Triplatin tetranitrate
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| ChEBI | |
| Chemical and physical data | |
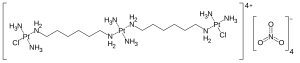
| Formula | C12H54Cl2N14O12Pt3 |
| Molar mass | 1242.8018 g/mol g·mol−1 |
| (verify) | |
Triplatin tetranitrate (rINN; also known as BBR3464) is a platinum-based cytotoxic drug that underwent clinical trials for the treatment of human cancer.[1] The drug acts by forming adducts with cellular DNA, preventing DNA transcription and replication, thereby inducing apoptosis. Other platinum-containing anticancer drugs include cisplatin, carboplatin, and oxaliplatin.
Drug development
Triplatin tetranitrate contains three platinum centres linked by amine ligands. Other ligands attached to this coordination complex, include chloride. Its invention arose from earlier work showing that diplatinum analogues of cisplatin were cytotoxic. BBR3464 was patented in the mid-1990s and was originally licensed to the pharmaceutical company Roche. In preclinical trials, it demonstrated cytotoxic activity in cancer cell lines that had either intrinsic or acquired resistance to cisplatin. On this basis it entered Phase I (Toxicity) clinical trials under the auspices of Novuspharma before the rights were transferred to Cell Therapeutics. It is currently undergoing Phase II (Efficacy) trials with mixed results. So far trials of the drug with patients suffering from ovarian cancer, small cell lung cancer and gastric or gastro-oesophageal adenocarcinomas have been reported in the literature.
Mode of action
The main target of triplatin is cellular DNA, similar to cisplatin. Outside of the cell, the concentration of chloride (approx. 100 millimolar) prevents the drugs from hydrolysing, but once inside the cell, where the concentration of chloride drops to between 4 and 20 millimolar, the chloride ligands of BBR3464 come off and the drug is capable of forming coordinate covalent bonds with purine bases on DNA. The novel adducts BBR3464 forms with DNA are thought to be the mechanism by which this drug acts; the adducts are able to prevent DNA transcription and replication, thus inducing cell apoptosis.
Side effects
All platinum based drugs, and particularly BBR3464, cause large dose limiting side-effects. For BBR3464 these are largely diarrhea, cramps and vomiting, but are so severe that the maximum tolerated dose (MTD) in humans is between 0.9 and 1.1 milligrams per square metre. This is considerably lower than the MTD for all the platinum based drugs currently used in the clinic, like cisplatin (60–120 mg) and carboplatin (approx. 800 mg).
References
- ^ Wheate, Nial J.; Walker, Shonagh; Craig, Gemma E.; Oun, Rabbab (2010). "The status of platinum anticancer drugs in the clinic and in clinical trials". Dalton Transactions. 39 (35): 8113–27. doi:10.1039/C0DT00292E. PMID 20593091.
