Australian sea lion
| Australian sea lion | |
|---|---|

| |
| Male Australian sea lion with a harem at Seal Bay Conservation Park, Kangaroo Island, South Australia | |

| |
| Female Australian sea lion with pup at Seal Bay Conservation Park, Kangaroo Island, South Australia | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Carnivora |
| Clade: | Pinnipedia |
| Family: | Otariidae |
| Genus: | Neophoca |
| Species: | N. cinerea
|
| Binomial name | |
| Neophoca cinerea (Péron, 1816)
| |
| |
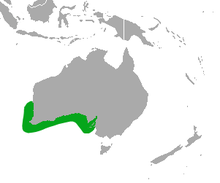
| Australian sea lion range | |
The Australian sea lion (Neophoca cinerea), also known as the Australian sea-lion or Australian sealion, is a species of sea lion that is the only endemic pinniped in Australia.[2] It is currently monotypic in the genus Neophoca, with the extinct Pleistocene New Zealand sea lion Neophoca palatina the only known congener.[3] With a population estimated at around 14,730 animals, the Wildlife Conservation Act of Western Australia (1950) has listed them as “in need of special protection”. Their Conservation status is listed as endangered. These pinnipeds are specifically known for their abnormal breeding cycles, which are varied between a 5-month breeding cycle and a 17-18 month aseasonal breeding cycle, compared to other pinnipeds which fit into a 12-month reproductive cycle.[2] Females are either silver or fawn with a cream underbelly and males are dark chocolate brown with a yellow mane and are bigger than the females.
Distribution
Australian sea lions are sparsely distributed across their range which reaches from the Houtman Arbrolhos Islands (28°S, 114°E) in Western Australia along the southern Australian coast to The Pages Islands (35°46’S, 138°18’E) in South Australia. 66 breeding colonies have been identified: 28 in Western Australia and 38 in South Australia.[4]
Most breeding colonies exist on offshore islands, with the exception of Point Labatt in South Australia, Baxter Cliffs (west of Twilight Cove) in Western Australia, and the Bunda Cliffs, Great Australian Bight, which straddles the border between the two states. Forty-two percent of the total known population occurs at the three largest colonies east of Port Lincoln: Seal Bay (on Kangaroo Island's south coast), The Pages and Dangerous Reef (in Spencer Gulf).[4]
The species' breeding range has contracted as the population has fallen. Now extinct breeding colonies existed in Bass Strait, particularly on Clarke Island and adjacent islands in the Furneaux Group. The Abrolhos Island breeding colony is believed to be much smaller today than it was prior to European settlement. Kangaroo Island's north and east coasts and islands near Perth and Albany hosted now-extinct breeding colonies.[4]
Phylogeny

The Australian sea lion is a pinniped, most closely related to other species of sea lions and fur seals making up the family Otariidae.[5] These mammals use their flippers to propel themselves in water and can walk on land with their flippers. Australian sea lions share distinct features with other sea lions. These include short fur, short flippers and a bulky body.[6]
Communication

In pinnipeds, mothers and pups are frequently separated throughout nursing and are thus expected to have evolved an efficient individual recognition system. Consequentially, in Australian sea lions, as in many social mammals, mothers and their offspring can identify each other. Individual recognition produces mutual benefits by avoiding misdirected maternal care and therefore energy expenditure for mothers, and the risk of injury for young approaching unrelated, potentially dangerous, adult females. Individual recognition can be accomplished with a combination of several sensory modalities, including olfaction, vision, and audition. The use of olfactory cues as a close range recognition mechanism allows mothers to further confirm their pup's identity. In contrast to recent olfactory studies in pinnipeds which showed the presence but not a natural function of olfaction in pinnipeds, the present study shows that wild Australian sea lions use their olfactory abilities in a functional manner, by discrimination between the scents of their own offspring and a non-filial pup.[7] However, in a dynamic, crowded colony, the acoustic channel seems to be the most reliable modality. For pinnipeds, neither visual nor olfactory cues are likely to be the primary modality for mother–pup recognition.[8]
Male Australian sea lions were observed producing three different call types: a barking call, a bleating call and a female-like call. The predominant call type produced by males of all ages was the barking call. The barking call of Australian sea lions was similar in structure to the barking calls described in some other species of otariid in that it was a short sound produced repetitively in a series. Mature Australian sea lion males were found to emit the barking call in almost all social interactions, despite the existence of at least three call types in their vocal repertoire, plus a guttural threat and growl.[9]
Diet
Australian sea lions have been described as opportunistic, benthic foragers. Limited stomach content and faecal analyses have identified a wide variety of prey in the diet of the Australian sea lion, including teleost fish, squid, cuttlefish, octopus, sharks (including Port Jackson sharks), Southern rock lobster, other small crustaceans and Little penguins. Regurgitate and stomach samples from Australian sea lions at Seal Bay contained hard parts consisting predominantly of benthic taxa. This supports previous evidence that this species forages primarily on neritic, benthic prey, many of which are non-migratory. For the cephalopod component of the Australian sea lion diet, octopus and giant Australian cuttlefish made up the greatest biomass of prey taxa. Although the Australian sea lion feeds off seasonally available prey such as semelparous cephalopods, it also exploits prey species that are available throughout the year, such as Southern rock lobster and many of the fish species.[10]
Breeding behaviour

As of 2020, 66 breeding colonies have been identified: 28 in Western Australia and 38 in South Australia.[4] The animals breed on at least 50 islands, 27 in Western Australia and 23 in Southern Australia. Prior to a study that took place from 1987 to 1992, thirty-one of the 50 islands were undiscovered, as well as 19 more islands considered additional breeding grounds.[11] On the basis of surveys conducted primarily in 1990, about 70% of the population was in Southern Australia and 30% in Western-Australia.[12] Pup production was estimated at 2,432 for these 50 islands in 1990. In 1994 and 1995 another 10 breeding colonies were recorded on the mainland in the Great Australian Bight region, only producing 161 pups.[12] Thus, reproduction is yielding less and less pups per breeding season. The four largest colonies, on The Pages Islands, at Seal Bay on Kangaroo Island, and at Dangerous Reef, produced 42% of the total pup numbers; they are at the eastern end of the range, east of Port Lincoln.[12]
The breeding cycle of the Australian sea lion is unusual within the pinniped family. It is a 17.6- to 18-month cycle and is 'not' synchronised between colonies. However, census data collected since 1973 shows that breeding events shift forward in time to 13.8 days earlier every 18 months.[13] The duration of the breeding season can range from five to seven months and has been recorded for up to nine months at Seal Bay on Kangaroo Island.
Bulls do not have fixed territories during the breeding season. The males fight other males from a very young age to establish their individual positions in the male hierarchy and during the breeding season, dominant males will guard females for the right to breed with her when she comes into oestrus. A female comes into season for about 24 hours within 7 to 10 days after she has given birth to her new pup. She will only look after the new pup and generally fights off the previous season's pup if it attempts to continue to suckle from her. Male Australian sea lions are also known to kill young as an act of defence of territory.
Australian sea lions also practice alloparental care, in which an adult may adopt the pup or pups of another. This might take place if the original parents die or are for some reason separated from them. This behaviour is common and is seen in many other animal species such as the elephant and fathead minnow.[14]

Population
In 2010, an estimated 14,730 Australian sea lions existed.[15] By 2014, the population had dropped to an estimated 6,500 and continues to decrease. The population is listed as Endangered on the IUCN Red List of Threatened Species.[16] The Australian sea lion population is naturally disadvantaged when compared to other pinnipeds in Australia. Its long and complicated breeding cycle, the high site fidelity of females, and high mortality rates all make the species more vulnerable to extinction.[4]
Threats
Sea lions were heavily hunted following European settlement which greatly reduced their numbers. Large-scale hunting ceased in the 1920s.[17] No baseline population data exists, but the species population and range have both decreased.
Major threatening processes in the 20th and 21st centuries were primarily interactions with commercial fishing gear and illegal shooting. Australian sealions are caught as bycatch by the Southern and Eastern Scalefish and Shark Fishery. Following the closure of the Commonwealth gillnet fishery in southern Spencer Gulf in 2001, pup production increased at Dangerous Reef (within Spencer Gulf) and that population began to recover.[4]
Researchers estimated that while the fishery was operating, 374 Australian sea lions were killed by the fishery each breeding cycle. While most sealion bycatch occurred close to breeding colonies, some occurred as far as 130 kilometres away.[4]
Some Australian sealions have drowned after becoming entangled or trapped in Southern rock lobster pots and gear. Commercial fishers have also illegally shot and killed Australian sealions which they believed were competing with their fishery.[4]
The transition for young mammals from dependence on milk to independent foraging can lead to increased risk of natural mortality. The Australian sea lion demonstrates one of the longest lactation periods in pinnipeds and pups begin diving before they are weaned. Australian sea lion adults work hard to forage at the seabed, demonstrating high field metabolic rates, and spending 58% of time at sea diving and 35% of time at sea on or near the bottom. Juveniles spend 67% of time at sea diving and 44% of time at sea on or near the bottom. Although many air-breathing vertebrates dive well within their estimated limit of oxygen stores, Australian sea lion adults and juveniles appear to operate close to their physiological maximum. The prolonged dependency period could provide extensive opportunities for foraging lessons, while the extreme diving behaviour required in the Australian sea lions' environment might necessitate it. Alternatively, it has hypothesised that female harbour seals accompanying pups demonstrate reduced foraging efficiency, and hence, the metabolic demands of foraging for Australian sea may preclude lactating females from performing suboptimal dives with their young. This becomes a preventative measure to maintain a population and avoid a complete extinction. However, as a result of small population size, small breeding colony size, low reproductive rate, exposure to human activities, and evidence of population declines in some areas, Australian sea lions have recently been listed as threatened and vulnerable.[18]
Conservation status & management
In 1909, field naturalists lobbied for the protection of three critical breeding sites for the Australian sea lion: Dangerous Reef, The Pages and the Casuarina Islets off Kangaroo Island.[19]
In 1918, the South Australian parliament debated the Birds and Animals Protection Bill, and the Legislative Council agreed that it would protect Australian sea lions from harm covering waters of Spencer Gulf, St Vincent Gulf, the Investigator Strait, Backstairs Passage and to the Murray Mouth.[20]
The introduction of the South Australian National Parks and Wildlife Act 1972 prohibited the killing of Australian sea lions statewide.
The Australian sea lion was listed as vulnerable under the Commonwealth Environment Protection and Biodiversity Conservation Act 1999 in 2005 and is also listed as a threatened species in each state in its range (South Australia and Western Australia). On 11 June 2013, the Recovery Plan for the Australian sea lion (Neophoca cinerea) was adopted by the Minister for Sustainability, Environment, Water, Population and Communities. The plan considers the conservation requirements of the species across its range and identifies the actions to be taken to ensure its long-term viability in nature and the parties that will undertake those actions.[21]
Fisheries Management
The Australian Fisheries Management Authority Commission has also finalised the Australian Sea Lion Management Strategy which came into force on 30 June 2010 which includes closures of waters around colonies, seasonal closures, increased observation of sea lion activity and trials of modified fishing techniques and equipment. The strategy was designed to meet the commission's obligations under the Fisheries Management Act 1991 and the Environment Protection and Biodiversity Conservation Act 1999. The strategy will significantly reduce the impact of fishing in the SESSF on Australian sea lions and enable the recovery of the species, including all sub-populations.[22]
Southern rock lobster fisheries in both South Australia and Western Australia now use pots which feature special collars designed to prevent Australian sealions from entering the pots or spikes which act as a deterrent.[4]
Ecology

Australian sea lions defecate nutrient-rich faeces which may provide an important nutrient source for coastal ecosystems. Metagenomic analysis of the bacterial consortia found in the faeces of Australian sea lions found very high levels of nutrient cycling and transport genes which may break down the nutrients defecated by sea lions into a bioavailable form for incorporation into marine food webs.[23]
Diving behaviours indicate that the Australian Sea Lions worked extremely hard to exploit the benefits of their surrounding habitats. The Australian sea lion exceeds the limit (calculated aerobic dive limit) on 79% of dives. Australian sea lions spend 58% of time at sea diving and demonstrate high field metabolism,[24] which allows the sea lions to maximise their time spent at or near the benthos, with 61% of each dive and 35% of their time at sea being spent at the deepest 20% of the dives.[25] When diving, these animals are spending 57.9% of their time at sea spent at depths greater than or equal to 6 m, which can be considered as continuous diving.[25] Seasonal variability in foraging energetics and dive behaviour is likely to be sensitive to regional oceanography, the maintenance costs of female sea lions and their offspring, and the distribution and behaviour of their prey.
The Australian sea lion exhibits strong site fidelity, with a foraging range of at most 300 km from their colony. Australian sea lions sometimes travel inland during tumultuous weather, and have been known to travel up to 9.4 km from the ocean.[26]
See also
- Uncinaria sanguinis, a hookworm parasite of the Australian sea lion.
References
- ^ Goldsworthy, S.; Gales, N. (2008). "Neophoca cinerea". IUCN Red List of Threatened Species. 2008. Retrieved 30 January 2009.
{{cite journal}}: Unknown parameter|last-author-amp=ignored (|name-list-style=suggested) (help) Listed as Endangered (EN A2bd+3d) - ^ a b Gales, NJ; Cheal, AJ; Pobar, GJ; Williamson, P (1992-01-01). "Breeding biology and movements of Australian sea lions, Neophoca cinerea, off the west coasst of Western Australia". Wildlife Research. 19 (4): 405–415. doi:10.1071/wr9920405.
- ^ Churchill, Morgan; Boessenecker, Robert W. (16 June 2016). "Taxonomy and biogeography of the Pleistocene New Zealand sea lion Neophoca palatina (Carnivora: Otariidae)". Journal of Paleontology. 90 (2): 375–388. doi:10.1017/jpa.2016.15.
- ^ a b c d e f g h i Environment, jurisdiction=Commonwealth of Australia; corporateName=Department of the. "Neophoca cinerea — Australian Sea-lion, Australian Sea Lion". www.environment.gov.au. Retrieved 2020-06-22.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Scheffer, Victor B. (1958-01-01). Seals, Sea Lions, and Walruses: A Review of the Pinnipedia. Stanford University Press. ISBN 9780804705448.
- ^ Shaughnessy, Peter (2009-01-17). "Australian sea lions Neophoca cinerea at colonies in South Australia: distribution and abundance, 2004 to 2008" (PDF). Endangered Species Research. 13 (2): 87–98. doi:10.3354/esr00317. Retrieved 2015-08-29.
- ^ Pitcher, Benjamin J.; Harcourt, Robert G.; Schaal, Benoist; Charrier, Isabelle (2011-02-23). "Social olfaction in marine mammals: wild female Australian sea lions can identify their pup's scent". Biology Letters. 7 (1): 60–62. doi:10.1098/rsbl.2010.0569. ISSN 1744-9561. PMC 3030890. PMID 20685695.
- ^ Charrier, Isabelle; Harcourt, Robert G. (2006-10-17). "Individual Vocal Identity in Mother and Pup Australian Sea Lions (Neophoca cinerea)". Journal of Mammalogy. 87 (5): 929–938. doi:10.1644/05-MAMM-A-344R3.1. ISSN 0022-2372.
- ^ Gwilliam, Jessica; Charrier, Isabelle; Harcourt, Robert G. (2008-07-15). "Vocal identity and species recognition in male Australian sea lions, Neophoca cinerea". Journal of Experimental Biology. 211 (14): 2288–2295. doi:10.1242/jeb.013185. ISSN 0022-0949. PMID 18587123.
- ^ McIntosh, Rebecca R.; Page, Brad; Goldsworthy, Simon D. (2006-12-19). "Dietary analysis of regurgitates and stomach samples from free-living Australian sea lions". Wildlife Research. 33 (8): 661. doi:10.1071/WR06025.
- ^ Gales, N. J.; Shaughnessy, P. D.; Dennis, T. E. (1994-11-01). "Distribution, abundance and breeding cycle of the Australian sea lion Neophoca cinerea (Mammalia: Pinnipedia)". Journal of Zoology. 234 (3): 353–370. doi:10.1111/j.1469-7998.1994.tb04853.x. ISSN 1469-7998.
- ^ a b c Shaughnessy, P.D.; Dennis, T.E.; Seager, P.G. (2005-01-01). "Status of Australian sea lions, Neophoca cinerea, and New Zealand fur seals, Arctocephalus forsteri, on Eyre Peninsula and the far west coast of South Australia". Wildlife Research. 32 (1): 85–101. doi:10.1071/wr03068.
- ^ Higgins, Lesley V. (1993-05-21). "The Nonannual, Nonseasonal Breeding Cycle of the Australian Sea Lion, Neophoca cinerea". Journal of Mammalogy. 74 (2): 270–274. doi:10.2307/1382381. ISSN 0022-2372. JSTOR 1382381.
- ^ Riedman, Marianne L. (1982). "The Evolution of Alloparental Care in Mammals and Birds". The Quarterly Review of Biology. 57 (4): 405–435. doi:10.1086/412936.
- ^ "Wildlife as Canon Sees It". National Geographic Magazine. 218 (6). December 2010.
Surviving number: Estimated at 14,730
- ^ Simon Goldsworthy (South Australian Research & Development Institute, Australia) (2014-11-03). "IUCN Red List of Threatened Species: Neophoca cinerea". IUCN Red List of Threatened Species. Retrieved 2020-06-22.
- ^ "Neophoca cinerea — Australian Sea-lion, Australian Sea Lion". Species Profile and Threats Database.
- ^ "Web of Science [v.5.19] - All Databases Full Record". apps.webofknowledge.com. Retrieved 2015-10-30.
- ^ "GENERAL NEWS". Advertiser (Adelaide, SA : 1889 - 1931). 1909-02-05. p. 8. Retrieved 2020-06-22.
- ^ "GENERAL NEWS". Advertiser (Adelaide, SA : 1889 - 1931). 1918-11-20. p. 6. Retrieved 2020-06-22.
- ^ "Recovery Plan for the Australian Sea Lion (Neophoca cinerea)". Commonwealth of Australia, Department of Sustainability, Environment, Water, Population and Communities. 2013. Retrieved 11 December 2014.
- ^ "Australian Sea Lion Management Strategy and SESSF Closure Direction No. 3". Australian Fisheries Management Authority Commission. Archived from the original on 13 February 2014. Retrieved 11 December 2014.
- ^ Lavery TJ, Roudnew B, Seymour J, Mitchell JG, Jeffries T (2012). "High nutrient transport and cycling potential revealed in the microbial metagenome of Australian sea lion (Neophoca cinerea) faeces". PLOS ONE. 7 (5). e36478. doi:10.1371/journal.pone.0036478. PMC 3350522. PMID 22606263.
- ^ Fowler, Shannon L.; Costa, Daniel P.; Arnould, John P. Y.; Gales, Nicholas J.; Kuhn, Carey E. (2006-03-01). "Ontogeny of diving behaviour in the Australian sea lion: trials of adolescence in a late bloomer". Journal of Animal Ecology. 75 (2): 358–367. doi:10.1111/j.1365-2656.2006.01055.x. ISSN 1365-2656. PMID 16637989.
- ^ a b Costa, Daniel P.; Gales, Nicholas J. (2003-02-01). "Energetics of a Benthic Diver: Seasonal Foraging Ecology of the Australian Sea Lion, Neophoca cinerea". Ecological Monographs. 73 (1): 27–43. doi:10.1890/0012-9615(2003)073[0027:eoabds]2.0.co;2. JSTOR 3100073.
- ^ Hoglund, Kara. "Neophoca cinerea Australian sea lion". Animal Diversity Web. Retrieved 2015-08-28.
Further reading
- "Saving Australia's sea lion population" (2019). The University of Sydney. https://sydney.edu.au/news-opinion/news/2019/11/08/saving-australia-sea-lion-population-vet-science.html
- Marcus, Alan David (2015). Hookworm infection in the Australian sea lion (Neophoca cinerea). PhD thesis. The University of Sydney. http://hdl.handle.net/2123/13606.
- Shannon Leone Fowler (2005). Ontogeny of diving in the Australian sea lion. Ph.D. thesis. University of California, Santa Cruz.
- Randall R. Reeves; Brent S. Stewart; Phillip J. Clapham; James A. Powell (2002). National Audubon Society Guide to Marine Mammals of the World. Alfred A. Knopf, Inc. ISBN 0-375-41141-0.

