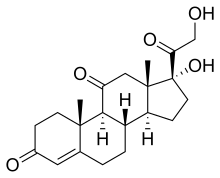
Cortisone
| |
| |
| Names | |
|---|---|
| Pronunciation | /ˈkɔːrtɪsoʊn/, /ˈkɔːrtɪzoʊn/ |
| IUPAC name
(8S,9S,10R,13S,14S,17R)-17-Hydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-1,2,6,7,8,9,12,14,15,16-decahydrocyclopenta[a]phenanthrene-3,11-dione
| |
| Other names
17α,21-Dihydroxypregn-4-ene-3,11,20-trione; 17α,21-Dihydroxy-11-ketoprogesterone; 17α-Hydroxy-11-dehydrocorticosterone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.149 |
| KEGG | |
| MeSH | Cortisone |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C21H28O5 | |
| Molar mass | 360.450 g·mol−1 |
| Melting point | 220 to 224 °C (428 to 435 °F; 493 to 497 K) |
| Pharmacology | |
| H02AB10 (WHO) S01BA03 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Cortisone is a pregnane (21-carbon) steroid hormone. It is one of the main hormones released by the adrenal gland in response to stress. In chemical structure, it is a corticosteroid closely related to cortisol. It is used to treat a variety of ailments and can be administered intravenously, orally, intra-articularly (into a joint), or transcutaneously. Cortisone suppresses the immune system, thus reducing inflammation and attendant pain and swelling at the site of the injury. Risks exist, in particular in the long-term use of cortisone.[1][2]
It was first described in 1949.[3]
Effects and uses
Cortisone, a glucocorticoid, and epinephrine (adrenaline) are the main substances released by the body as a reaction to stress. They elevate blood pressure and prepare the body for a fight or flight response.
A cortisone injection can be used to give short-term pain relief and reduce the swelling from inflammation of a joint, tendon, or bursa in, for example, the joints of the knee, elbow, and shoulder [1] and into a broken coccyx.[4]
Cortisone may also be used to deliberately suppress immune response in persons with autoimmune diseases or following an organ transplant to prevent transplant rejection.[medical citation needed] The suppression of the immune system may also be important in the treatment of inflammatory conditions.[5]
Cortisone is also used by dermatologists to treat keloids,[6] relieve the symptoms of eczema and atopic dermatitis,[7] and stop the development of sarcoidosis.[medical citation needed]
Side effects
Oral use of cortisone has a number of potential systemic side-effects: Asthma, hyperglycemia, insulin resistance, diabetes mellitus, osteoporosis, anxiety, depression, amenorrhoea, cataracts, Cushing's syndrome and glaucoma, among other problems.[1][2]
Local side effects are rare but can include: pain, infection, skin pigment changes, loss of fatty tissue, and tendon rupture.[8]
History
Cortisone was first identified by the American chemists Edward Calvin Kendall and Harold L. Mason while researching at the Mayo Clinic.[9][10][11] In 1929, Philip S. Hench and colleagues discovered that cortisone is effective in the treatment of rheumatoid arthritis.[12] Kendall was awarded the 1950 Nobel Prize for Physiology or Medicine along with Philip S. Hench and Tadeus Reichstein for the discovery of adrenal cortex hormones, their structures, and their functions. As it turns out, both Reichstein and the team of O. Wintersteiner and J. Pfiffner had separately isolated the compound prior to Mason and Kendall, but failed to recognize its biological significance.[10] Mason's contributions to the crystallization and characterization of the compound have generally been forgotten outside of the Mayo Clinic.[10]
Cortisone was first produced commercially by Merck & Co. in 1948 or 1949.[12][13] On September 30, 1949, Percy Julian announced an improvement in the process of producing cortisone from bile acids.[chronology citation needed] This eliminated the need to use osmium tetroxide, a rare, expensive, and dangerous chemical. In the UK in the early 1950s, John Cornforth and Kenneth Callow at the National Institute for Medical Research collaborated with Glaxo to produce cortisone from hecogenin from sisal plants.[14]
Production
Cortisone is one of several end-products of a process called steroidogenesis. This process starts with the synthesis of cholesterol, which then proceeds through a series of modifications in the adrenal gland (suprarenal) to become any one of many steroid hormones. One end-product of this pathway is cortisol. For cortisol to be released from the adrenal gland, a cascade of signaling occurs. Corticotropin-releasing hormone released from the hypothalamus stimulates corticotrophs in the anterior pituitary to release ACTH, which relays the signal to the adrenal cortex. Here, the zona fasciculata and zona reticularis, in response to ACTH, secrete glucocorticoids, in particular cortisol. In the peripheral tissues, cortisol is converted to cortisone by the enzyme 11-beta-steroid dehydrogenase.
Cortisone has marginally reduced glucocorticoid activity compared to cortisol (80-90%[15]), and thus, cortisone can be considered an active metabolite of cortisol. However, 11-beta-steroid dehydrogenase can catalyze the reverse reaction as well, and, thus, cortisone is also a precursor molecule of cortisol. Cortisone is activated through hydrogenation of the 11-keto-group, and cortisol is, thus, sometimes referred to as hydrocortisone.[citation needed]
Popular culture
Addiction to cortisone was the subject of the 1956 motion picture, Bigger Than Life, produced by and starring James Mason. Though it was a box-office flop upon its initial release,[16] many modern critics hail it as a masterpiece and brilliant indictment of contemporary attitudes towards mental illness and addiction.[17] In 1963, Jean-Luc Godard named it one of the ten best American sound films ever made.[18]
John F. Kennedy needed to regularly use corticosteroids such as cortisone as a treatment for Addison's disease.[19]
See also
Notes
- ^ a b c "Cortisone shots". MayoClinic.com. 2010-11-16. Retrieved July 31, 2013.
- ^ a b "Prednisone and other corticosteroids: Balance the risks and benefits". MayoClinic.com. 2010-06-05. Retrieved 2017-12-21.
- ^ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 484. ISBN 9783527607495.
- ^ "injections and needles for coccyx pain". www.coccyx.org.
- ^ Driver, Catherine; Shiel, William. "Cortisone Injection (Corticosteroid Injection) of Soft Tissues & Joints". MedicineNet.com. Retrieved August 7, 2013.
- ^ Zanon, E; Jungwirth, W; Anderl, H (1992). "Cortisone jet injection as therapy of hypertrophic keloids". Handchirurgie, Mikrochirurgie, Plastische Chirurgie : Organ der Deutschsprachigen Arbeitsgemeinschaft Fur Handchirurgie : Organ der Deutschsprachigen Arbeitsgemeinschaft Fur Mikrochirurgie der Peripheren Nerven und Gefasse : Organ der V. 24 (2): 100–2. PMID 1582609.
- ^ "All About Atopic Dermatitis". National Eczema Association.
- ^ Cole, BJ; Schumacher (Jan–Feb 2005). "Injectable Corticosteroids in Modern Practice". Journal of the American Academy of Orthopaedic Surgeons. 13: 37–46. CiteSeerX 10.1.1.562.1931. doi:10.5435/00124635-200501000-00006. PMID 15712981.
- ^ "Cortisone Discovery and the Nobel Prize". Retrieved 2009-07-04.
- ^ a b c "I Went to See the Elephant" autobiography of Dwight J. Ingle, published by Vantage Press (1963), pg 94, 109
- ^ "The chemistry of crystalline substances isolated from the suprarenal gland" (PDF). J. Biol. Chem. 114: 613. Retrieved 2014-09-07.
- ^ a b Thomas L. Lemke; David A. Williams (2008). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 889–. ISBN 978-0-7817-6879-5.
- ^ Calvert DN (1962). "Anti-inflammatory steroids". Wis. Med. J. 61: 403–4. PMID 13875857.
- ^ Quirke, Viviane (2005). "Making British Cortisone: Glaxo and the development of Corticosteroids in Britain in the 1950s–1960s". Studies in History and Philosophy of Science Part C. 36 (4): 645–674. doi:10.1016/j.shpsc.2005.09.001. PMID 16337555.
- ^ "Corticosteroid Dose Equivalents". Medscape. Retrieved 20 December 2016.
- ^ Cossar 2011, p. 273.
- ^ Halliwell 2013, pp. 159–162.
- ^ Marshall, Colin (December 2, 2013). "A Young Jean-Luc Godard Picks the 10 Best American Films Ever Made (1963)". Open Culture.
- ^ Altman, Lawrence (October 6, 1992). "The doctor's world; Disturbing Issue of Kennedy's Secret Illness". New York Times.
Bibliography
- Bonagura J., DVM; et al. (2000). Current Veterinary Therapy. Vol. 13. pp. 321–381.
- Ingle DJ (October 1950). "The biologic properties of cortisone: a review". J. Clin. Endocrinol. Metab. 10 (10): 1312–54. doi:10.1210/jcem-10-10-1312. PMID 14794756.[permanent dead link]
- Woodward R. B.; Sondheimer F.; Taub D. (1951). "The Total Synthesis of Cortisone". Journal of the American Chemical Society. 73 (8): 4057. doi:10.1021/ja01152a551.
