Coluracetam
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
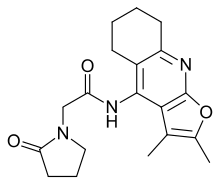
| Formula | C19H23N3O3 |
| Molar mass | 341.411 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Coluracetam (INN) (code name BCI-540; formerly MKC-231) is a nootropic agent of the racetam family.[1] It was initially developed and tested by the Mitsubishi Tanabe Pharma Corporation for Alzheimer's disease. After the drug failed to reach endpoints in its clinical trials it was in-licensed by BrainCells Inc for investigations into major depressive disorder (MDD), which was preceded by being awarded a "Qualifying Therapeutic Discovery Program Grant" by the state of California.[2] Findings from phase IIa clinical trials have suggested that it would be a potential medication for comorbid MDD with generalized anxiety disorder (GAD).[3] BrainCells Inc is currently[when?] out-licensing the drug for this purpose.[4][full citation needed] It may also have potential use in prevention and treatment of ischemic retinopathy and retinal and optic nerve injury.[medical citation needed]
Coluracetam has been shown to reverse the loss of choline acetyltransferase production in the medial septal nucleus of rats exposed to phencyclidine (PCP), and is considered a potential therapeutic drug for schizophrenia.[5]
Mechanism of action
Coluracetam enhances high-affinity choline uptake (HACU),[6] which is the rate-limiting step of acetylcholine (ACh) synthesis. Studies have shown coluracetam to improve learning impairment on a single oral dose given to rats which have been exposed to cholinergic neurotoxins. Subsequent studies have shown that it may induce long-lasting procognitive effects in cholinergic neurotoxin-treated rats by changing the choline transporter regulation system.[7]
Legality
Australia
Coluracetam is a schedule 4 substance in Australia under the Poisons Standard (February 2020).[8] A schedule 4 substance is classified as "Prescription Only Medicine, or Prescription Animal Remedy – Substances, the use or supply of which should be by or on the order of persons permitted by State or Territory legislation to prescribe and should be available from a pharmacist on prescription." [8]
See also
References
- ^ Bessho T, Takashina K, Tabata R, Ohshima C, Chaki H, Yamabe H, et al. (April 1996). "Effect of the novel high affinity choline uptake enhancer 2-(2-oxopyrrolidin-1-yl)-N-(2,3-dimethyl-5,6,7,8-tetrahydrofuro[2,3-b] quinolin-4-yl)acetoamide on deficits of water maze learning in rats". Arzneimittel-Forschung. 46 (4): 369–73. PMID 8740080.
- ^ Qualifying Therapeutic Discovery Project Grants for the State of California, IRS.gov.
- ^ BrainCells Inc. Announces Results From Exploratory Phase 2a Trial of BCI-540 Archived November 21, 2011, at the Wayback Machine
- ^ [Pipeline,BCI-540], BCI-540 (coluracetam).
- ^ Shirayama Y, Yamamoto A, Nishimura T, Katayama S, Kawahara R (September 2007). "Subsequent exposure to the choline uptake enhancer MKC-231 antagonizes phencyclidine-induced behavioral deficits and reduction in septal cholinergic neurons in rats". European Neuropsychopharmacology. 17 (9): 616–26. doi:10.1016/j.euroneuro.2007.02.011. PMID 17467960.
- ^ Murai S, Saito H, Abe E, Masuda Y, Odashima J, Itoh T (1994). "MKC-231, a choline uptake enhancer, ameliorates working memory deficits and decreased hippocampal acetylcholine induced by ethylcholine aziridinium ion in mice". Journal of Neural Transmission. General Section. 98 (1): 1–13. doi:10.1007/BF01277590. PMID 7710736.
- ^ Bessho T, Takashina K, Eguchi J, Komatsu T, Saito K (July 2008). "MKC-231, a choline-uptake enhancer: (1) long-lasting cognitive improvement after repeated administration in AF64A-treated rats". Journal of Neural Transmission. 115 (7): 1019–25. doi:10.1007/s00702-008-0053-4. PMID 18461272.
- ^ a b Poisons Standard February 2020. comlaw.gov.au
