Ciproxifan
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
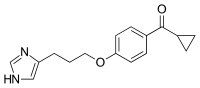
| Formula | C16H18N2O2 |
| Molar mass | 270.33 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Ciproxifan is an extremely potent histamine H3 inverse agonist/antagonist.
The histamine H3 receptor is an inhibitory autoreceptor located on histaminergic nerve terminals, and is believed to be involved in modulating the release of histamine in the brain. Histamine has an excitatory effect in the brain via H1 receptors in the cerebral cortex, and so drugs such as ciproxifan which block the H3 receptor and consequently allow more histamine to be released have an alertness-promoting effect.[1][2][3]
Ciproxifan produces wakefulness and attentiveness in animal studies, and produced cognitive enhancing effects without prominent stimulant effects at relatively low levels of receptor occupancy, and pronounced wakefulness at higher doses.[4] It has therefore been proposed as a potential treatment for sleep disorders such as narcolepsy and to improve vigilance in old age, particularly in the treatment of conditions such as Alzheimer's disease.[5][6] It also potentiated the effects of antipsychotic drugs, and has been suggested as an adjuvant treatment for schizophrenia.[7]
References
- ^ Passani MB, Lin JS, Hancock A, Crochet S, Blandina P (December 2004). "The histamine H3 receptor as a novel therapeutic target for cognitive and sleep disorders". Trends Pharmacol. Sci. 25 (12): 618–25. doi:10.1016/j.tips.2004.10.003. PMID 15530639.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Passani MB, Giannoni P, Bucherelli C, Baldi E, Blandina P (April 2007). "Histamine in the brain: beyond sleep and memory". Biochem. Pharmacol. 73 (8): 1113–22. doi:10.1016/j.bcp.2006.12.002. PMID 17241615.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Parmentier R, Anaclet C, Guhennec C, Brousseau E, Bricout D, Giboulot T, Bozyczko-Coyne D, Spiegel K, Ohtsu H, Williams M, Lin JS (April 2007). "The brain H3-receptor as a novel therapeutic target for vigilance and sleep-wake disorders". Biochem. Pharmacol. 73 (8): 1157–71. doi:10.1016/j.bcp.2007.01.002. PMID 17288995.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Le S, Gruner JA, Mathiasen JR, Marino MJ, Schaffhauser H (June 2008). "Correlation between ex vivo receptor occupancy and wake-promoting activity of selective H3 receptor antagonists". J. Pharmacol. Exp. Ther. 325 (3): 902–9. doi:10.1124/jpet.107.135343. PMID 18305012.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ LLigneau X, Lin J, Vanni-Mercier G, Jouvet M, Muir JL, Ganellin CR, Stark H, Elz S, Schunack W, Schwartz J (November 1998). "Neurochemical and behavioral effects of ciproxifan, a potent histamine H3-receptor antagonist". J. Pharmacol. Exp. Ther. 287 (2): 658–66. PMID 9808693.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Witkin JM, Nelson DL (July 2004). "Selective histamine H3 receptor antagonists for treatment of cognitive deficiencies and other disorders of the central nervous system". Pharmacol. Ther. 103 (1): 1–20. doi:10.1016/j.pharmthera.2004.05.001. PMID 15251226.
- ^ Pillot C, Ortiz J, Héron A, Ridray S, Schwartz JC, Arrang JM (August 2002). "Ciproxifan, a histamine H3-receptor antagonist/inverse agonist, potentiates neurochemical and behavioral effects of haloperidol in the rat". J. Neurosci. 22 (16): 7272–80. PMID 12177222.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
