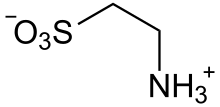
Taurine
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Aminoethane-1-sulfonic acid | |
| Other names
2-Aminoethanesulfonic acid
Tauric acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.168 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C2H7NO3S | |
| Molar mass | 125.14 g/mol |
| Appearance | colorless or white solid |
| Density | 1.734 g/cm3 (at −173.15 °C) |
| Melting point | 305.11 °C (581.20 °F; 578.26 K) |
| Acidity (pKa) | <0, 9.06 |
| Related compounds | |
Related compounds
|
Sulfamic acid Aminomethanesulfonic acid Homotaurine |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Taurine (/ˈtɔːriːn/), or 2-aminoethanesulfonic acid, is an organic compound that is widely distributed in animal tissues.[1] It is a major constituent of bile and can be found in the large intestine, and accounts for up to 0.1% of total human body weight. Taurine is named after the Latin taurus (a cognate of the Greek ταῦρος) which means bull or ox, as it was first isolated from ox bile in 1827 by German scientists Friedrich Tiedemann and Leopold Gmelin.[2] It was discovered in human bile in 1846 by Edmund Ronalds.[3]
Taurine has many biological roles, such as conjugation of bile acids, antioxidation, osmoregulation, membrane stabilization, and modulation of calcium signaling. It is essential for cardiovascular function, and development and function of skeletal muscle, the retina, and the central nervous system.
Taurine is an unusual example of a naturally occurring sulfonic acid.
Chemical and biochemical features
Taurine exists as a zwitterion H3N+CH2CH2SO3−, as verified by X-ray crystallography.[4] The sulfonic acid has a low pKa[5] ensuring that it is fully ionized to the sulfonate at the pH's found in the intestinal tract.
Synthesis
Synthetic taurine is obtained by the ammonolysis of isethionic acid (2-hydroxyethanesulfonic acid), which in turn is obtained from the reaction of ethylene oxide with aqueous sodium bisulfite. A direct approach involves the reaction of aziridine with sulfurous acid.[6]
In 1993, about 5,000–6,000 tons of taurine were produced for commercial purposes: 50% for pet food and 50% in pharmaceutical applications.[7] As of 2010, China alone has more than 40 manufacturers of taurine. Most of these enterprises employ the ethanolamine method to produce a total annual production of about 3,000 tons.[8]
In the laboratory taurine can be produced by alkylation of ammonia with bromoethanesulfonate salts.[9]
Biosynthesis
Taurine is naturally derived from cysteine. Mammalian taurine synthesis occurs in the pancreas via the cysteine sulfinic acid pathway. In this pathway, cysteine is first oxidized to its sulfinic acid, catalyzed by the enzyme cysteine dioxygenase. Cysteine sulfinic acid, in turn, is decarboxylated by sulfinoalanine decarboxylase to form hypotaurine. Hypotaurine is enzymatically oxidized to yield taurine by hypotaurine dehydrogenase.[10]
Taurine is also produced by the transsulfuration pathway, which converts homocysteine into cystathionine. The cystathionine is then converted to hypotaurine by the sequential action of three enzymes: cystathionine gamma-lyase, cysteine dioxygenase, and cysteine sulfinic acid decarboxylase. Hypotaurine is then oxidized to taurine as described above.[11]

Oxidative degradation of cysteine to taurine
Nutritional significance
Taurine occurs naturally in fish and meat.[12][13][14] The mean daily intake from omnivore diets was determined to be around 58 mg (range from 9 to 372 mg) and to be low or negligible from a strict vegan diet. In another study, taurine intake was estimated to be generally less than 200 mg/day, even in individuals eating a high-meat diet. According to a third study, taurine consumption was estimated to vary between 40 and 400 mg/day.[15]
The availability of taurine is affected depending on how the food is prepared, raw diets retaining the most taurine, and baking or boiling resulting in the greatest taurine loss.[16]
Taurine levels were found to be significantly lower in vegans than in a control group on a standard American diet. Plasma taurine was 78% of control values, and urinary taurine was 29%.[17]
Prematurely born infants are believed to lack the enzymes needed to convert cystathionine to cysteine, and may, therefore, become deficient in taurine. Taurine is present in breast milk, and has been added to many infant formulas, as a measure of prudence, since the early 1980s. However, this practice has never been rigorously studied, and as such it has yet to be proven to be necessary, or even beneficial.[18]
Energy drinks
Taurine is an ingredient in energy drinks. Many contain 1000 mg per serving,[19] and some as much as 2000 mg.[20] The addition of taurine is used as a nervous system depressant.[21][unreliable medical source?]
Physiological functions
Taurine is essential for cardiovascular function and development and function of skeletal muscle, the retina, and the central nervous system.[22] It is a biosynthetic precursor to the bile salts sodium taurochenodeoxycholate and sodium taurocholate.
Taurine functions as an antioxidant, suppressing the toxicity of hypochlorite and hypobromite produced physiologically. Taurine reacts with these halogenating agents to form N-chloro- and N-bromotaurine, which are less toxic than their precursors hypohalides.[23]
Role in nutrition and cardiovascular health
Taurine has been shown to reduce the secretion of apolipoprotein B100 and lipids in HepG2 cells.[24] High concentrations of serum lipids and apolipoprotein B100 (essential structural component of VLDL and LDL) are major risk factors of atherosclerosis and coronary heart disease. Hence, taurine supplementation is possibly beneficial for the prevention of these diseases.
Role in the muscular system
Taurine is necessary for normal skeletal muscle functioning.[25] Mice with a genetic taurine deficiency had a nearly complete depletion of skeletal and cardiac muscle taurine levels and a reduction of more than 80% of exercise capacity compared to control mice. Taurine can influence (and possibly reverse) defects in nerve blood flow, motor nerve conduction velocity, and nerve sensory thresholds in experimental diabetic neuropathic rats.[26][27]
Pharmacology
Taurine crosses the blood–brain barrier[28][29][30] and has been implicated in a wide array of physiological phenomena including inhibitory neurotransmission,[31] long-term potentiation in the striatum/hippocampus,[32] membrane stabilization,[33][unreliable medical source?] feedback inhibition of neutrophil/macrophage respiratory burst, adipose tissue regulation and possible prevention of obesity,[34][35] calcium homeostasis,[36] recovery from osmotic shock,[37] protection against glutamate excitotoxicity,[38] and prevention of epileptic seizures.[39]
According to the single study on human subjects, daily administration of 1.5 g of taurine had no significant effect on insulin secretion or insulin sensitivity.[40] There is evidence that taurine may exert a beneficial effect in preventing diabetes-associated microangiopathy and tubulointerstitial injury in diabetic nephropathy.[41][42]
According to animal studies, taurine produces an anxiolytic effect and may act as a modulator or antianxiety agent in the central nervous system by activating the glycine receptor.[43][44][45]
Taurine acts as a glycation inhibitor. Taurine-treated diabetic rats had a decrease in the formation of advanced glycation end products (AGEs) and AGEs content.[46][47] The United States Department of Agriculture has found a link between cataract development and lower levels of vitamin B6, folate, and taurine in the diets of the elderly.[48]
Animal physiology and nutrition
In diabetic rats, taurine supplementation slighly reduced abdominal body fat while improving glucose tolerance.[49] Taurine is effective in removing fatty liver deposits in rats, preventing liver disease, and reducing cirrhosis in tested animals.[50][51] Evidence indicates taurine may be beneficial for blood pressure in male rats. A single intravenous taurine supplementation resulted in measurable decreases in blood pressure. However, when rats were supplemented with taurine in their drinking water, only female rats showed an increase in blood pressure. Both genders showed significant tachycardia.[52]
Likewise, taurine administration to diabetic rabbits resulted in 30% decrease in serum glucose levels.[53]
Cats lack the enzymatic machinery to produce taurine and must therefore acquire it from their diet.[54] A taurine deficiency in cats can lead to retinal degeneration and eventually blindness. Other effects of a diet lacking in this essential amino acid are dilated cardiomyopathy and reproductive failure in females.[55] The absence of taurine causes a cat's retina to slowly degenerate, causing eye problems and (eventually) irreversible blindness – a condition known as central retinal degeneration (CRD),[56][57] as well as hair loss and tooth decay. Decreased plasma taurine concentration has been demonstrated to be associated with feline dilated cardiomyopathy.[58] Unlike CRD, the condition is reversible with supplementation. Taurine is now a requirement of the Association of American Feed Control Officials (AAFCO) and any dry or wet food product labeled approved by the AAFCO should have a minimum of 0.1% taurine in dry food and 0.2% in wet food.[59] Studies suggest the amino acid should be supplied at 10 mg/kg of bodyweight/day for domestic cats.[60]
Taurine appears essential to the development of passerine birds. Many passerines seek out taurine-rich spiders to feed their young, particularly just after hatching. Researchers compared the behaviours and development of birds fed a taurine-supplemented diet to a control diet and found the juveniles fed taurine-rich diets as neonates were much larger risk takers and more adept at spatial learning tasks.[61]
Taurine has been used in some cryopreservation mixes for animal artificial insemination.[62]
Safety and toxicity
A substantial increase in the plasma concentration of growth hormone was reported in some epileptic patients during taurine tolerance testing (oral dose of 50 mg per kg body mass per day), suggesting a potential to stimulate the hypothalamus and to modify neuroendocrine function.[63] A 1966 study found an indication that taurine (2 g/day) has some function in the maintenance and possibly in the induction of psoriasis.[15] Three later studies failed to support that finding.[64][65][66] It may also be necessary to take into consideration that absorption of taurine from beverages may be more rapid than from foods.[15]
Taurine has an observed safe level of supplemental intake in normal healthy adults at up to 3 g/day.[67] Even so, a study by the European Food Safety Authority found no adverse effects for up to 1,000 mg of taurine per kilogram of body weight per day.[68]
A review published in 2008 found no documented reports of negative or positive health effects associated with the amount of taurine used in energy drinks, concluding, "The amounts of guarana, taurine, and ginseng found in popular energy drinks are far below the amounts expected to deliver either therapeutic benefits or adverse events".[69]
Other uses
In cosmetics and contact lens solutions
Since the 2000s cosmetic compositions containing taurine have been introduced, possibly due to its antifibrotic properties. It has been shown to prevent the damaging effects of TGFB1 to hair follicles.[70] It also helps to maintain skin hydration.[71]
Taurine is also used in some contact lens solutions.[72]
- Taurine is used in the preparation of the anthelmintic drug netobimin (Totabin).
- Taurolidine
- Taurocholic acid and tauroselcholic acid
- Tauromustine
- 5-Taurinomethyluridine and 5-taurinomethyl-2-thiouridine are modified uridines in (human) mitrochondrial tRNA.[73]
- Tauryl is the functional group attaching at the sulfur, 2-aminoethylsulfonyl.[74]
- Taurino is the functional group attaching at the nitrogen, 2-sulfoethylamino.
See also
- Homotaurine (tramiprosate), precursor to acamprosate
- Taurates, a substance group.
References
- ^ Schuller-Levis, Georgia B.; Park, Eunkyue (2003). "Taurine: new implications for an old amino acid". FEMS Microbiology Letters. 226 (2): 195–202. doi:10.1016/S0378-1097(03)00611-6. PMID 14553911.
- ^ F. Tiedemann, L. Gmelin; Gmelin (1827). "Einige neue Bestandtheile der Galle des Ochsen". Annalen der Physik. 85 (2): 326–37. Bibcode:1827AnP....85..326T. doi:10.1002/andp.18270850214.
- ^ Ronalds, B.F. (2019). "Bringing Together Academic and Industrial Chemistry: Edmund Ronalds' Contribution". Substantia. 3 (1): 139–152.
- ^ Görbitz, Carl Henrik; Prydz, Kristian; Ugland, Sigurd (2000). "Taurine". Acta Crystallographica Section C. 56: e23–e24. doi:10.1107/S0108270199016029.
- ^ Irving CS, Hammer BE, Danyluk SS, Klein PD (1980). "13C Nuclear Magnetic Resonance Study of the Complexation of Calcium by Taurine". Journal of Inorganic Biochemistry. 13 (2): 137–50. doi:10.1016/S0162-0134(00)80117-8. PMID 7431022.
- ^ Kosswig, K. (2000). "Sulfonic Acids, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a25_503. ISBN 978-3527306732.
- ^ Tully PS, ed. (2000). "Sulfonic Acids". Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc. doi:10.1002/0471238961.1921120620211212.a01. ISBN 978-0471238966.
- ^ Amanda Xia (2010-01-03). "China Taurine Market Is Expected To Recover". Press release and article directory: technology. Archived from the original on 2018-09-20. Retrieved 2010-05-24.
- ^ Marvel, C. S.; Bailey, C. F.; Cortese, Frank (1938). "Taurine". Org. Synth. 18: 77. doi:10.15227/orgsyn.018.0077.
- ^ Sumizu K (1962). "Oxidation of hypotaurine in rat liver". Biochim. Biophys. Acta. 63: 210–212. doi:10.1016/0006-3002(62)90357-8. PMID 13979247.
- ^ Harris Ripps, Wen Shen (2012). "Review: Taurine: A "very essential" amino acid". Molecular Vision. 18: 2673–2686. PMC 3501277. PMID 23170060.
- ^ Bouckenooghe T, Remacle C, Reusens B (2006). "Is taurine a functional nutrient?". Current Opinion in Clinical Nutrition and Metabolic Care. 9 (6): 728–33. doi:10.1097/01.mco.0000247469.26414.55. PMID 17053427.
- ^ Brosnan J, Brosnan M (2006). "The sulfur-containing amino acids: an overview". J Nutr. 136 (6 Suppl): 1636S–40S. doi:10.1093/jn/136.6.1636S. PMID 16702333.
- ^ Huxtable RJ (1992). "Physiological actions of taurine". Physiol Rev. 72 (1): 101–163. doi:10.1152/physrev.1992.72.1.101. PMID 1731369.
- ^ a b c "Opinion on Caffeine, Taurine and D-Glucurono — g -Lactone as constituents of so-called 'energy' drinks". Directorate-General Health and Consumers, European Commission, European Union. 1999-01-21. Archived from the original on 2006-06-23.
- ^ Jacobson, Samuel G.; Kemp, Colin M.; Borruat, François-Xavier; Chaitin, Michael H.; Faulkner, David J. (1987-10-01). "Rhodopsin topography and rod-mediated function in cats with the retinal degeneration of taurine deficiency". Experimental Eye Research. 45 (4): 481–490. doi:10.1016/S0014-4835(87)80059-3. PMID 3428381.
- ^ Laidlaw S, Shultz T, Cecchino J, Kopple J (1988). "Plasma and urine taurine levels in vegans". American Journal of Clinical Nutrition. 47 (4): 660–3. doi:10.1093/ajcn/47.4.660. PMID 3354491.
- ^ Heird, W.C. (2004). "Taurine in neonatal nutrition—revisited". Archives of Disease in Childhood: Fetal Neonatal. 89 (6): F473–F474. doi:10.1136/adc.2004.055095. PMC 1721777. PMID 15499132.
- ^ rockstar69.com Original Rockstar Ingredients
- ^ Chang, PL (2008-05-03). "Nos Energy Drink – Review". energyfanatics.com. Archived from the original on 2008-06-17. Retrieved 2010-05-21.
- ^ "Why Is Taurine in Energy Drinks and Pre Workouts? - 4 Gauge". 4 Gauge. 2017-11-16. Retrieved 2018-03-01.
- ^ Huxtable, RJ (1992). "Physiological Actions of Taurine". Physiol Rev. 72 (1): 101–63. doi:10.1152/physrev.1992.72.1.101. PMID 1731369.
- ^ Marcinkiewicz, Janusz; Kontny, Ewa (2014). "Taurine and inflammatory diseases". Amino Acids. 46 (1): 7–20. doi:10.1007/s00726-012-1361-4. PMC 3894431. PMID 22810731.
- ^ Yanagita, T; Han, SY; Hu, Y; Nagao, K; Kitajima, H; Murakami, S (2008). "Taurine reduces the secretion of apolipoprotein B100 and lipids in HepG2 cells". Lipids in Health and Disease. 7: 38. doi:10.1186/1476-511x-7-38. PMC 2579289. PMID 18925970.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Warskulat U, Flögel U, Jacoby C, Hartwig HG, Thewissen M, Merx MW, Molojavyi A, Heller-Stilb B, Schrader J, Häussinger D (2004). "Taurine transporter knockout depletes muscle taurine levels and results in severe skeletal muscle impairment but leaves cardiac function uncompromised" (PDF). The FASEB Journal. 18 (3): 03–0496fje. doi:10.1096/fj.03-0496fje. PMID 14734644.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Li F, Abatan OI, Kim H, Burnett D, Larkin D, Obrosova IG, Stevens MJ (2006). "Taurine reverses neurological and neurovascular deficits in Zucker diabetic fatty rats". Neurobiology of Disease. 22 (3): 669–76. doi:10.1016/j.nbd.2006.01.012. PMID 16624563.
- ^ Pop-Busui R, Sullivan KA, Van Huysen C, Bayer L, Cao X, Towns R, Stevens MJ (2001). "Depletion of taurine in experimental diabetic neuropathy: implications for nerve metabolic, vascular, and functional deficits". Exp Neurol. 168 (2): 259–72. doi:10.1006/exnr.2000.7591. PMID 11259114.
- ^ Urquhart, N; Perry, TL; Hansen, S; Kennedy, J (1974). "Passage of taurine into adult mammalian brain". Journal of Neurochemistry. 22 (5): 871–2. doi:10.1111/j.1471-4159.1974.tb04309.x. PMID 4407108.
- ^ Tsuji, A; Tamai, I (1996). Sodium- and chloride-dependent transport of taurine at the blood–brain barrier. Advances in Experimental Medicine and Biology. Vol. 403. pp. 385–91. doi:10.1007/978-1-4899-0182-8_41. ISBN 978-1-4899-0184-2. PMID 8915375.
- ^ Salimäki, J; Scriba, G; Piepponen, TP; Rautolahti, N; Ahtee, L (2003). "The effects of systemically administered taurine and N-pivaloyltaurine on striatal extracellular dopamine and taurine in freely moving rats". Naunyn-Schmiedeberg's Archives of Pharmacology. 368 (2): 134–41. doi:10.1007/s00210-003-0776-6. PMID 12898127.
- ^ Olive, MF (2002). "Interactions between taurine and ethanol in the central nervous system". Amino Acids. 23 (4): 345–57. doi:10.1007/s00726-002-0203-1. PMID 12436202.
- ^ Dominy, J Jr; Thinschmidt, JS; Peris, J; Dawson, R Jr; Papke, RL (2004). "Taurine-induced long-lasting potentiation in the rat hippocampus shows a partial dissociation from total hippocampal taurine content and independence from activation of known taurine transporters". Journal of Neurochemistry. 89 (5): 1195–205. doi:10.1111/j.1471-4159.2004.02410.x. PMID 15147512.
- ^ Birdsall, TC (1998). "Therapeutic applications of taurine". Alternative Medicine Review. 3 (2): 128–36. PMID 9577248.
- ^ Ide T, Kushiro M, Takahashi Y, Shinohara K, Cha S. "mRNA expression of enzymes involved in taurine biosynthesis in rat adipose tissues. Metabolism: Clinical and Experimental 2002 Sep;51(9):1191-7.
- ^ Tsuboyama-Kasaoka, N; Shozawa, C; Sano, K; Kamei, Y; Kasaoka, S; Hosokawa, Y; Ezaki, O (2006). "Taurine (2-aminoethanesulfonic acid) deficiency creates a vicious circle promoting obesity". Endocrinology. 147 (7): 3276–84. doi:10.1210/en.2005-1007. PMID 16627576.
- ^ Foos, TM; Wu, JY (2002). "The role of taurine in the central nervous system and the modulation of intracellular calcium homeostasis". Neurochemical Research. 27 (1–2): 21–6. doi:10.1023/A:1014890219513. PMID 11926272.
- ^ Stummer W, Betz AL, Shakui P, Keep RF (1995). "Blood–brain barrier taurine transport during osmotic stress and in focal cerebral ischemia". Journal of Cerebral Blood Flow and Metabolism. 15 (5): 852–9. doi:10.1038/jcbfm.1995.106. PMID 7673378.
- ^ Leon R, Wu H, Jin Y, Wei J, Buddhala C, Prentice H, Wu JY (2008). "Protective function of taurine in glutamate-induced apoptosis in cultured neurons". Journal of Neuroscience Research. 87 (5): 1185–1194. doi:10.1002/jnr.21926. PMID 18951478.
- ^ El Idrissi A, Messing J, Scalia J, Trenkner E (2003). "Prevention of epileptic seizures by taurine". Taurine 5. Advances in Experimental Medicine and Biology. Vol. 526. pp. 515–25. doi:10.1007/978-1-4615-0077-3_62. ISBN 978-1-4613-4913-6. PMID 12908638.
- ^ C. Brøns, C. Spohr, H. Storgaard, J. Dyerberg, A. Vaag. "Effect of taurine treatment on insulin secretion and action, and on serum lipid levels in overweight men with a genetic predisposition for type II diabetes mellitus. European Journal of Clinical Nutrition (2004) 58, 1239–1247.
- ^ Wu QD, Wang JH, Fennessy F, Redmond HP, Bouchier-Hayes D (1999). "Taurine prevents high-glucose-induced human vascular endothelial cell apoptosis". The American Journal of Physiology. 277 (6 Pt 1): C1229–38. doi:10.1152/ajpcell.1999.277.6.C1229. PMID 10600775.
- ^ Verzola, D; Bertolotto, MB; Villaggio, B; Ottonello, L; Dallegri, F; Frumento, G; Berruti, V; Gandolfo, MT; Garibotto, G; et al. (2002). "Taurine prevents apoptosis induced by high ambient glucose in human tubule renal cells". Journal of Investigative Medicine. 50 (6): 443–51. doi:10.2310/6650.2002.32504.
- ^ Kong WX, Chen SW, Li YL, et al. (2006). "Effects of taurine on rat behaviors in three anxiety models". Pharmacol. Biochem. Behav. 83 (2): 271–6. doi:10.1016/j.pbb.2006.02.007. PMID 16540157.
- ^ Zhang, CG; Kim, SJ (2007). "Taurine induces anti-anxiety by activating strychnine-sensitive glycine receptor in vivo". Annals of Nutrition & Metabolism. 51 (4): 379–86. doi:10.1159/000107687. PMID 17728537.
- ^ Chen, SW; Kong, WX; Zhang, YJ; Li, YL; Mi, XJ; Mu, XS (2004). "Possible anxiolytic effects of taurine in the mouse elevated plus-maze". Life Sciences. 75 (12): 1503–11. doi:10.1016/j.lfs.2004.03.010. PMID 15240184.
- ^ Effects of taurine on advanced glycosylation end products and expression of TGF-β in renal cortex of, TsingHua, 2005, http://www.shvoong.com/medicine-and-health/1599878-effects-taurine-advanced-glycosylation-end/
- ^ Huang JS, Chuang LY, Guh JY, Yang YL, Hsu MS (2008). "Effect of taurine on advanced glycation end products-induced hypertrophy in renal tubular epithelial cells". Toxicology and Applied Pharmacology. 233 (2): 220–6. doi:10.1016/j.taap.2008.09.002. PMID 18834896.
- ^ "ARS: 50 Years of Research for the Growing World". Ars.usda.gov. Retrieved 2012-10-27.
- ^ Nakaya Y, Minami A, Harada N, Sakamoto S, Niwa Y, Ohnaka M (2000). "Taurine improves insulin sensitivity in the Otsuka Long-Evans Tokushima Fatty rat, a model of spontaneous type 2 diabetes". American Journal of Clinical Nutrition. 71 (1): 54–8. doi:10.1093/ajcn/71.1.54. PMID 10617946.
- ^ Kerai, MDJ; Catherine J. Waterfield; S. H. Kenyon; D. S. Asker; J. A. Timbrell (1998). "Taurine: Protective properties against ethanol-induced hepatic steatosis and lipid peroxidation during chronic ethanol consumption in rats". Amino Acids. 15 (1–2): 53–76. doi:10.1007/BF01345280. PMID 9871487.
- ^ McCall, B (2005-12-28). "The ultimate hangover cure?". bbc.co.uk. Retrieved 2008-09-01.
- ^ El Idrissi A, Okeke E, Yan X, Sidime F, Neuwirth LS (2013). Taurine regulation of blood pressure and vasoactivity. Advances in Experimental Medicine and Biology. Vol. 775. pp. 407–25. doi:10.1007/978-1-4614-6130-2_31. ISBN 978-1-4614-6129-6. PMID 23392950.
{{cite book}}:|journal=ignored (help) - ^ Winiarska K, Szymanski K, Gorniak P, Dudziak M, Bryla J (2008). "Hypoglycaemic, antioxidative and nephroprotective effects of taurine in alloxan diabetic rabbits". Biochimie. 91 (2): 261–270. doi:10.1016/j.biochi.2008.09.006. PMID 18957317.
- ^ KNOPF, Karen (2011). "Taurine: An Essential Nutrient for the Cat". The Journal of Nutrition. 108 (5): 773–778. doi:10.1093/jn/108.5.773. PMID 641594 – via Primo.
- ^ Hayes KC, Carey RE, Schmidt SY (1975). "Retinal degeneration associated with taurine deficiency in the cat". Science. 188 (4191): 949–51. Bibcode:1975Sci...188..949H. doi:10.1126/science.1138364. PMID 1138364.
- ^ "Taurine And Its Importance In Cat Foods". Iams Cat Nutrition Library. 2004. Archived from the original on 2006-10-19. Retrieved 2006-08-22.
- ^ "Nutrient Requirements of Cats". Nutrient Requirements of Cats, Revised Edition, 1986. 1986. Archived from the original on 2006-09-01. Retrieved 2006-09-10.
- ^ PD Pion (1987). "Myocardial failure in cats associated with low plasma taurine: a reversible cardiomyopathy". Science. 237 (4816): 764–768. Bibcode:1987Sci...237..764P. doi:10.1126/science.3616607. PMID 3616607.
- ^ "AAFCO CAT FOOD NUTRIENT PROFILES". Archived from the original on 2015-05-29. Retrieved 30 May 2015.
- ^ Burger, I. H.; Barnett, K. C. (1982). "The taurine requirement of the adult cat". Journal of Small Animal Practice. 23 (9): 533–537. doi:10.1111/j.1748-5827.1982.tb02514.x.
- ^ Arnold, KE; Ramsay, S.L.; Donaldson, C.; Adam, A (2007). "Parental prey selection affects risk-taking behaviour and spatial learning in avian offspring" (PDF). Proceedings of the Royal Society B. 274 (1625): 2563–9. doi:10.1098/rspb.2007.0687. PMC 2275882. PMID 17698490.[permanent dead link]
- ^ Shiva Shankar Reddy, N.; Jagan Mohanarao, G.; Atreja, S.K. (2010). "Effects of adding taurine and trehalose to a tris-based egg yolk extender on buffalo (Bubalus bubalis) sperm quality following cryopreservation". Animal Reproduction Science. 119 (3–4): 183–190. doi:10.1016/j.anireprosci.2010.01.012. PMID 20197223.
- ^ John Mantovani, MD; Darryl C. DeVivo, MD (November 1979). "Effects of Taurine on Seizures and Growth Hormone Release in Epileptic Patients". Archives of Neurology. 36 (11): 672–674. doi:10.1001/archneur.1979.00500470042006. PMID 508122.
- ^ Zackheim, Herschel S; Farber, Eugene M (1968). "Taurine and Psoriasis". The Journal of Investigative Dermatology. 50 (3): 227–230. doi:10.1038/jid.1968.32. PMID 5644896. Retrieved 2013-11-23.
- ^ Zackheim, Herschel S. (1982). "Taurine and Diet in Psoriasis". Archives of Dermatology. 118 (12): 961. doi:10.1001/archderm.1982.01650240005005.
- ^ Zackheim, Herschel S.; Farber, Eugene M. (1969). "Low-Protein Diet and Psoriasis A Hospital Study". Archives of Dermatology. 99 (5): 580–586. doi:10.1001/archderm.1969.01610230072012. PMID 5780964.
- ^ Shao A, Hathcock JN (2008). "Risk assessment for the amino acids taurine, L-glutamine and L-arginine". Regulatory Toxicology and Pharmacology. 50 (3): 376–99. doi:10.1016/j.yrtph.2008.01.004. PMID 18325648.
the newer method described as the Observed Safe Level (OSL) or Highest Observed Intake (HOI) was utilized. The OSL risk assessments indicate that based on the available published human clinical trial data, the evidence for the absence of adverse effects is strong for Tau at supplemental intakes up to 3 g/d, Gln at intakes up to 14 g/d and Arg at intakes up to 20 g/d, and these levels are identified as the respective OSLs for normal healthy adults.
- ^ "EFSA adopts opinion on two ingredients commonly used in some energy drinks". 12 February 2009. efsa.europa.eu.
- ^ Clauson, KA; Shields, KM; McQueen, CE; Persad, N (2008). "Safety issues associated with commercially available energy drinks". Journal of the American Pharmacists Association. 48 (3): e55–63, quiz e64–7. doi:10.1331/JAPhA.2008.07055. PMID 18595815.
- ^ Collin, C; Gautier, B; Gaillard, O; Hallegot, P; Chabane, S; Bastien, P; Peyron, M; Bouleau, M; et al. (2006). "Protective effects of taurine on human hair follicle grown in vitro". International Journal of Cosmetic Science. 28 (4): 289–98. doi:10.1111/j.1467-2494.2006.00334.x. ISSN 0142-5463. PMID 18489269.
We showed that taurine [...] prevented TGF-β1-induced deleterious effects on hair follicle.
- ^ Janeke G, Siefken W, Carstensen S, Springmann G, Bleck O, Steinhart H, Höger P, Wittern KP, Wenck H, Stäb F, Sauermann G, Schreiner V, Doering T (2003). "Role of taurine accumulation in keratinocyte hydration". The Journal of Investigative Dermatology. 121 (2): 354–61. doi:10.1046/j.1523-1747.2003.12366.x. PMID 12880428.
- ^ James, TJ; Hansen D; Nolfi, J (2004-04-01). "Ocular Health and Next Generation Solutions". Optometric Management. Archived from the original on 2005-04-19. Retrieved 2008-01-10.
- ^ Suzuki, T.; Suzuki, T.; Wada, T.; Saigo, K.; Watanabe, K. (2002). "Taurine as a constituent of mitochondrial tRNAs: new insights into the functions of taurine and human mitochondrial diseases". EMBO J. 21 (23): 6581–6589. doi:10.1093/emboj/cdf656. PMC 136959. PMID 12456664.
- ^ Bünzli-Trepp, Ursula (2007). Systematic nomenclature of organic, organometallic and coordination chemistry. EPFL Press. p. 226. ISBN 9781420046151.
