Aphthous stomatitis
| Aphthous stomatitis | |
|---|---|
| Other names | recurrent aphthous stomatitis, recurring oral aphthae, recurrent aphthous ulceration |
| Specialty | Oral medicine, dermatology |
Aphthous stomatitis is a common condition characterized by the repeated formation of benign and non-contagious mouth ulcers (aphthae) in otherwise healthy individuals. The informal term canker sores is also used, mainly in North America, although this may also refer to any mouth ulcer.
The cause is not completely understood, but involves a T cell-mediated immune response triggered by a variety of factors. Different individuals have different triggers, which may include nutritional deficiencies, local trauma, stress, hormonal influences, allergies, genetic predisposition or other factors.
These ulcers occur periodically and heal completely between attacks. In the majority of cases, the individual ulcers last about 7–10 days, and ulceration episodes occur 3–6 times per year. Most appear on the non-keratinizing epithelial surfaces in the mouth (i.e. anywhere except the attached gingiva, the hard palate and the dorsum of the tongue), although the more severe forms, which are less common, may also involve keratinizing epithelial surfaces. Symptoms range from a minor nuisance to interfering with eating and drinking. The severe forms may be debilitating, even causing weight loss due to malnutrition.
The condition is very common, affecting about 20% of the general population to some degree. The onset is often during childhood or adolescence, and the condition usually lasts for several years before gradually disappearing. There is no cure, and treatments aim to manage pain, reduce healing time and reduce the frequency of episodes of ulceration. The term is from Greek: αφθα, translit. aphtha meaning "mouth ulcer".
Signs and symptoms
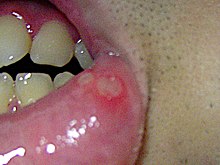
Persons with aphthous stomatitis have no detectable systemic symptoms or signs (i.e., outside the mouth).[1] Generally, symptoms may include prodromal sensations such as burning, itching, or stinging, which may precede the appearance of any lesion by some hours; and pain, which is often out of proportion to the extent of the ulceration and is worsened by physical contact, especially with certain foods and drinks (e.g., acidic). Pain is worst in the days immediately following the initial formation of the ulcer, and then recedes as healing progresses.[2] If there are lesions on the tongue, speaking and chewing can be uncomfortable, and ulcers on the soft palate, oropharynx, or esophagus can cause odynophagia (painful swallowing).[2] Signs are limited to the lesions themselves.
Ulceration episodes usually occur about 3–6 times per year.[3] However, severe disease is characterized by virtually constant ulceration (new lesions developing before old ones have healed) and may cause debilitating chronic pain and interfere with comfortable eating. In severe cases, this prevents adequate nutrient intake leading to malnutrition and weight loss.[2]
Aphthous ulcers typically begin as erythematous macules (reddened, flat area of mucosa) which develop into ulcers that are covered with a yellow-grey fibrinous membrane that can be scraped away. An erythematous "halo" surrounds the ulcer.[4] The size, number, location, healing time, and periodicity between episodes of ulcer formation are all dependent upon the subtype of aphthous stomatitis.
Causes
The cause is not entirely clear,[1] but is thought to be multifactorial.[5] It has even been suggested that aphthous stomatitis is not a single entity but rather a group of conditions with different causes.[1] Multiple research studies have attempted to identify a causative organism, but aphthous stomatitis appears to be non-contagious, non-infectious and not sexually transmissible.[1] The mucosal destruction is thought to be the result of a T cell (T lymphocyte) mediated immune response which involves the generation of interleukins and tumor necrosis factor alpha (TNF-α).[5] Mast cells and macrophages are also involved, secreting TNF-α along with the T cells. When early aphthous ulcers are biopsied, the histologic appearance shows a dense inflammatory infiltrate, 80% of which is made up of T cells.[4] Persons with aphthous stomatitis also have circulating lymphocytes which react with peptides 91–105 of heat shock protein 65-60,[1] and the ratio of CD4+ T cells to CD8+ T cells in the peripheral blood of individuals with aphthous stomatitis is decreased.[4]
Despite this preferred theory of immuno-dysregulation held by most researchers,[6] aphthous stomatitis behaves dissimilarly to autoimmune diseases in many regards. There is no association between aphthous stomatitis and other autoimmune diseases, which often accompany each other; common autoantibodies are not detected, the condition tends to resolve spontaneously with advancing age rather than worsen, and usually serum immunoglobulins are at normal levels.[1]
Evidence for the T cell-mediated mechanism of mucosal destruction is strong, but the exact triggers for this process are unknown and are thought to be multiple and varied from one person to the next. This suggests that there are a number of possible triggers, each of which is capable of producing the disease in different subgroups. In other words, different subgroups appear to have different causes for the condition. These can be considered in three general groups, namely primary immuno-dysregulation, decrease of the mucosal barrier and states of heightened antigenic sensitivity (see below).[4][6] Etiologic factors in aphthous stomatitis are also sometimes considered as either host-related or environmental.[7]
Immunity
At least 40% of people with aphthous stomatitis have a positive family history, suggesting that some people are genetically predisposed to suffering with oral ulceration.[5] HLA-B12, HLA-B51, HLA-Cw7, HLA-A2, HLA-A11, and HLA-DR2 are examples of human leukocyte antigen types associated with aphthous stomatitis.[1][4] However, these HLA types are inconsistently associated with the condition, and also vary according to ethnicity.[8] People who have a positive family history of aphthous stomatitis tend to develop a more severe form of the condition, and at an earlier age than is typical.[8]
Stress has effects on the immune system,[6] which may explain why some cases directly correlate with stress. It is often stated that ulceration is exacerbated during examination periods and lessened during periods of vacation.[1][4] Alternatively, it has been suggested that oral parafunctional activities such as lip or cheek chewing become more pronounced during periods of stress, and hence the mucosa is subjected to more minor trauma.[8]
Aphthous-like ulceration also occurs in conditions involving systemic immuno-dysregulation, e.g. cyclic neutropenia and human immunodeficiency virus infection. In cyclic neutropenia, more severe oral ulceration occurs during periods of severe immuno-dysregulation, and resolution of the underlying neutropenia prevents the cycle of ulceration. The relative increase in percentage of CD8+ T cells, caused by a reduction in numbers of CD4+ T cells may be implicated in RAS-type ulceration in HIV infection.[4]
Mucosal barrier
The thickness of the mucosa may be an important factor in aphthous stomatitis. Usually, ulcers form on the thinner, non-keratinizing mucosal surfaces in the mouth. Factors which decrease the thickness of mucosa increase the frequency of occurrence, and factors which increase the thickness of the mucosa correlate with decreased ulceration.[4]
The nutritional deficiencies associated with aphthous stomatitis (B12, folate, and iron) can all cause a decrease in the thickness of the oral mucosa (atrophy).[4]
Local trauma is also associated with aphthous stomatitis, and it is known that trauma can decrease the mucosal barrier. Trauma could occur during injections of local anesthetic in the mouth, or otherwise during dental treatments, frictional trauma from a sharp surface in the mouth such as broken tooth, or from tooth brushing.[8]
Hormonal factors are capable of altering the mucosal barrier. In one study, a small group of females with apthous stomatitis had fewer occurrences of aphthous ulcers during the luteal phase of the menstrual cycle or with use of the contraceptive pill.[1][4] This phase is associated with a fall in progestogen levels, mucosal proliferation and keratinization. This subgroup often experiences remission during pregnancy. However, other studies report no correlation between aphthous stomatitis and menstrual period, pregnancy or menopause.[8]
Aphthous stomatitis is uncommon in people who smoke,[5] and there is also a correlation between habit duration and severity of the condition.[9] Tobacco use is associated with an increase in keratinization of the oral mucosa.[4] In extreme forms, this may manifest as leukoplakia or stomatitis nicotina (smoker's keratosis). This increased keratinization may mechanically reinforce the mucosa and reduce the tendency of ulcers to form after minor trauma, or present a more substantial barrier to microbes and antigens, but this is unclear. Nicotine is also known to stimulate production of adrenal steroids and reduce production of TNF-α, interleukin-1 and interleukin-6.[8] Smokeless tobacco products also seem to protect against aphthous stomatitis.[9] Cessation of smoking is known to sometimes precede the onset of aphthous stomatitis in people previously unaffected, or exacerbate the condition in those who were already experiencing aphthous ulceration.[1] Despite this correlation, starting smoking again does not usually lessen the condition.[10]
Antigenic sensitivity
It has been hypothesized that the condition represents a state of heightened sensitivity to antigenic stimuli, with cross-reactivity of the resulting cell-mediated immune response with cells of the epithelium. Some hypothesize that aphthous stomatitis is caused by expression of HLA class II antigens along with the normally found HLA class I antigens in epithelial cells, which results in them being recognized by the immune system as foreign cells rather than self.[6] Various antigenic triggers have been implicated as a trigger, including L forms of streptococci, herpes simplex virus, varicella-zoster virus, adenovirus, and cytomegalovirus.[4]
Others argue that there is no available evidence that demonstrates that any of these organisms are capable of causing aphthous stomatitis by themselves.[6] Some people with aphthous stomatitis may show herpes virus within the epithelium of the mucosa, but without any productive infection. In some persons, attacks of ulceration occur at the same time as asymptomatic viral shedding and elevated viral titres.[4] However, antiviral medication has no effect on aphthous stomatitis.[6]
In some instances, recurrent mouth ulcers may be a manifestation of an allergic reaction.[11] Possible allergens include certain foods (e.g., chocolate, coffee, strawberries, eggs, nuts, tomatoes, cheese, citrus fruits, benzoates, cinnamaldehyde, and highly acidic foods), toothpastes, and mouthwashes.[7][11] Where dietary allergens are responsible, mouth ulcers usually develop within about 12–24 hours of exposure.[7]
Sodium lauryl sulphate (SLS), a detergent present in some brands of toothpaste and other oral healthcare products, may produce oral ulceration in some individuals.[1] It has been shown that aphthous stomatitis is more common in people using toothpastes containing SLS, and that some reduction in ulceration occurs when a SLS-free toothpaste is used.[8] Some have argued that since SLS is almost ubiquitously used in oral hygiene products, there is unlikely to be a true predisposition for aphthous stomatitis caused by SLS.[8]
Systemic disease
| Systemic disorders associated with aphthous-like ulceration[4] |
|---|
Aphthous-like ulceration may occur in association with several systemic disorders (see table). These ulcers are clinically and histopathologically identical to the lesions of aphthous stomatitis, but this type of oral ulceration is not considered to be true aphthous stomatitis by some sources.[5][12] Some of these conditions may cause ulceration on other mucosal surfaces in addition to the mouth such as the conjunctiva or the genital mucous membranes. Resolution of the systemic condition often leads to decreased frequency and severity of the oral ulceration.[4]
Behçet's disease is a triad of mouth ulcers, genital ulcers and anterior uveitis.[7] The main feature of Behçet's disease is aphthous-like ulceration, but this is usually more severe than seen in aphthous stomatitis without a systemic cause, and typically resembles major or herpetiforme ulceration or both.[5][13] Aphthous-like ulceration is the first sign of the disease in 25–75% of cases.[4] Behçet's is more common in individuals whose ethnic origin is from regions along the Silk Road (between the Mediterranean and the Far East).[14] It tends to be rare in other countries such as the United States and the United Kingdom.[7] MAGIC syndrome is a possible variant of Behçet disease, and is associated with aphthous-like ulceration. The name stands for "mouth and genital ulcers with inflamed cartilage" (relapsing polychondritis).[8]
PFAPA syndrome is a rare condition that tends to occur in children.[8] The name stands for "periodic fever, aphthae, pharyngitis (sore throat) and cervical adenitis" (inflammation of the lymph nodes in the neck). The fevers occur periodically about every 3–5 weeks. The condition appears to improve with tonsillectomy or immunosuppression, suggesting an immunologic cause.[13]
In cyclic neutropenia, there is a reduction in the level of circulating neutrophils in the blood that occurs about every 21 days. Opportunistic infections commonly occur and aphthous-like ulceration is worst during this time.[13]
Hematinic deficiencies (vitamin B12, folic acid and iron), occurring singly or in combination,[7] and with or without any underlying gastrointestinal disease, may be twice as common in people with RAS. However, iron and vitamin supplements only infrequently improve the ulceration.[13] The relationship to vitamin B12 deficiency has been the subject of many studies. Although these studies found that 0–42% of those with recurrent ulcers suffer from vitamin B12 deficiency, an association with deficiency is rare. Even in the absence of deficiency, vitamin B12 supplementation may be helpful due to unclear mechanisms.[15] Hematinic deficiencies can cause anemia, which is also associated with aphthous-like ulceration.[5]
Gastrointestinal disorders are sometimes associated with aphthous-like stomatitis, e.g. most commonly Celiac disease, but also inflammatory bowel disease such as Crohn's disease or ulcerative colitis.[5] The link between gastrointestinal disorders and aphthous stomatitis is probably related to nutritional deficiencies caused by malabsorption.[13] Less than 5% of people with RAS have Celiac disease, which usually presents with severe malnutrition, anemia, abdominal pain, diarrhea and glossitis (inflammation of the tongue).[8] Sometimes aphthous-like ulcerations can be the only sign of celiac disease.[8] Despite this association, a gluten-free diet does not usually improve the oral ulceration.[13]
Other examples of systemic conditions associated with aphthous-like ulceration include Reactive arthritis (Reiter's syndrome),[5] and recurrent erythema multiforme.[5]
Diagnosis
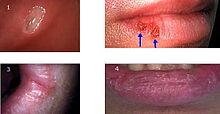

Diagnosis is mostly based on the clinical appearance and the medical history.[1] The most important diagnostic feature is a history of recurrent, self healing ulcers at fairly regular intervals.[17] Although there are many causes of oral ulceration, recurrent oral ulceration has relatively few causes, most commonly aphthous stomatitis, but rarely Behçet's disease, erythema multiforme, ulceration associated with gastrointestinal disease,[10][17] and recurrent intra-oral herpes simplex infection. A systemic cause is more likely in adults who suddenly develop recurrent oral ulceration with no prior history.[13]
Special investigations may be indicated to rule out other causes of oral ulceration. These include blood tests to exclude anemia, deficiencies of iron, folate or vitamin B12 or celiac disease.[7] However, the nutritional deficiencies may be latent and the peripheral blood picture may appear relatively normal.[7] Some suggest that screening for celiac disease should form part of the routine work up for individuals complaining of recurrent oral ulceration.[8] Many of the systemic diseases cause other symptoms apart from oral ulceration, which is in contrast to aphthous stomatitis where there is isolated oral ulceration. Patch testing may be indicated if allergies are suspected (e.g. a strong relationship between certain foods and episodes of ulceration). Several drugs can cause oral ulceration (e.g. nicorandil), and a trial substitution to an alternative drug may highlight a causal relationship.[1]
Tissue biopsy is not usually required, unless to rule out other suspected conditions such as oral squamous cell carcinoma.[17] The histopathologic appearance is not pathognomonic (the microscopic appearance is not specific to the condition). Early lesions have a central zone of ulceration covered by a fibrinous membrane. In the connective tissue deep to the ulcer there is increased vascularity and a mixed inflammatory infiltrate composed of lymphocytes, histiocytes and polymorphonuclear leukocytes. The epithelium on the margins of the ulcer shows spongiosis and there are many mononuclear cells in the basal third. There are also lymphocytes and histiocytes in the connective tissue surrounding deeper blood vessels near to the ulcer, described histologically as "perivascular cuffing".[4][17]
Classification
Aphthous stomatitis has been classified as a type of non-infectious stomatitis (inflammation of the mouth).[17] One classification distinguishes "common simple aphthae", accounting for 95% of cases, with 3–6 attacks per year, rapid healing, minimal pain and restriction of ulceration to the mouth; and "complex aphthae", accounting for 5% of cases, where ulcers may be present on the genital mucosa in addition to mouth, healing is slower and pain is more severe.[3] A more common method of classifying aphthous stomatitis is into three variants, distinguished by the size, number and location of the lesions, the healing time of individual ulcers and whether a scar is left after healing (see below).
Minor aphthous ulceration
This is the most common type of aphthous stomatitis, accounting for about 80–85% of all cases.[7] This subtype is termed minor aphthous ulceration (MiAU),[1] or minor recurrent aphthous stomatitis (MiRAS). The lesions themselves may be referred to as minor aphthae or minor aphthous ulcers. These lesions are generally less than 10 mm in diameter (usually about 2–3 mm),[7] and affect non-keratinized mucosal surfaces (i.e. the labial and buccal mucosa, lateral borders of the tongue and the floor of the mouth). Usually several ulcers appear at the same time, but single ulcers are possible. Healing usually takes seven to ten days and leaves no scar. Between episodes of ulceration, there is usually an ulcer-free period of variable length.[5]
Major aphthous ulceration
This subtype makes up about 10% of all cases of aphthous stomatitis.[4] It is termed major aphthous ulceration (MaAU) or major recurrent aphthous stomatitis (MaRAS). Major aphthous ulcers (major aphthae) are similar to minor aphthous ulcers, but are more than 10 mm in diameter and the ulceration is deeper.[4][5] Because the lesions are larger, healing takes longer (about twenty to thirty days), and may leave scars. Each episode of ulceration usually produces a greater number of ulcers, and the time between attacks is less than seen in minor aphthous stomatitis.[4] Major aphthous ulceration usually affects non keratinized mucosal surfaces, but less commonly keratinized mucosa may also be involved, such as the dorsum (top surface) of the tongue or the gingiva (gums).[8] The soft palate or the fauces (back of the throat) may also be involved,[8] the latter being part of the oropharynx rather than the oral cavity. Compared to minor aphthous ulceration, major aphthae tend to have an irregular outline.[7]
Herpetiform ulceration
Herpetiform ulcers,[5] (also termed stomatitis herpetiformis,[18] or herpes-like ulcerations) is a subtype of aphthous stomatitis so named because the lesions resemble a primary infection with herpes simplex virus (primary herpetic gingivostomatitis).[4] However, herpetiform ulceration is not caused by herpes viruses. As with all types of aphthous stomatitis, it is not contagious. Unlike true herpetic ulcers, herpetiforme ulcers are not preceded by vesicles (small, fluid filled blisters).[8] Herpetiforme ulcers are less than 1 mm in diameter and occur in variably sized crops up to one hundred at a time. Adjacent ulcers may merge to form larger, continuous areas of ulceration. Healing occurs within fifteen days without scarring.[7] The ulceration may affect keratinized mucosal surfaces in addition to non keratinized. Herpetiform ulceration is often extremely painful, and the lesions recur more frequently than minor or major aphthous ulcers. Recurrence may be so frequent that ulceration is virtually continuous. It generally occurs in a slightly older age group than the other subtypes,[8] and females are affected slightly more frequently than males.[1]
RAS type ulceration
Recurrent oral ulceration associated with systemic conditions is termed "RAS type ulceration", "RAS like ulceration", or "aphthous-like ulcers".[1] Aphthous stomatitis occurs in individuals with no associated systemic disease.[5] Persons with certain systemic diseases may be prone to oral ulceration, but this is secondary to the underlying medical condition (see the systemic disease section).[5] This kind of ulceration is considered by some to be separate from true aphthous stomatitis.[5][12] However, this definition is not strictly applied. For example, many sources refer to oral ulceration caused by anemia and/or nutritional deficiencies as aphthous stomatitis, and some also consider Behçet's disease to be a variant.[4][7]
Treatment
The vast majority of people with aphthous stomatitis have minor symptoms and do not require any specific therapy. The pain is often tolerable with simple dietary modification during an episode of ulceration such as avoiding spicy and acidic foods and beverages.[2] Many different topical and systemic medications have been proposed (see table), sometimes showing little or no evidence of efficacy when formally investigated.[5] Some of the results of interventions for RAS may in truth represent a placebo effect.[13] No therapy is curative, with treatment aiming to relieve pain, promote healing and reduce the frequency of episodes of ulceration.[5]
The first line therapy for aphthous stomatitis is topical agents rather than systemic medication,[5] with topical corticosteroids being the mainstay treatment.[1][13] Systemic treatment is usually reserved for severe disease due to the risk of adverse side effects associated with many of these agents. A systematic review found that no single systemic intervention was found to be effective.[5] Good oral hygiene is important to prevent secondary infection of the ulcers.[1]
Occasionally, in females where ulceration is correlated to the menstrual cycle or to an oral contraceptive, progestogen or a change in oral contraceptive may be beneficial.[1] Use of nicotine replacement therapy for people who have developed oral ulceration after stopping smoking has also been reported.[8] Starting smoking again does not usually lessen the condition.[10] Trauma can be reduced by avoiding rough or sharp foodstuffs and by brushing teeth with care. If sodium lauryl sulfate is suspected to be the cause, avoidance of products containing this chemical may be useful and prevent recurrence in some individuals.[20] Similarly patch testing may indicate that food allergy is responsible, and the diet modified accordingly.[1] If investigations reveal deficiency states, correction of the deficiency may result in resolution of the ulceration. For example, there is some evidence that vitamin B12 supplementation may prevent recurrence in some individuals.[20]
Surgical excision of aphthous ulcers has been described, but it is an ineffective and inappropriate treatment.[4] Silver nitrate has also been used as a chemical cauterant.[13] Apart from the mainstream approaches detailed above, there are numerous treatments of unproven effectiveness, ranging from herbal remedies to otherwise alternative treatments, including aloe vera, myrtus communis, Rosa damascena, zinc sulfate, nicotine, polio virus vaccine and prostaglandin E2.[1]
Prognosis
By definition, there is no serious underlying medical condition, and most importantly, the ulcers do not represent oral cancer nor are they infectious. However, aphthae are capable of causing significant discomfort. There is a spectrum of severity, with symptoms ranging from a minor nuisance to disabling.[2] Due to pain during eating, weight loss may develop as a result of not eating in severe cases of aphthous stomatitis. Usually, the condition lasts for several years before spontaneously disappearing in later life.[1]
Epidemiology
Reported prevalence ranges from 5–66%, but in most populations, about 20% of individuals are affected to some degree,[4][7] making it the most common disease of the oral mucosa.[17] Aphthous stomatitis occurs worldwide, but is more common in developed countries.[1]
Within nations, there is a slightly higher prevalence in higher socioeconomic groups.[1] Males and females are affected in an equal ratio, and the peak age of onset between 10 and 19 years.[5] About 80% of people with aphthous stomatitis first developed the condition before the age of 30.[4] There have been reports of ethnic variation. For example, in the United States, aphthous stomatitis may be three times more common in white-skinned people than black-skinned people.[13]
History, society and culture
"Aphthous affectations" and "aphthous ulcerations" of the mouth are mentioned several times in the treatise "Of the Epidemics" (part of the Hippocratic corpus, in the 4th century B.C),[21] although it seems likely that this was oral ulceration as a manifestation of some infectious disease, since they are described as occurring in epidemic-like patterns, with concurrent symptoms such as fever.
Aphthous stomatitis was once thought to be a form of recurrent herpes simplex virus infection, and some clinicians still refer to the condition as "herpes" despite this etiology having been disproven.[22]
The informal term "canker sore" is sometimes used, mainly in North America,[23] either to describe this condition generally,[6] or to refer to the individual ulcers of this condition,[24] or mouth ulcers of any cause unrelated to this condition. The origin of the word "canker" is thought to have been influenced by Latin, Old English, Middle English and Old North French.[25] In Latin, cancer translates to "malignant tumor" or literally "crab" (related to the likening of sectioned tumors to the limbs of a crab). The closely related word in Middle English and Old North French, chancre, now more usually applied to syphilis, is also thought to be involved.[25] Despite this etymology, aphthous stomatitis is not a form of cancer but rather entirely benign.
An aphtha (plural aphthae) is a non specific term that refers to an ulcer of the mouth. The word is derived from the Greek word aphtha meaning "eruption" or "ulcer".[8] The lesions of several other oral conditions are sometimes described as aphthae, including Bednar's aphthae (infected, traumatic ulcers on the hard palate in infants),[26] oral candidiasis, and foot-and-mouth disease. When used without qualification, aphthae commonly refers to lesions of recurrent aphthous stomatitis. Since the word aphtha is often taken to be synonymous with ulcer, it has been suggested that the term "aphthous ulcer" is redundant, but it remains in common use.[6][27] Stomatitis is also a non-specific term referring to any inflammatory process in the mouth, with or without oral ulceration.[28] It may describe many different conditions apart from aphthous stomatitis such as angular stomatitis.
The current most widely used medical term is "recurrent aphthous stomatitis" or simply "aphthous stomatitis".[2] Historically, many different terms have been used to refer to recurrent aphthous stomatitis or its sub-types, and some are still in use. Mikulicz' aphthae is a synonym of minor RAS,[8] named after Jan Mikulicz-Radecki. Synonyms for major RAS include Sutton's ulcers (named after Richard Lightburn Sutton), Sutton's disease,[29] Sutton's syndrome and pariadenitis mucosa necrotica recurrens.[1][8] Synonyms for aphthous stomatitis as a whole include (recurrent) oral aphthae, (recurrent) aphthous ulceration and (oral) aphthosis.[4][12]
In traditional Chinese medicine, treatments for aphthae focus on clearing heat and nourishing Yin.[30]
Rembrandt Gentle White toothpaste did not contain sodium lauryl sulfate, and was specifically marketed as being for the benefit of "canker sore sufferers". When the manufacturer Johnson & Johnson discontinued the product in 2014, it caused a backlash of anger from long term customers, and the toothpaste began to sell for many times the original price on the auction website eBay.[31][32]
References
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad Scully C (2013). Oral and maxillofacial medicine: the basis of diagnosis and treatment (3rd ed.). Edinburgh: Churchill Livingstone. pp. 226–234. ISBN 978-0-7020-4948-4.
- ^ a b c d e f Treister JM, Bruch NS (2010). Clinical oral medicine and pathology. New York: Humana Press. pp. 53–56. ISBN 978-1-60327-519-4.
- ^ a b Altenburg A, Zouboulis CC (September 2008). "Current concepts in the treatment of recurrent aphthous stomatitis". Skin Therapy Letter. 13 (7): 1–4. PMID 18839042.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z Neville BW, Damm DD, Allen CM, Bouquot JE (2008). Oral & maxillofacial pathology (3rd ed.). Philadelphia: W.B. Saunders. pp. 331–336. ISBN 978-1-4160-3435-3.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah Brocklehurst P, Tickle M, Glenny AM, Lewis MA, Pemberton MN, Taylor J, Walsh T, Riley P, Yates JM (September 12, 2012). "Systemic interventions for recurrent aphthous stomatitis (mouth ulcers)". Cochrane Database of Systematic Reviews. 9: CD005411. doi:10.1002/14651858.CD005411.pub2. PMID 22972085.
- ^ a b c d e f g h Swain N, Pathak J, Poonja LS, Penkar Y (September–December 2012). "Etiological Factors of Recurrent Aphthous Stomatitis: A Common Perplexity". Journal of Contemporary Dentistry 2 (3): 96–100.
{{cite journal}}: Italic or bold markup not allowed in:|journal=(help) - ^ a b c d e f g h i j k l m n Millet D, Welbury R (2004). Clinical problem solving in orthodontics and paediatric dentistry. Edinburgh: Churchill Livingstone. pp. 143–144. ISBN 978-0-443-07265-9.
- ^ a b c d e f g h i j k l m n o p q r s t u Preeti L, Magesh KT, Rajkumar K, Karthik R (January 1, 2011). "Recurrent aphthous stomatitis". Journal of Oral and Maxillofacial Pathology. 15 (3): 252–6. doi:10.4103/0973-029X.86669. PMID 22144824.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Ślebioda, Zuzanna; Szponar, Elżbieta; Kowalska, Anna (November 12, 2013). "Etiopathogenesis of Recurrent Aphthous Stomatitis and the Role of Immunologic Aspects: Literature Review". Archivum Immunologiae et Therapiae Experimentalis. 62 (3): 205–215. doi:10.1007/s00005-013-0261-y. PMC 4024130. PMID 24217985.
- ^ a b c Odell W (2010). Clinical problem solving in dentistry (3rd ed.). Edinburgh: Churchill Livingstone. pp. 87–90. ISBN 978-0-443-06784-6.
- ^ a b "Canker sore". Mayo Foundation for Medical Education and Research. March 24, 2012. Retrieved July 7, 2014.
- ^ a b c Riera Matute G, Riera Alonso E (September–October 2011). "[Recurrent aphthous stomatitis in Rheumatology]". Reumatología Clinica. 7 (5): 323–328. doi:10.1016/j.reuma.2011.05.003. PMID 21925448.
- ^ a b c d e f g h i j k l m n o p q Scully C, Porter S (March 31, 2008). "Oral mucosal disease: Recurrent aphthous stomatitis". British Journal of Oral and Maxillofacial Surgery. 46 (3): 198–206. doi:10.1016/j.bjoms.2007.07.201.
- ^ Dalvi SR, Yildirim R, Yazici Y (December 3, 2012). "Behcet's Syndrome". Drugs. 72 (17): 2223–2241. doi:10.2165/11641370-000000000-00000. PMID 23153327.
- ^ Baccaglini L, Lalla RV, Bruce AJ, Sartori-Valinotti JC, Latortue MC, Carrozzo M, Rogers RS (2011). "Urban legends: recurrent aphthous stomatitis". Oral Diseases. 17 (8): 755–770. doi:10.1111/j.1601-0825.2011.01840.x. PMC 3192917. PMID 21812866.
- ^ Dorfman J, The Center for Special Dentistry
- ^ a b c d e f Cawson RA, Odell EW, Porter S (2008). Cawson's essentials of oral pathology and oral medicine (8th ed.). Edinburgh: Churchill Livingstone. pp. 220–224. ISBN 978-0-443-10125-0.
- ^ "International Classification of Diseases-10". World Health Organization. Retrieved February 16, 2013.
- ^ a b c d e McBride DR (July 1, 2000). "Management of aphthous ulcers". American Family Physician. 62 (1): 149–154, 160. PMID 10905785.
- ^ a b c Bailey J, McCarthy C, Smith RF (2011). "Clinical inquiry. What is the most effective way to treat recurrent canker sores?". The Journal of Family Practice. 60 (10): 621–632. PMID 21977491.
- ^ Wikisource:Of the Epidemics
- ^ Greenberg MS, Glick M (2003). Burket's oral medicine diagnosis & treatment (10th ed.). Hamilton, Ont.: BC Decker. p. 63. ISBN 1-55009-186-7.
- ^ "Canker". Oxford dictionaries. Retrieved July 12, 2014.
- ^ "Aphthous stomatitis". Merriam-Webster, Incorporated. Retrieved July 12, 2014.
- ^ a b "Chancre and Canker". Douglas Harper. Retrieved September 1, 2013.
- ^ Tricarico A, Molteni G, Mattioli F, Guerra A, Mordini B, Presutti L, Iughetti L (November–December 2012). "Nipple trauma in infants? Bednar aphthae". American journal of otolaryngology. 33 (6): 756–757. doi:10.1016/j.amjoto.2012.06.009. PMID 22884485.
- ^ Fischman SL (June 1994). "Oral ulcerations". Seminars in Dermatology. 13 (2): 74–77. PMID 8060829.
- ^ Stewart MG, Selesnick S (editors) (January 1, 2011). Differential diagnosis in otolaryngology – head and neck surgery. New York: Thieme. ISBN 978-1-60406-279-3.
{{cite book}}:|last=has generic name (help) - ^ Burruano F, Tortorici S; Tortorici, S (January–February 2000). "[Major aphthous stomatitis (Sutton's disease): etiopathogenesis, histological and clinical aspects]". Minerva Stomatologica. 49 (1–2): 41–50. PMID 10932907.
- ^ Liu C, Tseng A, Yang S (2004). Chinese Herbal Medicine Modern Applications of Traditional Formulas. London: CRC Press. p. 533. ISBN 978-0-203-49389-2.
- ^ Deardorff J (March 5, 2014). "Loss of canker sore toothpaste angers loyal users". Chicago Tribune. Retrieved April 12, 2014.
- ^ Graedon J, Graedon T (2002). The people's pharmacy guide to home and herbal remedies. New York: St. Martin's Press. p. 122. ISBN 978-0-312-98139-6.
External links
