Evolutionary anachronism
Evolutionary anachronism is a concept in evolutionary biology, named by Connie C. Barlow in her book The Ghosts of Evolution (2000),[1] to refer to attributes of living species that are best explained as a result of having been favorably selected in the past due to coevolution with other biological species that have since become extinct. When this context is removed, the said attributes appear as unexplained energy investments on the part of the living organism, with no apparent benefit extracted from them, and perhaps are prejudicial to the continued reproduction of the surviving species.
The general theory was formulated by Costa Rican-based American botanist Daniel Janzen and University of Arizona-based geologist Paul S. Martin (a prominent defender of the overkill hypothesis to explain the Quaternary extinction event) in a Science article published in 1982, titled Neotropical Anachronisms: The fruit the gomphotheres ate.[1][2] Previously, in 1977, Stanley Temple had proposed a similar idea to explain the decline of the Mauritius endemic tree tambalacoque following the extinction of the iconic dodo.[3]
Janzen, Martin and Barlow mainly discussed evolutionary anachronisms in the context of seed dispersal and passive defense strategies exhibited by plants that had evolved alongside disappeared megaherbivores. However, some examples have also been described in animal species. John Byers used the name relict behavior for animal behavior examples.[4]
Evolutionary anachronisms should not be confused with examples of vestigiality. Though both concepts refer ultimately to organs that evolved to deal with pressures that are no longer present today, in the case of anachronisms, the original function of the organ and the capacity of the organism to use it are retained intact. An example is the absence of gomphotheres eating avocados does not render the avocado's pulp vestigial, rudimentary or incapable of playing its original function of seed dispersal if a new suitable ecological partner appears. A truly vestigial organ like the python's pelvic spurs cannot be used to walk again.
Megafauna dispersal syndrome
Dispersal syndromes are complexes of fruit traits that enable plants to disperse seeds. The kind of fruits that birds are attracted to are usually small, with only a thin protective skin, and the colors are red or dark shades of blue or purple. Fruits categorized as mammal syndrome are bigger than bird fruits. They possess a tough rind or husk, emit a strong odor when ripe but retain a dull coloration of brown, burnished yellow, orange or remain green, because most mammals have a powerful sense of smell but poor color vision in general, primates being the most notable exception. The megafauna dispersal syndrome refers to those attributes of fruits that evolved in order to attract megafauna (animals that weigh or weighed more than 44 kilograms) as primary dispersal agents. Since the Holocene extinction, large herbivores have become extinct outside Africa and to a lesser extent Asia, leaving these fruits without a suitable dispersal mechanism in the absence of agriculture.
Common megafaunal dispersal traits

- Large fruit, best suited to be consumed whole by large animals without seed loss.
- Fruit grows on or close to the trunk, or on stout branches.
- Indehiscent fruit that retains its seeds upon ripening.
- Seeds deter or elude being ground up by teeth through having a thick, tough or hard endocarp; or bitter, peppering or nauseating toxins. They are also difficult to separate from the pulp, which is tasty and soft, to deter seed spitting.
- The seeds benefit from—or even require—physical or chemical abrasion to germinate.
- If tropical, the fruit drops on or just before ripening, stopping monkeys from eating them. In colder climates, the fruit stays on the branch for a prolonged time, keeping it away from predation by ineffectual seed dispersers like rodents.
- "Looks, feels, smells, and tastes" like other fruits known to be dispersed by megafauna where megafauna still exists.[1]
Ecological indicators of missing dispersal partners

- The fruit either rots where it falls or is ineffectually disseminated by current dispersal agents.
- The plant is more common where livestock (proxy for megafauna) are present.
- The seeds germinate and grow well in upland habitats where planted, but the species almost exclusively inhabits floodplains (where water flow disperses the seeds) in the wild.
- The geographic range is inexplicably patchy or restricted.[1]
Proposed examples in plants
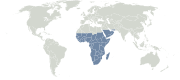
Afrotropical realm
-
Extent of the Afrotropical biogeographical realm
| Example | Binomial name | Native range | Anachronism description | Suggested extinct coevolutionary partners |
|---|---|---|---|---|
| Balanites | Balanites wilsoniana | West and Central Africa | Described as an "anachronism in the making", with seed dispersal being extremely limited or even unrecorded in areas where elephants have been extirpated. At least one forest in Kenya is known to lack seedlings and young balanites altogether, with all trees present being older than the local extinction of elephants.[1] | Forest elephant[1] Bush elephant[1] |
 Double coconut |
Lodoicea maldivica | Praslin and Curieuse islands (Seychelles) | The fruit weighs over 20 kg and contains the largest seeds in the world. No known animal eats the fruit, and the surviving trees appear to be the result of vegetative reproduction. Mature fruits don't float and are killed by sea water, unlike real coconuts.[5] The species is not thought to have dispersed over water, but to have evolved locally in the Seychelles after they broke off from the Indian plate 66 million years ago.[6] | |
 Makak |
Mimusops petiolaris | Mauritius | In decline due to the absence of animals removing its pulp. As a result, the fruit is colonized by fungi hyphae and the seeds rot without germinating. The fruit is only sporadically consumed by the Mauritian flying fox, which doesn't ingest the seeds.[7] | |
  Tambalacoque |
Sideroxylon grandiflorum | Mauritius | Peach-sized fruit that ripens from green to brown, much larger than its relatives present in the island and which are eaten by flying birds. The seed is in fact too large to be ingested by flying birds, and introduced pigs and monkeys destroy the seeds rather than disperse them. The tambalacoque evolved locally from smaller-seeded species in the genus Calvaria, which is found in Africa and Madagascar. Stanley Temple reported in 1977 that only 13 trees were left, all of them over three hundred years old, and that seeds could not germinate at all without being ingested and abraded first. However, these claims have since been debunked.[1] | Temple proposed that the tambalacoque had a strict mutualist relation with the dodo, extinct since c.1662.[1] Critics of Temple proposed that the seeds were originally dispersed by a giant tortoise instead, and that the tambalacoque might even have descended from seeds contained in a tortoise drifting from Madagascar, since tortoises are buoyant and colonize islands easily. In the Galapagos, ingestion by giant tortoises reduces seed dormancy in the Galapagos wild tomato, Solanum galapagense. Two species of giant tortoise were originally present in Mauritius and went extinct around the same time, the Domed Mauritius giant tortoise and the Saddle-backed Mauritius giant tortoise. However, tambalacoque seeds have harder covers than seeds usually eaten by tortoises, which have no gizzard; this might imply to a mutualistic relation with a bird after all, and the dodo was the only bird large enough to ingest the seeds. In any case, it was later found that germination was not favored by ingestion and abrasion of the seeds, but by pulp removal. As with Mimusops, the fruit that remains whole is colonized by fungi and its seeds rot.[1] The broad-billed parrot was also a large bird, although a flying one, and had an even more powerful beak than the dodo.[7] The Mauritian giant skink is presumed to have been omnivorous.[7] The coconut crab existed formerly in Mauritius, but has since disappeared from the island.[7] |
Madagascar
-
Hadropithecus restoration
-
Elephant bird restoration
-
Archaeolemur restoration
-
Pachylemur restoration
| Example | Binomial name | Native range | Anachronism description | Suggested extinct coevolutionary partners |
|---|---|---|---|---|
 |
Alluaudia spp. | Southwestern Madagascar | Heavily spined stems, apparently as defense against climbing browsers, but browsing lemurs are rare in their area of distribution. The only known living predator is the ring-tailed lemur.[8] | Isotope testing found that the extinct monkey lemur genera Mesopropithecus and Hadropithecus likely fed on these plants.[8] |
 Borassoid and arecoid palms |
Borassus spp. Hyphaene spp. Bismarckia spp. Satranala decussilvae Voanioala gerardii Orania spp. Lemurophoenix halleuxii |
Madagascar | Large seeded palms. Their relatives outside Madagascar are dispersed by elephants, bats, orangutans, baboons, capuchin monkeys, peccaries and tapirs.[5] | Elephant bird[5][9] |
| Canarium paniculatum | Mauritius | Hard seeds and fleshy pulp. Though common in the high forest vegetation, it has a poor regeneration rate.[7] | ||
| Commiphora guillaminii | Western Madagascar | Endozoochorous dry forest tree with high genetic variation among subpopulations at the local scale but similar genetic differentiation among populations at the regional scale as relatives in South Africa, suggesting that the dispersal distance shrunk in the recent past.[10] | Giant lemurs[10] | |
| Dilobeia | Dilobeia tenuinervis D. thouarsii |
Eastern Madagascar | Fruit with a single seed measuring 3–4 cm by 2-2.5 cm, too large to be dispersed by any extant animal in Madagascar.[5] | |
 Grandidier's baobab  Suarez baobab |
Adansonia grandidieri A. suarezensis |
Madagascar | Fruit with fragile pericarp, tasty and nutritious pulp, and seeds with a tough, thick testa, clearly adapted for animal dispersal but lacking any known disperser. Relatives in continental Africa dispersed by elephants and baboons. Very restricted geographic distribution.[5] | Archaeolemur,[5][10] a semiterrestrial, generalist lemur similar to a baboon, extinct since the Middle Ages Pachylemur[10] |
 Malagasy pandan |
Pandanus utilis | Madagascar, Mauritius and Seychelles | Seeds of variable size, the largest suited to be eaten by lemurs slightly larger than the extant species. Hard cover.[5] | |
| Malagasy wire plants | Several unrelated species | Madagascar | Plants convergent with New Zealand's divaricating plants, adapted to resist browsing by large birds, rather than like their continental African relatives, which have defenses against ungulate browsers.[11] | Elephant bird[11] |
| Ramy nut | Canarium madagascariense | Madagascar | Fruits 6–7 cm long and 4–5 cm wide, with substantial flesh and a single seed 4 cm long and 2 cm wide. The flesh is eaten by aye-ayes but rarely whole, and they may be satiated without removing all the flesh from the seed, indicating that they are not the intended disperser. Its Asian relatives are dispersed by large parrots and hornbills.[5] | Elephant bird[5] Pachylemur, a close relative of the living black-and-white ruffed lemur, but larger and more robust.[5] |
 Traveller's tree |
Ravenala madagascariensis | Madagascar | Plants often thrive and even form monocultures in degraded areas, due to their efficient vegetative reproduction. Hard, one centimeter long seeds, not adapted for wind or water dispersal, surrounded by odoriferous, light blue arils. The only viable seeds were found in the dung of the black-and-white ruffed lemur, the largest living lemur.[5] | Pachylemur[5] |
Australasian realm
-
Extent of the Australasian biogeographical realm
-
Genyornis restoration
-
Diprotodon restoration
-
South Island giant moa restoration
-
Naturalized Kawekaweau
| Example | Binomial name | Native range | Anachronism description | Suggested extinct coevolutionary partners |
|---|---|---|---|---|
| Birds-nest wattle Needle wattle |
Acacia pickardii A. carneorum |
Central Australia | Endangered spiny plants with extremely patchy populations. Both have low seed regeneration and reproduce mainly clonally.[12] | |
 Bowgada |
Acacia ramulosa | Central Australia | Unlike related species, the seeds are too large to be dispersed by ants and their low energy-to-water ratio make them unattractive to birds. The large legumes can be found directly beneath the shrub, in abundance and unopened, months after the end of the fruiting season.[1] Defensive spines are also common[citation needed], despite consumption of Acacia leaves by living marsupials being generally rare.[13] | |
 Burrawang |
Macrozamia spp. | Australia | Poor seed dispersal in spite of bright red, fleshy coatings. Brushtail possums eat the flesh but rarely carry the seeds. Many fruits fall in place and rot on the ground.[14] | Genyornis[14] |
 Bush tomato |
Solanum spp. | Australia | Several species with a variable amount of defensive spines in the branches. Strikingly, the most spiny species live in the Australian desert, where browsing marsupials are most rare.[13] | |
 Crystal Creek walnut |
Endiandra floydii | Queensland-New South Wales border | Rare rainforest species with a massive seed per fruit[12] | Cassowaries[12] |
 Cypress-pine |
Callitris spp. | Australia | Fossil pollen records show a great abundance of this species 50,000 years ago (after the extinction of the megafauna) compared to 100,000 years ago, despite the climate being similar and in contrast to other tree species which declined.[13] | There is direct evidence of predation by Diprotodon[13] |
| Dacrydium guillauminii | New Caledonia (Holocene) | Critically endangered and limited to New Caledonia in the present, but pollen records show that it was also present in Australia before the Last Glacial Maximum. It is mostly found in the margins of streams and the seeds are dispersed by large birds.[13] | Extinct flightless birds[13] | |
 Desert lime |
Citrus glauca | Eastern and southern Australia | Defensive spines up to seven centimeters long.[15] | Giant marsupials[15] |
 Durobby |
Syzygium moorei | Mount Warning, New South Wales | Large fruit and very small distribution.[12] | Cassowaries[12] |
 Hairy walnut Hairy walnut |
Endiandra pubens | New South Wales and Queensland | Massive red fruit compared to other rainforest fruits[12] | Cassowaries[12] |
 Idiot fruit Idiot fruit |
Idiospermum australiense | Daintree lowlands, Mount Bellenden Ker and Mount Bartle Frere in Tropical North Queensland | Largest seeds of any plant in Australia (225 grams), which are only sporadically dispersed by gravity and water. As a result, its range is extremely limited and largely restricted to lowly elevations and the margins of streams. However, translocation experiments have found that the species germinates easily in upland rainforests. The seeds are nutritious but contain toxins that make them severely poisonous to small mammals. The fruit has no pulp, but the seeds are easily divided into cotyledons, each of which can produce a different seedling. A large-jawed mammal currently absent might be able to feed on the seeds and disperse some of the seedlings uphill, if these fell from its mouth while chewing the seeds.[13] | Diprotodon[citation needed] |
| Lady apple | Syzygium suborbiculare | Northern Australia and Papua New Guinea | Tasty, red, apple-sized fruits encasing big round seeds, with no animals in their native range suited to eat them.[14] | Genyornis[12][14] |
 Leopardwood |
Flindersia dissosperma F. maculosa |
Inland Australia | Several defensive measures against large browsers, including wide, divaricate angle of branching, stiff and spiky twig tips, and small leaves widely separated along branchlets.[13] The defensive measures are lost when the plant reaches four meters, way above the reach of the largest local browsers - swamp and rock wallabies.[12] | Browsing flightless birds[12] |
| Myall Creek wattle | Acacia atrox | Tamworth, New South Wales | Spiny species found only in two stands. Low seed regeneration and mostly clonal reproduction.[12] | |
 Narrow-leafed bumble tree |
Capparis loranthifolia | Australia | [13] | |
| Nutwood | Terminalia arostrata | Western Australia, Northern Territory and Queensland[16] | Defenses against browsers lost around four meters tall, like the divaricate growth pattern.[12] | Browsing flightless birds[12] |
| Oldenlandia gibsonii | Gladstone, Queensland | Spiny and divaricate shrub, also the only woody member of its genus in Australia.[12] | Browsing megafauna[12] | |
| Omphalea | Omphalea queenslandiae | Queensland | 12.5 cm wide fruit similar to African and Asian fruits dispersed by elephants.[12] | Giant marsupials[12] |
 Pincushion tree |
Hakea spp. | Australia | Spiny leaves that are not eaten by any living mammal.[13] At least one species (H. eyreana) has cammouflaged flowers, despite no living animal browsing it.[17] | Dromornithids[17] |
 Rosewood tree |
Alectryon oleifolius | Australia | Trees growing in semi-circular stands sprouted around ancient burrow systems, possibly in soil once covered by dung of digging megafauna.[15] | Giant rat kangaroos[15] Phascolonus[15] |
| Scrub guava | Siphonodon australis | Northeastern Australia[18] | Big musky fruit.[15] | Diprotodon[15] |
 Southern ironwood |
Acacia estrophiolata | Central Australia | Intricately branched and tangled with small phyllodes at shrub level; erect and with long pendulous phyllodes at tree level.[13] | |
 Spiny everlasting |
Acanthocladium dockeri | Laura, South Australia | Woody, spiny herbaceous species with relatives that are neither woody nor spiny. Presumed extinct until 1992, when a few clonal populations were discovered.[12] | Browsing megafauna[12] |
| Spiny peppercress | Lepidium archersonii | Eastern and Western Australia[19] | Woody, spiny herbaceous species with relatives that are neither woody nor spiny. Only a few widely scattered populations remaining.[12] | Browsing megafauna[12] |
| Touriga | Mammea touriga | Tropical Queensland | Large-fruited plant with a restricted range. A close relative, M. africana, is dispersed by elephants in Congo.[12] | Giant marsupials[12] |
| Vicious hairy Mary | Calamus radicalis | Daintree rainforest[20] | Defensive spines.[15] | Giant marsupials[15] |
 Waddywood |
Acacia peuce | Margins of the Simpson Desert | Three anti-browser responses depending of its height: at grass level, the plant is soft but has a strong smell similar to stale urine, and induces headaches in humans; at shrub level, the plant is densely branched and has rigid, sharply pointed and outwardly reaching phyllodes; and at tree level (starting between two and three meters), the plant grows vertically, with soft phyllodes, and sheds all the rigid ones. However, the largest mammal in the area, the red kangaroo, rarely reaches two meters and is a grazer, not a browser. There are only three disjunct populations, but genetic testing shows that each is highly diverse, and similar in its genetic makeup to the others, indicating that they are recent remnants of a larger range area.[13] Seed regeneration is low and the species reproduces mainly clonally. The dense phyllodes of the shrub stage make it very vulnerable to fire, which might be another reason for its decline, as forest fires increased after the extinction of the megafauna.[12] | Browsing megafauna[12] |
 White bark |
Endiandra compressa | Eastern Australia | Northern populations widespread and dispersed by cassowaries; southern populations restricted to stream banks.[12] | Pygmy cassowary[12] |
 Wild orange |
Capparis mitchellii | Australia | Large, round fruits, with drab color and alluring aroma, typical of fruits ingested by mammals. Hooked spines also present.[14] | Diprotodon[14] |
 Wild pomegranate |
Capparis canescens | Northeastern Australia[21] | [13] |
New Zealand
| Example | Binomial name | Native range | Anachronism description | Suggested extinct coevolutionary partners |
|---|---|---|---|---|
 Divaricating plants of New Zealand |
54 unrelated species[1][22] | New Zealand | 10% of New Zealand plants have a divaricating pattern of growth (i.e. they grow in thickets), a much larger proportion than elsewhere in the world. Like spines, a divaricating growth pattern reduces the action of large browsers, but it is more effective against browsing birds, while spines are more effective against browsing mammals. However, the only large browsers in New Zealand today are introduced deer.[1] These defenses disappear three meters above ground, at most.[12] | Moas - the larger species in particular, which have been identified as browsers from their preserved gizzard contents.[1] The largest species of all, the South Island giant moa, matches the height where the plant defenses disappear.[12] |
 Karaka |
Corynocarpus laevigatus | New Zealand, including the Chatham Islands | Fruit with typical lizard dispersal syndrome features like most plants in New Zealand, but too large to be swallowed by any wild animal in the islands.[1] | New Zealand's kawekaweau was the second largest gecko in the world after the Rodrigues giant day gecko (also extinct). It was last observed in 1870.[1][23] |
 Tussock grass |
Several unrelated species | New Zealand | [1] | Moas[1] |
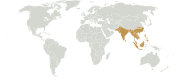
Indomalayan realm
-
Extent of the Indomalayan biogeographical realm
-
Ptilodus restoration
| Example | Binomial name | Native range | Anachronism description | Suggested extinct coevolutionary partners |
|---|---|---|---|---|
  Ginkgo |
Ginkgo biloba | China (Holocene) Northern Hemisphere (Jurassic related forms) |
An extreme living fossil, the same genus existed already in the Jurassic and the species might go back to the Middle Cretaceous. Ginkgos were widespread through the Northern Hemisphere until the Paleocene, survived in North America until the end of the Miocene, and in Europe and Japan until the Pleistocene. Seeds are protected by a shell too fragile to deter mammals, since they are capable of mastication, but the pulp is poisonous to frugivores (including humans). Red-bellied tree squirrels (in China) and eastern gray squirrels (in North American parks and plantations) are known to extract seeds from the pulp and store them, but are only secondary dispersers. Fallen diaspores smell like rotten flesh after a few days on the ground, attracting carnivorans like the masked palm civet, the leopard cat and the raccoon dog which eat them whole; however, their marking of their territory through defecation also limits their ability as seed dispersers.[1] The species is entirely pollinated by the wind in the present, but the chemical profile of its pollination drops is similar to those of insect-pollinated, or mixed wind and insect-pollinated Gnetophyta[24] | Squirrel-like multituberculates, particularly Ptilodus[1] Small carrion-eating dinosaurs both lived on the ground and lacked the more powerful masticatory apparatus and gizzard stones of the vegetarian species[1] Several extinct, early pollinating insect lineages are known from the Middle Jurassic to the Early Cretaceous, before modern flowers evolved. Most of these are long-proboscid scorpion flies (Mecoptera), and include Juracimbrophlebia, whose shape mimicked ginkgo leaves.[24] The unusual trunk and root growth pattern may have evolved in a pre-angiosperm world where the main competitors of the ginkgo were tree ferns, cycads and cycadeoids[25] |
 Plum-Yew |
Cephalotaxus spp. | East Asia | Gymnosperm widespread through the Northern Hemisphere in the Tertiary. | Multituberculates[1] |
 Rafflesia |
Rafflesia spp. | Southeast Asia | Between 14 and 28 species of dioecious parasitic plants with no visible stems, branches or leaves, but that produce enormous red flowers with a fetid, carrion-like smell. The smell attracts flies but they are poor pollinators. The fruits are giant berries around 14 centimeters long, with woody, cryptic cover; and smooth, oily flesh which smells and tastes like ripe (or rotten) coconut. The only observed dispersers are small rodents and treeshrews that eat part of the pulp and sometimes swallow seeds. Most species are endangered and have disjunct and extremely limited ranges.[1] | The original main pollinators might have been dung or carrion-eating beetles that became rarer as the megafauna declined.[1] The Asian elephant, Javan rhinoceros and Sumatran rhinoceros all used to, but are no longer present in Rafflesia's range, and might have been its intended seed dispersers.[1] |
Nearctic realm
-
Extent of the Nearctic realm
-
American mastodon restoration
-
Western camel restoration
-
Columbian mammoth restoration
| Example | Binomial name | Native range | Anachronism description | Suggested extinct coevolutionary partners |
|---|---|---|---|---|
 American persimmon |
Diospyros virginiana | Southeastern United States | Seeds difficult to separate from the pulp, like in its Old World relatives, and highly toxic unless swallowed whole. Gray foxes, raccoons and American black bears are well known dispersers of seeds, but they used to be less abundant before their natural predators and competitors like gray wolves, cougars and grizzlies were extirpated by humans, and they tend to defecate in certain places in order to mark their territory, limiting their dispersal potential. Virginia opossums consume the pulp but never swallow the seeds. The fruit is edible for a month before it falls from the tree and remains so for several months afterward.[1] | American Mastodon[1] |
 Buffalo gourd |
Cucurbita foetidissima | Southwestern United States and Mexico | Squash relative with orange-sized fruit that often rots and dries on the ground next to the plant while the next year's fruit is already ripening. The plant grows well in dry uplands, yet is more commonly encountered in floodplains where flash floods provide occasional dispersal, to the point that hydrochory was proposed once as the main dispersal syndrome of buffalo gourd, but this has been rejected since. High concentrations of cucurbitacin in its pulp and, to a lesser extent, seeds, makes it bitter to most animals. Domestic cattle and donkeys eat it rarely and mostly as a last resource. If eaten by a cow, the cow's milk will become bitter to humans, and it is deadly to sheep and cattle if eaten in enough quantity. In Africa and Asia, such bitter fruit is most commonly eaten by the largest megaherbivores of all, elephants and rhinoceros. Its distribution is also extremely patchy.[1] | Proboscideans[1] American horses[1] Toxodon[1] Camelids[1] Hesperotestudo[1] |
 Cocklebur |
Xanthium spp. | Americas and Eastern Asia | One of the best known examples of zoochory that does not involve eating the fruit (and direct inspiration of velcro for instance). In New Mexico, the burs, each containing two seeds, adhere to horse fur with such tenacity that they will remain there until they are retrieved by humans or the fur is shed. However, the burs fail to adhere to the fur of the largest wild ungulates in the area, deer.[1] | |
 Creosote bush |
Larrea tridentata | Western United States and Mexico | One of the plants readily eaten by the animals of the United States Camel Corps, a 19th-century experimental unit of the United States Cavalry active in Texas and California.[1] | Western camel[1] |
 Devil's walkingstick |
Aralia spinosa | Southeastern United States | Defensive spines appear at a particular height, but neither above nor below. However, this height is considerably higher than that of the current tallest browser in the area, the white-tailed deer.[26] | Columbian mammoth[26] Ground sloths[26] |
 Florida nutmeg |
Torreya taxifolia | Apalachicola river | Historically reduced to northern Florida's Apalachicola river, which acted as a refuge for many temperate trees during the ice ages. Unlike other species, the Florida nutmeg did not expand north again when the climate became warmer in the Holocene, and successive blights killed all trees starting in the 1950s. The species survives mostly through asexual reproduction, generating new trees from surviving roots, and it is estimated that it will become extinct when the roots run out of reserves in about 50 years. However, trees introduced to colder, mountain areas in North Carolina thrive and are free of disease, suggesting that the species is better adapted to the climate currently found there than in its Pleistocene refuge.[27] | The Florida nutmeg might have depended on an unknown large mammal for long range seed dispersal, which became extinct before the ice age ended. Living squirrels are known to provide some dispersal, but this was only enough to ensure the species's survival up to recent times, not its re-expansion after the glaciers retired north.[27] Because the genus Torreya goes back to the Eocene, it's been suggested that squirrel-like multituberculates dispersed the seeds before squirrels evolved.[1] |
 Hawthorn |
Crataegus spp. | Temperate Northern Hemisphere | Long, widely spaced and insufficiently densed thorns, better at dissuading larger African browsers like rhinoceroses and kudus than the local, narrow-muzzled white-tailed deer.[1] | Ground sloths[1] American mastodon[1] |
  Honey locust |
Gleditsia triacanthos | Mississippi river basin | Weather-resistant fruit (pods) that remains on the tree or the ground from one year to another, too large to be eaten by any wild animal in the area, but the seeds need abrasion to germinate. Horses ignore the fruit, but donkeys and mules will eat it on occasion. Large defensive thorns sometimes up to 20 cm are also present, usually high above ground.[1] | Columbian mammoth[1] American mastodon[1] American horses[1] Ground sloths[26] Brontotheres[1] Indricotherium[1] Aepycamelus[1] |
   Joshua tree |
Yucca brevifolia | Mojave desert | The fruit is much larger than in related species dispersed by birds and fruit-eating bats, a considerable investment in a desert. Fruit-eating bats are not present in the Mojave, and birds eat parasitic insects living in the Joshua tree's fruit but not the fruit itself. Among rodents, ground squirrels eat the seeds but only sporadically, and pack rats eat the fruits both on the tree and the ground, but avoid the seeds, leaving them in place and not acting as seed dispersers. The fruit is eaten full both by the largest wild mammals in the area (mule deer and bighorn sheep) and livestock species including horses, donkeys and cattle, but adult trees are so tall that they are only able to eat fruit from the ground or the lowest branches, leaving the numerous spines on the rest of the plant unexplained.[28] The fruit may grow at three meters above ground.[1] | The western camel was 20% larger than the modern dromedary, allowing it to browse up to 4 meters. Although dromedaries have trouble swallowing whole seeds and are very selective eaters and poor seed dispersers as a result, this might have been different in western camels due to their greater size. However, known western camel fossil dung contains only finely chewed plant remains, like in modern camels.[28] The American mastodon, Columbian mammoth and Gomphotherium all lived within the modern range of the Joshua tree and could reach even its tallest branches. Like modern elephants, they are presumed to have had an inefficient digestive system, making them both voracious eaters and perfect seed dispersers.[28] The Shasta ground sloth was common in western North America during the Pleistocene and has been identified as a primary yucca feeder from its fossil feces, which are commonly found in caves of the desert. However, it was only the size of an American black bear and would have been limited to eating only Joshua tree fruits from the lower branches or already on the ground. It probably fed more on smaller species of yucca.[28] |
 Jumping cholla |
Cylindropuntia fulgida | Arizona and Sonora | The defensive spines have backward-pointing teeth that attach to passing animals and the stems detach easily. The portions of the stem are transported for a while until they fall to the ground and grow into a new plant. The fruit is also ingested by many desert animals, but it grows above their reach as often as it does below. The fruit that grows in higher branches may remain in place for months after ripening. It falls after desiccating, when it is no longer attractive for potential seed dispersers.[1] | Western camel[1] Shasta ground sloth[1] Gomphotheres[1] |
 Kentucky coffeetree |
Gymnocladus dioicus | Midwestern United States | Large distribution area but very low density in its whole range. Like the buffalo gourd, it is more common in floodplains even though it grows upland with no problem. The seeds are the largest of any species in the contiguous United States, but they are not harvested by rodents because they can't break the pod's tough walls. They need abrasion to germinate. The pulp is very sweet and slightly bitter, similar in taste to the honey locust, but also poisonous to both livestock and humans because of its high content in saponin and alkaloids (nevertheless, it was used historically as a substitute for coffee in the Kentucky area, hence the name, because the toxins are destroyed in the roasting process). The seeds are more poisonous than the pulp, and often, large numbers of fallen pods and non-germinated seeds from preceding years can be found on the ground around a tree, trampled and rotten. The seeds die if they are not removed from the pod in time. Similar, related species in Africa are dispersed by elephants.[1][26][27] | American mastodon[1] |
 Mesquite |
Prosopis spp. | Tamaulipan Mezquital | Sweet and nutritious pods edible to humans and livestock. Horses and cattle both act as dispersers and also abrade the seeds walls, helping it germinate; foxes and coyotes eat the pods and disperse the seeds but don't abrade them. As a result, the mesquite's range began to expand after European colonization. The rest of the plant, however, is armed with thorns and is poisonous to livestock, which makes it unpopular with farmers. Mesquite also limits the growth of grass and favors the establishment of nopales, and once it grows to tree size, it is very hard to kill because it will grow back from the root after being knocked down (currently only possible with tractors).[26][1] | Western camel. One of the species sought by the animals of the United States Camel Corps while ignoring the grasses. Along with their resistance to drought, this makes camel ranching a viable (though unexplored) alternative to horse and cattle ranching in the Mezquital.[1] Gomphotheres were large enough to knock adult trees, like elephants do to similar species in Africa, and might have fed on mesquite pods and prickly pears during their respective fruiting seasons.[1] |
 Nopal |
Opuntia ficus-indica | Central Mexico | Defensive spines at heights far above the range of current browsers. The whole plant is consumed by camels in North Africa and Australia, where animal and plant alike have been introduced and are now feral, and was sought by the camels of the United States Camel Corps. Camels and other livestock also disperse the seeds.[1] | Western camel[1] Gomphotheres[1] |
 Osage orange |
Maclura pomifera | East Texas | The orange-sized fruit is eaten in place by mice, rabbits, tree squirrels and deer, but they don't swallow nor store the seeds. It is eaten less discriminately by domestic horses and mules.[27] The defensive spines on its branches are also too wide spaced to dissuade deer-sized ungulates from eating the leaves, making them only effective against larger animals that aren't alive in the wild in Texas. In addition, fossils show that this species used to be distributed all the way to southern Canada during previous interglacials, suggesting that its range area shrank dramatically after its seed dispersal capacity was diminished.[26][27] Distribution might have been even smaller before the introduction of horses to Texas in the 16th century, even though the wood was favored by many Native American peoples to fashion bows and the local tribes profited greatly from its trade.[27] A close African relative is dispersed by forest elephants in Gabon.[1] | Columbian mammoth[26] Ground sloths[26] American mastodon[27] American horses[27] Gomphotheres[1] |
  Pawpaw |
Asimina triloba | Eastern North America | The species largely reproduces asexually today, sprouting patches of small, clonal trees that live around 50 years, from a root system that can live tens of thousands. Its sexual reproduction is elaborate but ineffective. The flower mimics carrion or dung (brown color, fetid odor), but it is rarely visited and pollinated by flies. The downward-facing flower is better suited to be pollinated by beetles, as it is known to happen in related species, all of which live in warmer climates. The fruit is similar in taste and nutritious value to cherimoya and it is the largest edible and the most fleshy in the United States. However, the fruiting season is short and the fruit rots soon after falling from the tree; for this reason the pawpaw's consumption was abandoned when commercial tropical fruits became available. The seeds are also large and encased in a sweet, but slippery aril that is difficult to remove from them. The species distribution is very patchy and it is more abundant in floodplains and where it was cared for by indigenous peoples of the Eastern Woodlands. However, the plant grows with no problem in uplands and humans eat the pulp without swallowing the seeds. The seed dispersal capacity of foxes, raccoons, skunks and American black bears is unclear.[1] | American mastodon[1] Dung beetles could have been the main pollinators of the pawpaw before they became rarer after the extinction of the megafauna[1] |
 Red Devil's claw |
Proboscidea parviflora | Southwestern United States and northern Mexico | Sticky, repugnant leaves invulnerable to herbivore predation. The fruit divides in two opposite claws when it browns and hardens, the circumference of each being larger than a human leg. Though an obvious zoochoric mechanism, this is far larger than the leg thickness of the largest wild mammals in the area (deer, peccaries, coyotes), and as a result the seed is mostly dispersed by humans, horses and cattle. Though already cultivated by Native Americans to make baskets, the species greatly expanded its range after the Europeans introduced livestock in the area. The range now expands into Louisiana and Iowa.[1] | |
 Squash |
Cucurbita pepo | Mexico, Texas, and the Eastern United States | Unlike its many domestic varieties, the wild form is bitter to humans.[1] | Seeds found in association with American mastodon fossils in Florida, including stomach contents.[1] |
 Yellow tomato  Wild tomato |
Solanum elaeagnifolium S. carolinense |
Western North America and South America Southeastern United States |
Mostly found in disturbed sites and floodplains. Fruit often remains on the branch for months or over a year after ripening, when it is already rotten or desiccated, holding the seeds trapped in its interior. Mammals and birds shun the fruit for its high glycoalkaloid content, which is even lethal to livestock. Reptiles, on the other hand, are not affected by them, and the fruit has features that makes it attractive to turtles (yellow-orange color and right height of fructification), just like other related plants.[1] | The Box turtle and Gopher tortoise inhabited many areas where wild tomatoes are found, before they went locally extinct.[1] Hesperotestudo[1] |
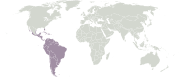
Neotropical realm
-
Extent of the Neotropical realm
-
Cuvieronius restoration
-
Toxodon restoration
-
Restoration of Glyptotherium, a glyptodont
-
Restoration of Amerhippus, a subgenus of modern horses that was endemic to South America in the Pleistocene
| Example | Binomial name | Native range | Anachronism description | Suggested extinct coevolutionary partners |
|---|---|---|---|---|
| Acacia riparia | Central America, South America and the Caribbean[29] | Recurved thorns on twigs and leaves.[2] | Ground sloths[2] Gomphotheres[2] | |
| Almendro | Dipteryx panamensis | Honduras to Colombia[30] | [2] | Gomphotheres[2] |
 American figs |
Ficus spp. | Neotropics | Excessive fruit yield, more than bats and spider monkeys can take.[2] | |
 Ara a gato |
Senegalia tenuifolia | California to Bolivia and Brazil, including the Caribbean | Recurved thorns on twigs and leaves.[2] | Ground sloths[2] Gomphotheres[2] |
 Avocado |
Persea americana | Mesoamerica | Although the pulp is nutritive and eaten by many animals (even carnivores), the seeds are too large to be swallowed by most. Zoochory is limited to seeds hoarded by agoutis or eaten by jaguars, but this is more occasional than common. Avocado relatives in different latitudes have smaller fruits and seeds, and are eaten by vegetarians. The pulp is so soft that it doesn't need chewing, but the seeds are poisonous. Forest Elephants have been observed entering plantations in Cameroon and feeding on avocados.[1][31] | Reaching up to six meters tall, the adults of the giant ground sloth Eremotherium could have gained access to the ripe avocados before any other mammal (and the juveniles, small enough to climb trees, might have reached even higher). The soft, fatty pulp might have made avocados more attractive to ground sloths than other fruits, because ground sloths lacked both incisors and canines[1] Cuvieronius[1] Toxodon[1] Glyptodonts[1] Brontotheres[1] |
 Baboonwood |
Virola surinamensis | Costa Rica to Brazil and Peru | Fruit with typical features of those dispersed by birds and monkeys (bright red, dehiscent, with seeds individually coated with fleshy aril), if slightly larger than usual. However, its known assemblage of bird and mammal dispersal agents is anomalously small and the fruit is often found rotting on the ground. The plant sprouts better from larger seeds, but the seeds better dispersed are the smaller ones that can be ingested by birds.[1] | Protopithecus, a distant relative of howler and spider monkeys but twice the size of the largest living New World monkey.[1][32] |
 Black calabash |
Amphitecna macrophylla | Small patches of Mexico and Guatemala | [2] | Gomphotheres[2] |
 Black palm |
Astrocaryum standleyanum | Nicaragua to Ecuador | [2] | Gomphotheres[2] |
 Black sapote |
Diospyros nigra | Eastern Mexico, the Caribbean, Central America, and Colombia | [1] | |
| Boat-spine acacia | Acacia cochliacantha | Mexico | Extremely thorny at shrub level, almost entirely unarmed at tree level.[2] | |
| Bunchosia biocellata | Southeastern Mexico to Nicaragua[33] | [2] | ||
 Cabbage tree |
Andira inermis | Southern Mexico to Northern South America | Fruit eaten by bats but often found felled under the tree; passed over by domestic pigs, horses and cattle, possibly due to high antibiotic content in its pulp. The seeds of the uneaten fruit are in turn killed by weevil larvae.[2] | Gomphotheres[2] Toxodon[2] |
 Calabash tree |
Crescentia cujete | Central and South America | Fruit the size of a soccer ball, with a hard rind that is tough to crack. The largest living native mammal, Baird's tapir, cannot open its mouth wide enough to position its incisors in a way capable of biting it. The only animals ever witnessed feeding on the fruit are domestic horses, which step on top of the fruit and must employ as much as two hundred kilograms of pressure to open it. The seeds are rubbery and surrounded by slimy black tissue that is both fetid and very sweet. The fruit falls to the ground while it still is green, and ripens after a month on the forest floor.[1] | American horses[1] Toxodon, a rhinoceros-sized tropical notoungulate with enormous, unusually oriented incisors whose function is poorly understood. These might have evolved specifically to peel fruits of this type[1] |
 Carao |
Cassia grandis | Southern Mexico to Venezuela and Ecuador | Hard, cylindrical, half-meter long fruit with an inch and a half of diameter, containing large seeds 2 centimeters long, 1.5 cm wide and 0.5 cm thick, embedded in sweet molasses-like pulp. Currently, the fruit often remains on the tree long enough for bean weevils and moths to kill all the seeds, making it an obvious maladaptation.[1] | Ground sloths[1] Cuvieronius[1] |
| Cedron | Simaba cedron | Colombia and Central America | [2] | Gomphotheres[2] |
 Ceiba tree |
Ceiba aesculifolia[2] C. pentandra[2] C. speciosa[citation needed] |
Tropics, mostly in America but also Africa and southeast Asia | Prominent trunk spines (only saplings in C. pentandra's case).[2] | Browsing megafauna[2] |
 Central American burs[34] |
Aeschynomene spp. Bidens riparia Desmodium spp. Krameria cuspidata Petiveria alliacea Pisonia macrunthocarpa Triumfetta lappula |
Central America | Burs stick to the dense hair of horses and cattle, but not to native wild mammals like tapirs, pacas, collared peccaries or white-lipped peccaries. Excluding Pisonia and Krameria, all are herbaceous species that occur on open, well-trampled habitats.[2] | Gomphotheres Toxodon Ground sloths |
 Cherimoya  Custard apple and relatives |
Annona cherimola[1] A. reticulata[1] A. muricata[1] A. squamosa[1] A. purpurea[2] A. holosericea[2] A. reticulata[2] Sapranthus palanga[2] |
Neotropics | Cuvieronius[1] | |
 Chilean mesquite |
Prosopis chilensis | Peru, eastern Argentina and central Chile | Sweet fruit with hard seeds. Grows mostly in floodplains and stream margins, in natural corridors followed by livestock herds.[2] | |
 Christ's Crown of Thorns |
Gleditsia amorphoides | Argentina | Defensive trunk spines up to forty centimeters long.[1] | American horses[1] Proboscideans[1] |
 Cocoa tree |
Theobroma spp. | Central and South America | [2] | Gomphotheres[2] |
 Divi-divi |
Caesalpina coriacea | Caribbean, Mexico, Central and Northern South America | [2] | |
 Dyer's mulberry |
Maclura tinctoria | Mexico to Argentina | Saplings with trunk spines.[2] | Browsing megafauna.[2] |
 Genipapo |
Genipa americana | Southern Mexico to Peru | [2] | |
| Grangel | Randia echinocarpa | Mexico | Sweet fruit with hard seeds. Grows mostly in floodplains and stream margins, in natural corridors followed by livestock herds.[2] | |
 Grugru |
Acrocomia aculeata[2] | Southern Mexico and the Caribbean to Paraguay and northern Argentina | Large fruit and seeds, with tough epicarp, sticky pulp and very hard endocarp. The fruit grows at heights suitable for terrestrial mammals, but it is often found in piles on the ground under the tree, uneaten, and accompanied by thousands of even older, ungerminated seeds. Young trees are heliophilous, requiring the clearing of older trees to grow. Domestic cattle ingest the fruit, dispersing the seeds when they regurgitate them during rumination, and also help the establishment of new plants through trampling of older vegetation.[35] Long trunk and leaf spines ill-suited to dissuade smaller predators like rodents.[2] | Browsing megafauna[2] |
  Guanacaste tree |
Enterolobium cyclocarpum | Central Mexico to northern Brazil and Venezuela | The flowers grow rapidly into a large, fleshy, ear-shaped pod during the dry season a year after they are fertilized. The ripe pods are brown and cacao-flavored, and fall to the ground over the space of a month. Though many wild animals eat the pods' flesh, only tapirs are large enough to also swallow and disperse the seeds. The pods are also eaten and dispersed with ease by domestic horses and cattle, however, and as a result the trees are common in areas cleared for pasture or near them.[1][2][36] | American horses[36] Gomphotheres[36] Glyptodonts[36] Ground sloths[36] Columbian mammoth[36] Toxodonts[36] |
 Guapinol |
Hymenaea courbaril | Caribbean, Central and South America | Thick woody pod with dry sugary pulp of the same color as the honey locust. Although showing obvious signs of megafaunal dispersal syndrome, the species is currently dispersed almost exclusively by a seed-hoarding rodent, the agouti.[1] | Gomphotheres[2] |
| Guatemalan zizfum | Ziziphus guatemalensis | Chiapas to Costa Rica[37] | [2] | |
| Guayabillo | Chloroleucon mangense | Central, Northern South America and the Caribbean | Sweet fruit with hard seeds. Grows mostly in floodplains and stream margins, in natural corridors followed by livestock herds.[2] | |
| Indigoberry | Randia echinocarpa | Mexico | [2] | |
| Ixtle | Aechmea magdalenae | Southern Mexico to Ecuador | [2] | Gomphotheres[2] |
 |
Jacquinia pungens | Southern Mexico to Costa Rica | Produces leaves with needle-sharp tips only in the dry season. Spines best developed within four to six meters of the ground.[2] | Ground sloths[2] Gomphotheres[2] |
 Locust bean |
Parkia pendula | Honduras to Bolivia and Brazil[38] | [2] | Gomphotheres[2] |
 Manchineel Manchineel |
Hippomane mancinella | Southern North America and Northern South America | Small seeds imbedded in a hard core.[2] | |
 Maya nut Maya nut |
Brosimum alicastrum | Yucatan and Guatemala to the Amazon | [2] | |
 Mexican calabash |
Crescentia alata | Mesoamerica and Central America | Close relative of the calabash tree, with white, orange-sized fruit. If not mechanically broken, the seeds will die either from desiccation (in a dry environment) or when the pulp ferments (in moist).[1] The fruit is often consumed by free-ranging horses, and the tree's size (3–4 meters tall) and shape is similar to an African tree typically dispersed by megafauna.[2] | Fossils of the native horse Amerhippus have been found in the plant's current range area.[2] |
| Mexican ebony | Pithecellobium mexicanum | Sonora, Sinaloa and Baja California Sur[39] | Sweet fruit with hard seeds. Grows mostly in floodplains and stream margins, in natural corridors followed by livestock herds.[2] | |
| Mimosa | Mimosa eurycarpa M. guanacastensis |
Central and South America | Recurved thorns in twigs and leaves.[2] | Ground sloths[2] Gomphotheres[2] |
 Monkeypod |
Pithecellobium dulce | Pacific coast of Mexico, Central and northern South America | Sweet fruit with hard seeds. Grows mostly in floodplains and stream margins, in natural corridors followed by livestock herds.[2] | |
 Nance |
Byrsonima crassifolia | Central Mexico to Bolivia and Brazil, including the Caribbean | [2] | |
| Nicaragua persimmon | Diospyros nicaraguensis | Eastern Yucatan, southern Nicaragua and northern Costa Rica[40] | Large fruit production that just rots on the ground.[1] | |
 Forest palm |
Attalea rostrata | Central America[41] | Large fruit and seeds, with tough epicarp, sticky pulp and very hard endocarp. The fruit grows at heights suitable for terrestrial mammals, but it is often found in piles on the ground under the tree, uneaten, and accompanied by thousands of even older, ungerminated seeds. Young trees are heliophilous, requiring the clearing of older trees to grow. Domestic cattle ingest the fruit, dispersing the seeds when they regurgitate them during rumination, and also help the establishment of new plants through trampling of older vegetation.[35] | Cuvieronius[2] |
 Jobo |
Spondias mombin S. purpurea S. radlkoferi |
Neotropics | Excessive fruit crop with small seeds imbedded in a hard core.[1][2] | |
| Ojo de Buey | Dioclea megacarpa | Western Nicaragua[42] | [2] | |
 Papaya |
Carica papaya | Central and northern South America | The fruit is already large in the wild form, reaching about ten centimeters. The pulp is soft and doesn't require chewing, but the seeds are poisonous. The seeds are small but clustered at the center, and have a pungent, peppery taste. Forest Elephants have been observed entering plantations in Cameroon and feeding on papayas.[26][27] | Cuvieronius[1] Ground sloths[1] Toxodon[1] |
 Peine de mico |
Apeiba tibourbou | Caatinga, Cerrado and Costa Rica | [2] | |
 Piñuela |
Bromelia karatas B. pinguin |
Sinaloa to Brazil | [2] | |
 Pochote |
Pachira quinata | Costa Rica to Colombia and Venezuela | Prominent trunk spines, especially in younger trees.[2] | Browsing megafauna[2] |
 Pouteria tree |
Pouteria spp. | Neotropics | [2] | Gomphotheres[2] |
 Pupunha |
Bactris guineensis[2] B. major[2] |
Mexico to Colombia, Venezuela and Trinidad | Large fruit and seeds, with tough epicarp, sticky pulp and very hard endocarp. The fruit grows at heights suitable for terrestrial mammals, but it is often found in piles on the ground under the tree, uneaten, and accompanied by thousands of even older, ungerminated seeds. Young trees are heliophilous, requiring the clearing of older trees to grow. Domestic cattle ingest the fruit, dispersing the seeds when they regurgitate them during rumination, and also help the establishment of new plants through trampling of older vegetation.[35] Long leaf spines ill-suited to dissuade smaller predators like rodents.[2] | |
 Purui |
Alibertia edulis | Caribbean coast of Central America | [2] | |
 Rain tree |
Albizia saman | Mexico to Peru and Brazil | Fruit eaten by domestic horses and cattle.[2] | |
 Sachamango |
Gustavia superba | Central and Northwestern South America | [1] | |
| Sali | Tetragastris panamensis | Guatemala to Bolivia and Brazil[43] | Fruit very similar to Baboonwood. Seed waste deemed "enormous" and known dispersal agents "inefficient".[1] | Protopithecus[1] |
 Sandbox tree |
Hura crepitans | Tropical North and South America | Prominent trunk spines, especially in young trees.[2] | Browsing megafauna[2] |
 Sapodilla |
Manilkara zapota | Mexico, Central America and the Caribbean | [2] | |
| Shinglewood | Nectandra hihua | Southern Sonora | Sweet fruit with hard seeds. Grows mostly in floodplains and stream margins, in natural corridors followed by livestock herds.[2] | |
| Sphinga platyloba | Central America | Recurved thorns on twigs and leaves.[2] | Ground sloths Gomphotheres[2] | |
 Sweet acacia |
Vachellia farnesiana | Mexico and Central America | Fruit sought by domestic cattle and horses.[2] | |
| Taruma | Vitex mollis | Southern Sonora | Sweet fruit with hard seeds. Grows mostly in floodplains and stream margins, in natural corridors followed by livestock herds.[2] | |
 Tempisque |
Sideroxylon capiri | Mesoamerica and the West Indies | [2] | |
| Velvetseed | Guettarda macrosperma | Chiapas to Costa Rica[44] | [2] | |
 West Indian elm |
Guazuma ulmifolia | Neotropics | Sweet fruit with hard seeds, which is eaten by domestic horses and cattle. Grows mostly in floodplains and stream margins, in natural corridors followed by livestock herds.[2] The pulp has woody obstacles that prevent mastication.[1] | |
 White bayahonda |
Prosopis juliflora | Mexico, South America and the Caribbean | Very localized and patchy distribution along margins of mangrove swamps and beaches. Ingested by cattle and horses.[2] | |
 |
Zamia spp. | Mexico to Bolivia, including the West Indies | [2] | Gomphotheres[2] |
| Zanthoxylum setulosum | Costa Rica to Colombia and Venezuela[45] | Prominent trunk spines, especially in young trees.[2] | Browsing megafauna[2] |
Oceanian realm
-
Restoration of the Maui Nui large-billed moa-nalo and the small-billed moa-nalo
-
Restoration of the turtle-jawed moa-nalo
-
Restoration of the O'ahu moa-nalo
-
Stuffed Hawaii mamo, an extinct Hawaiian honeycreeper
-
Contemporary depiction of Laysan honeycreepers, also extinct
| Example | Binomial name | Native range | Anachronism description | Suggested extinct coevolutionary partners |
|---|---|---|---|---|
 Hawaiian lobelioids |
Cyanea spp. | Hawaii | Defensive thorns in leaves and stems despite no native browsers being found in the islands. | Moa-nalo, four extinct species of flightless ducks identified as browsers from their beak morphology and fossil excrements |
 Mountain hibiscus |
Hibiscadelphus spp. | Hawaii | Eight extinct or endangered species of Hibiscus relatives whose flowers remain folded in a tube, limiting pollination | Several species of Hawaiian honeycreepers, some extinct and others endangered, with varying beak lengths and curvatures suited to feed in the nectar of different tubular flowers |
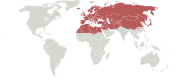
Palearctic realm
-
Extent of the Palearctic biogeographical realm
-
Restoration of Hippopotamus gorgops
-
Straight-tusked elephant restoration
-
Megaloceros restoration
-
Woolly mammoth restoration
| Example | Binomial name | Native range | Anachronism description | Suggested extinct coevolutionary partners |
|---|---|---|---|---|
 European holly |
Ilex aquifolium | Western Europe | Leaves with defensive spiny edges up to four or five meters, when they are replaced by smooth leaves.[1] This is more than twice the reach of the current largest browsers in the area, the red deer and the wisent. | |
 Hazel  Oak |
Corylus spp. Quercus spp. |
Temperate Northern Hemisphere | Inability to regenerate in either the deep shade of a forest canopy or under heavy browsing in the open. Though some Eurasian megafauna capable of clearing forests survived into the Holocene (red deer, aurochs, tarpan, wisent, Eurasian beaver and wild boar), differences in the composition of pollen records between the earliest Holocene previous to large human-induced clearing and the interglacial MIS 5 suggests that further clearing was done by even larger megaherbivores disappeared in the Late Pleistocene.[46] | Hippopotamus[46][47] Straight-tusked elephant[46][47] Narrow-nosed rhinoceros[46][47] |
 Juniper Juniper |
Juniperus spp. | Northern Hemisphere | Reduction of fossil pollen concentration in Ireland and subsequent increase unrelated to climate change.[46] | The giant deer Megaloceros colonized Ireland right around the time juniper numbers went down and became extinct when they went up.[46] Megaloceros browsed juniper and other shrubs because of their high phosphorus concentration, which was needed in turn to grow the giant deer's massive antlers for the mating season.[48] This predation caused in turn the descent of juniper and its replacement by grasses.[46] |
 Mammoth steppe |
Several unrelated species | Altai-Sayan Mountains | Dry, but botanically diverse biome, composed of grasses, forbs and sedges, which occupied most of northern Eurasia and North America during the Pleistocene and was associated with high concentrations of large grazers. Starting about 13,000 years ago, the steppe was replaced by wet mossy and shrub tundra, taiga and deciduous forests with reduced plant diversity. The change has been traditionally attributed to a climatic shift to warmer, wetter, less continental conditions in the transition to the Holocene, and in turn used to explain the extinction of the local megafauna. Sergey Zimov proposes the opposite: That the extinction of the fauna caused the change in vegetation, and that this wouldn't have happened if the megafauna was still around, just like it didn't happen in previous interglacials.[46] | Woolly mammoth[46][1] Muskox[1] Steppe bison[1] Wild horse[1] |
 |
Chamaerops humilis | Southwest Mediterranean | Successful seed dispersal by introduced species (feral goat and pine marten) as probable proxies for the extinct Myotragus balearicus in the Balearic Islands.[49] | Myotragus balearicus |
| Argan tree | Argania spinosa | Morocco | Thorns actually protect against goats climbing the trees, but there's no native herbivores capable of reaching the branches. | Nord-African elephant |
Proposed examples in animals
-
Shrub-ox restoration
-
Teratornis restoration
-
Stegodon restoration
-
Giant short-faced bear restoration
-
American lion restoration
| Example | Binomial name | Native range | Anachronism description | Suggested extinct coevolutionary partners |
|---|---|---|---|---|
| Australian bush fly | Musca vetustissima | Australia | Native dung fly dependent on introduced cattle, and before cattle was introduced, on human dung. The flies ignore kangaroo dung because it is drier and not as abundant.[15] | Dung of Australian megafauna |
  Brown-headed cowbird |
Molothrus ater | North America | Flocks follow horse and cattle herds, feeding on insects stirred up by the ungulates' trampling. Their numbers and eastern range expanded greatly after these were introduced to the area with European colonization; however, fossils show that they were just as numerous or more in the Pleistocene, and also that there were two other species in North America that disappeared during the transition to the Holocene.[50] | American bison[50] Harlan's muskox and shrub-ox[50] American horses[50] North American llama[50] Western camel[50] Columbian mammoth[50] American mastodon[50] |
 California condor |
Gymnogyps californianus | Western North America | Critically endangered and only found in a few areas of California and Arizona. Before the human settlement of the Americas, however, the same species (or others very closely related) were commonly found through North America, Cuba and South America as far south as Peru. | North American megafauna It was suggested that condors survived near the Pacific by feeding mostly on beached whales and elephant seal carcasses, which provide a lot of meat, but have skin soft enough to be pierced by the condor's weak beak. Elsewhere, the condor would have fed on terrestrial megafauna, but only after larger carrion birds like Teratornis had pierced their tough, furry skin, mirroring the symbiotic relationship between African white-backed vultures and the larger lappet-faced vultures and white-headed vultures.[1] Coincidentally, the only other living condor, the Andean condor, is also limited to the Pacific coast of South America and is known to feed on beached whales, but the lack of a fossil record for this species means that it is impossible to know if it existed previously in other areas. |
 Cuban crocodile |
Crocodylus rhombifer | Cuba's Zapata Swamp and Isle of Youth | Critically endangered species that was once widespread through Cuba and also present in the Cayman Islands and the Bahamas. One of the smallest crocodiles in the world, it is also among the most terrestrial and intelligent. Observations in captivity revealed previously unknown pack-hunting behavior, which would make it capable of taking down animals larger than the largest ones currently found in Cuba.[51] | Six Caribbean ground sloths,[51] the largest of which was the size of an American black bear[1] |
 |
Helictopleurus giganteus | Eastern Madagascar | The largest and most rare of native dung beetle species in Madagascar, apparently entirely dependent on human feces. Yet humans arrived in Madagascar for the first time only 2000 years ago.[52] | Giant lemurs[52] |
 Hyacinth macaw  Indigo macaw Indigo macaw |
Anodorhynchus hyacinthinus A. leari |
South America | Both species follow cattle herds in Brazil (mostly of the zebu-crossed Brahman race, which is a bigger fruit eater) and extract partially digested seeds from their dung. They have adaptations to terrestrial locomotion not present in other macaws, and they ignore the same fruit species while still on the tree, even when ripe, suggesting that this behavior is an ancient adaptation rather than recently learned. Grey parrots do the same with dung of African elephants.[35] It is unknown if the same behavior was exhibited by the third Anodorhynchus species, A. glaucus, which was originally present in Paraguay and northern Argentina and is probably extinct. | Cuvieronius[1] |
  Komodo dragon |
Varanus komodensis | Flores and other Indonesian islands, such as Komodo | Though an endemic species, this large reptile survives largely by hunting or scavenging artiodactyls like Javan rusa deer, banded pig and water buffalo, all of which were introduced to the islands by humans. | Dwarf stegodonts (Stegodon florensis),[53] pygmy elephants around the same size as bovines, horses, and large pigs. More recently, it was suggested that the Komodo dragon's ancestors, including Megalania the largest terrestrial lizard ever, evolved their large size in northern Australia and then spread north to colonize Indonesian islands including Flores.[54] If true, this would make the Komodo dragon a double example, as they would have originally preyed on giant marsupials such as Diprotodon. Interestingly, pigs, cattle, deer, camels, horses, and buffaloes have all been introduced to Australia, where they have no predators and are now overpopulated and causing ecological and agricultural concerns. It has been suggested to introduce Komodo dragons as part of rewilding efforts and to control populations of these introduced feral livestock species[55] |
| Merobruchus columbinus | Central America and the Caribbean[56] | Bean weevil parasiting the fruit of Albizia saman. The animals leave the fruit just before the fall, even though it is still nutritive then.[2] | The rapid exit may be an adaptation to avoid accidental ingestion by large mammals, now extinct[2] | |
  Pronghorn |
Antilocapra americana | Western North America | Capable of sustaining speeds of 60 miles per hour, making it the second fastest land animal in the world, after the cheetah, and the fastest long-endurance runner. No carnivores found in its range approach this speed.[4] Cougars are the only regular predators of adult pronghorns, but can only hunt them when the terrain allows for a stealthy approach. Wolves and coyotes may prey on the young but are poorly suited to hunt adults. American black bears have also been known to attempt ambushes on pronghorns on occasion, typically unsuccessfully.[4] The leg muscles are so overbuilt towards sustained speed that pronghorns cannot jump and will try to cross fences by going under rather than above them.[1] | Both the giant short-faced bear and the extinct American lion were larger and better built for sustained speed than their living relatives, the spectacled bear and the lion, respectively[4] The jaguar was present in large areas of the United States during the Pleistocene and might have hunted pronghorns by stealth, just like the cougar[4] The extinct American cheetahs (Miracinonyx inexpectatus and particularly M. trumani) were explosive runners very similar to the living cheetah, though not closely related to it. If they could reach the same speed (70 mph), they would have been the most successful predators of pronghorns in short distances, and also explain the pronghorn's evolution towards sustained running, since modern cheetahs can't keep running for long[4] Chasmaporthetes, the only hyena that ever colonized North America successfully, had cheetah-like proportions and was better built for speed than its living relatives[4] |
 Ring-tailed lemur  Sifakas |
Lemur catta Propithecus diadema P. verreauxi |
Madagascar | The adults practice measures against predation by birds of prey, even though they are too large to be hunted by birds currently found on the island.[57][58] | Malagasy crowned eagle, a relative of the African crowned eagle extinct since c. 1500 AD Extinct Malagasy Aquila eagle |
See also
- Keystone species
- Coevolution
- Evolutionary arms race
- Evolutionary trap
- Coextinction
- Pleistocene extinction
- Holocene extinction
- Pleistocene rewilding
- Youtube video "Why are Lemurs Terrified of Predators that don't Exist?"
- Gourds and squashes (Cucurbita spp.) adapted to megafaunal extinction and ecological anachronism through domestication
References
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar as at au av aw ax ay az ba bb bc bd be bf bg bh bi bj bk bl bm bn bo bp bq br bs bt bu bv bw bx by bz ca cb cc cd ce cf cg ch ci cj ck cl cm cn co cp cq cr cs ct cu cv cw cx cy cz da db dc dd de df dg dh di dj dk Barlow, Connie C. (2000). The Ghosts of Evolution: Nonsensical Fruit, Missing Partners, and Other Ecological Anachronisms. New York: Basic Books. ISBN 9780465005512.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar as at au av aw ax ay az ba bb bc bd be bf bg bh bi bj bk bl bm bn bo bp bq br bs bt bu bv bw bx by bz ca cb cc cd ce cf cg ch ci cj ck cl cm cn co cp cq Janzen, D. H.; Martin, P. S. (1982). "Neotropical Anachronisms: The Fruits the Gomphotheres Ate" (PDF). Science. 215 (4528). American Association for the Advancement of Science (AAAS): 19–27. Bibcode:1982Sci...215...19J. doi:10.1126/science.215.4528.19. ISSN 0036-8075. PMID 17790450. S2CID 19296719.
- ^ TEMPLE, S. A. (1977-08-26). "Plant-Animal Mutualism: Coevolution with Dodo Leads to Near Extinction of Plant". Science. 197 (4306). American Association for the Advancement of Science (AAAS): 885–886. Bibcode:1977Sci...197..885T. doi:10.1126/science.197.4306.885. ISSN 0036-8075. PMID 17730171. S2CID 2392411.
- ^ a b c d e f g Byers, John (1997). American Pronghorn: Social Adaptations and the Ghosts of Predators Past. Chicago: University of Chicago Press. ISBN 9780226086996.
- ^ a b c d e f g h i j k l Godfrey, Laurie R.; Jungers, William L.; Schwartz, Gary T.; Irwin, Mitchell T. (2008). "Ghosts and Orphans". Developments in Primatology: Progress and Prospects. New York, NY: Springer New York. pp. 361–395. doi:10.1007/978-0-387-73896-3_24. ISBN 978-0-387-73895-6.
- ^ Barras, Colin (2019-02-26). "The secret of the world's largest seed revealed". New Scientist.
- ^ a b c d e Jackson, P.S.W.; Cronk, Q.C.B.; Parnell, J.A.N. (1988). "Notes on the regeneration of two rare Mauritian endemic trees". Tropical Ecology (78): 56–65.
- ^ a b Crowley, Brooke E.; Godfrey, Laurie R. (1990-01-06). "Why all those spines?: Anachronistic defences in the Didiereoideae against now extinct lemurs". South African Journal of Science. 109 (1–2): 1–7. doi:10.1590/sajs.2013/1346. ISSN 0038-2353.
- ^ Dransfield, John; Beentje, Henk (1995). The Palms of Madagascar. Kew, Victoria, Australia: Royal Botanic Gardens. ISBN 0-947643-82-6.
- ^ a b c d Crowley, Brooke E.; Godfrey, Laurie R.; Irwin, Mitchell T. (2010-12-15). "A glance to the past: subfossils, stable isotopes, seed dispersal, and lemur species loss in Southern Madagascar". American Journal of Primatology. 73 (1). Wiley: 25–37. doi:10.1002/ajp.20817. ISSN 0275-2565. PMID 20205184. S2CID 25469045.
- ^ a b Bond, William J; Silander, John A (2007-05-29). "Springs and wire plants: anachronistic defences against Madagascar's extinct elephant birds". Proceedings of the Royal Society B: Biological Sciences. 274 (1621). The Royal Society: 1985–1992. doi:10.1098/rspb.2007.0414. ISSN 0962-8452. PMC 2275176. PMID 17535797.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac Weber, Lui (Spring 2013). "Plants that miss the megafauna". Wildlife Australia: 22–25. ISSN 0043-5481.
{{cite journal}}: CS1 maint: date and year (link) - ^ a b c d e f g h i j k l m Johnson, Chris (2006). Australia's Mammal Extinctions: A 50,000-Year History. Melbourne: Cambridge University Press. ISBN 978-0-521-68660-0.
- ^ a b c d e f Low, Tim (2017-03-07). "What the Giants Ate". australiangeographic.com.au. Archived from the original on 2018-07-09.
- ^ a b c d e f g h i j Low, Tim (2017). The new nature. Docklands, Vic: Penguin Random House. ISBN 978-0-14-378363-3. OCLC 956766454.
- ^ "Terminalia arostrata". Useful Tropical Plants.
- ^ a b Murray, Peter F.; Vickers-Rich, Patricia (2004). Magnificent Mihirungs: The Colossal Flightless Birds of the Australian Dreamtime. Bloomington: Indiana University Press. ISBN 0-253-34282-1.
- ^ "Siphonodon australis".
- ^ "Lepidium aschersonii — Spiny Pepper-cress".
- ^ "Calamus radicalis". Palmpedia - Palm Grower's Guide.
- ^ "Capparis canescens".
- ^ Coprosma acerosa pictured
- ^ Bauer, Aaron M. [in French]; Russell, Anthony P. (1986). "Hoplodactylus delcourti n. sp. (Reptilia: Gekkonidae), the largest known gecko" (PDF). New Zealand Journal of Zoology. 13 (1). Informa UK Limited: 141–148. doi:10.1080/03014223.1986.10422655. ISSN 0301-4223.
- ^ a b Nepi, Massimo; Little, Stefan; Guarnieri, Massimo; Nocentini, Daniele; et al. (2017-10-16). "Phylogenetic and functional signals in gymnosperm ovular secretions". Annals of Botany. 120 (6). Oxford University Press (OUP): 923–936. doi:10.1093/aob/mcx103. ISSN 0305-7364. PMC 5710648. PMID 29045531.
- ^ Royer, Dana L.; Hickey, Leo J.; Wing, Scott L. (2003). "Ecological conservatism in the "living fossil" Ginkgo" (PDF). Paleobiology. 29 (1). Cambridge University Press (CUP): 84–104. doi:10.1666/0094-8373(2003)029<0084:ecitlf>2.0.co;2. ISSN 0094-8373.
- ^ a b c d e f g h i j Bronaugh, Whit (2010). "The Trees That Miss The Mammoths". American Forests. 115 (4): 38–43.
- ^ a b c d e f g h i Barlow, Connie (2001). "Anachronistic Fruits and the Ghosts Who Haunt Them" (PDF). Arnoldia. 61 (2): 14–21.
- ^ a b c d Lenz, Lee W. (2001). "Seed dispersal in Yucca brevifolia (Agavaceae) - Present and past, with considerations of the future of the species". Aliso. 20 (2): 61–74. doi:10.5642/aliso.20012002.03.
- ^ Groom, A. (2012). "Acacia riparia". IUCN Red List of Threatened Species. 2012: e.T19892630A20123577. doi:10.2305/IUCN.UK.2012.RLTS.T19892630A20123577.en.
- ^ "Dipteryx oleifera". Useful Tropical Plants.
- ^ B. N. Wolstenholme; A. W. Whiley (1999). "ECOPHYSIOLOGY OF THE AVOCADO (Persea americana Mill.) TREE AS A BASIS FOR PRE-HARVEST MANAGEMENT" (PDF). Revista Chapingo Serie Horticultura. 5: 77–88.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hartwig, Walter Carl; Cartelle, Castor (1996-05-23). "A complete skeleton of the giant South American primate Protopithecus". Nature. 381 (6580): 307–311. Bibcode:1996Natur.381..307H. doi:10.1038/381307a0. ISSN 1476-4687. PMID 8692267. S2CID 4262976.
- ^ "Bunchosia biocellata SCHLTDL". Tropicos.
- ^ Triumfetta lappula pictured
- ^ a b c d Yamashita, Carlos (2013-05-17). "Anodorhynchus macaws as followers of extinct megafauna: an hypothesis". Revista Brasileira de Ornitologia - Brazilian Journal of Ornithology. 5 (7). ISSN 2178-7875.
- ^ a b c d e f g Cherfas, J (1987). "A tropical tree that travels by horse". New Scientist (1564): 46–52. ISSN 0262-4079.
- ^ "!Ziziphus guatemalensis Hemsl". Tropicos.
- ^ "Parkia pendula". Useful Tropical Plants.
- ^ "Pithecellobium mexicanum". Arid Zone Trees.
- ^ "Diospyros nicaraguensis (Standl.)". Tropicos.
- ^ "Attalea rostrata". Useful Tropical Plants.
- ^ "!Dioclea megacarpa Rolfe". Tropicos.
- ^ "T. panamensis (Engl.) Kuntze". Herbario Panamá (in Spanish).
- ^ "Guettarda macrosperma Donn. Sm". Tropicos.
- ^ "Zanthoxylum setulosum". Useful Tropical Plants.
- ^ a b c d e f g h i Johnson, C.N. (2009-03-18). "Ecological consequences of Late Quaternary extinctions of megafauna". Proceedings of the Royal Society B: Biological Sciences. 276 (1667). The Royal Society: 2509–2519. doi:10.1098/rspb.2008.1921. ISSN 0962-8452. PMC 2684593. PMID 19324773.
- ^ a b c Stuart, A.J.; Lister, A.M. (2007). "Patterns of Late Quaternary megafaunal extinctions in Europe and northern Asia". Courier-Forschungsinstitut Senckenberg. 259: 287–297.
- ^ Moen, Ron A.; Pastor1, John; Cohen2, Yosef (1999). "Antler growth and extinction of Irish elk". Evolutionary Ecology Research. 1 (2): 235–249. CiteSeerX 10.1.1.525.2990. ISSN 1522–0613.
{{cite journal}}: Check|issn=value (help)CS1 maint: numeric names: authors list (link) - ^ Muñoz-Gallego, Raquel; Fedriani, José M.; Traveset, Anna (2019). "Non-native Mammals Are the Main Seed Dispersers of the Ancient Mediterranean Palm Chamaerops humilis L. in the Balearic Islands: Rescuers of a Lost Seed Dispersal Service?". Frontiers in Ecology and Evolution. 7. doi:10.3389/fevo.2019.00161. ISSN 2296-701X.
- ^ a b c d e f g h PEER, Brian D.; RIVERS, James W.; ROTHSTEIN, Stephen I. (2013-03-20). "Cowbirds, conservation, and coevolution: potential misconceptions and directions for future research". Chinese Birds. 4 (1): 15–30. doi:10.5122/cbirds.2013.0009. ISSN 1674-7674.
- ^ a b Alexander, Marc (2006-01-01). "Last of the Cuban crocodile?". Americas (English Edition). Organization of American States. ISSN 0379-0940. Retrieved 2010-07-09.
- ^ a b Viljanen, Heidi; Wirta, Helena; Montreuil, Olivier; Rahagalala, Pierre; Johnson, Steig; Hanski, Ilkka (2010-07-30). "Structure of local communities of endemic dung beetles in Madagascar". Journal of Tropical Ecology. 26 (5): 481–496. doi:10.1017/S0266467410000325. ISSN 1469-7831.
- ^ Diamond, Jared M. (1987). "Did Komodo dragons evolve to eat pygmy elephants?". Nature. 326 (6116). Springer Science and Business Media LLC: 832. Bibcode:1987Natur.326..832D. doi:10.1038/326832a0. ISSN 0028-0836. S2CID 37203256.
- ^ Clarke, Sarah (2009-09-30). "Australia was 'hothouse' for killer lizards". ABC News.
- ^ "Rewilding: should we introduce lions and Komodo dragons to Australia?". ABC Radio National. 2013-07-03.
- ^ Arguedas, Marcela (1997). Plagas de semillas forestales en America Central y el Caribe (in Spanish). Bib. Orton IICA / CATIE. ISBN 978-9977-57-284-0.
- ^ Wright, P. C. (June 1998). "Impact of Predation Risk on the Behaviour of Propithecus diadema edwardsi in the Rain Forest of Madagascar". Behaviour. 135 (4). Brill Publishers: 483–512. doi:10.1163/156853998793066186. JSTOR 4535540.
- ^ Goodman, S. M. (1994). "The enigma of antipredator behavior in lemurs: evidence of a large extinct eagle on Madagascar". International Journal of Primatology. 15 (1). Springer: 129–134. doi:10.1007/BF02735238. S2CID 6129168.