Lead
 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lead | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ˈlɛd/ | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | metallic gray | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Pb) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lead in the periodic table | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 82 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | group 14 (carbon group) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | p-block | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Xe] 4f14 5d10 6s2 6p2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 18, 4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 600.61 K (327.46 °C, 621.43 °F) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 2022 K (1749 °C, 3180 °F) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (at 20 °C) | 11.348 g/cm3 [3] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 10.66 g/cm3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 4.77 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 179.5 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 26.650 J/(mol·K) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | common: +2, +4 −4,[4] −2,? −1,? 0,[5] +1,? +3? | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 2.33 (in +4), 1.87 (in +2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 175 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 146±5 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 202 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | face-centered cubic (fcc) (cF4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lattice constant | a = 494.99 pm (at 20 °C)[3] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 28.73×10−6/K (at 20 °C)[3] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 35.3 W/(m⋅K) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 208 nΩ⋅m (at 20 °C) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | −23.0×10−6 cm3/mol (at 298 K)[6] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 16 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 5.6 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 46 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 1190 m/s (at r.t.) (annealed) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.44 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 1.5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 38–50 MPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7439-92-1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | Middle East (7000 BCE) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symbol | "Pb": from Latin plumbum | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes of lead | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Isotopic abundances vary greatly by sample[8] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Lead (/lɛd/) is a chemical element with atomic number 82 and symbol Pb (from Template:Lang-la). It is a soft, malleable, and heavy metal. Freshly cut solid lead has a bluish-white color that soon tarnishes to a dull grayish color when exposed to air; the liquid metal has shiny chrome-silver luster. Lead's density of 11.34 g/cm3 exceeds that of most common materials. Lead has the second highest atomic number of all practically stable elements. As such, lead is located at the end of some decay chains of heavier elements, which in part accounts for the relative abundance of lead: it exceeds those of other similarly-numbered elements.
Lead is a post-transition metal, and is relatively inert unless powdered. Its weakened metallic character is illustrated by its general amphoteric nature: it and its oxides react with both acids and bases. It also displays a marked tendency toward covalent bonding. Its compounds are most commonly found in the +2 oxidation state, rather than +4, unlike the lighter group 14 elements. Exceptions are mostly limited to organolead compounds, where the positive charge on lead is dispersed and stabilized. Like the lighter group 14 elements, lead shows a tendency to bond to itself, forming complicated chain, ring, or polyhedral structures.
Lead is relatively easy to extract, and the metal was known to prehistoric people in Western Asia. While its softness and dullness prevented it from high demand, galena—a principle ore of lead—often bore silver in it, which helped initiate production of lead. Lead production peaked in ancient Rome, and lead became easily available to common people. After the fall of Rome, levels of lead production fell, and those of Rome were not surpassed anywhere until as late as the Industrial Revolution. The metal was established as poisonous as late as in the late nineteenth century, which led to its eventual displacement from many uses, and it has been or is being phased out from those which include immediate contact to people.
Lead has several properties that make it advantageous to use, alongside its commonness: high density, low melting point, ductility, and relative inertness against oxygen attack. Lead minerals are easier to mine and lead is easier to extract from its ores than many other metals, which makes the resulting metal relatively inexpensive. For example, lead is used in building construction, lead–acid batteries, bullets and shot, weights, as part of solders, pewters, fusible alloys, and as a radiation shield. Currently, lead is produced in quantities of around ten thousand tonnes annually; secondary production from recycling is gaining ground, accounting for around half of that figure. Lead's toxicity has been a reason why lead was or is being phased out for some uses. If ingested or inhaled, lead and its compounds are poisonous to animals and humans. Lead is a neurotoxin which accumulates in soft tissues and bones, damaging the nervous system and causing brain disorders. Excessive lead also causes blood disorders in mammals.
Etymology
The modern English word "lead" is of Germanic origin; it comes from the Middle English leed and Old English lēad (with the macron above the "e" signifying that the vowel sound of that letter is long).[9] The Old English word is derived from the hypothetical Proto-Germanic *lauda- ("lead").[10] According to the linguistic hypothesis, this word bore descendants in most Germanic languages (with a major exception being German) of exactly the same meaning.
The origin of the Proto-Germanic *lauda- is not agreed on in the linguistic community. One hypothesis suggests it is derived from Proto-Indo-European *lAudh- ("lead"; capitalization of the vowel is equivalent to the macron).[11] Another hypothesis suggests it is borrowed from Proto-Celtic *ɸloud-io- ("lead"). This word is related to the Latin plumbum, which gave the element its chemical symbol Pb. The origin of *ɸloud-io- presumably pre-dates the Proto-Indo-European language;[10] while it is yet unknown, it is suggested it is also the origin of Proto-Germanic *bliwa- (which also means "lead"), from which stemmed the German Blei ("lead").[12]
The name of the chemical element is not related to the verb of the same spelling, which is instead derived from (eventually) Proto-Germanic *laidijan- ("to lead").[13]
Properties
Atomic
A lead atom has 82 electrons, arranged in an electronic configuration of [Xe]4f145d106s26p2. The combined first and second ionization energy of lead—the total energy required to remove the two 6p electrons from a neutral lead atom—is close to that of tin, its upper neighbor in group 14. This is unusual since ionization energies generally fall going down a group as an element's electrons become more distant from its nucleus. The similarity is attributable to the lanthanide contraction. This describes the greater-than-expected decrease in the radii of elements in the lanthanide series from atomic number 57, lanthanum, to 71, lutetium, and causes smaller than otherwise expected ionic radii for the subsequent elements starting with 72, hafnium. It results from poor shielding of the nucleus by the lanthanide 4f electrons; the outer electrons are drawn towards the nucleus, thus resulting in a smaller atomic radius. The combined first four ionization energies of lead exceed those of tin[14] contrary to what the periodic trends would predict. For this reason lead, unlike tin,[15] rarely has a +4 oxidation state in inorganic compounds.[15] Such behavior is attributable to relativistic effects, which become particularly prominent at the bottom of the periodic table;[15] the result is that the 6s electrons of lead become reluctant to participate in bonding,[a] a phenomenon referred to as the inert pair effect.
All the lighter elements in group 14 have a stable or metastable allotrope in which they crystallize in the diamond cubic structure, involving covalent bonds. In this structure, each atom is tetrahedrally coordinated, indicating that all four bonds are equivalent, having each attained the lowest possible energy. To explain this, in spite of the fact that two of the electrons are in s-orbitals and the other two in higher-energy p-orbitals, orbital hybridization is invoked, in which one of the electrons is "promoted" from an s-orbital to a p-orbital, and then all form four intermediate hybrid orbitals in a process called sp3 hybridization. The inert pair effect affects the crystal structure of lead, because the promotion energy of a 6s-electron becomes larger than the amount of energy that would be released from the additional bonds formed.[17] Thus, rather than having the diamond-cubic covalent structure, lead forms metallic bonds, in which only the p-electrons are delocalized and shared between the Pb2+ ions, resulting in a face-centered cubic structure like those of the similarly-sized divalent calcium and strontium.[18]
Physical

Lead is a bright silvery metal with a very slight shade of blue in a dry atmosphere.[19] It tarnishes on contact with moist air, forming a complex surface mixture of compounds whose color and composition will vary depending on the prevailing conditions. Lead's characteristic properties include high density, softness, malleability, ductility, poor electrical conductivity compared to other metals, high resistance to corrosion (conferred by its surface patina), and a propensity to react with organic reagents.[19]
Lead's face-centered cubic structure and high atomic weight give it a characteristically high density[20] of 11.34 g/cm3, hence the idiom go down like a lead balloon.[21] Its density exceeds that of common metals such as iron (7.87 g/cm3), copper (8.93 g/cm3), zinc (7.14 g/cm3).[22] Some rarer metals are denser: tungsten and gold are both 19.3 g/cm3, while the densest metal known—osmium—has a density of 22.59 g/cm3, almost twice that of lead.[23]
Lead is a very soft material with a Mohs hardness of 1.5; it can be scratched with a fingernail.[24] It is malleable and ductile[b] metal, with its malleability exceeding its ductility.[25] Lead easily changes its shape, compared to most metals.[26] Its compressive strength is high and it can therefore be rolled into extremely thin sheets.[25] The bulk modulus—a measure of the ease of compressibility of a material—of lead is 45.8 GPa. (For comparison, that of aluminium is 75.2 GPa; copper 137.8 GPa; and mild steel 160–169 GPa.)[27] Lead has to be treated carefully when being elongated into wire as its tensile strength is comparatively low: 12–17 MPa (that of aluminium is 6 times higher; copper 10 times higher; mild steel 15 times higher); this value is easily improved by adding small concentrations of other metals (such as antimony or copper).[26]
The melting point of lead is 327.5 °C (621.5 °F),[28] which is considered low from an industrial perspective.[29][c] Its boiling point is 1749 °C (3180 °F).[31] The electrical resistivity of lead at 20 °C is 208 nano-ohm-meters; this almost an order of magnitude higher than those of industrially applied metals (that of copper is 17.12 nΩ·m; gold 22.55 nΩ·m; aluminium 27.09 nΩ·m).
Chemical

As with many metals, finely divided powdered lead exhibits pyrophoricity.[32] It burns with a bluish-white flame.
Bulk lead exposed to moist air forms a protective layer of varying composition. A common reaction is the formation of the oxide which in turn reacts with carbon dioxide to give lead carbonate.[33][34][35] Other insoluble compounds, such the sulfate or chloride, may form the protective layer if lead is exposed to a different chemical environment.[36]
Fluorine reacts with lead at room temperature, forming lead(II) fluoride. The reaction with chlorine is similar, but requires heating: the chloride layer diminishes the reactivity of the elements.[37][36] Molten lead reacts with the chalcogens to give lead(II) chalcogenides.[38]
The presence of carbonates or sulfates results in the formation of insoluble lead salts, which protect the metal from corrosion. So does carbon dioxide, as the insoluble lead carbonate is formed; however an excess of this gas will result in the formation of the soluble bicarbonate, which makes the use of lead pipes dangerous.[39] Water in the presence of oxygen attacks lead to start an accelerating reaction.[40] Lead also dissolves in concentrated alkalis (≥10%) because of the amphoteric character and solubility of plumbites.[39]
The metal is not attacked by dilute sulfuric acid; the concentrated acid dissolves lead thanks to complexation.[40] Lead reacts slowly with hydrochloric acid; nitric acid reacts vigorously to form nitrogen oxides and lead(II) nitrate.[40] Organic acids, such as acetic acid, also dissolve lead, but this reaction requires oxygen as well.[36]
Isotopes
Lead has four stable isotopes, lead-204, lead-206, lead-207, and lead-208.[41] The high number of stable isotopes relies on the fact that lead's atomic number of 82 is even, and is a magic number.[d] With its high atomic number, lead is the second-heaviest element that occurs naturally in the form of isotopes regarded as stable: bismuth has a higher atomic number of 83, but its only primordial isotope was found in 2003 to be very slightly radioactive.[e] The four stable isotopes of lead could theoretically undergo alpha decay to isotopes of mercury with a release of energy, but this has not been observed for any of them:[42] accordingly, their predicted half-lives are extremely long, ranging up to over 10100 years.[45][f] As such, lead is often quoted as the heaviest stable element.

Three of these isotopes are also found in three of the four major decay chains: lead-206, lead-207, and lead-208, are the final decay products of uranium-238, uranium-235, and thorium-232, respectively; the decay chains are called the uranium series, actinium series, and thorium series. Since the amounts of them in nature depend on the presence of other elements, the isotopic composition of natural lead varies between samples: in particular, the relative amount of lead-206 may vary between 20.84% and 27.78%,[41] and the abundance of lead-208 may vary between 52.4% in normal samples to 90% in thorium ores.[46] (For this reason, the atomic weight of lead is given to only one decimal place.[47]) As time passes, the ratio of lead-206 and lead-207 to lead-204 increase, since the former two are supplemented by radioactive decay of heavier elements and the latter is not; this allows for lead–lead dating. Analogously, as uranium decays (eventually) into lead, their relative amounts change; this allows for uranium–lead dating.[48]
Apart from the stable isotopes, which make up almost all lead that exists naturally, there are trace quantities of a few radioactive isotopes. One of them is lead-210; although it has a half-life of 22.3 years,[42] a period too short to allow any primordial lead-210 to exist, some small non-primordial quantities of it occur in nature, because lead-210 is found in the uranium series: thus, even though it constantly decays away, it is constantly regenerated by decay of its parent, polonium-214, which, while also constantly decaying, is also supplied by decay of its parent, and so on, all the way up to original uranium-238, which has been present for billions of years on Earth. Lead-210 is particularly useful for helping to identify ages of samples containing it, which is performed by measuring lead-210 to lead-206 ratios (both isotopes are present in a single decay chain).[49][50] Lead-214 is also present in the decay chain of natural uranium-238, lead-212 is present in that of natural thorium-232, and lead-211 is present in that of natural uranium-235; therefore, traces of all three of these isotopes exist naturally as well. Lastly, very minute traces of lead-209 are also present from the cluster decay of radium-223, one of the daughter products of natural uranium-235. Hence, natural lead consists of not only the four stable isotopes, but also minute traces of another five short-lived radioisotopes.[51]
In total, thirty-eight isotopes of lead have been synthesized, with mass numbers of 178–215.[42] Lead-205 is the most stable radioisotope of lead, with a half-life of around 1.5×107 years.[g] The second-most stable radioisotope is the synthetic lead-202, which has a half-life of about 53,000 years, longer than any of the natural trace radioisotopes. Additionally, 47 nuclear isomers (long-lived excited nuclear states), of 24 lead isotopes, have been characterized. The longest-lived isomer is lead-204m2 with a half-life of about 1.1 hours).[42]
Chemistry
Lead shows two main oxidation states: +4 and +2. The tetravalent state is common for group 14. Tne divalent state is rare for carbon and silicon, minor for germanium, important (but not prevailing) for tin, and is the more important for lead: even the strongest oxidizing agents, oxygen and fluorine, initially oxidize lead only to lead(II).[36] This is caused by relativistic effects, specifically the inert pair effect, which manifests itself when there is a large difference in electronegativity between lead and, for example, oxide, halide, or nitride anions, leading to a significant partial positive charge on lead. The result is a stronger contraction of the lead 6s orbital than is the case for the 6p orbital, making it rather inert in ionic compounds. This is not quite as applicable to compounds in which lead forms covalent bonds to elements of similar electronegativity such as carbon in organolead compounds. Here the 6s and 6p orbitals remain similarly sized and sp3 hybridization in compounds is still energetically favorable; as such, lead, like carbon, is predominantly tetravalent in organolead compounds.[53] The 5s electron pair tends to be stereochemically active in tin(II) compounds, but is much less so in lead(II) compounds. Consequently, there are often structural similarities between lead(II) compounds and analogous compounds of the divalent cations of calcium, strontium, barium, europium, and ytterbium.[54]
The electrode potential of lead shows that it is only slightly easier to oxidize than hydrogen. Lead can therefore dissolve in acids, but this is often impossible due to factors such as the formation of insoluble salts.[39] Electronegativity, although often thought to be constant for each element, is a variable property; lead shows a high electronegativity difference between values for lead(II) and lead(IV) of —1.87 and 2.33, respectively. This difference marks a reversal in the trend of increasing stability of the +4 oxidation state down group 14; tin, by comparison, has electronegativities of 1.80 and 1.96 in the +2 and +4 oxidation states.[55]
Inorganic compounds
Lead(II)

Lead (II) compounds are characteristic of the inorganic chemistry of lead. Even strong oxidizing agents like fluorine and chlorine react with lead at room temperature to give only PbF2 and PbCl2.[56] Lead forms binary compounds with many nonmetals, but not all of them; for example there is no known lead carbide.[57]
Most lead(II) compounds are ionic, but they are not as ionic as those of many other metals. In particular, many lead(II) compounds are water-insoluble. In solution, lead(II) ions are colorless, but under specific conditions, lead is capable of changing its color.[58] Unlike tin(II) ions, these do not react as reducing agents in solution. Lead(II) ions partially hydrolyze in aqueous solution to form Pb(OH)+ and finally Pb4(OH)4 (in which the hydroxyls ions act as bridging ligands).[59]
Lead monoxide exists in two allotropes, red α-PbO and yellow β-PbO, the latter being stable only above around 488 °C. It is the most commonly applicable compound of lead.[60] Its hydroxide counterpart, lead(II) hydroxide, is not capable of existence outside of solution; in solution, it is known to form plumbite anions. Lead commonly reacts with the heavier chalcogens. Lead sulfide can only be dissolved in strong acids.[61] It is a semiconductor, a photoconductor, and an extremely sensitive infrared radiation detector. A mixture of the monoxide and the monosulfide, when heated, forms the metal.[62] The other two chalcogenides are likewise photo-conducting. They are quite unusual in that their color becomes lighter down the group.[56]

Lead dihalides are well-characterized; this includes the diastatide,[63] and mixed examples, such as PbFCl. The relative insolubility of the latter forms a useful basis for the gravimetric determination of fluorine. The difluoride was the first ionically conducting compound to be discovered (in 1838, by Michael Faraday). The other dihalides decompose on exposure to ultraviolet or visible light, especially the diiodide.[54] Many pseudohalides are also known.[56] Lead(II) forms a tremendous variety of coordination complexes, such as [PbCl4]2−, [PbCl6]4−, and the chain anion [Pb2Cl9]n5n−, although most of them are not yet adequately characterized structurally.[54]
Lead(II) sulfate is well known for its insolubility in water, like the sulfates of the other heavy divalent cations; lead(II) nitrate and lead(II) acetate, in contrast, are very soluble, and this facility is exploited in the synthesis of other lead compounds.[64]
Lead(IV)
Few inorganic lead(IV) compounds are known, and they are typically strong oxidants or exist only in highly acidic solutions.[15] Lead(II) oxide gives a mixed oxide on further oxidation, Pb
3O
4. It is described as lead(II,IV) oxide, or structurally 2PbO•PbO
2, and is the best-known mixed valence lead compound. Lead dioxide is a strong oxidizing agent, capable of oxidizing hydrochloric acid to chlorine gas. This is because the expected PbCl4 that would be produced is unstable and spontaneously decomposes to PbCl2 and Cl2. Analogously to lead monoxide, lead dioxide is capable of forming plumbate anions. Lead tetrafluoride, a yellow crystalline powder, is stable, but less stable than the difluoride. Lead tetrachloride (a yellow oil) decomposes at room temperature, lead tetrabromide is less stable still and the existence of lead tetraiodide is questionable.[65][66] Lead disulfide, like the monosulfide, is a semiconductor.[67] Lead(IV) selenide is also known.[68]
Other oxidation states

Some lead compounds exist in formal oxidation states other than +4 or +2. Lead(III) may be obtained as an intermediate between lead(II) and lead(IV), in larger organolead complexes (rather than on its own).[70][71] This oxidation state is not specifically stable, as the lead(III) ion (and, consequently, the larger complexes containing it) is a radical; the same applies for lead(I), which can also be found in such species.[72]
Negative oxidation states can occur as Zintl phases, as either free lead anions, for example, in Ba
2Pb, with lead formally being lead(−IV),[73] or in oxygen-sensitive cluster ions, for example, in a trigonal bipyramidal Pb5−
2 ion, where two lead atoms are lead(−I) and three are lead(0):[74] this illustrates lead's proclivity towards catenation (the ability to form chains of atoms of the same element), an ability shared with all the lighter members of group 14 and more generally with most of the heavy p-block elements.[38] In such anions, each atom is at a polyhedral vertex and contributes two electrons to each covalent bond along an edge from their sp3 hybrid orbitals, the other two being an external lone pair. The shapes of such anions may be determined by Wade's rules.[59] They may be made by reduction of lead by sodium in liquid ammonia.[75]
Many mixed lead(II,IV) oxides are known. When PbO2 is heated in air, it becomes Pb12O19 at 293 °C, Pb12O17 at 351 °C, Pb3O4 at 374 °C, and finally PbO at 605 °C. A further sesquioxide Pb2O3 can be obtained at high pressure, along with several non-stoichiometric phrases. Many of them show defect fluorite structures in which some oxygen atoms are replaced by vacancies: for instance, PbO can be considered as such a structure with every alternate layer of oxygen atoms absent.[76]
Organolead
Carbon
Hydrogen
Lead
Lead can form long singly- or multiply-bonded chains—catenas—and so shares some covalent chemistry with its lighter homolog carbon. This tendency is much lower for lead because the Pb–Pb bond energy (98 kJ/mol) is much lower than for the C–C bond (356 kJ/mol).[38] Lead atoms can build metal–metal bonds of an order up to three.[77] Lead also forms covalent bonds with carbon to produce organolead compounds similar to but generally less stable than typical organic compounds,[78] as the Pb–C bond is rather weak.[59] Nevertheless, the organometallic chemistry of lead is far less wide-ranging than that of tin.[79] Almost all are organolead(IV) compounds. Very few organolead(II) compounds are known: even starting with inorganic lead(II) reactants always results in organolead(IV) products. The most well-characterized exceptions are the purple Pb[CH(SiMe)3)2]2 and lead cyclopentadienide, Pb(η5-C5H5)2.[79]
The simplest lead analog of an organic compound is plumbane, the lead analog of methane. It is unstable against heat, decaying in heated tubes,[80] and thermodynamically;[81] little is known about the chemistry of plumbane, due to its instability. A lead analog of the next alkane, ethane, is not known.[80] Two simple plumbane derivatives, tetramethyllead and tetraethyllead, are the best-known organolead compounds. They may be made by the addition (hydroplumbation) of trimethyllead or triethyllead to alkenes or alkynes; these precursors may themselves be made from the corresponding lead halides and lithium aluminium hydride at −78 °C. These compounds are relatively stable against heating—tetraethyllead only starts to decompose at 100 °C (210 °F)[78]—as well as sunlight or ultraviolet light.[82] (Tetraphenyllead is even more thermally stable, decomposing only at 270 °C.)[79] The general oxidizing nature of organolead compounds find use in chemistry: tetraethyllead is produced in larger quantities than any other organometallic compound;[83] lead tetraacetate is an important laboratory reagent for oxidation in organic chemistry.[84] Other organolead compounds, including homologs of the said compounds, are still less chemically stable.[78] Polyplumbanes are not well-characterized and are generally highly thermally unstable and reactive.[79]
Lead readily forms an equimolar alloy with sodium metal that reacts with alkyl halides to form organometallic compounds of lead such as tetraethyllead.[85] Plumbane may be obtained in a reaction between metallic lead and atomic (not molecular) hydrogen.[80] Atoms of chlorine or bromine displace alkyl groups in tetramethyllead and tetraethyllead; hydrogen chloride, a by-product of the previous reaction, further reacts with the halogenated molecules to complete mineralization—a chemical reaction or a series of reactions transforming an organic compound into an inorganic one—of the original compounds, yielding lead dichloride.[82]
Origin and occurrence
In space

Primordial lead—which comprises the isotopes lead-204, lead-206, lead-207, and lead-208—was largely created as a result of repetitive neutron capture processes occurring in stars. The two main modes of capture are the s-process and the r-process.
In the s-process (s is for "slow"), captures are separated by years or decades, allowing less stable nuclei to beta decay. For example, a stable thallium-203 nucleus captures a neutron and becomes thallium-204, which is unstable. The thallium-204 nucleus undergoes beta decay to give stable lead-204; on capturing another neutron, it becomes lead-205, which, while unstable, is stable enough to generally last longer than a capture takes (its half-life is around 15 million years). Further captures result in lead-206, lead-207, and lead-208. On capturing another neutron, lead-208 becomes lead-209, which quickly decays into bismuth-209, which on capturing another neutron becomes bismuth-210, which either undergoes an alpha decay to result in thallium-206, which would beta decay into lead-206, or a beta decay to yield polonium-210, which would inevitably alpha decay into lead-206 as well, and the cycle ends at lead-206, lead-207, lead-208, and bismuth-209.
In the r-process (r is for "rapid"), captures happen faster than nuclei can decay. This occurs in environments with a high neutron density, possibly in a supernova or during the merger of two neutron stars. The neutron flux involved may be on the order of 1022 neutrons/cm2/second.[86] The r-process does not form as much lead as the s-process. This is because the r-process tends to subside once very neutron-rich nuclei reach 126 neutrons. At this point the neutrons are arranged in complete shells within the atomic nucleus and it becomes harder to accommodate more of them. When the neutron flux subsides, the nuclei beta decay into stable isotopes of osmium, iridium and platinum. This effect is nevertheless masked, because the lead isotopes involved are located at the end of three major decay chains (see above), and are supplemented by the decay of the fewer heavier elements created by the r-process.[87]
Most synthesized nuclides with mass numbers just above those of stable lead and bismuth, quickly undergo alpha and beta decay to stable lead and bismuth isotopes. Nuclides with mass numbers 232, 235, and 238 and above soon decay to the extremely long-lived isotopes of thorium and uranium, which decay very slowly to lead. These decay away quite slowly and, in some quantities, still exist to this day; therefore, the amount of lead in the universe is still increasing, although noticeably only from the perspective of millions of years.[88]
| Atomic number |
Element | Relative amount |
|---|---|---|
| 42 | Molybdenum | 1.0 |
| 46 | Palladium | 0.3 |
| 50 | Tin | 0.9 |
| 52 | Tellurium | 1.6 |
| 56 | Barium | 1.2 |
| 80 | Mercury | 0.1 |
| 82 | Lead | 1 |
| 92 | Uranium | 0.0052 |
The isotopes at the end of the chains make up around 98.02% lead in the universe, with non-radiogenic lead-204 making up slightly less than two percent.[89] Lead is not an abundant element in general—its per-particle abundance in the Universe is 0.06 ppb (parts per billion)[90]—still, it is an order of magnitude more abundant than mercury, and further exceeds those of most other elements of similar atomic numbers. After element 40 (zirconium), no element is at least twofold as abundant as lead, and there is no element as abundant as lead starting after element 56 (barium). Lead is three times as abundant as platinum, ten times as mercury, and twenty times as gold.[89] Per mass, lead's abundance is 10 ppb[90]—the difference between the per-mass and per-particle abundances is justified by mass difference between lead isotopes and the most common elements: the most common nuclide in the Universe, hydrogen-1, has a mass of approximately one atomic mass unit, while those of lead isotopes have masses of over 200 atomic mass units.
On Earth

Since lead commonly reacts with sulfur (see above), it is classified as a chalcophile using the Goldschmidt classification. Lead is likely to form minerals that do not sink into the core but that stay above on Earth in its crust. Lead's abundance in the Earth's crust is 16 ppm.[91] This results in a great availability of lead minerals and easy extraction of the metal; for this reason, the mineral form of its sulfide, galena, has been known for millennia, as was the metal itself (see below). Lead's pronounced chalcophilic character is close to those of zinc and copper; as such, it is usually found in ore and extracted together with these metals.[91] Metallic lead does occur in nature, but it is rare. As a result of lead's chemistry, it occurs in primary minerals exclusively as lead(II), unlike tin, which always occurs as tin(IV).[47][h] Lead deposits can be hydrothermal vein, impregnation, and replacement deposits; volcanogenic sedimentary deposits; and hydrothermal or marine sedimentary deposits. World resources of lead exceed 2 billion tons.[92] Massive resources are located in Australia, China, Ireland, Mexico, Peru, Portugal, Russia, and the United States. World reserves—resources ready to be mined for which that would be economically feasible—totaled 89 million tons in 2015, of which Australia had 35 million, China had 15.8 million, and Russia had 9.2 million.[92]
The main lead mineral is galena (PbS). Galena is mostly found with other minerals, mostly zinc ores.[91]
Most other lead minerals are normally related to galena in some way; for example, boulangerite, Pb
5Sb
4S
11, is a mixed sulfide derived from galena; anglesite, PbSO
4, is a product of galena oxidation; cerussite or white lead ore, PbCO
3, is a decomposition product of galena. Zinc, copper, arsenic, tin, anitmony, silver, gold, and bismuth are common impurities in lead minerals.[91]
History

(present on the picture = 1980)[93]
Lead has been commonly used for thousands of years because it is widespread, easy to extract, and easy to work with. It is highly malleable and easily smeltable. Metallic lead beads dating back to 7000–6500 BCE, if not before that, have been found in Asia Minor; this indicates lead was the first metal ever to be smelted.[94] At that point, lead had little, if any at all, applications, as it is soft and dull.[94] The major reason for the spread of lead production, rather than its usefulness, was its association with silver, which may be obtained by burning galena, a widespread lead mineral,[95][96] even though a few minor uses for lead appeared over time: The Ancient Egyptians are thought to have used lead for sinkers in fishing nets, in glazes, glasses and enamels, and for ornaments;[95] they also were the first to use lead for cosmetics, a use that would continue through millennia to Ancient Greece and far beyond.[97] Various civilizations of the Fertile Crescent used lead as a writing material, as currency, and for construction.[95] The Ancient Chinese used lead as a stimulant in the royal court,[95] a currency,[98] and a contraceptive;[99] lead also had a few uses, such as making amulets, for the Indus Valley civilization and the Mesoamericans.[95] Peoples of eastern and southern Africa are known to exercise wire drawing.[100]

As silver was extensively used as a decoration material and a means for exchange, lead deposits were first worked in Asia Minor in 3000 BCE.[96] Lead deposits were worked in 2000 BCE in the Iberian peninsula by the Phoenicians;[101] and also in Athens, Carthage, and Sicily.[95] With the development of mining and its territorial expansion in Europe and across the Mediterranean, Rome became the greatest producer of lead during the classical era, with an estimated annual output equaling 80,000 tonnes at its peak. The Romans obtained lead mostly as a by-product of extensive silver smelting.[93][102][103] Lead mining occurred in Central Europe, Britain, the Balkans, Greece, Anatolia, and Hispania, which alone accounted for 40% of world production.[93] Lead was used for making water pipes in the Roman Empire, as lead was formable and resistant to corrosion,[104] and, consequently, the Latin word for the metal, plumbum, was the origin of the periodic table symbol Pb and the English word "plumbing" and its derivatives—even though some Romans, such as Vitruvius, were able to recognize its danger for health.[105] Some researchers suggest lead poisoning was one of the reasons behind the fall of Rome.[j] Lead poisoning—a condition in which one becomes dark and cynical—was called "saturnine", after the ghoulish god of Saturn; the metal was also considered the father of all metals. It was easily available in the Roman society, and as such, its social status was low.[107]
During the ancient and classical eras, (and even far beyond them, until the 17th century), tin was often not distinguished from lead or seen as a different kind of the metal that lead is: Romans called lead plumbum nigrum (literally, "black lead"), while tin was called plumbum candidum (literally, "bright lead"). Their association through history can also be seen in other languages: the word olovo in Czech translates to "lead", but in Russian the cognate олово (olovo) means "tin".[108] In addition to that, lead also bore a close relation to antimony: Both elements commonly occur as sulfides (galena and stibnite), often together. Pliny declared stibnite would give lead on heating, whereas the mineral on heating actually produces antimony.[109] The originally South Asian surma—"galena" in English—spread across Asia with that meaning, and also gave its name to antimony in a number of Central Asian languages, as well as Russian.

Lead plumbing in Western Europe may have been continued beyond the fall of the Western Roman Empire into the medieval era,[111] but lead mining in Europe in general fell into decline, with the only region with noticeable production being the Arabian Iberia,[112][113] and the largest lead production was conducted in South and East Asia, especially China and India, where lead output underwent a strong growth.[113] In Europe, lead production only began to revive in the 11th and 12th centuries, and lead was again used for roofing and piping; from the 13th century, it was used to create stained glass.[113] During the period, lead was used increasingly for wine adulteration. This practice was declared forbidden in 1498 by a papal bull, but it continued long past the date, being a reason of various mass poisonings up to late 18th century.[112] In the wake of the Renaissance, the printing press was invented, and lead served as a key material for its parts, starting with the Johannes Gutenberg's press;[114] lead dust was commonly inhaled by operators, causing lead poisoning.[115] Additionally, firearms were invented approximately at the same time, and lead, despite its expense over iron, became a chief material for making bullets, because it made less damage to iron gun barrels, had a higher density (which allowed for better retention of velocity and energy), and its lower melting point made production much easier: bullets could be made on wooden fire.[116][117] Lead was extensively used in cosmetics at the time in Western Europe by the aristocracy, as whitened faces were seen as a sign of modesty.[97][118] The practice eventually expanded to white wigs and eyeliners, and it only faded out with the French Revolution in the late 18th century; one effect of such prolonged contacts with lead was teeth rotting and teeth replacements often were also made of lead (which temporarily gave sweet breath), inducing further damage to the organism.[119] (A similar fashion appeared in Japan in the 18th century with the emergence of the geishas, with the practice continuing long into the 20th century and the white face becoming a "symbol of a Japanese woman"; lead was commonly used as a face whitener.[120][121][122])
In the New World, lead was first produced soon after the European settlers had arrived; the earliest recorded lead production dates to 1621, in the Colony of Virginia that had been founded fourteen years earlier.[123] In Australia, mining was introduced by the colonists as well, and they opened the first mine on the continent—a lead mine—in 1841.[124] Centuries before the Europeans were able to start the colonization of Africa in the late 19th century, lead mining was known in the Benue Trough[125] and the lower Congo basin, where lead was used for trade with the Europeans and as a currency.[126][k]

In the second half of the 18th century, Britain and later continental Europe and then the United States entered the Industrial Revolution. During the period, lead mining proved important; the Industrial Revolution was the first time to have greater lead production rates than those of Rome.[93] Britain was the leading producer during the period, losing the status of the greatest producer by the mid-19th century with depletion of its mines and development of lead mining in Germany, Spain, and the United States.[127] Lead production in the United States dominated by 1900;[128] other non-European nations—in particular, Canada, Mexico, and Australia—started their massive lead production, and by 1900, Europe's output of lead fell below that elsewhere.[129] A great share of demand of lead came from plumbing and painting—lead paints had been invented and regularly used; with invention of gasoline in the late 19th century, lead was extensively used as an additive.[130] At this time, more people—the working class—contacted the metal, and this led to the increase of the numbers of those poisoned by lead. This also led to research of effects of lead intake: lead was proven to be more dangerous in its fume form than as a solid metal; lead poisoning and gout were linked (Alfred Baring Garrod noted a third of his gout patients was plumbers and painters); effects of chronic ingestion of lead, including mental disorders, were all studied in the 19th century. The first political acts to decrease the degree of lead poisoning in factories followed in the 1870s and 1880s in the United Kingdom.[130]

Further evidence of the threat lead posed to human organisms were revealed in the late 19th and early 20th centuries—mechanisms of the harm were better realized, and lead blindness was documented[131]—and countries in Europe and the United States started efforts to reduce the amount of lead a regular person contacts with. The last major innovation to impose contact with lead on humans was adding tetraethyllead to gasoline, invented in the United States in 1921; it was phased out in the U.S. and the European Union by 2000.[130] Most European countries banned usage of lead paint for interiors by 1930.[132] The result of many regulations and bans put on lead products was significant: in the last quarter of the 20th century, percentage of people with excessive lead blood levels dropped from over three quarters of the population to slightly over two percent in the U.S.[130] By the end of the 20th century, the main good made of lead was the lead–acid battery,[133] which possesses no direct threat to humans. This allowed for a consistent lead production in the industrialized countries. From 1960 to 1990, lead output in the Western Bloc grew by 31%.[134] The share of the world's lead production of the Eastern Bloc increased from 10% and 30% from 1950 to 1990, with the Soviet Union being world's largest producer during the mid- and late 1970s and the 1980s, and China started a massive lead production in the late 20th century.[135] Unlike the European communist countries, China was largely unindustrialized by mid-20th century; in 2004, China surpassed Australia as the largest producer of lead.[136] Similarly to European industrialization, lead has a negative effect on health in the country.[137]
Production
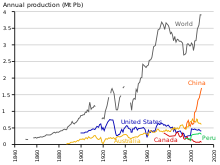
Production and consumption of lead is increasing worldwide. Lead production generally is divided into two major categories, primary and secondary: the primary production is the production from concentrate from the previously mined ores, and the secondary production is the production from scrap. In 2013, 4.74 million metric tons came from the primary production, and 5.74 million tons came from secondary production. The top mining countries for lead in 2013 were China, Australia, Russia, India, Bolivia, Sweden, North Korea, South Africa, Poland, and Ireland. The top lead producing countries were China, United States, India, South Korea, Germany, Mexico, United Kingdom, Canada, Japan, and Australia.[138] According to the International Resource Panel's Metal Stocks in Society report of 2010, the global per capita stock of lead in use in society is 8 kg. Much of this is in more developed countries (20–150 kg per capita) rather than less developed countries (1–4 kg per capita).[139]
Processes of production of primary and secondary lead are similar, despite using different sources of lead—ores and scrap—and some primary production plants now also use scrap, and this trend is likely increase in the future. Given adequate techniques, secondary lead is indistinguishable from primary lead. Scrap lead from the building trade is usually fairly clean and is re-melted without the need for smelting, though some refining operations may be necessary; as such, secondary lead is also cheaper to produce than primary in terms of energy spent on production, often twice or more so.[140]
Primary production
Most lead ores contain only a very low percentage of lead, which must be concentrated during processing.[141] During initial ore processing, ores typically undergo crushing, dense-medium separation, grinding, froth flotation, and drying of the resulting concentrate. The resulting concentrate is the initial quantitative metric of mined lead.[138] Sulfide concentrate is more common for subsequent lead production than oxide concentrate; it commonly has a lead content fraction of 50%–60%, occasionally varying to up to 30% or 80%.[142]
| Country | Output (thousand tons) |
|---|---|
| 2,300 | |
| 633 | |
| 385 | |
| 300 | |
| 240 | |
| 130 | |
| 90 | |
| 82 | |
| 76 | |
| 54 | |
| 45 | |
| 40 | |
| 40 | |
| 38 | |
| 33 | |
| Other countries | 226 |
The resulting concentrate is then turned into (impure) lead metal. The main route for doing so is the two-stage process: The sulfide concentrate is roasted in the air, the main reaction occurring is oxidation of lead sulfide with oxygen:[143]
- 2PbS + 3O2 → 2PbO + 2SO2↑
This reaction releases heat once it started. As the original concentrate was not pure lead sulfide, roasting does not yield pure oxide, producing primarily lead oxide and a mixture of sulfates and silicates of lead and other metals contained in the ore.[142] This impure lead oxide reduced in a coke-fired blast furnace to the (again, impure) metal by a reaction with that very coke:[144][145]
- 2PbO + C → Pb + CO2↑
Research on a process cheaper in terms of energy spent and pollution introduced into the environment than the described one continues, with some success; a major drawback is that the alternative results in either an exceedingly high sulfur content of the resulting lead metal or too much lead lost as waste. An alternative gaining ground involves direct smelting without an intermediate compound involved; another promising alternative involves hydrometallurgical means (it is based on anodes of impure lead and cathodes of pure lead dissolved in an electrolyte).[146]
Impurities in the resulting metal are still significant; these are mostly contaminants of arsenic, antimony, bismuth, zinc, copper, silver, and gold. The melt is treated in a reverberatory furnace with air, steam, and sulfur, which oxidizes the contaminants except silver, gold, and bismuth. The oxidized contaminants are removed by drossing, where they float to the top and are skimmed off.[147][148] Since lead ores contain significant concentrations of silver, the smelted metal also is commonly contaminated with silver. Metallic silver as well as gold is removed and recovered economically by means of the Parkes process, in which zinc is added to lead and adsorbs silver, which dissolves in zinc many times more actively than in lead.[62][148] Desilvered lead is freed of bismuth according to the Betterton–Kroll process by treating it with metallic calcium and magnesium, which forms a bismuth dross that can be skimmed off.[148] Very pure lead can be obtained by processing smelted lead electrolytically by means of the Betts process. The process uses anodes of impure lead and cathodes of pure lead in an electrolyte of silica fluoride. Once electrical potential is applied, impure lead at the anode dissolves and then plates out in the cathode, while the impurities remain in the solution.[148][149]
Secondary production
Smelting, an essential part of the primary production, is often skipped in the secondary production. The reason for that is that scrap lead itself is commonly reproduced to its metallic form. As such, smelting is only performed when metallic lead had undergone significant chemical transformation, such as oxidation/rusting.[140] When smelting is performed, it is performed in a fashion similar to that of the primary production in either a blast furnace or a rotary furnace (though both, with the essential difference being the greater variability of what could be extracted as the final product after the latter). The Isasmemt process is a more recent method that possesses a possibility of extension to the primary production; the essence of this process is that the input battery paste is deprived of its sulfur content (by, for example, treating it with alkalies) and then treated in a coal-fueled furnace in presence of oxygen, which eventually yields impure lead with antimony being the most common impurity.[150]
Refining of secondary lead is similar to that of primary lead; some refining processes may be skipped depending on the material recycled and its potential contamination, with bismuth and silver being skipped especially often of all major impurities.[150]
Of the sources of lead for recycling, lead–acid batteries is the most important one; lead pipe, sheet, and cable sheathing are also significant sources.[140]
Applications
Contrary to popular belief, pencil leads in wooden pencils have never been made from lead. The term comes from the Roman stylus, called the penicillus, a small brush used for painting.[151] When the pencil originated as a wrapped graphite writing tool, the particular type of graphite being used was named plumbago (lit. act for lead, or lead mockup).[152]
Elemental form

Lead metal has a number of mechanical properties that make using it advantageous in comparison with many alternatives: high density, low melting point, ductility, and relative inertness against oxygen attacks. While many metals are superior to lead in some of these aspects, lead is also more common than most of these metals; moreover, lead minerals are easier to mine and then lead is easier to extract from its ores than many other metals, which makes the resulting metal relatively inexpensive. One disadvantage of using lead is its chemical toxicity, and it has been a reason why lead was or is being phased out for some uses.
Lead has been used for bullets since their invention (see above); with the development of firearms, round bullets became pointed and later, lead was jacketed with, for example, copper. The low melting point makes casting of lead easy, and therefore small arms ammunition and shotgun pellets can be cast with minimal technical equipment. It is also inexpensive and denser than other common metals.[153] Lead is sometimes alloyed with tin or antimony: this increases the cost and time of making the bullet, but increasing the hardness of the bullet, this makes the bullet more effective against hard targets, eases the tension on the gun barrel and does not contaminate it with lead, as simple lead bullets do.[154] Concerns have been raised over whether lead bullets used for hunting can damage the environment.[l]
Because of its high density and resistance to corrosion, lead is used for the ballast keel of sailboats.[156] Its high density allows it to counterbalance the heeling effect of wind on the sails while at the same time occupying a small volume and thus offering the least underwater resistance. For the same reason, it is used in scuba diving weight belts to counteract the diver's natural buoyancy and that of his equipment.[157]

Lead is alloyed with copper and its alloys (namely, brass and bronze) to increase their machinability and to reduce machine tool wear. Lead does not form a solid solution with copper and is found as granules within copper. It acts as a lubricant in copper; in low concentrations, it also acts as a chip breaker.[158]
It is also used to form glazing bars for stained glass or other multi-lit windows. The practice has become less common, not for danger but for stylistic reasons. Lead, or sheet-lead, is used as a sound deadening layer in some areas in wall, floor and ceiling design in sound studios where levels of airborne and mechanically produced sound are targeted for reduction or virtual elimination.[159][160] It is the traditional base metal of organ pipes, mixed with varying amounts of tin to control the tone of the pipe.[161][162]
Lead has many uses in the construction industry (e.g., lead sheets are used as architectural metals in roofing material, cladding, flashing, gutters and gutter joints, and on roof parapets). Detailed lead moldings are used as decorative motifs used to fix lead sheet. Lead is still widely used in statues and sculptures. Lead is often used to balance the wheels of a car; this use is being phased out in favor of other materials for environmental reasons.
Apart from its mechanical properties, lead is also useful in batteries, namely lead–acid batteries. The reactions in the battery between lead, lead dioxide, and sulfuric acid provides a reliable source of voltage.[m] This, since lead in batteries undergoes no direct contact with humans (and thus no toxicity), is a use not threatened by toxicity concerns, and has been the largest use of lead in early 21st century.

Lead is also used as electrodes in the process of electrolysis. It is used in solder for electronics, although this usage is being phased out by some countries to reduce the amount of environmentally hazardous waste, and in high voltage power cables as sheathing material to prevent water diffusion into insulation. Lead is one of three metals used in the Oddy test for museum materials, helping detect organic acids, aldehydes, and acidic gases. It is also used as shielding from radiation (e.g., in X-ray rooms).[164] Molten lead is used as a coolant (e.g., for lead cooled fast reactors).[165]
Compounds
Lead compounds are used as a coloring element in ceramic glazes, notably for the colors red and yellow.[166]
Lead tetraacetate (LTA) and lead dioxide have been used as oxidizing agents in organic chemistry. Geminal diols are cleaved to a pair of carbonyl compounds by stoichiometric LTA. LTA also is a selective oxidant of 5-methyl groups in 5-methylpyrrole-2-carboxylic esters, leading to 5-acetoxymethyl groups or 5-formyl groups with one or two equivalents of oxidant, respectively, to provide important intermediates for porphyrin synthesis.[167]
Lead is frequently used in polyvinyl chloride (PVC) plastic, which coats electrical cords.[168][169]
Lead is used in some candles to treat the wick to ensure a longer, more even burn. Because of the dangers, European and North American manufacturers use alternatives such as zinc.[170][171] Lead glass is composed of 12–28% lead oxide. It changes the optical characteristics of the glass and reduces the transmission of ionizing radiation.[172]
Lead-based semiconductors, such as lead telluride, lead selenide and lead antimonide are finding applications in photovoltaic (solar energy) cells and infrared detectors.[173]
Biological and environmental effects
Biological
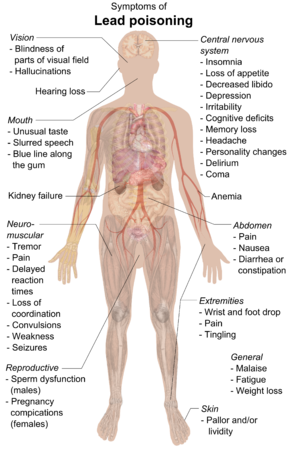
Lead is a highly poisonous metal (whether inhaled or swallowed), affecting almost every organ and system in the body. The component limit of lead (1.0 μg/g) is a test benchmark for pharmaceuticals, representing the maximum daily intake an individual should have. Even at this level, a prolonged intake can be hazardous to human beings.[174] Exposure to lead and lead chemicals occurs primarily through ingestion, to a lesser extent through inhalation and occasionally by direct contact.[175]
The main target for lead toxicity is the nervous system, both in adults and children, in which it crosses the blood-brain barrier by mimicking calcium. Lead causes loss of neurons' myelin sheaths, reduces numbers of neurons, interferes with neurotransmission, and decreases neuronal growth.[176] In a child's developing brain, lead interferes with synapse formation in the cerebral cortex, neurochemical development (including that of neurotransmitters), and organization of ion channels.[177] The primary cause of lead's toxicity is its interference with a variety of enzymes because it binds to sulfhydryl groups found on many enzymes.[176] Part of lead's toxicity results from its ability to mimic other metals that take part in biological processes, which act as cofactors in many enzymatic reactions, displacing them at the enzymes on which they act.[178] Lead salts are thus very quickly and efficiently absorbed by the body, accumulating in it and leading to both chronic and acute poisoning.[179] Lead is able to bind to and interact with many of the same enzymes as these metals but, due to its differing chemistry, does not properly function as a cofactor, thus interfering with the enzyme's ability to catalyze its normal reaction or reactions. Among the essential metals with which lead interacts are calcium, iron, and zinc.[180] Thus high levels of calcium and iron tend to protect one somewhat from lead poisoning, while low levels of these metals render one more susceptible.[179] According to the United States Agency for Toxic Substance and Disease Registry, a small amount of ingested lead (1%) will be stored in bones, and the rest will be excreted by an adult through urine and feces within a few weeks of exposure. Only about a third of lead will be excreted by a child.[181]
Long-term exposure of adults can result in decreased performance in some tests that measure functions of the nervous system.[182] Long-term exposure to lead or its salts (especially soluble salts or the strong oxidant PbO2) can cause nephropathy, and colic-like abdominal pains. It may also cause weakness in fingers, wrists, or ankles. Lead exposure also causes small increases in blood pressure, particularly in middle-aged and older people and can cause anemia. Exposure to high lead levels can cause severe damage to the brain and kidneys in adults or children and ultimately cause death. In pregnant women, high levels of exposure to lead may cause miscarriage. Chronic, high-level exposure has been shown to reduce fertility in males.[183] Lead also damages nervous connections (especially in young children) and causes blood and brain disorders. Lead poisoning typically results from ingestion of food or water contaminated with lead, but may also occur after accidental ingestion of contaminated soil, dust, or lead-based paint.[184] It is rapidly absorbed into the bloodstream and is believed to have adverse effects on the central nervous system, the cardiovascular system, kidneys, and the immune system.[185]
| NFPA 704 safety square | |
|---|---|
Fire diamond for lead granules |
The treatment for lead poisoning consists of dimercaprol and succimer.[186] Acute lead poisoning is treated using disodium calcium edetate: the calcium chelate of the disodium salt of ethylenediaminetetraacetic acid (EDTA). This chelating agent has a greater affinity for lead than for calcium and so the lead chelate is formed by exchange. This is then excreted in the urine leaving behind harmless calcium.[187]
The concern about lead's role in cognitive deficits in children has brought about widespread reduction in its use (lead exposure has been linked to learning disabilities).[188] Most cases of adult elevated blood lead levels are workplace-related.[189] High blood levels are associated with delayed puberty in girls.[190] Lead has been shown many times to permanently reduce the cognitive capacity of children at extremely low levels of exposure.[191]
Despite the toxicity of lead in significant amounts, there is some evidence that trace amounts of lead are beneficial in pigs and rats, and that its absence causes deficiency signs including depressed growth, anemia, and disturbed iron metabolism. If true in humans as well, this would make lead an essential element; nevertheless, these findings are still uncertain, and even if lead does turn out to be beneficial in small quantities, the threshold of toxicity is so low that lead toxicity would remain a much higher priority to address than lead deficiency.[192][193][194]
Sources of exposure

Ingestion of lead-based paint is the major source of lead exposure for children. As lead paint deteriorates, it peels, is pulverized into dust and then enters the body through hand-to-mouth contact or through contaminated food, water, or alcohol. Ingesting certain home remedy medicines may also expose people to lead or lead compounds.[175] Lead can be ingested through fruits and vegetables contaminated by high levels of lead in the soils they were grown in. Soil is contaminated through particulate accumulation from lead in pipes, lead paint and residual emissions from leaded gasoline that was used before the Environment Protection Agency issued the regulation in 1980.[195] The use of lead for water pipes is problematic in areas with soft or (and) acidic water. Hard water forms insoluble layers in the pipes while soft and acidic water dissolves the lead pipes.[196]
Inhalation is the second major pathway of exposure, especially for workers in lead-related occupations. Almost all inhaled lead is absorbed into the body, the rate is 20–70% for ingested lead; children absorb more than adults.[175]
Dermal exposure may be significant for a narrow category of people working with organic lead compounds, but is of little concern for general population, as most countries stopped using leaded gasoline by 2007. The rate of skin absorption is also low for inorganic lead.[175]
Environmental
The extraction, production, use, and disposal of lead and its products have caused significant contamination of the Earth's soils and waters, posing a hazard to living organisms because of its toxicity. Atmospheric emissions of lead were at their peak during the Industrial Revolution and the period of leaded petrol in the second half of the twentieth century; although these periods are over, elevated concentrations of lead persist in soils and sediments in post-industrial and urban areas. Meanwhile, industrial emissions continue in many parts of the world.[197]

Lead accumulates in soil, especially in soil with high organic contents, where it remains for a long time, hundreds and thousand of years. According to the U.S. Environmental Protection Agency, lead from soils may take places of other metals within organic matter, particularly plants. In plants, lead accumulates on the surface, thus covering the plant from the incoming carbon dioxide, reducing the rate of photosynthesis, which trump the growth of the plant or kills it. Contamination of soils and plants, in turn, affects microorganisms and animals. Affected animals have reduced ability to synthesize red blood cells. Sources of contamination of the environment with lead are thus being limited.[198][n]
Research has been conducted on how to remove lead from biosystems by the means of biological organisms: Fish bones are being researched for their ability to bioremediate lead in contaminated soil.[200][201] The fungus Aspergillus versicolor is known as both greatly effective and fast at removing lead ions.[202] Several bacteria have been researched for their ability to reduce lead; including the sulfate reducing bacteria Desulfovibrio and Desulfotomaculum, which are highly effective in aqueous solutions.[203]
Restriction of lead usage
During the 20th century, the use of lead in paint pigments was sharply reduced because of the danger of lead poisoning, especially to children.[204] By the mid-1980s, a significant shift in lead end-use patterns had taken place. Much of this shift was a result of the U.S. lead consumers' compliance with environmental regulations that significantly reduced or eliminated the use of lead in non-battery products, including gasoline, paints, solders, and water systems. Lead use is being further curtailed by the European Union's RoHS directive.[205] Lead may still be found in harmful quantities in stoneware,[206] vinyl[207] (such as that used for tubing and the insulation of electrical cords), and Chinese brass. Old houses may still contain substantial amounts of lead paint.[207] White lead paint has been withdrawn from sale in industrialized countries, but the yellow lead chromate is still in use. Old paint should not be stripped by sanding, as this produces inhalable dust.[208]
People can be exposed to lead in the workplace by breathing it in, swallowing it, skin contact, and eye contact. In the United States, the Occupational Safety and Health Administration has set the permissible exposure limit for lead exposure in the workplace as 0.05 mg/m3 over an 8-hour workday, which applies to metallic lead, inorganic lead compounds, and lead soaps. The National Institute for Occupational Safety and Health has set a recommended exposure limit of 0.05 mg/m3 over an 8-hour workday, and recommends that workers' blood concentrations of lead stay below 0.06 mg per 100 g blood. At levels of 100 mg/m3, lead is immediately dangerous to life and health.[209]
See also
- 2009 Chinese lead poisoning scandal
- Adult Blood Lead Epidemiology and Surveillance
- Alquifou
- Consumer Product Safety Improvement Act
- Devon colic
- Flint water crisis
- Medical geology
- Plumbosolvency
- Restriction of Hazardous Substances Directive
- Roman lead pipe inscription
Notes
- ^ About 10% of the lanthanide contraction has also been attributed to relativistic effects.[16]
- ^ The difference between the two terms is that malleability refers to deformability under compression (i.e., pressing a tablet of a material into a sheet) while ductility refers to its ability to stretch (i.e., elongating a rod of a material into a wire).
- ^ In addition to that, this, for example, allows dipping a finger into molten lead without burning it.[30]
- ^ An even number of either protons or neutrons generally increases the nuclear stability of isotopes, compared to isotopes with odd numbers. For example, elements with odd atomic numbers have no more than two stable isotopes, while even-numbered elements have multiple stable isotopes, with tin (element 50) having the highest number of isotopes of all elements, ten.[42] See Even and odd atomic nuclei for more details.
- ^ The half-life found in the experiment was 1.9×1019 years.[43] A kilogram of natural bismuth would thus be radioactive with an activity value of approximately 0.003 becquerels—decays per second. For comparison, the natural radiation within human body would make an adult human have radioactivity of 65 becquerels per kilogram of body weight (around 4500 becquerels on average).[44]
- ^ The predicted half-lives of lead isotopes are expected to be as follows:[45]
- 204Pb: 2.3×1035–1.2×1037 y
- 206Pb: 1.8×1065–6.7×1068 y
- 207Pb: 3.6×10152–3.4×10189 y
- 208Pb: 1.2×10124–7.4×10132 y
- ^ It decays solely via electron capture, which means when there are no electrons available and lead is accordingly fully ionized—has all 82 electrons removed—it cannot decay and becomes stable. Fully ionized thallium-205, the isotope lead-205 would decay to, becomes unstable with respect to decaying into a bound state of lead-205.[52]
- ^ In the oxidized zones of lead deposits, small quantities of lead(IV) species can be found, including the oxide minerals plattnerite, scrutinyite, and murdochite.
- ^ "Made when the Emperor Vespasian was consul for the ninth time and the Emperor Titus was consul for the seventh time, when Gnaeus Iulius Agricola was imperial governor [of Britain]".
- ^ It is suggested that the sweeteners the Romans made were often prepared in lead vessels; this led to the formation of poisonous lead(II) acetate, which accumulated in the sweeteners and, accordingly, the products they were used for; in particular, wine. Lead containers did further sweeten their contents as well as help to preserve them.[106] In comparison, copper vessels spoiled the taste of wine in them. The fact that Julius Caesar managed to father only one child, as well as the alleged sterility of his successor, Caesar Augustus, have also been attributed to lead poisoning.[98] The Romans were aware of the potential health problems lead could cause, as well as the fact that copper was used far more commonly for Roman vessels than lead.
- ^ It is not known when mining was first performed in the region because no tradition of keeping written records was in place, but there are European 17th century records of trade with the Congolese, which indicates lead was first smelted no later than then.[126]
- ^ For instance, the U.S. state of California banned lead bullets for hunting on that basis in April 2015.[155]
- ^ See [163] for details on how a lead–acid battery works.
- ^ For example, in the Netherlands, the use of lead shot for hunting and sport shooting was banned in 1993, which caused a large drop in lead emission, from 230 tonnes in 1990 to 47.5 tonnes in 1995, two years after the ban.[199]
References
- ^ "Standard Atomic Weights: Lead". CIAAW. 2020.
- ^ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (4 May 2022). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- ^ a b c Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 28. ISBN 978-0-08-037941-8.
- ^ Pb(0) carbonyls have been observered in reaction between lead atoms and carbon monoxide; see Ling, Jiang; Qiang, Xu (2005). "Observation of the lead carbonyls PbnCO (n=1–4): Reactions of lead atoms and small clusters with carbon monoxide in solid argon". The Journal of Chemical Physics. 122 (3): 034505. 122 (3): 34505. Bibcode:2005JChPh.122c4505J. doi:10.1063/1.1834915. ISSN 0021-9606. PMID 15740207.
- ^ Weast, Astle & Beyer 1983, p. E110.
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ Meija et al. 2016.
- ^ Merriam-Webster. "Definition of LEAD". www.merriam-webster.com. Retrieved 12 August 2016.
- ^ a b Kroonen 2013, *lauda-.
- ^ Nikolayev, Sergei, ed. (2012). "*lAudh-". Indo-European etymology. Retrieved 21 August 2016.
{{cite book}}:|website=ignored (help) - ^ Kroonen 2013, *bliwa- 2.
- ^ Kroonen 2013, *laidijan-.
- ^ Lide 2004, p. 10-179.
- ^ a b c d Polyanskiy 1986, pp. 14–15.
- ^ Pyykko, P. (1988). "Relativistic effects in structural chemistry". Chemical Reviews. 88 (3): 563–594. doi:10.1021/cr00085a006.
- ^ Greenwood & Earnshaw 1998, p. 227.
- ^ Christensen, N. E. (2002). "Relativistic Solid State Theory". In Schwerdtfeger, P. (ed.). Relativistic Electronic Structure Theory - Fundamentals. Elsevier. pp. 867–868. ISBN 9780080540467.
- ^ a b Polyanskiy 1986, p. 18.
- ^ Thornton, Radu & Brush 2001, p. 6.
- ^ Cambridge University Press. "Go down like a lead balloon". Cambridge Dictionary. Retrieved 22 August 2016.
- ^ Lide 2004, pp. 12-35—12–37.
- ^ Lide 2004, pp. 4-39—4–96.
- ^ Vogel, N. A.; Achilles, R. (2013). The Preservation and Repair of Historic Stained and Leaded Glass (PDF) (Report). U.S. Department of the Interior. p. 8. Retrieved 30 October 2016.
- ^ a b Anderson, J. (1869). "Malleability and Ductility of Metals". Scientific American. 21 (22): 341–343. doi:10.1038/scientificamerican11271869-341.
- ^ a b Thornton, Radu & Brush 2001, p. 8.
- ^ Gale, W. F.; Totemeier, T. C. (2003). Smithells Metals Reference Book. Butterworth-Heinemann. pp. 15-2–15-3. ISBN 9780080480961.
- ^ Lide 2004, p. 12-220.
- ^ Koshal, D. (2014). Manufacturing Engineer's Reference Book. Butterworth-Heinemann. p. 1/92. ISBN 9780080523958.
- ^ "The Physics Behind Four Amazing Demonstrations - CSI". Skeptical Inquirer. 23 (6). 1999. Retrieved 6 September 2016.
- ^ Lide 2004, p. 12-219.
- ^ Charles, J.; Kopf, P. W.; Toby, S. (1966). "The Reaction of Pyrophoric Lead with Oxygen". Journal of Physical Chemistry. 70 (5): 1478–1482. doi:10.1021/j100877a023.
- ^ Thornton, Radu & Brush 2001, pp. 10–11.
- ^ Thurmer, K.; Williams, E; Reutt-Robey, J. (2002). "Autocatalytic Oxidation of Lead Crystallite Surfaces". Science. 297 (5589): 2033–5. Bibcode:2002Sci...297.2033T. doi:10.1126/science.297.5589.2033. PMID 12242437.
- ^ Tétreault, J.; Sirois, J.; Stamatopoulou, E. (1998). "Studies of Lead Corrosion in Acetic Acid Environments". Studies in Conservation. 43 (1): 17–32. doi:10.2307/1506633. JSTOR 1506633.
- ^ a b c d Greenwood & Earnshaw 1998, p. 373.
- ^ Polyanskiy 1986, p. 19.
- ^ a b c Greenwood & Earnshaw 1998, p. 374.
- ^ a b c Polyanskiy 1986, p. 20.
- ^ a b c Polyanskiy 1986, p. 32.
- ^ a b Polyanskiy 1986, p. 16.
- ^ a b c d e Audi, G.; Wapstra, A. H.; Thibault, C.; et al. (2003). "The NUBASE evaluation of nuclear and decay properties" (PDF). Nuclear Physics A. 729 (1): 3–128. Bibcode:2003NuPhA.729....3A. doi:10.1016/j.nuclphysa.2003.11.001.
- ^ Marcillac, P. de; Coron, N.; Dambier, G.; et al. (2003). "Experimental detection of α-particles from the radioactive decay of natural bismuth". Nature. 422 (6934): 876–878. Bibcode:2003Natur.422..876D. doi:10.1038/nature01541. PMID 12712201.
- ^ "Nuclear Radiation and Health Effects". World Nuclear Association. 2015. Retrieved 12 November 2015.
- ^ a b Beeman, J. W.; Bellini, F.; Cardani, L. (2013). "New experimental limits on the α decays of lead isotopes". The European Physical Journal A. Vol. 49, no. 50. Retrieved 21 August 2016.
{{cite news}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ Smirnov, A. Yu.; Borisevich, V. D.; Sulaberidze, A. (2012). "Evaluation of specific cost of obtainment of lead-208 isotope by gas centrifuges using various raw materials". Theoretical Foundations of Chemical Engineering. 46 (4): 373–378. doi:10.1134/s0040579512040161.
- ^ a b Greenwood & Earnshaw 1998, p. 368.
- ^ Boltwood, B. B. (1907). "On the ultimate disintegration products of the radio-active elements. Part II. The disintegration products of uranium". American Journal of Science. 23: 77–88.
- ^ Fiorini, E. "2.000 years-old Roman Lead for physics" (PDF). ASPERA. Retrieved 29 October 2016.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Nosengo, N. (2010). "Roman ingots to shield particle detector". Nature News. doi:10.1038/news.2010.186.
- ^ University of California Berkeley Nuclear Forensic Search Project. "Decay Chains". Nuclear Forensics: A Scientific Search Problem. Retrieved 23 November 2015.
- ^ Takahashi, K.; Boyd, R. N.; Mathews, G. J.; et al. (October 1987). "Bound-state beta decay of highly ionized atoms" (PDF). Physical Review C. 36 (4). American Institute of Physics for the American Physical Society. ISSN 0556-2813. OCLC 1639677. Retrieved 27 August 2013.
- ^ Kaupp, M. (2014). "Chemical Bonding of Main-Group Elements". In Frenking, G.; Shaik, S. (eds.). The Chemical Bond : Chemical Bonding Across the Periodic Table (PDF). John Wiley & Sons. pp. 9–10. doi:10.1002/9783527664658.ch1.
- ^ a b c Greenwood & Earnshaw 1998, p. 382.
- ^ Rappoport, Zvi; Marek, Ilan (2010). The Chemistry of Organocopper Compounds. John Wiley & Sons. p. 509. ISBN 978-0-470-77296-6.
- ^ a b c Greenwood & Earnshaw 1998, p. 389.
- ^ Percy, J. (1870). The Metallurgy of Lead, including Desiverization and Cupellation. Murray, J. p. 67.
- ^ Ensafi, A. A.; Far, A. K.; Meghdadi, S. (2009). "Highly selective optical-sensing film for lead(II) determination in water samples". Journal of Hazardous Materials. 172 (2–3): 1069–75. doi:10.1016/j.jhazmat.2009.07.112. PMID 19709813.
- ^ a b c King, R. B. (1995). Inorganic Chemistry of Main Group Elements. Wiley-VCH. pp. 43–63. ISBN 0-471-18602-3.
- ^ Greenwood & Earnshaw 1998, p. 384.
- ^ Lewis, A. E. (2010). "Review of metal sulphide precipitation" (PDF). Hydrometallurgy. 104 (2): 222–234. doi:10.1016/j.hydromet.2010.06.010. Retrieved 14 October 2014.
- ^ a b Pauling, L. (1947). General Chemistry. W. H. Freeman and Company. ISBN 0-486-65622-5.
- ^ Zuckerman, J. J.; Hagen, A. P. (1989). Inorganic Reactions and Methods, the Formation of Bonds to Halogens. John Wiley & Sons. p. 426. ISBN 978-0-471-18656-4.
- ^ Greenwood & Earnshaw 1998, p. 388.
- ^ Greenwood & Earnshaw 1998, p. 398.
- ^ Macomber 1996, p. 230
- ^ Cava, R. J.; Hor, Y. S.; Cava, R. J. (2011). "Pressure Stabilized Se–Se Dimer Formation in PbSe2". Solid State Sciences. 13: 38–41. Bibcode:2011SSSci..13...38B. doi:10.1016/j.solidstatesciences.2010.10.003.
- ^ Silverman, M. S. (1966). "High-pressure (70-kilobar) Synthesis of New Crystalline Lead Dichalcogenides". Inorganic Chemistry. 5 (11): 2067–2069. doi:10.1021/ic50045a056.
- ^ Yong, L.; Hoffmann, S. D.; Fässler, T. F. (2006). "A low-dimensional arrangement of [Pb9]4− clusters in [K(18-crown-6)]2K2Pb9·(en)1.5". Inorganica Chimica Acta. 359 (15). Elsevier: 4774–4778. doi:10.1016/j.ica.2006.04.017.
- ^ Becker, M.; Förster, C.; Franzen, C.; et al. (2008). "Persistent Radicals of Trivalent Tin and Lead". Inorganic Chemistry. 47 (21): 9965–9978. doi:10.1021/ic801198p. PMID 18823115.
- ^ Mosseri, S.; Henglein, A.; Janata, E. (1990). "Trivalent lead as an intermediate in the oxidation of lead(II) and the reduction of lead(IV) species". The Journal of Physical Chemistry. 94 (6): 2722–2726. doi:10.1021/j100369a089.
- ^ Chia, S.-P.; X., H.-W.; Li, Y.; et al. (2013). "A Base-Stabilized Lead(I) Dimer and an Aromatic Plumbylidenide Anion". Angewandte Chemie International Edition. 52 (24): 6298–6301. doi:10.1002/anie.201301954.
- ^ Universität Freiburg. "Binäre Zintl-Phasen" [Binary Zintl Phases] (in German).
- ^ Alsfasser, R. (2007). Moderne anorganische Chemie [Modern inorganic chemistry] (in German). Walter de Gruyter. pp. 261–263. ISBN 978-3-11-019060-1.
- ^ Greenwood & Earnshaw 1998, p. 393.
- ^ Greenwood & Earnshaw 1998, p. 386.
- ^ Stabenow, F.; Saak, W.; Weidenbruch, M. (2003). "Tris(triphenylplumbyl)plumbate: An anion with three stretched lead–lead bonds". Chemical Communications (18): 2342. doi:10.1039/B305217F.
- ^ a b c Polyanskiy 1986, p. 43.
- ^ a b c d Greenwood & Earnshaw 1998, p. 404.
- ^ a b c Wiberg, E.; Wiberg, N.; Holleman, A. F. (2001). Inorganic Chemistry. Academic Press. p. 918. ISBN 978-0-12-352651-9.
{{cite book}}: Invalid|ref=harv(help) - ^ Hein, T. A.; Thiel, W.; Lee, T. J. (1993). "Ab initio study of the stability and vibrational spectra of plumbane, methylplumbane, and homologous compounds". The Journal of Physical Chemistry. 97 (17): 4381–4385. doi:10.1021/j100119a021.
- ^ a b Polyanskiy 1986, p. 44.
- ^ Greenwood & Earnshaw 1998, p. 405.
- ^ Zýka, J. (1966). "Analytical study of the basic properties of lead tetraacetate as oxidizing agent" (PDF). Pure and Applied Chemistry. 13 (4): 569–581. doi:10.1351/pac196613040569. Retrieved 19 December 2013.
- ^ Windholz, M. (1976). Merck Index of Chemicals and Drugs (9th ed.). Merck & Co. ISBN 0-911910-26-3. Monograph 8393.
- ^ Frebel, A. (2015). Searching for the Oldest Stars: Ancient Relics from the Early Universe. Princeton University. pp. 114–115. ISBN 978-0-691-16506-6.
- ^ Burbidge, E. M.; Burbidge, G. R.; Fowler, W. A.; et al. (1957). "Synthesis of the Elements in Stars" (PDF). Reviews of Modern Physics. 29 (4): 547–650. Bibcode:1957RvMP...29..547B. doi:10.1103/RevModPhys.29.547.
- ^ Roederer, I. U.; Kratz, K.-L.; Frebel, A.; et al. (2009). "The End of Nucleosynthesis: Production of Lead and Thorium in the Early Galaxy". The Astrophysical Journal. 698 (2). The American Astronomical Society: 1963–1980. Bibcode:2009ApJ...698.1963R. doi:10.1088/0004-637X/698/2/1963. Retrieved 18 July 2016.
- ^ a b c Cameron, A. G. W. (1973). "Abundance of the Elements in the Solar System" (PDF). Space Science Review. 15: 121–146. Bibcode:1973SSRv...15..121C. doi:10.1007/BF00172440.
- ^ a b Winter, Mark. "Lead»geological information [WebElements Periodic Table]". www.webelements.com. Retrieved 9 March 2016.
- ^ a b c d Sutherland et al. 2005, p. 5.
- ^ a b c U.S. Geological Survey (2016). Lead (PDF) (Report). Retrieved 20 February 2016.
- ^ a b c d Hong, Sungmin; Candelone, Jean-Pierre; Patterson, Clair Cameron; Boutron, Claude F. (1994). "Greenland Ice Evidence of Hemispheric Lead Pollution Two Millennia Ago by Greek and Roman Civilizations" (PDF). Science. 265 (5180): 1841–1843. Bibcode:1994Sci...265.1841H. doi:10.1126/science.265.5180.1841. PMID 17797222.
- ^ a b Rich 1994, p. 4.
- ^ a b c d e f Winder, C. (1993). "The History of Lead - Part 3". LEAD Action News. 2 (3). Archived from the original on 31 August 2007. Retrieved 12 February 2016.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b Rich 1994, p. 5.
- ^ a b "A History of Cosmetics from Ancient Times | Cosmetics Info". www.cosmeticsinfo.org. Retrieved 18 July 2016.
- ^ a b "Lead in history". corrosion-doctors.org. Retrieved 13 February 2016.
- ^ "Toronto museum explores history of contraceptives". ABC News. 2003. Retrieved 13 February 2016.
- ^ Bisson & Vogel 2000, p. 105.
- ^ Sutherland et al. 2005, p. 2.
- ^ Callataÿ, F. de (2005). "The Graeco-Roman Economy in the Super Long-Run: Lead, Copper, and Shipwrecks". Journal of Roman Archaeology. 18: 361–372.
- ^ Settle, D. M.; Patterson, C. C. (1980). "Lead in Albacore: Guide to Lead Pollution in Americans". Science. 207 (4436): 1167–1176. Bibcode:1980Sci...207.1167S. doi:10.1126/science.6986654. PMID 6986654. see 1170f.
- ^ Rich 1994, p. 6.
- ^ Hodge, T. A. (1981). "Vitruvius, Lead Pipes and Lead Poisoning". American Journal of Archaeology. 85 (4). Archaeological Institute of America: 486–491. doi:10.2307/504874. JSTOR 504874.
- ^ Winder, C. (1994). "The History of Lead Part 4". LEAD Action News. Archived from the original on 5 April 2015. Retrieved 12 February 2016.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ EPA,OA,OEAEE,OWC, US. "Lead Poisoning: A Historical Perspective". www.epa.gov. Retrieved 7 February 2016.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Polyanskiy 1986, p. 8.
- ^ Thomson, T. (1830). The History of Chemistry. Colburn, H. and Bentley, R. p. 74.
- ^ "Queen Elizabeth I". medicalbag.com. 2012. Retrieved 2 October 2016.
- ^ Squatriti, P., ed. (2000). Working with water in medieval Europe: technology and resource use. Brill. pp. 134 ff. ISBN 978-90-04-10680-2.
- ^ a b Winder, C. (1993). "The History Of Lead part 1". LEAD Action News. The LEAD Group. Archived from the original on 31 August 2007. Retrieved 5 February 2016.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b c Rich 1994, p. 7.
- ^ Rubinstein, G. (1999). "Printing: History and Development". karmak.org. Retrieved 13 February 2016.
- ^ Sinha, S. P.; Shelly; Sharma, V.; et al. (1993). "Neurotoxic effects of lead exposure among printing press workers". Bulletin of Environmental Contamination and Toxicology. 51 (4). doi:10.1007/BF00192162.
- ^ Harris, W. (2013). "10 Innovations That Led to the Modern Bullet". HowStuffWorks. Retrieved 13 February 2016.
- ^ Ramage, C. K. (1980). Lyman Cast Bullet Handbook (3rd ed.). Lyman Publications. p. 8.
- ^ Hessa (2009). "History lesson: lead, mercury and leeches were used to whiten complexions in the 1400s". whiterskin.info. Retrieved 15 July 2016.
- ^ Angeloglou, M. (1970). A History of Make-Up. Studio Vista. p. 71.
- ^ Nakashima, T.; Matsuno, K.; Matsushita, T. (2007). "Lifestyle-determined gender and hierarchical differences in the lead contamination of bones from a feudal town of the Edo period". Journal of Occupational Health. 49 (2): 134–139. doi:10.1539/joh.49.134. PMID 17429171.
- ^ Nakashima, T.; Hayashi, H.; Tashiro, H.; et al. (1998). "Gender and Hierarchical Differences in Lead-Contaminated Japanese Bone from the Edo Period". Journal of Occupational Health. 40: 55–60. doi:10.1539/joh.40.55.
- ^ Ashikari, M. (2003). "The memory of the women's white faces: Japaneseness and the ideal image of women". Japan Forum. 15: 55–79. doi:10.1080/0955580032000077739.
- ^ Beard, M. E. (1995). Lead in Paint, Soil, and Dust: Health Risks, Exposure Studies, Control Measures, Measurement Methods, and Quality Assurance. ASTM International. p. 66. ISBN 9780803118843.
- ^ "Mining History". www.mininghistory.asn.au. Retrieved 15 February 2016.
- ^ Bisson & Vogel 2000, p. 85.
- ^ a b Bisson & Vogel 2000, pp. 131–132.
- ^ "Lead Mining". The Northern Echo. Retrieved 16 February 2016.
- ^ Sohn, E. "Lead: Versatile Metal, Long Legacy". Dartmouth Toxic Metals Superfund Research Program. Retrieved 16 February 2016.
- ^ Rich 1994, p. 11.
- ^ a b c d Riva, M. A.; Lafranconi, A.; d'Orso, M. I.; et al. (2012). "Lead Poisoning: Historical Aspects of a Paradigmatic "Occupational and Environmental Disease"". Safety and Health at Work. 3 (1): 11–16. doi:10.5491/SHAW.2012.3.1.11. PMC 3430923. PMID 22953225.
- ^ Hernberg, S. (2000). "Lead poisoning in a historical perspective" (PDF). American Journal of Industrial Medicine. 38: 244–254. doi:10.1002/1097-0274(200009)38:3<244::AID-AJIM3>3.0.CO;2-F. PMID 10940962.
- ^ Markowitz, G.; Rosner, D. (2000). ""Cater to the children": the role of the lead industry in a public health tragedy, 1900-1955". American Journal of Public Health. 90: 36–46. PMC 1446124. PMID 10630135.
- ^ Rich 1994, p. 117.
- ^ Rich 1994, p. 17.
- ^ Rich 1994, pp. 91–92.
- ^ U.S. Geological Survey (2005). Lead (PDF) (Report). Retrieved 20 February 2016.
- ^ Zhang, X.; Yang, L.; Li, Y.; Li, H.; Wang, W.; Ye, B. (2012). "Impacts of lead/zinc mining and smelting on the environment and human health in China". Environmental Monitoring and Assessment. 184 (4): 2261–2273. doi:10.1007/s10661-011-2115-6. ISSN 1573-2959. PMID 21573711.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ a b Guberman, D. E. (2015). "Lead". 2013 Minerals Yearbook (PDF) (Report). U.S. Geological Survey. Retrieved 2 November 2016.
- ^ Graedel, T. E.; et al. (2010). "Metal stocks in Society – Scientific Synthesis" (PDF). International Resource Panel. Retrieved 2 July 2012.
- ^ a b c Thornton, Radu & Brush 2001, p. 56.
- ^ Greenwood & Earnshaw 1998, p. 369.
- ^ a b Sutherland et al. 2005, pp. 7–19.
- ^ Thornton, Radu & Brush 2001, p. 51.
- ^ Thornton, Radu & Brush 2001, pp. 51–52.
- ^ "Primary Extraction of Lead Technical Notes". LDA International. Archived from the original on 22 March 2007. Retrieved 7 April 2007.
- ^ Thornton, Radu & Brush 2001, pp. 52–53.
- ^ Sutherland et al. 2005, pp. 19–27.
- ^ a b c d "Primary Lead Refining Technical Notes". LDA International. Archived from the original on 22 March 2007. Retrieved 7 April 2007.
- ^ Sutherland et al. 2005, p. 26.
- ^ a b Thornton, Radu & Brush 2001, p. 57.
- ^ "A history of pencils". pencils.com. Retrieved 7 April 2007.
- ^ Evans, J. W. (1908). "V.— the Meanings and Synonyms of Plumbago". Transactions of the Philological Society. 26 (2): 133–179. doi:10.1111/j.1467-968X.1908.tb00513.x.
- ^ Rooney, C. "Contamination at Shooting Ranges" (PDF). The Lead Group Inc. Retrieved 7 April 2007.
- ^ "About Us". thebulletworks.net. 2010. Retrieved 4 July 2016.
- ^ Bastasch, M. (2015). "California Officially Bans Hunters From Using Lead Bullets". The Daily Caller. Retrieved 4 July 2016.
- ^ Parker, R. B. (2005). The New Cold-Molded Boatbuilding: From Lofting to Launching. WoodenBoat Books. pp. 194–195. ISBN 9780937822890.
- ^ Krestovnikoff, M.; Halls, M. (2006). Scuba Diving. Penguin. p. 70. ISBN 9780756640637.
- ^ Copper Development Association. "Leaded Coppers". copper.org. Retrieved 10 July 2016.
- ^ Guruswamy, S. (2000). Engineering properties and applications of lead alloys. Marcel Dekker. p. 31. ISBN 978-0-8247-8247-4.
- ^ Lansdown, R.; Yule, William, eds. (1986). The Lead debate : the environment, toxicology, and child health. Croom Helm. p. 240. ISBN 978-0-7099-1653-6.
- ^ Audsley, G. A. (1988). The Art of Organ Building, Volume 2. pp. 250–251. ISBN 978-0-486-21315-6.
- ^ Palmieri, R., ed. (2006). The Organ. Garland. pp. 412–413. ISBN 978-0-415-94174-7.
- ^ Progressive Dynamics, Inc. "How Lead Acid Batteries Work: Battery Basics". progressivedyn.com. Retrieved 3 July 2016.
- ^ National Council on Radiation Protection and Measurements (2004). Structural shielding design for medical X-ray imaging facilities. National Council on Radiation Protection and Measurement. pp. 16–17. ISBN 978-0-929600-83-3.
- ^ Tuček, K.; Carlsson, J.; Wider, H. (2006). "Comparison of sodium and lead-cooled fast reactors regarding reactor physics aspects, severe safety and economical issues" (PDF). Nuclear Engineering and Design. 236 (14–16): 1589–1598. doi:10.1016/j.nucengdes.2006.04.019.
- ^ Leonard, A. R.; Lynch, G. (1958). "Dishware as a Possible Source for Lead Poisoning". California Medicine. 89 (6): 414–416. PMC 1512529. PMID 13608300.
- ^ Siedel, W.; Winkler, F. (1943). "Oxydation von Pyrrolderivaten mit Bleitetraacetat. Neuartige Porphyrinsynthesen" [Oxidation of pyrrole deratives with lead tetraacetate. New porphyrin syntheses]. Justus Liebig's Annalen der Chemie (in German). 554 (1): 162–201. doi:10.1002/jlac.19435540111. ISSN 0075-4617.
- ^ Zweifel, H. (2009). Plastics Additives Handbook. Hanser. p. 438. ISBN 978-3-446-40801-2.
- ^ Wilkes, C. E.; Summers, J. W.; Daniels, C. A.; et al. (2005). PVC handbook. Hanser. p. 106. ISBN 978-1-56990-379-7.
- ^ Randerson, J. (2002). "Candle pollution". newscientist.com (2348). Retrieved 7 April 2007.
- ^ Nriagu, J.; Kim, M. J. (2000). "Emissions of lead and zinc from candles with metal-core wicks". The Science of the Total Environment. 250 (1–3): 37–41. doi:10.1016/S0048-9697(00)00359-4. PMID 10811249.
- ^ Amstock, J. S. (1997). Handbook of glass in construction. McGraw-Hill Professional. pp. 116–119. ISBN 978-0-07-001619-4.
- ^ Rogalski, Antonio (2010). Infrared Detectors, Second Edition. CRC Press. pp. 485–541. ISBN 978-1-4200-7672-1. Retrieved 19 November 2016.
- ^ Editors of Encyclopædia Britannica. "Pharmaceutical". britannica.com. Britannica Online Encyclopedia. Retrieved 23 January 2012.
{{cite web}}:|author=has generic name (help) - ^ a b c d "Case Studies in Environmental Medicine Lead (Pb) Toxicity: How are People Exposed to Lead?". Agency for Toxic Substances and Disease Registry. Archived from the original on 6 June 2011.
- ^ a b Abraham M. Rudolph; Colin D. Rudolph; Margaret K. Hostetter; George E. Lister; Norman J. Siegel (2003). "Lead". Rudolph's Pediatrics (21st ed.). McGraw-Hill Professional. p. 369. ISBN 0-8385-8285-0.
- ^ Mycyk, M.; Hryhorczuk, D.; Amitai, Y. (2005). "Lead". In Erickson, TB; Ahrens, WR; Aks, S; Ling, L. Pediatric Toxicology: Diagnosis and Management of the Poisoned Child. McGraw-Hill Professional. ISBN 0-07-141736-2. p. 462.
- ^ Dart, R. C.; Hurlbut, K. M.; Boyer-Hassen, L. V. (2004). "Lead". In Dart, R. C. (ed.). Medical Toxicology (3rd ed.). Lippincott Williams & Wilkins. p. 1426. ISBN 0-7817-2845-2.
- ^ a b Venugopal, B. (2013). Physiologic and Chemical Basis for Metal Toxicity. Springer. pp. 177–178. ISBN 9781468429527.
- ^ Kosnett, M. J. (2006). "Lead". In Olson, K. R. (ed.). Poisoning and Drug Overdose (5th ed.). McGraw-Hill Professional. p. 238. ISBN 0-07-144333-9.
- ^ "Toxic Substances Portal – Lead". Agency for Toxic Substance and Disease Registry. Archived from the original on 6 June 2011.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ US Environmental Protection Agency (2016). "Basic Information about Lead Air Pollution". epa.gov. Retrieved 9 November 2016.
- ^ Sokol, R. C. (2005). "Summary". In Golub, M. S. (ed.). Metals, fertility, and reproductive toxicity. Taylor and Francis. p. 153. ISBN 978-0-415-70040-5.
- ^ "ToxFAQs: CABS/Chemical Agent Briefing Sheet: Lead" (PDF). Agency for Toxic Substances and Disease Registry/Division of Toxicology and Environmental Medicine. 2006. Archived from the original (PDF) on 4 March 2010.
- ^ Bergeson, Lynn L. (2008). "The proposed lead NAAQS: Is consideration of cost in the clean air act's future?". Environmental Quality Management. 18: 79–84. doi:10.1002/tqem.20197.
- ^ Jagadish Prasad, P. (2010). Conceptual Pharmacology. Universities Press. p. 652. ISBN 978-81-7371-679-9. Retrieved 21 June 2012.
- ^ Masters, S. B.; Trevor, A. J.; Katzung, B. G. (2008). Katzung & Trevor's Pharmacology: Examination & Board Review (8th ed.). McGraw Hill Medical. pp. 481–483. ISBN 0071488693.
- ^ Hu, H. (1991). "Knowledge of diagnosis and reproductive history among survivors of childhood plumbism". American Journal of Public Health. 81 (8): 1070–1072. doi:10.2105/AJPH.81.8.1070. PMC 1405695. PMID 1854006.
- ^ "NIOSH Adult Blood Lead Epidemiology and Surveillance". United States National Institute for Occupational Safety and Health. Retrieved 4 October 2007.
- ^ Schoeters, G.; Den Hond, E.; Dhooge, W.; et al. (2008). "Endocrine Disruptors and Abnormalities of Pubertal Development". Basic & Clinical Pharmacology & Toxicology. 102 (2): 168–175. doi:10.1111/j.1742-7843.2007.00180.x. PMID 18226071.
- ^ Needleman, H. L.; Schell, A.; Bellinger, D.; et al. (1990). "The long-term effects of exposure to low doses of lead in childhood. An 11-year follow-up report". New England Journal of Medicine. 322 (2): 83–88. doi:10.1056/NEJM199001113220203. PMID 2294437.
- ^ Berdanier, C. D.; Dwyer, J. T.; Heber, D. (2016). Handbook of Nutrition and Food (3rd ed.). CRC Press. pp. 211–226. ISBN 978-1-4665-0572-8. Retrieved 3 July 2016.
- ^ Gottschlich, M. M. (2001). The Science and Practice of Nutrition Support: A Case-based Core Curriculum. Kendall Hunt. p. 98. ISBN 978-0-7872-7680-5. Retrieved 9 July 2016.
- ^ Insel, P. M.; Turner, R. Elaine; Ross, Don (2004). Nutrition. Jones & Bartlett Learning. p. 499. ISBN 978-0-7637-0765-1. Retrieved 10 July 2016.
- ^ "Information for the Community Lead Toxicity". Agency for Toxic Substances and Disease Registry.
- ^ Moore, M. R. (1977). "Lead in drinking water in soft water areas—health hazards". Science of the Total Environment. 7 (2): 109–115. doi:10.1016/0048-9697(77)90002-X. PMID 841299.
- ^ United Nations Environment Programme, Chemicals Branch, Division of Technology, Industry and Economics (2010). Final review of scientific information on lead (PDF). United Nations Environment Programme. pp. 11–33.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Ratcliffe, D. J. "Effects of lead on the environment". lead.org.au. Retrieved 30 October 2016.
- ^ Deltares [in Dutch]; Netherlands Organisation for Applied Scientific Research (2016). "Lood en zinkemissies door jacht" [Lead and zinc emissions from hunting] (PDF) (in Dutch).
- ^ Freeman, K. S. (January 2012). "Remediating Soil Lead with Fishbones". Environmental Health Perspectives. 120 (1): a20–a21. doi:10.1289/ehp.120-a20a. PMC 3261960. PMID 22214821.
- ^ "Battling lead contamination, one fish bone at a time". Compass.
- ^ Bairagi, H.; Khan, M.; Ray, L.; et al. (February 2011). "Adsorption profile of lead on Aspergillus versicolor: A mechanistic probing". Journal of Hazardous Materials. 186 (1): 756–764. doi:10.1016/j.jhazmat.2010.11.064. PMID 21159429.
- ^ Park, J. H.; Bolan, N.; Meghara, M.; et al. (2011). "Bacterial-Assisted Immobilization of Lead in Soils: Implications for Remediation" (PDF). Pedologist: 162–174.
- ^ "Lead Paint Information". Master Painters Australia. Archived from the original on 12 February 2008. Retrieved 7 April 2007.
- ^ Smith, D. R.; Flegal, A. R. "Lead in the Biosphere: Recent Trends". 24: 21–23. JSTOR 4314280.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Grandjean, P. (1978). "Widening perspectives of lead toxicity". Environmental Research. 17 (2): 303–321. doi:10.1016/0013-9351(78)90033-6. PMID 400972.
- ^ a b Levin, R.; Brown, M. J.; Kashtock, M. E.; et al. (2008). "Lead Exposures in U.S. Children, 2008: Implications for Prevention". Environmental Health Perspectives. 116 (10): 1285–1293. doi:10.1289/ehp.11241. PMC 2569084. PMID 18941567.
- ^ Marino, P. E.; Landrigan, P. J.; Graef, J.; Nussbaum, A.; Bayan, G.; Boch, K.; Boch, S. (1990). "A case report of lead paint poisoning during renovation of a Victorian farmhouse". American Journal of Public Health. 80 (10): 1183–1185. doi:10.2105/AJPH.80.10.1183. PMC 1404824. PMID 2119148.
- ^ The National Institute for Occupational Safety and Health. "CDC – NIOSH Pocket Guide to Chemical Hazards - Lead". www.cdc.gov. Retrieved 18 November 2016.
Bibliography
- Rieuwerts, John (2015). "Chapter 13. Lead". The Elements of Environmental Pollution. Abingdon, UK: Routledge. pp. 224–34. ISBN 9780415859202.
{{cite book}}: CS1 maint: ref duplicates default (link) - Bisson, Michael S.; Vogel, Joseph O. (2005). Ancient African Metallurgy: The Sociocultural Context. Rowman & Littlefield. ISBN 978-0-742-50261-1.
- Sutherland, Charles A.; Milner, Edward F.; Kerby, Robert C.; Teindl, Herbert; Melin, Albert; Bolt, Hermann M. (2005). "Lead". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a15_193.pub2. ISBN 3-527-30673-0.
- Lide, D. R., ed. (2004). CRC Handbook of Chemistry and Physics (84th ed.). CRC Press. ISBN 978-0-8493-0484-2.
{{cite book}}: Invalid|ref=harv(help) - Greenwood, N. N.; Earnshaw, A. (1998). Chemistry of the Elements (2nd ed.). Butterworth Heinemann. ISBN 0-7506-3365-4.
{{cite book}}: Invalid|ref=harv(help) - Kroonen, Guus (2013). Etymological Dictionary of Proto-Germanic. Leiden Indo-European Etymological Dictionary Series. Leiden, Boston: Brill.
- Rich, Vincent (1994). The International Lead Trade. Woodhead Publishing. ISBN 9781855731035.
{{cite book}}: CS1 maint: ref duplicates default (link) - Polyanskiy, N. G. (1986). Fillipova, N. A (ed.). Аналитическая химия элементов: Свинец (in Russian). Nauka.
{{cite book}}: Invalid|ref=harv(help); Unknown parameter|trans_title=ignored (|trans-title=suggested) (help) - Thornton, Iain; Rautiu, Radu; Brush, Susan (2001). Lead: the facts. Hersham, Surrey: Ian Allan Printing. ISBN 0-9542496-0-7.
Further reading
- Keisch, B.; Feller, R. L.; Levine, A. S.; Edwards, R. R. (1967). "Dating and Authenticating Works of Art by Measurement of Natural Alpha Emitters". Science. 155 (3767): 1238–1242. Bibcode:1967Sci...155.1238K. doi:10.1126/science.155.3767.1238. PMID 17847535.
- Keisch, B (1968). "Dating Works of Art Through their Natural Radioactivity: Improvements and Applications". Science. 160 (3826): 413–415. Bibcode:1968Sci...160..413K. doi:10.1126/science.160.3826.413. PMID 17740234.
- Keisch, B (1968). "Discriminating Radioactivity Measurements of Lead: New Tool for Authentication". Curator. 11 (1): 41–52. doi:10.1111/j.2151-6952.1968.tb00884.x.
- Casas, Jose S.; Sordo, Jose, eds. (2006). Lead Chemistry, Analytical Aspects. Environmental Impacts and Health Effects. Elsevier. ISBN 0-444-52945-4.
External links
- National Lead Free Wheel Weight Initiative| Waste Minimization|Wastes|US EPA
- CDC – NIOSH Pocket Guide to Chemical Hazards – Lead



