Natural gas: Difference between revisions
→Domestic use: updated data |
m →Domestic use: slight error correction |
||
| Line 65: | Line 65: | ||
[[Image:WMATA 3006.jpg|thumb|right|A [[Metrobus (Washington, D.C.)|Metrobus]] using natural gas]] |
[[Image:WMATA 3006.jpg|thumb|right|A [[Metrobus (Washington, D.C.)|Metrobus]] using natural gas]] |
||
[[Compressed natural gas]] ([[methane]]) is a cleaner alternative to other [[automobile]] fuels such as [[gasoline]] (petrol) and [[diesel]]. As of December 2008, the countries with the highest number of CNG vehicles, ranked numerically, were [[Pakistan]] <ref>{{cite web|url=http://www.iangv.org/tools-resources/statistics.html|title=NATURAL GAS VEHICLE STATISTICS|publisher=International Association for Natural Gas Vehicles]]|date=2008-12-31 |accessdate=2009-06-11}}</ref>, [[Argentina]], [[Brazil]], |
[[Compressed natural gas]] ([[methane]]) is a cleaner alternative to other [[automobile]] fuels such as [[gasoline]] (petrol) and [[diesel]]. As of December 2008, the countries with the highest number of CNG vehicles, ranked numerically, were [[Pakistan]] <ref>{{cite web|url=http://www.iangv.org/tools-resources/statistics.html|title=NATURAL GAS VEHICLE STATISTICS|publisher=International Association for Natural Gas Vehicles]]|date=2008-12-31 |accessdate=2009-06-11}}</ref>, [[Argentina]], [[Brazil]], [[Iran]] and [[India]]. The energy efficiency is generally equal to that of gasoline engines, but lower compared with modern diesel engines. Gasoline/petrol vehicles converted to run on natural gas suffer because of the low [[compression ratio]] of their engines, resulting in a cropping of delivered power while running on natural gas (10%-15%). CNG-specific engines, however, use a higher compression ratio due to this fuel's higher [[octane number]] of 120–130.<ref>[http://www.imrt.ethz.ch/research/engine/CNG/cev Clean Engine Vehicle], Measurement and Control Laboratory</ref> |
||
===Fertilizer=== |
===Fertilizer=== |
||
Revision as of 02:27, 11 June 2009
Natural gas is a gas consisting primarily of methane. It is found associated with fossil fuels, in coal beds, as methane clathrates, and is created by methanogenic organisms in marshes, bogs, and landfills. It is an important fuel source, a major feedstock for fertilizers, and a potent greenhouse gas.
Natural gas is often informally referred to as simply gas, especially when compared to other energy sources such as electricity. Before natural gas can be used as a fuel, it must undergo extensive processing to remove almost all materials other than methane. The by-products of that processing include ethane, propane, butanes, pentanes and higher molecular weight hydrocarbons, elemental sulfur, and sometimes helium and nitrogen.
Sources
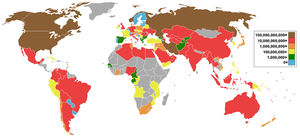
Fossil natural gas
Fossil natural gas can be "associated" (found in oil fields) or "non-associated" (isolated in natural gas fields), and is also found in coal beds (as coalbed methane). It sometimes contains significant quantities of ethane, propane, butane, and pentane—heavier hydrocarbons removed prior to use as a consumer fuel—as well as carbon dioxide, nitrogen, helium and hydrogen sulfide.[1] Natural gas is commercially produced from oil fields and natural gas fields. Gas produced from oil wells is called casinghead gas or associated gas. The natural gas industry is producing gas from increasingly more challenging resource types: sour gas, tight gas, shale gas and coalbed methane.
The world's largest proven gas reserves are located in Russia, with 4.757×1013 m³ (1.6×1015 cu ft). Russia is also the world's largest natural gas producer, through the Gazprom company. Major proven resources (with year of estimate) (in billion cubic metres) are world 175,400 (2006), Russia 47,570 (2006), Iran 26,370 (2006), Qatar 25,790 (2007), Saudi Arabia 6,568 (2006) and United Arab Emirates 5,823 (2006).
The world's largest gas field is Qatar's offshore North Field, estimated to have 25 trillion cubic metres[2] (9.0×1014 cu ft) of gas in place—enough to last more than 200 years at optimum production levels. The second largest natural gas field is the South Pars Gas Field in Iranian waters in the Persian Gulf. Connected to Qatar's North Field, it has estimated reserves of 8 to 14 trillion cubic metres[3] (2.8×1014 to 5.0×1014 cu ft) of gas.
Because natural gas is not a pure product, when non-associated gas is extracted from a field under supercritical (pressure/temperature) conditions, it may partially condense upon isothermic depressurizing—an effect called retrograde condensation. The liquids thus formed may get trapped by depositing in the pores of the gas reservoir. One method to deal with this problem is to reinject dried gas free of condensate to maintain the underground pressure and to allow reevaporation and extraction of condensates.
Town gas
Town gas is a mixture of methane and other gases, mainly the highly toxic carbon monoxide, that can be used in a similar way to natural gas and can be produced by treating coal chemically. This is a historic technology, still used as 'best solution' in some local circumstances, although coal gasification is not usually economic at current gas prices. However, depending upon infrastructure considerations, it remains a future possibility.
Most town "gashouses" located in the eastern United States in the late nineteenth and early twentieth centuries were simple by-product coke ovens which heated bituminous coal in air-tight chambers. The gas driven off from the coal was collected and distributed through town-wide networks of pipes to residences and other buildings where it was used for cooking and lighting purposes. (Gas heating did not come into widespread use until the last half of the twentieth century.) The coal tar that collected in the bottoms of the gashouse ovens was often used for roofing and other water-proofing purposes, and also, when mixed with sand and gravel, was used for creating Bitumen for the surfacing of local streets.
Biogas
When methane-rich gases are produced by the anaerobic decay of non-fossil organic matter (biomass), these are referred to as biogas (or natural biogas). Sources of biogas include swamps, marshes, and landfills (see landfill gas), as well as sewage sludge and manure[4] by way of anaerobic digesters, in addition to enteric fermentation particularly in cattle.
Methanogenic archaea are responsible for all biological sources of methane, some in symbiotic relationships with other life forms, including termites, ruminants, and cultivated crops. Methane released directly into the atmosphere would be considered a pollutant, however, methane in the atmosphere is oxidised, producing carbon dioxide and water. Methane in the atmosphere has a half life of seven years, meaning that every seven years, half of the methane present is converted to carbon dioxide and water.

Future sources of methane, the principal component of natural gas, include landfill gas, biogas and methane hydrate. Biogas, and especially landfill gas, are already used in some areas, but their use could be greatly expanded. Landfill gas is a type of biogas, but biogas usually refers to gas produced from organic material that has not been mixed with other waste.
Landfill gas is created from the decomposition of waste in landfills. If the gas is not removed, the pressure may get so high that it works its way to the surface, causing damage to the landfill structure, unpleasant odor, vegetation die-off and an explosion hazard. The gas can be vented to the atmosphere, flared or burned to produce electricity or heat. Experimental systems were being proposed for use in parts Hertfordshire, UK and Lyon in France.
Once water vapor is removed, about half of landfill gas is methane. Almost all of the rest is carbon dioxide, but there are also small amounts of nitrogen, oxygen and hydrogen. There are usually trace amounts of hydrogen sulfide and siloxanes, but their concentration varies widely. Landfill gas cannot be distributed through natural gas pipelines unless it is cleaned up to the same quality. It is usually more economical to combust the gas on site or within a short distance of the landfill using a dedicated pipeline. Water vapor is often removed, even if the gas is combusted on site. If low temperatures condense water out of the gas, siloxanes can be lowered as well because they tend to condense out with the water vapor. Other non-methane components may also be removed in order to meet emission standards, to prevent fouling of the equipment or for environmental considerations. Co-firing landfill gas with natural gas improves combustion, which lowers emissions.
Biogas is usually produced using agricultural waste materials, such as otherwise unusable parts of plants and manure. Biogas can also be produced by separating organic materials from waste that otherwise goes to landfills. This is more efficient than just capturing the landfill gas it produces. Using materials that would otherwise generate no income, or even cost money to get rid of, improves the profitability and energy balance of biogas production.
Anaerobic lagoons produce biogas from manure, while biogas reactors can be used for manure or plant parts. Like landfill gas, biogas is mostly methane and carbon dioxide, with small amounts of nitrogen, oxygen and hydrogen. However, with the exception of pesticides, there are usually lower levels of contaminants.
Hydrates
Huge quantities of natural gas (primarily methane) exist in the form of hydrates under sediment on offshore continental shelves and on land in arctic regions that experience permafrost such as those in Siberia (hydrates require a combination of high pressure and low temperature to form). However, as of 2009[update] no technology has been developed to produce natural gas economically from hydrates.
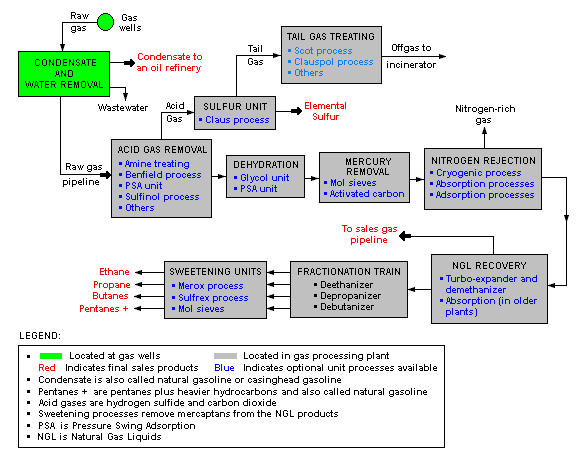
Natural gas processing

The image below is a schematic block flow diagram of a typical natural gas processing plant. It shows the various unit processes used to convert raw natural gas into sales gas pipelined to the end user markets.
The block flow diagram also shows how processing of the raw natural gas yields byproduct sulfur, byproduct ethane, and natural gas liquids (NGL) propane, butanes and natural gasoline (denoted as pentanes +).[5][6][7][8][9]
Uses of natural gas
Power generation
Natural gas is a major source of electricity generation through the use of gas turbines and steam turbines. Most grid peaking power plants and some off-grid engine-generators use natural gas. Particularly high efficiencies can be achieved through combining gas turbines with a steam turbine in combined cycle mode. Natural gas burns more cleanly than other fossil fuels, such as oil and coal, and produces less carbon dioxide per unit energy released. For an equivalent amount of heat, burning natural gas produces about 30% less carbon dioxide than burning petroleum and about 45% less than burning coal.[10] Combined cycle power generation using natural gas is thus the cleanest source of power available using fossil fuels, and this technology is widely used wherever gas can be obtained at a reasonable cost. Fuel cell technology may eventually provide cleaner options for converting natural gas into electricity, but as yet it is not price-competitive. (Please note: some algal fuel producers are considering feeding the carbon dioxide resulting from natural gas burning to algae to promote growth.)
Domestic use
Natural gas is supplied to homes, where it is used for such purposes as cooking in natural gas-powered ranges and/or ovens, natural gas-heated clothes dryers, heating/cooling and central heating. Home or other building heating may include boilers, furnaces, and water heaters. CNG is used in rural homes without connections to piped-in public utility services, or with portable grills. However, due to CNG being less economical than LPG, LPG (Propane) is the dominant source of rural gas.

Compressed natural gas (methane) is a cleaner alternative to other automobile fuels such as gasoline (petrol) and diesel. As of December 2008, the countries with the highest number of CNG vehicles, ranked numerically, were Pakistan [11], Argentina, Brazil, Iran and India. The energy efficiency is generally equal to that of gasoline engines, but lower compared with modern diesel engines. Gasoline/petrol vehicles converted to run on natural gas suffer because of the low compression ratio of their engines, resulting in a cropping of delivered power while running on natural gas (10%-15%). CNG-specific engines, however, use a higher compression ratio due to this fuel's higher octane number of 120–130.[12]
Fertilizer
Natural gas is a major feedstock for the production of ammonia, via the Haber process, for use in fertilizer production.
Aviation
Russian aircraft manufacturer Tupolev is currently running a development program to produce LNG- and hydrogen-powered aircraft.[13] The program has been running since the mid-1970s, and seeks to develop LNG and hydrogen variants of the Tu-204 and Tu-334 passenger aircraft, and also the Tu-330 cargo aircraft. It claims that at current market prices, an LNG-powered aircraft would cost 5,000 roubles (~ $218/ £112) less to operate per ton, roughly equivalent to 60%, with considerable reductions to carbon monoxide, hydrocarbon and nitrogen oxide emissions.
The advantages of liquid methane as a jet engine fuel are that it has more specific energy than the standard kerosene mixes and that its low temperature can help cool the air which the engine compresses for greater volumetric efficiency, in effect replacing an intercooler. Alternatively, it can be used to lower the temperature of the exhaust.
Hydrogen
Natural gas can be used to produce hydrogen, with one common method being the hydrogen reformer. Hydrogen has various applications: it is a primary feedstock for the chemical industry, a hydrogenating agent, an important commodity for oil refineries, and a fuel source in hydrogen vehicles.
Other
Natural gas is also used in the manufacture of fabrics, glass, steel, plastics, paint, and other products.
Storage and transport

The major difficulty in the use of natural gas is transportation and storage because of its low density. Natural gas pipelines are economical, but are impractical across oceans. Many existing pipelines in North America are close to reaching their capacity, prompting some politicians representing colder areas to speak publicly of potential shortages.
LNG carriers can be used to transport liquefied natural gas (LNG) across oceans, while tank trucks can carry liquefied or compressed natural gas (CNG) over shorter distances. They may transport natural gas directly to end-users, or to distribution points such as pipelines for further transport. These may have a higher cost, requiring additional facilities for liquefaction or compression at the production point, and then gasification or decompression at end-use facilities or into a pipeline.

In the past, the natural gas which was recovered in the course of recovering petroleum could not be profitably sold, and was simply burned at the oil field (known as flaring). This wasteful practice is now illegal in many countries[14]. Additionally, companies now recognize that value for the gas may be achieved with LNG, CNG, or other transportation methods to end-users in the future. The gas is now re-injected back into the formation for later recovery. This also assists oil pumping by keeping underground pressures higher. In Saudi Arabia, in the late 1970s, a "Master Gas System" was created, ending the need for flaring. Satellite observation unfortunately shows that some large gas-producing countries still use flaring[15] and venting[16] routinely. The natural gas is used to generate electricity and heat for desalination. Similarly, some landfills that also discharge methane gases have been set up to capture the methane and generate electricity.
Natural gas is often stored underground inside depleted gas reservoirs from previous gas wells, salt domes, or in tanks as liquefied natural gas. The gas is injected during periods of low demand and extracted during periods of higher demand. Storage near the ultimate end-users helps to best meet volatile demands, but this may not always be practicable.
With 15 nations accounting for 84% of the worldwide production, access to natural gas has become a significant factor in international economics and politics. In this respect, control over the pipelines is a major strategic factor.[17] In particular, in the 2000s, Gazprom, the Russian national energy company, has engaged in disputes with Ukraine and Belarus over the price of its natural gas, which have created worries that gas deliveries to parts of Europe could be cut off for political reasons.[18]
Environmental effects
Global Climate Change
Natural gas is often described as the cleanest fossil fuel, producing less carbon dioxide per joule delivered than either coal or oil.[10], and far fewer pollutants than other fossil fuels. However, in absolute terms it does contribute substantially to global carbon emissions, and this contribution is projected to grow. According to the IPCC Fourth Assessment Report (Working Group III Report, Chapter 4), in 2004 natural gas produced about 5,300 Mt/yr of CO2 emissions, while coal and oil produced 10,600 and 10,200 respectively (Figure 4.4); but by 2030, according to an updated version of the SRES B2 emissions scenario, natural gas would be the source of 11,000 Mt/yr, with coal and oil now 8,400 and 17,200 respectively.[19] (Total global emissions for 2004 were estimated at over 27,200 Mt.)
In addition, natural gas itself is a greenhouse gas far more potent than carbon dioxide when released into the atmosphere but is not of large concern due to the small amounts in which this occurs.
When drilled in the US, the CO2 pumped out with the natural gas is released directly into the atmosphere. This amount of CO2 is not counted with the release of the CO2 when natural gas is burned.[citation needed]
Pollutants
Natural gas produces far less amounts of sulfur dioxide and nitrous oxides than any other fossil fuel.
Safety

In any form, a minute amount of odorant such as t-butyl mercaptan, with a rotting-cabbage-like smell, is added to the otherwise colorless and almost odorless gas, so that leaks can be detected before a fire or explosion occurs. Sometimes a related compound, thiophane is used, with a rotten-egg smell. Adding odorant to natural gas began in the United States after the 1937 New London School explosion. The buildup of gas in the school went unnoticed, killing three hundred students and faculty when it ignited. Odorants are considered non-toxic in the extremely low concentrations occurring in natural gas delivered to the end user.
In mines, where methane seeping from rock formations has no odor, sensors are used, and mining apparatuses have been specifically developed to avoid ignition sources, e.g., the Davy lamp.
Explosions caused by natural gas leaks occur a few times each year. Individual homes, small businesses and boats are most frequently affected when an internal leak builds up gas inside the structure. Frequently, the blast will be enough to significantly damage a building but leave it standing. In these cases, the people inside tend to have minor to moderate injuries. Occasionally, the gas can collect in high enough quantities to cause a deadly explosion, disintegrating one or more buildings in the process. The gas usually dissipates readily outdoors, but can sometimes collect in dangerous quantities if weather conditions are right. However, considering the tens of millions of structures that use the fuel, the individual risk of using natural gas is very low.
Some gas fields yield sour gas containing hydrogen sulfide (H2S). This untreated gas is toxic. Amine gas treating, an industrial scale process which removes acidic gaseous components, is often used to remove hydrogen sulfide from natural gas.[20]
Extraction of natural gas (or oil) leads to decrease in pressure in the reservoir. This in turn may lead to subsidence at ground level. Subsidence may affect ecosystems, waterways, sewer and water supply systems, foundations, etc.
Natural gas heating systems are a minor source of carbon monoxide deaths in the United States. According to the US Consumer Product Safety Commission (2008), 56% of unintentional deaths from non-fire CO poisoning were associated with engine-driven tools like gas-powered generators and lawn mowers. Natural gas heating systems accounted for 4% of these deaths. Improvements in natural gas furnace designs have greatly reduced CO poisoning concerns. Detectors are also available that warn of carbon monoxide and/or explosive gas (methane, propane, etc.).
Energy content, statistics and pricing

Quantities of natural gas are measured in normal cubic meters (corresponding to 0°C at 101.325 kPa) or in standard cubic feet (corresponding to 60 °F (16 °C) and 14.73 PSIA). The gross heat of combustion of one normal cubic meter of commercial quality natural gas is around 39 megajoules (≈10.8 kWh), but this can vary by several percent.
The price of natural gas varies greatly depending on location and type of consumer. In 2007, a price of $7 per 1,000 cubic feet (28 m3) was typical in the United States. The typical caloric value of natural gas is roughly 1,000 BTU per cubic foot, depending on gas composition. This corresponds to around $7 per million BTU, or around $7 per gigajoule. In April 2008, the wholesale price was $10 per 1,000 cubic feet (28 m3) ($10/MMBTU).[21] The residential price varies from 50% to 300% more than the wholesale price. At the end of 2007, this was $12-$16 per 1,000 cu ft (28 m3).[22] Natural gas in the United States is traded as a futures contract on the New York Mercantile Exchange. Each contract is for 10,000 MMBTU (gigajoules), or 10 billion BTU. Thus, if the price of gas is $10 per million BTUs on the NYMEX, the contract is worth $100,000.
United Kingdom
Natural gas is also traded as a commodity in Europe, principally at the United Kingdom NBP and related European hubs, such as the TTF in the Netherlands.
Rest of the world
In the rest of the world, LNG (liquified natural gas) and LPG (liquified petroleum gas) is traded in metric tons or mmBTU as spot deliveries. Long term contracts are signed in metric tons. The LNG and LPG is transported by specialized transport ships, as the gas is liquified at cryogenic temperatures. The specification of each LNG/LPG cargo will usually contain the energy content, but this information is in general not available to the public.
United States
In US units, one standard cubic foot of natural gas produces around 1,028 British Thermal Units (BTU). The actual heating value when the water formed does not condense is the net heat of combustion and can be as much as 10% less.[23]
In the United States, retail sales are often in units of therms (th); 1 therm = 100,000 BTU. Gas meters measure the volume of gas used, and this is converted to therms by multiplying the volume by the energy content of the gas used during that period, which varies slightly over time. Wholesale transactions are generally done in decatherms (Dth), or in thousand decatherms (MDth), or in million decatherms (MMDth). A million decatherms is roughly a billion cubic feet of natural gas.
See also
|
References
- ^ Natural gas overview
- ^ Background note: Qatar
- ^ "Pars Special Economic Energy Zone". Pars Special Economic Energy Zone. Retrieved 2007-07-17.
- ^ http://www.manure.umn.edu/
- ^ Natural Gas Processing: The Crucial Link Between Natural Gas Production and Its Transportation to Market
- ^ Example Gas Plant
- ^ From Purification to Liquefaction Gas Processing
- ^ Feed-Gas Treatment Design for the Pearl GTL Project
- ^ Benefits of integrating NGL extraction and LNG liquefaction
- ^ a b Natural Gas and the Environment
- ^ "NATURAL GAS VEHICLE STATISTICS". International Association for Natural Gas Vehicles]]. 2008-12-31. Retrieved 2009-06-11.
- ^ Clean Engine Vehicle, Measurement and Control Laboratory
- ^ PSC Tupolev - Development of Cryogenic Fuel Aircraft
- ^ Hyne, Norman J. (1991). Dictionary of petroleum exploration, drilling & production. pg. 190: PennWell Books. p. 625. ISBN 0878143521.
{{cite book}}: CS1 maint: location (link) - ^ Satellite observation of flares in the world
- ^ satellite observation of methane in earth's atmosphere
- ^ The Contours of the New Cold War
- ^ Gazprom and Russian Foreign Policy
- ^ [1]
- ^ NaturalGas.org - Processing Natural Gas
- ^ Graph of Natural Gas Futures Prices - NYMEX
- ^ Natural Gas Prices published by the US government
- ^ Heat value definitions. WSU website. Retrieved 2008-05-19.
External links
- LNG Unlimited The worlds leading LNG publication
- CERA - collection of market and industry reports
- DOE/EIA EIA Natural Gas Weekly Update - current NG prices and market analysis
- American Gas Association - information portal from distributor advocacy group
- Natural Gas Supply Association - information portal from producer advocacy group
- National Grid - information portal from UK's largest utility company

