Miroprofen
Appearance
From Wikipedia, the free encyclopedia
This is the current revision of this page, as edited by FK1954 (talk | contribs) at 13:04, 16 August 2023 (who mistakes "carbocyclic" for "carboxylic???). The present address (URL) is a permanent link to this version.
(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)
Analgesic and NSAID
 | |
| Clinical data | |
|---|---|
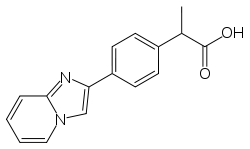
| Other names | Antopen; BRN 0888858; NSC 261037; Ro 07-0582; Y 9213; 2-[4-(1,7-diazabicyclo[4.3.0]nona-2,4,6,8-tetraen-8-yl)phenyl]propanoic acid |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C16H14N2O2 |
| Molar mass | 266.300 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| | |
Miroprofen (INN) is an analgesic and NSAID, meaning that it has anti-inflammatory, antipyretic and antiplatelet aggregation activity. Chemically it is a carboxylic acid belonging to the group of phenylpropanoic acids.[1]
References
[edit]- ^ Mikashima H, Goto K (January 1982). "[Inhibitory effect of 2-(4-(2-imidazo(1,2-a)pyridyl)phenyl) propionic acid (miroprofen) on platelet aggregation and prostaglandin I2 generation (author's transl)]". Yakugaku Zasshi. 102 (1): 99–103. doi:10.1248/yakushi1947.102.1_99. PMID 7045328.
| pyrazolones / pyrazolidines | |
|---|---|
| salicylates | |
| acetic acid derivatives and related substances | |
| oxicams | |
| propionic acid derivatives (profens) |
|
| n-arylanthranilic acids (fenamates) | |
| COX-2 inhibitors (coxibs) | |
| other | |
| NSAID combinations | |
Key: underline indicates initially developed first-in-class compound of specific group; #WHO-Essential Medicines; †withdrawn drugs; ‡veterinary use. | |
This drug article relating to the musculoskeletal system is a stub. You can help Wikipedia by expanding it. |
