Cafestol: Difference between revisions
m Bot: Deprecating Template:Cite pmid and some minor fixations |
Cyberbot II (talk | contribs) Rescuing 1 sources, flagging 0 as dead, and archiving 0 sources. #IABot |
||
| Line 31: | Line 31: | ||
A typical bean of ''[[Coffea arabica]]'' contains about 0.4-0.7% cafestol by weight.<ref>Kitzberger C, Scholz M, Benassi M. Bioactive compounds content in roasted coffee from traditional and modern Coffea arabica cultivars grown under the same edapho-climatic conditions. Food Research International [serial online]. July 2014;61:61-66. Available from: Academic Search Premier, Ipswich, MA. Accessed June 7, 2014.</ref> Cafestol is present in highest quantity in unfiltered coffee drinks such as [[French press]] coffee or [[Turkish coffee]]/[[Greek coffee]]. In filtered coffee drinks such as [[drip brew]]ed coffee, it is present in only negligible amounts. |
A typical bean of ''[[Coffea arabica]]'' contains about 0.4-0.7% cafestol by weight.<ref>Kitzberger C, Scholz M, Benassi M. Bioactive compounds content in roasted coffee from traditional and modern Coffea arabica cultivars grown under the same edapho-climatic conditions. Food Research International [serial online]. July 2014;61:61-66. Available from: Academic Search Premier, Ipswich, MA. Accessed June 7, 2014.</ref> Cafestol is present in highest quantity in unfiltered coffee drinks such as [[French press]] coffee or [[Turkish coffee]]/[[Greek coffee]]. In filtered coffee drinks such as [[drip brew]]ed coffee, it is present in only negligible amounts. |
||
Studies have shown that regular consumption of boiled coffee increases [[serum cholesterol]] by 8% in men and 10% in women. For those drinking filter coffee, the effect was only significant for women.<ref name=ntp>[[National Toxicology Program]] (NTP): [http://ntp.niehs.nih.gov/ntp/htdocs/Chem_Background/ExSumPdf/Cafestol.pdf ''Cafestol (CASRN 469-83-0) and Kahweol (CASRN 6894-43-5) - Review of Toxicological Literature.'' (PDF)] October 1999{{ |
Studies have shown that regular consumption of boiled coffee increases [[serum cholesterol]] by 8% in men and 10% in women. For those drinking filter coffee, the effect was only significant for women.<ref name=ntp>[[National Toxicology Program]] (NTP): [http://ntp.niehs.nih.gov/ntp/htdocs/Chem_Background/ExSumPdf/Cafestol.pdf ''Cafestol (CASRN 469-83-0) and Kahweol (CASRN 6894-43-5) - Review of Toxicological Literature.'' (PDF)] October 1999 {{wayback|url=http://ntp.niehs.nih.gov/ntp/htdocs/Chem_Background/ExSumPdf/Cafestol.pdf |date=20041101034550 }}</ref> |
||
== Potential biological activity == |
== Potential biological activity == |
||
Revision as of 15:24, 11 January 2016
| |
| Names | |
|---|---|
| IUPAC name
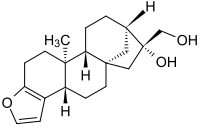
(3bS,5aS,7R,8R,10aR,10bS)-3b,4,5,6,7,8,9,10,10a,10b,11,12-Dodecahydro-7-hydroxy-10b-methyl-5a,8-methano-5aH-cyclohepta[5,6]naphtho[2,1-b]furan-7-methanol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H28O3 | |
| Molar mass | 316.441 g·mol−1 |
| Melting point | 158 to 162 °C (316 to 324 °F; 431 to 435 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Cafestol is a diterpene molecule present in coffee.
A typical bean of Coffea arabica contains about 0.4-0.7% cafestol by weight.[1] Cafestol is present in highest quantity in unfiltered coffee drinks such as French press coffee or Turkish coffee/Greek coffee. In filtered coffee drinks such as drip brewed coffee, it is present in only negligible amounts.
Studies have shown that regular consumption of boiled coffee increases serum cholesterol by 8% in men and 10% in women. For those drinking filter coffee, the effect was only significant for women.[2]
Potential biological activity
Cafestol has also shown anticarcinogenic properties in rats.[2] Cafestol may act as an agonist ligand for the nuclear receptor farnesoid X receptor and pregnane X receptor, blocking cholesterol homeostasis.[3] Cafestol also has neuroprotective effects in a Drosophila fruit fly model of Parkinson's disease.[4]
See also
References
- ^ Kitzberger C, Scholz M, Benassi M. Bioactive compounds content in roasted coffee from traditional and modern Coffea arabica cultivars grown under the same edapho-climatic conditions. Food Research International [serial online]. July 2014;61:61-66. Available from: Academic Search Premier, Ipswich, MA. Accessed June 7, 2014.
- ^ a b National Toxicology Program (NTP): Cafestol (CASRN 469-83-0) and Kahweol (CASRN 6894-43-5) - Review of Toxicological Literature. (PDF) October 1999 Template:Wayback
- ^ Ricketts ML, Boekschoten MV, Kreeft AJ, Hooiveld GJ, Moen CJ, Müller M, Frants RR, Kasanmoentalib S, Post SM, Princen HM, Porter JG, Katan MB, Hofker MH, Moore DD (2007). "The cholesterol-raising factor from coffee beans, cafestol, as an agonist ligand for the farnesoid and pregnane X receptors". Molecular Endocrinology (Baltimore, Md.). 21 (7): 1603–16. doi:10.1210/me.2007-0133. PMID 17456796. Retrieved 2015-06-14.
- ^ Trinh K, Andrews L, Krause J, Hanak T, Lee D, Gelb M, Pallanck L (April 2010). "Decaffeinated coffee and nicotine-free tobacco provide neuroprotection in Drosophila models of Parkinson's disease through an NRF2-dependent mechanism". J. Neurosci. 30 (16): 5525–32. doi:10.1523/JNEUROSCI.4777-09.2010. PMID 20410106.
{{cite journal}}: Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help)CS1 maint: multiple names: authors list (link)
