]
Acne treatment
Hydrogen peroxide and benzoyl peroxide are sometimes used to treat acne.[55] This too has been challenged by the medical establishment, after research showed that hydrogen peroxide even at minute quantities is harmful to the healing process.[2][50] A fundamental difference from benzoyl peroxide is that hydrogen peroxide is not lipid soluble. Benzoyl peroxide selectively concentrates in the follicles and sebaceous glands because of its lipophilic properties, but hydrogen peroxide is much less soluble in lipids and is a much smaller molecule. This allows it to penetrate at any point in the skin and enter damaged cells relatively easily, leading to the aforementioned harm to the healing process.
Disinfecting wounds
Commonly used as treatment for disinfecting wounds, there is a body of evidence, e.g.[3] and many reports since, that the use of large volumes of hydrogen peroxide over substantial tissue areas can lead to a dangerous oxygen embolism (gas embolism). While judicious use on surface wounds can utilize the catalase-caused "fizzing" effect to assist debridement and cleaning of soil from the wound, clinicians are advised to consult the literature before using peroxide on wounds and tissue cavities. It has also been shown that hydrogen peroxide, even in dilute solutions and minute quantities can slow the healing process on wounds.[2][56][57][58] Further, hydrogen peroxide applied to wounds can impede healing and lead to scarring because it destroys newly formed skin cells.[59]
Cure for cancer
Following the call by alternative medicine advisors for drinking diluted hydrogen peroxide, and using it in various ways such as in shampoo and as an additive to toothpaste, as a treatment to illness in general and cancer in particular, the American Cancer Society states that "there is no scientific evidence that hydrogen peroxide is a safe, effective or useful cancer treatment", and advises cancer patients to "remain in the care of qualified doctors who use proven methods of treatment and approved clinical trials of promising new treatments."[60]
Inhalation, Oral administration and Injection
For treatment of various illnesses, another alternative medical procedure advocated by this group of medical doctors (now banned from publishing their material) is intravenous injection of hydrogen peroxide, which has been linked to several deaths.[61][62] Also advocated by this group and its followers are oral administration of "dilute quantities" of H2O2, and inhalation of hydrogen peroxide at a concentration of about 1%. These practices have been challenged by the medical establishment as well.[50]
Domestic uses
Bleach
2O
2
- Bleaching hair
Diluted H
2O
2 (between 3% and 8%) is used to bleach human hair when mixed with ammonium hydroxide. The chemical's bleaching property lends its name to the phrase "peroxide blonde".[63]
- Bleaching skin
It is absorbed by skin upon contact and creates a local skin capillary embolism that appears as a temporary whitening of the skin.
- Bleaching bones for display
It is used to whiten bones that are to be put on display.
- Cleaning blood stains
3% H
2O
2 is effective at treating fresh (red) blood-stains in clothing and on other items. It must be applied to clothing before blood stains are "set" with heated water. Cold water and soap are then used to remove the peroxide-treated blood.
- As floor bleach
Hydrogen peroxide can be used to clean tile and grout on floors. It is sometimes recommended to clean with both hydrogen peroxide and baking soda together.[64]
- Skunk odor removal
Mixed with baking soda and a small amount of hand soap, hydrogen peroxide is effective at removing skunk odor.[65]
Other uses
Hydrogen peroxide is used with phenyl oxalate ester and an appropriate dye in glow sticks as an oxidizing agent. It reacts with the ester to form an unstable CO
2 dimer, which excites the dye to an excited state; the dye emits a photon (light) when it spontaneously relaxes back to the ground state.
- In the Chemical industry
Hydrogen peroxide can be combined with vinegar and table salt to form a substitute for industrial chemicals such as ferric chloride, ammonium persulfate, or hydrochloric acid as a hobbyist's printed circuit board etchant.[66]
- Alcoholic beverage industry
Hydrogen peroxide may be used in accelerated aging of alcoholic spirits. Some hobby distillers advocate adding small amounts of hydrogen peroxide to distilled spirits, on the theory that the oxygen released will accelerate the oxidation of compounds that occurs naturally when spirits are aged in somewhat permeable oak barrels.[67] This has not been proven scientifically.
Propellant

High concentration H
2O
2 is referred to as High Test Peroxide (HTP). It can be used either as a monopropellant (not mixed with fuel) or as the oxidizer component of a bipropellant rocket. Use as a monopropellant takes advantage of the decomposition of 70–98+% concentration hydrogen peroxide into steam and oxygen. The propellant is pumped into a reaction chamber where a catalyst, usually a silver or platinum screen, triggers decomposition, producing steam at over 600 °C (1,112 °F), which is expelled through a nozzle, generating thrust. H
2O
2 monopropellant produces a maximum specific impulse (Isp) of 161 s (1.6 kN·s/kg), which makes it a low-performance monopropellant. Peroxide generates much less thrust than hydrazine. The Bell Rocket Belt used hydrogen peroxide monopropellant.
As a bipropellant H
2O
2 is decomposed to burn a fuel as an oxidizer. Specific impulses as high as 350 s (3.5 kN·s/kg) can be achieved, depending on the fuel. Peroxide used as an oxidizer gives a somewhat lower Isp than liquid oxygen, but is dense, storable, noncryogenic and can be more easily used to drive gas turbines to give high pressures using an efficient closed cycle. It can also be used for regenerative cooling of rocket engines. Peroxide was used very successfully as an oxidizer in World War II German rocket motors (e.g. T-Stoff, containing oxyquinoline stabilizer, for the Me 163B), most often used with C-Stoff in a self-igniting hypergolic combination, and for the low-cost British Black Knight and Black Arrow launchers.
In the 1940s and 1950s, the Walter turbine used hydrogen peroxide for use in submarines while submerged; it was found to be too noisy and require too much maintenance compared to diesel-electric power systems. Some torpedoes used hydrogen peroxide as oxidizer or propellant, but this was dangerous and has been discontinued by most navies. Hydrogen peroxide leaks were blamed for the sinkings of HMS Sidon and the Russian submarine Kursk. It was discovered, for example, by the Japanese Navy in torpedo trials, that the concentration of H
2O
2 in right-angle bends in HTP pipework can often lead to explosions in submarines and torpedoes. SAAB Underwater Systems is manufacturing the Torpedo 2000. This torpedo, used by the Swedish navy, is powered by a piston engine propelled by HTP as an oxidizer and kerosene as a fuel in a bipropellant system.[68][69]
While rarely used now as a monopropellant for large engines, small hydrogen peroxide attitude control thrusters are still in use on some satellites.They are easy to throttle, and safer to fuel and handle before launch than hydrazine thrusters. However, hydrazine is more often used in spacecraft because of its higher specific impulse and lower rate of decomposition.
Improvised explosive device / home-made bomb precursor
Hydrogen peroxide was the main ingredient in the 7 July 2005 London bombings that killed 52 London Underground and bus passengers. The bomb-making ingredients are reported to be easier to buy than large numbers of aspirin pills.[70] It was used again in other terrorist attacks.[45]
Safety
Regulations vary, but low concentrations, such as 6%, are widely available and legal to buy for medical use. Most over-the-counter peroxide solutions are not suitable for ingestion. Higher concentrations may be considered hazardous and are typically accompanied by a Material Safety Data Sheet (MSDS). In high concentrations, hydrogen peroxide is an aggressive oxidizer and will corrode many materials, including human skin. In the presence of a reducing agent, high concentrations of H
2O
2 will react violently.
High-concentration hydrogen peroxide streams, typically above 40%, should be considered hazardous due to concentrated hydrogen peroxide's meeting the definition of a DOT oxidizer according to U.S. regulations, if released into the environment. The EPA Reportable Quantity (RQ) for D001 hazardous wastes is 100 pounds (45 kg), or approximately 10 US gallons (38 L), of concentrated hydrogen peroxide.
Hydrogen peroxide should be stored in a cool, dry, well-ventilated area and away from any flammable or combustible substances.[71] It should be stored in a container composed of non-reactive materials such as stainless steel or glass (other materials including some plastics and aluminium alloys may also be suitable).[72] Because it breaks down quickly when exposed to light, it should be stored in an opaque container, and pharmaceutical formulations typically come in brown bottles that filter out light.[73]
Hydrogen peroxide, either in pure or diluted form, can pose several risks, the main one being that if forms explosive mixtures upon contact with organic compounds.[24] Highly concentrate hydrogen peroxide itself is unstable, and can then cause a boiling liquid expanding vapor explosion (BLEVE) of the remaining liquid. Distillation of hydrogen peroxide at normal pressures is thus highly dangerous. It is also corrosive especially when concentrated but even domestic-strength solutions can cause irritation to the eyes, mucous membranes and skin.[74] Swallowing hydrogen peroxide solutions is particularly dangerous, as decomposition in the stomach releases large quantities of gas (10 times the volume of a 3% solution) leading to internal bleeding. Inhaling over 10% can cause severe pulmonary irritation.[75]
With a significant vapor pressure (1.2 kPa at 50 °C[CRC Handbook of Chemistry and Physics, 76th Ed, 1995–1996]), hydrogen peroxide vapor is potentially hazardous. According to the U.S. NIOSH Immediately dangerous to life and health limit (IDLH) is only 75 ppm.[76] The U.S. Occupational Safety and Health Administration (OSHA) has established a permissible exposure limit of 1.0 ppm calculated as an eight hour time weighted average (29 CFR 1910.1000, Table Z-1)[24] and hydrogen peroxide has also been classified by the American Conference of Governmental Industrial Hygienists (ACGIH) as a "known animal carcinogen, with unknown relevance on humans."[77] Information on the hazards of hydrogen peroxide is available from OSHA[24] and from the ATSDR.[78]
Dubious health claims
Hydrogen peroxide therapy, a purported treatment where small, highly diluted amounts of food-grade hydrogen peroxide are taken orally or administered intravenously, is claimed to cure everything from acne to HIV and cancer. Several books on the topic advocate this therapy. This therapy is not approved by the U.S. FDA. Large oral doses of hydrogen peroxide at a 3% concentration may cause "irritation and blistering to the mouth, throat, and abdomen", as well as "abdominal pain, vomiting, and diarrhea".[79] Deaths and serious injury have been reported after intravenous injection of hydrogen peroxide.[80]
Historical incidents
- On 16 July 1934, in Kummersdorf, Germany, a rocket engine using hydrogen peroxide exploded, killing three people. As a result of this incident, Wernher von Braun decided not to use hydrogen peroxide as an oxidizer in the rockets he developed afterward.
- During the Second World War, doctors in German concentration camps experimented with the use of hydrogen peroxide injections in the killing of human subjects.[81]
- Several people received minor injuries after a hydrogen peroxide spill on board a flight between the U.S. cities Orlando and Memphis on 28 October 1998.[82]
- Hydrogen peroxide was said to be one of the ingredients in the bombs that failed to explode in the July 21, 2005 London bombings.[83]
- The Russian submarine K-141 Kursk sailed out to sea to perform an exercise of firing dummy torpedoes at the Pyotr Velikiy, a Kirov class battlecruiser. On 12 August 2000 at 11:28 local time (07:28 UTC), there was an explosion while preparing to fire the torpedoes. The only credible report to date is that this was due to the failure and explosion of one of the Kursk's hydrogen peroxide-fueled torpedoes. It is believed that HTP, a form of highly concentrated hydrogen peroxide used as propellant for the torpedo, seeped through rust in the torpedo casing. A similar incident was responsible for the loss of HMS Sidon in 1955.
- On 15 August 2010 a spill of about 30 US gallons (110 L) of cleaning fluid occurred on the 54th floor of 1515 Broadway, in Times Square, New York City. The spill, which a spokesperson for the New York City fire department said was of hydrogen peroxide, shut down Broadway between West 42nd and West 48th streets as fire engines responded to the hazmat situation. There were no reported injuries.[84]
See also
References
Notes
- ^ Hill, C. N. (2001). A Vertical Empire: The History of the UK Rocket and Space Programme, 1950–1971. Imperial College Press. ISBN 978-1-86094-268-6.
- ^ a b c Effects of Hydrogen Peroxide on Wound Healing in Mice in Relation to Oxidative Damage - The research concluded: "In our study, we found... implies that H2O2 can cause poor healing by other mechanisms besides causing oxidation of these biological substrates."
- ^ a b Shaw A, Cooperman A, Fusco J. Gas embolism produced by hydrogen peroxide. N Engl J Med 1967; 277: 238–41.
- ^ Dougherty, Dennis A. (2005). Modern Physical Organic Chemistry. University Science. p. 122. ISBN 1-891389-31-9.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Journal of Chemical Physics, Volume 111, No. 4, July 1999 (at the Korean Advanced Institute of Science and Technology website)
- ^ Landolt-Börnstein Substance – Property Index
- ^ 60% hydrogen peroxide msds 50% H2O2 MSDS
- ^ What is the pH of H2O2 solutions? | H2O2.com – US Peroxide – Technologies for Clean Environment
- ^ L. J. Thénard (1818). "Observations sur des nouvelles combinaisons entre l'oxigène et divers acides". Annales de chimie et de physique, 2nd series. 8: 306–312.
- ^ C. W. Jones, J. H. Clark. Applications of Hydrogen Peroxide and Derivatives. Royal Society of Chemistry, 1999.
- ^ Richard Wolffenstein (1894). "Concentration und Destillation von Wasserstoffsuperoxyd". Berichte der deutschen chemischen Gesellschaft. 27 (3): 3307–12. doi:10.1002/cber.189402703127.
- ^ a b Jose M. Campos-Martin, Gema Blanco-Brieva, Jose L. G. Fierro (2006). "Hydrogen Peroxide Synthesis: An Outlook beyond the Anthraquinone Process". Angewandte Chemie International Edition. 45 (42): 6962–6984. doi:10.1002/anie.200503779. PMID 17039551.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ H. Riedl and G. Pfleiderer, U.S. Patent 2,158,525 (2 October 1936 in USA, and 10 October 1935 in Germany) to I. G. Farbenindustrie, Germany
- ^ a b Ronald Hage, Achim Lienke (2005). "Applications of Transition-Metal Catalysts to Textile and Wood-Pulp Bleaching". Angewandte Chemie International Edition. 45 (2): 206–222. doi:10.1002/anie.200500525. PMID 16342123.
- ^ Hydrogen Peroxide Synthesis researchgate
- ^ research for Ministry of Science and Technology, Government of India
- ^ 5 October 2011 Solvay commissions the largest hydrogen peroxide plant in the world in Thailand
- ^ a b c d e Hydrogen Peroxide 07/08-03 Report, ChemSystems, May 2009.
- ^ a b G.J. Hutchings et al, Science, 2009, 323, 1037
- ^ "Gold-palladium Nanoparticles Achieve Greener, Smarter Production Of Hydrogen Peroxide". Sciencedaily.com. 3 March 2009. Retrieved 5 September 2010.
- ^ Jennifer K. Edwards, Benjamin Solsona, Edwin Ntainjua N, Albert F. Carley (February 2009). "Switching off hydrogen peroxide hydrogenation in the direct synthesis process". Science. 323 (5917): 1037–41. doi:10.1126/science.1168980. PMID 19229032.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ New role for fuel cells, August 2003, University of Liverpool website
- ^ Petrucci, Ralph H. (2007). General Chemistry: Principles & Modern Applications (9th ed.). Prentice Hall. p. 606. ISBN 0-13-149330-2.
- ^ a b c d Occupational Safety and Health Guideline for Hydrogen Peroxide
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi: 10.1055/s-2004-44387, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi= 10.1055/s-2004-44387instead. - ^ "Natural bleach 'key to healing'". BBC News. 6 June 2009. Retrieved 2 July 2009.
- ^ Niethammer, Philipp (3 June 2009). "A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish". Nature. 459 (7249): 996–9. doi:10.1038/nature08119. PMC 2803098. PMID 19494811. Retrieved 2 July 2009.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Veal EA, Day AM, Morgan BA (April 2007). "Hydrogen peroxide sensing and signaling". Mol. Cell. 26 (1): 1–14. doi:10.1016/j.molcel.2007.03.016. PMID 17434122.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Weindruch, Richard (January 1996). "Calorie Restriction and Aging". Scientific American: 49–52.
- ^ Giorgio M, Trinei M, Migliaccio E, Pelicci PG (September 2007). "Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals?". Nat. Rev. Mol. Cell Biol. 8 (9): 722–8. doi:10.1038/nrm2240. PMID 17700625.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ López-Lázaro M (July 2007). "Dual role of hydrogen peroxide in cancer: possible relevance to cancer chemoprevention and therapy". Cancer Lett. 252 (1): 1–8. doi:10.1016/j.canlet.2006.10.029. PMID 17150302.
- ^ Rapoport, R.; Hanukoglu, I.; Sklan, D. (May 1994). "A fluorimetric assay for hydrogen peroxide, suitable for NAD(P)H-dependent superoxide generating redox systems". Anal Biochem. 218 (2): 309–13. doi:10.1006/abio.1994.1183. PMID 8074285.
- ^ Water treatment US Peroxide Solutions company website
- ^ Fagan, Michael (17 March 1999). "Hydrogen Sulfide Control – Headworks Odor Control with Hydrogen Peroxide". US Peroxide. Retrieved 15 September 2012.
{{cite web}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Case Study: Cost Effective Control of H2S in Municipal Sludge with Hydrogen Peroxide". US Peroxide. Retrieved 15 September 2012.
- ^ Sulfide Oxidation
- ^ BOD-COD Removal
- ^ a b c d Nitrogen Oxide Abatement (NOx)
- ^ High Strength Wastewater Pretreatment
- ^ Fredrickson, Bryce. "Hydrogen Peroxide and Horticulture" (PDF). Retrieved 25 January 2009.
- ^ Ways to use hydrogen peroxide in the garden
- ^ Bhattarai SP, Su N, Midmore DJ (2005). "Oxygation Unlocks Yield Potentials of Crops in Oxygen-Limited Soil Environments". Advances in Agronomy. Advances in Agronomy. 88: 313–377. doi:10.1016/S0065-2113(05)88008-3. ISBN 9780120007868.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Great-lakes.org
- ^ fws.gov
- ^ a b Tiny cheap deadly hydrogen peroxide bombs, 2009 article about home made bombs and their use in terror attacks. (NBC News)
- ^ This view is usually advocated by the environmental movement and found on blogs and websites affiliated with this movement, for example a comparison of the two on the 'Green Living Tips' blog
- ^ a b Bleach vs. Hydrogen Peroxide (Canadian Agency for Durgs and Technology in Health website)
- ^ Instant steam puts heat on MRSA, Society Of Chemical Industry
- ^ "Sec. 184.1366 Hydrogen peroxide". U.S. Government Printing Office via GPO Access. 1 April 2001. Retrieved 7 July 2007.
- ^ a b c Pitfalls in Regulating Physicians (Science Based Medicine organization website)
- ^ Jeffrey Pommerville, Alcamo's Fundamentals of Microbiology, ISBN 076376258X, page 213.
- ^ "Drugs to Control or Stimulate Vomiting". Merck Veterinary manual. Merck & Co., Inc. 2006.
- ^ How to Induce Vomiting (Emesis) in Dogs
- ^ Shepherd, Steven. "Brushing Up on Gum Disease". FDA Consumer. Archived from the original on 14 May 2007. Retrieved 7 July 2007.
- ^ Milani, Massimo (2003). "Efficacy and safety of stabilised hydrogen peroxide cream (Crystacide) in mild-to-moderate acne vulgaris: a randomised, controlled trial versus benzoyl peroxide gel". Current Medical Research and Opinion. 19 (2): 135–138(4). doi:10.1185/030079902125001523. PMID 12740158.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) [dead link] - ^ O’Connor, Anahd (19 June 2007). "Really? The Claim: Hydrogen Peroxide Is a Good Treatment for Small Wounds". New York Times. Retrieved 13 July 2011.
- ^ Carroll, Aaron E. (12 July 2011). "Medical myths don't die easily". CNN. Retrieved 13 July 2011.
{{cite news}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Joseph M. Ascenzi, Handbook of Disinfectant and Antiseptics, CRC Press, 1996, ISBN 0824795245, page 161.
- ^ Wilgus TA, Bergdall VK, Dipietro LA, Oberyszyn TM (2005). "Hydrogen peroxide disrupts scarless fetal wound repair". Wound Repair Regen. 13 (5): 513–9. doi:10.1111/j.1067-1927.2005.00072.x. PMID 16176460.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Questionable methods of cancer management: hydrogen peroxide and other 'hyperoxygenation' therapies". CA: a cancer journal for clinicians. 43 (1): 47–56. 1993. doi:10.3322/canjclin.43.1.47. PMID 8422605.
- ^ Cooper, Anderson (12 January 2005). "A Prescription for Death?". CBS News. Retrieved 7 July 2007.
- ^ Mikkelson, Barbara (30 April 2006). "Hydrogen Peroxide". Snopes.com. Retrieved 7 July 2007.
- ^ Lane, Nick (2003). Oxygen : the molecule that made the world (First issued in paperback, repr. ed.). Oxford: Oxford University Press. p. 117. ISBN 0198607830.
- ^ "Tile and Grout Cleaning".
- ^ Chemist Paul Krebaum claims to have originated the formula for use on skunked pets at Skunk Remedy
- ^ PCB Etchant from household materials
- ^ "Making Fine Spirits using simple, easy-to-build gear" by Zymurgy Bob, available from The Amphora Society
- ^ Scott, Richard (November 1997). "Homing Instincts". Jane's Navy Steam generated by catalytic decomposition of 80–90% hydrogen peroxide was used for driving the turbopump turbines of the V-2 rockets, the X-15 rocketplanes, the early Centaur RL-10 engines and is still used on Soyuz for that purpose to-day. International.
- ^ Soyuz using hydrogen peroxide propellant (NASA website)
- ^ BBC News – 7/7 inquests: Coroner warns over bomb ingredient
- ^ Hydrogen Peroxide MSDS
- ^ Ozonelab Peroxide compatibility
- ^ "Hydrogen Peroxide Mouthwash is it Safe?". Retrieved 30 October 2013.
- ^ For example, see an MSDS for a 3% peroxide solution.
- ^ H2O2 toxicity and dangers Agency for Toxic Substances and Disease Registry website
- ^ Documentation for Immediately Dangerous to Life or Health Concentrations (IDLH): NIOSH National Institute for Occupational Safety and Health] Chemical Listing and Documentation of Revised IDLH Values (as of 3/1/95)]
- ^ Threshold Limit Values for Chemical Substances and Physical Agents & Biological Exposure Indices, ACGIH
- ^ Agency for Toxic Substances and Disease Registry
- ^ Hydrogen Peroxide, 3%. 3. Hazards Identification Southeast Fisheries Science Center, daughter agency of NOAA.
- ^ [http://www.thedenverchannel.com/news/naturopath-sentenced-for-injecting-teen-with-hydrogen-peroxide}
- ^ "The Nazi Doctors: Medical Killing and the Psychology of Genocide". Robert Jay Lifton. Retrieved 1 November 2007.
- ^ Hazardous Materials Incident Brief DCA-99-MZ-001, "Spill of undeclared shipment of hazardous materials in cargo compartment of aircraft". pub: National Transportation Safety Board. 28 October 1998; adopted 17 May 2000.
- ^ Four Men Found Guilty in Plot to Blow Up London's Transit System, "FOXNews.com". (9 July 2007)
- ^ "Bleach Spill Shuts Part of Times Square". 16 August 2010.
Bibliography
- J. Drabowicz; et al. (1994). G. Capozzi; et al. (eds.). The Syntheses of Sulphones, Sulphoxides and Cyclic Sulphides. Chichester UK: John Wiley & Sons. pp. 112–6. ISBN 0-471-93970-6.
{{cite book}}: Explicit use of et al. in:|author=(help); Explicit use of et al. in:|editor=(help) - N.N. Greenwood, A. Earnshaw (1997). Chemistry of the Elements (2nd ed.). Oxford UK: Butterworth-Heinemann. A great description of properties & chemistry of H
2O
2. - J. March (1992). Advanced Organic Chemistry (4th ed.). New York: Wiley. p. 723.
- W.T. Hess (1995). "Hydrogen Peroxide". Kirk-Othmer Encyclopedia of Chemical Technology. Vol. 13 (4th ed.). New York: Wiley. pp. 961–995.
External links
- Hydrogen Peroxide at The Periodic Table of Videos (University of Nottingham)
- Material Safety Data Sheet
- ATSDR Agency for Toxic Substances and Disease Registry FAQ
- International Chemical Safety Card 0164
- NIOSH Pocket Guide to Chemical Hazards
- Process flow sheet of Hydrogen Peroxide Production by anthrahydroquinone autoxidation