Portal:Minerals
Portal maintenance status: (May 2019)
|
The Minerals Portal

In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid substance with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.
The geological definition of mineral normally excludes compounds that occur only in living organisms. However, some minerals are often biogenic (such as calcite) or organic compounds in the sense of chemistry (such as mellite). Moreover, living organisms often synthesize inorganic minerals (such as hydroxylapatite) that also occur in rocks.
The concept of mineral is distinct from rock, which is any bulk solid geologic material that is relatively homogeneous at a large enough scale. A rock may consist of one type of mineral or may be an aggregate of two or more different types of minerals, spacially segregated into distinct phases.
Some natural solid substances without a definite crystalline structure, such as opal or obsidian, are more properly called mineraloids. If a chemical compound occurs naturally with different crystal structures, each structure is considered a different mineral species. Thus, for example, quartz and stishovite are two different minerals consisting of the same compound, silicon dioxide. (Full article...)
Mineralogy is a subject of geology specializing in the scientific study of the chemistry, crystal structure, and physical (including optical) properties of minerals and mineralized artifacts. Specific studies within mineralogy include the processes of mineral origin and formation, classification of minerals, their geographical distribution, as well as their utilization. (Full article...)
Selected articles
-
Image 1

Green fluorite with prominent cleavage
Cleavage, in mineralogy and materials science, is the tendency of crystalline materials to split along definite crystallographic structural planes. These planes of relative weakness are a result of the regular locations of atoms and ions in the crystal, which create smooth repeating surfaces that are visible both in the microscope and to the naked eye. If bonds in certain directions are weaker than others, the crystal will tend to split along the weakly bonded planes. These flat breaks are termed "cleavage". The classic example of cleavage is mica, which cleaves in a single direction along the basal pinacoid, making the layers seem like pages in a book. In fact, mineralogists often refer to "books of mica".
Diamond and graphite provide examples of cleavage. Each is composed solely of a single element, carbon. In diamond, each carbon atom is bonded to four others in a tetrahedral pattern with short covalent bonds. The planes of weakness (cleavage planes) in a diamond are in four directions, following the faces of the octahedron. In graphite, carbon atoms are contained in layers in a hexagonal pattern where the covalent bonds are shorter (and thus even stronger) than those of diamond. However, each layer is connected to the other with a longer and much weaker van der Waals bond. This gives graphite a single direction of cleavage, parallel to the basal pinacoid. So weak is this bond that it is broken with little force, giving graphite a slippery feel as layers shear apart. As a result, graphite makes an excellent dry lubricant.
While all single crystals will show some tendency to split along atomic planes in their crystal structure, if the differences between one direction or another are not large enough, the mineral will not display cleavage. Corundum, for example, displays no cleavage. (Full article...) -
Image 2
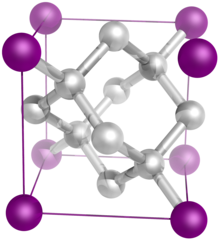
The diamond crystal structure belongs to the face-centered cubic lattice, with a repeated two-atom pattern.
In crystallography, a crystal system is a set of point groups (a group of geometric symmetries with at least one fixed point). A lattice system is a set of Bravais lattices. Space groups are classified into crystal systems according to their point groups, and into lattice systems according to their Bravais lattices. Crystal systems that have space groups assigned to a common lattice system are combined into a crystal family.
The seven crystal systems are triclinic, monoclinic, orthorhombic, tetragonal, trigonal, hexagonal, and cubic. Informally, two crystals are in the same crystal system if they have similar symmetries (though there are many exceptions). (Full article...) -
Image 3

Garnets ( /ˈɡɑːrnɪt/) are a group of silicate minerals that have been used since the Bronze Age as gemstones and abrasives.
All species of garnets possess similar physical properties and crystal forms, but differ in chemical composition. The different species are pyrope, almandine, spessartine, grossular (varieties of which are hessonite or cinnamon-stone and tsavorite), uvarovite and andradite. The garnets make up two solid solution series: pyrope-almandine-spessartine (pyralspite), with the composition range [Mg,Fe,Mn]3Al2(SiO4)3; and uvarovite-grossular-andradite (ugrandite), with the composition range Ca3[Cr,Al,Fe]2(SiO4)3. (Full article...) -
Image 4Malachite from the Democratic Republic of the Congo
Malachite is a copper carbonate hydroxide mineral, with the formula Cu2CO3(OH)2. This opaque, green-banded mineral crystallizes in the monoclinic crystal system, and most often forms botryoidal, fibrous, or stalagmitic masses, in fractures and deep, underground spaces, where the water table and hydrothermal fluids provide the means for chemical precipitation. Individual crystals are rare, but occur as slender to acicular prisms. Pseudomorphs after more tabular or blocky azurite crystals also occur. (Full article...) -
Image 5

Zeolite exhibited in the Estonian Museum of Natural History
Zeolite is a family of several microporous, crystalline aluminosilicate materials commonly used as commercial adsorbents and catalysts. They mainly consist of silicon, aluminium, oxygen, and have the general formula Mn+
1/n(AlO
2)−
(SiO
2)
x・yH
2O where Mn+
1/n is either a metal ion or H+.
The term was originally coined in 1756 by Swedish mineralogist Axel Fredrik Cronstedt, who observed that rapidly heating a material, believed to have been stilbite, produced large amounts of steam from water that had been adsorbed by the material. Based on this, he called the material zeolite, from the Greek ζέω (zéō), meaning "to boil" and λίθος (líthos), meaning "stone".
Zeolites occur naturally, but are also produced industrially on a large scale. As of December 2018[update], 253 unique zeolite frameworks have been identified, and over 40 naturally occurring zeolite frameworks are known. Every new zeolite structure that is obtained is examined by the International Zeolite Association Structure Commission (IZA-SC) and receives a three-letter designation. (Full article...) -
Image 6

Turquoise is an opaque, blue-to-green mineral that is a hydrous phosphate of copper and aluminium, with the chemical formula CuAl6(PO4)4(OH)8·4H2O. It is rare and valuable in finer grades and has been prized as a gemstone for millennia due to its hue.
Like most other opaque gems, turquoise has been devalued by the introduction of treatments, imitations, and synthetics into the market. The robin egg blue or sky blue color of the Persian turquoise mined near the modern city of Nishapur, Iran, has been used as a guiding reference for evaluating turquoise quality. (Full article...) -
Image 7
Graphite (/ˈɡræfaɪt/) is a crystalline allotrope (form) of the element carbon. It consists of many stacked layers of graphene, typically in the excess of hundreds of layers. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on a large scale (1.3 million metric tons per year in 2022) for uses in many critical industries including refractories (50%), lithium-ion batteries (18%), foundries (10%), lubricants (5%), among others (17%). Under extremely high pressures and extremely high temperatures it converts to diamond. Graphite's low cost, thermal and chemical inertness and characteristic conductivity of heat and electricity finds numerous applications in high energy and high temperature processes. (Full article...) -
Image 8
Mineralogy applies principles of chemistry, geology, physics and materials science to the study of minerals
Mineralogy is a subject of geology specializing in the scientific study of the chemistry, crystal structure, and physical (including optical) properties of minerals and mineralized artifacts. Specific studies within mineralogy include the processes of mineral origin and formation, classification of minerals, their geographical distribution, as well as their utilization. (Full article...) -
Image 9Beachy Head is a part of the extensive Southern England Chalk Formation.
Chalk is a soft, white, porous, sedimentary carbonate rock. It is a form of limestone composed of the mineral calcite and originally formed deep under the sea by the compression of microscopic plankton that had settled to the sea floor. Chalk is common throughout Western Europe, where deposits underlie parts of France, and steep cliffs are often seen where they meet the sea in places such as the Dover cliffs on the Kent coast of the English Channel.
Chalk is mined for use in industry, such as for quicklime, bricks and builder's putty, and in agriculture, for raising pH in soils with high acidity. It is also used for "blackboard chalk" for writing and drawing on various types of surfaces, although these can also be manufactured from other carbonate-based minerals, or gypsum. (Full article...) -
Image 10

Borax (also referred to as sodium borate, tincal (/ˈtɪŋkəl/) and tincar (/ˈtɪŋkər/)) is a salt (ionic compound), a hydrated or anhydrous borate of sodium, with the chemical formula Na2H20B4O17 (also written as Na2B4O7·10H2O).
It is a colorless crystalline solid that dissolves in water to make a basic solution.
It is commonly available in powder or granular form and has many industrial and household uses, including as a pesticide, as a metal soldering flux, as a component of glass, enamel, and pottery glazes, for tanning of skins and hides, for artificial aging of wood, as a preservative against wood fungus, and as a pharmaceutic alkalizer. In chemical laboratories, it is used as a buffering agent.
The terms tincal and tincar refer to native borax, historically mined from dry lake beds in various parts of Asia. (Full article...) -
Image 11

Rutile is an oxide mineral composed of titanium dioxide (TiO2), the most common natural form of TiO2. Rarer polymorphs of TiO2 are known, including anatase, akaogiite, and brookite.
Rutile has one of the highest refractive indices at visible wavelengths of any known crystal and also exhibits a particularly large birefringence and high dispersion. Owing to these properties, it is useful for the manufacture of certain optical elements, especially polarization optics, for longer visible and infrared wavelengths up to about 4.5 micrometres. Natural rutile may contain up to 10% iron and significant amounts of niobium and tantalum.
Rutile derives its name from the Latin rutilus ('red'), in reference to the deep red color observed in some specimens when viewed by transmitted light. Rutile was first described in 1803 by Abraham Gottlob Werner using specimens obtained in Horcajuelo de la Sierra, Madrid (Spain), which is consequently the type locality. (Full article...) -
Image 12A ruby crystal from Dodoma Region, Tanzania
Ruby is a pinkish red to blood-red colored gemstone, a variety of the mineral corundum (aluminium oxide). Ruby is one of the most popular traditional jewelry gems and is very durable. Other varieties of gem-quality corundum are called sapphires. Ruby is one of the traditional cardinal gems, alongside amethyst, sapphire, emerald, and diamond. The word ruby comes from ruber, Latin for red. The color of a ruby is due to the element chromium.
Some gemstones that are popularly or historically called rubies, such as the Black Prince's Ruby in the British Imperial State Crown, are actually spinels. These were once known as "Balas rubies".
The quality of a ruby is determined by its color, cut, and clarity, which, along with carat weight, affect its value. The brightest and most valuable shade of red, called blood-red or pigeon blood, commands a large premium over other rubies of similar quality. After color follows clarity: similar to diamonds, a clear stone will command a premium, but a ruby without any needle-like rutile inclusions may indicate that the stone has been treated. Ruby is the traditional birthstone for July and is usually pinker than garnet, although some rhodolite garnets have a similar pinkish hue to most rubies. The world's most valuable ruby to be sold at auction is the Sunrise Ruby, which sold for US$34.8 million. (Full article...) -
Image 13
A rock containing three crystals of pyrite (FeS2). The crystal structure of pyrite is primitive cubic, and this is reflected in the cubic symmetry of its natural crystal facets.
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties of these crystals:- Primitive cubic (abbreviated cP and alternatively called simple cubic)
- Body-centered cubic (abbreviated cI or bcc)
- Face-centered cubic (abbreviated cF or fcc)
Note: the term fcc is often used in synonym for the cubic close-packed or ccp structure occurring in metals. However, fcc stands for a face-centered-cubic Bravais lattice, which is not necessarily close-packed when a motif is set onto the lattice points. E.g. the diamond and the zincblende lattices are fcc but not close-packed.
Each is subdivided into other variants listed below. Although the unit cells in these crystals are conventionally taken to be cubes, the primitive unit cells often are not. (Full article...) -
Image 14Halite from the Wieliczka salt mine, Małopolskie, Poland
Halite (/ˈhælaɪt, ˈheɪlaɪt/ HAL-yte, HAY-lyte), commonly known as rock salt, is a type of salt, the mineral (natural) form of sodium chloride (NaCl). Halite forms isometric crystals. The mineral is typically colorless or white, but may also be light blue, dark blue, purple, pink, red, orange, yellow or gray depending on inclusion of other materials, impurities, and structural or isotopic abnormalities in the crystals. It commonly occurs with other evaporite deposit minerals such as several of the sulfates, halides, and borates. The name halite is derived from the Ancient Greek word for "salt", ἅλς (háls). (Full article...) -
Image 15

Micas (/ˈmaɪkəz/ MY-kəz) are a group of silicate minerals whose outstanding physical characteristic is that individual mica crystals can easily be split into fragile elastic plates. This characteristic is described as perfect basal cleavage. Mica is common in igneous and metamorphic rock and is occasionally found as small flakes in sedimentary rock. It is particularly prominent in many granites, pegmatites, and schists, and "books" (large individual crystals) of mica several feet across have been found in some pegmatites.
Micas are used in products such as drywalls, paints, and fillers, especially in parts for automobiles, roofing, and in electronics. The mineral is used in cosmetics and food to add "shimmer" or "frost". (Full article...) -
Image 16

Tourmaline (/ˈtʊərməlɪn, -ˌliːn/ TOOR-mə-lin, -leen) is a crystalline silicate mineral group in which boron is compounded with elements such as aluminium, iron, magnesium, sodium, lithium, or potassium. This gemstone comes in a wide variety of colors.
The name is derived from the Sinhalese tōramalli (ටෝරමල්ලි), which refers to the carnelian gemstones. (Full article...) -
Image 17Deep green isolated fluorite crystal resembling a truncated octahedron, set upon a micaceous matrix, from Erongo Mountain, Erongo Region, Namibia (overall size: 50 mm × 27 mm, crystal size: 19 mm wide, 30 g)
Fluorite (also called fluorspar) is the mineral form of calcium fluoride, CaF2. It belongs to the halide minerals. It crystallizes in isometric cubic habit, although octahedral and more complex isometric forms are not uncommon.
The Mohs scale of mineral hardness, based on scratch hardness comparison, defines value 4 as fluorite.
Pure fluorite is colourless and transparent, both in visible and ultraviolet light, but impurities usually make it a colorful mineral and the stone has ornamental and lapidary uses. Industrially, fluorite is used as a flux for smelting, and in the production of certain glasses and enamels. The purest grades of fluorite are a source of fluoride for hydrofluoric acid manufacture, which is the intermediate source of most fluorine-containing fine chemicals. Optically clear transparent fluorite has anomalous partial dispersion, that is, its refractive index varies with the wavelength of light in a manner that differs from that of commonly used glasses, so fluorite is useful in making apochromatic lenses, and particularly valuable in photographic optics. Fluorite optics are also usable in the far-ultraviolet and mid-infrared ranges, where conventional glasses are too opaque for use. Fluorite also has low dispersion, and a high refractive index for its density. (Full article...) -
Image 18

Gypsum is a soft sulfate mineral composed of calcium sulfate dihydrate, with the chemical formula CaSO4·2H2O. It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, drywall and blackboard or sidewalk chalk. Gypsum also crystallizes as translucent crystals of selenite. It forms as an evaporite mineral and as a hydration product of anhydrite. The Mohs scale of mineral hardness defines gypsum as hardness value 2 based on scratch hardness comparison.
Fine-grained white or lightly tinted forms of gypsum known as alabaster have been used for sculpture by many cultures including Ancient Egypt, Mesopotamia, Ancient Rome, the Byzantine Empire, and the Nottingham alabasters of Medieval England. (Full article...) -
Image 19Brazilian trigonal hematite crystal
Hematite (/ˈhiːməˌtaɪt, ˈhɛmə-/), also spelled as haematite, is a common iron oxide compound with the formula, Fe2O3 and is widely found in rocks and soils. Hematite crystals belong to the rhombohedral lattice system which is designated the alpha polymorph of Fe
2O
3. It has the same crystal structure as corundum (Al
2O
3) and ilmenite (FeTiO
3). With this it forms a complete solid solution at temperatures above 950 °C (1,740 °F).
Hematite occurs naturally in black to steel or silver-gray, brown to reddish-brown, or red colors. It is mined as an important ore mineral of iron. It is electrically conductive. Hematite varieties include kidney ore, martite (pseudomorphs after magnetite), iron rose and specularite (specular hematite). While these forms vary, they all have a rust-red streak. Hematite is not only harder than pure iron, but also much more brittle. Maghemite is a polymorph of hematite (γ-Fe
2O
3) with the same chemical formula, but with a spinel structure like magnetite.
Large deposits of hematite are found in banded iron formations. Gray hematite is typically found in places that have still, standing water, or mineral hot springs, such as those in Yellowstone National Park in North America. The mineral may precipitate in the water and collect in layers at the bottom of the lake, spring, or other standing water. Hematite can also occur in the absence of water, usually as the result of volcanic activity.
Clay-sized hematite crystals also may occur as a secondary mineral formed by weathering processes in soil, and along with other iron oxides or oxyhydroxides such as goethite, which is responsible for the red color of many tropical, ancient, or otherwise highly weathered soils. (Full article...) -
Image 20

Chalcopyrite (/ˌkælkəˈpaɪˌraɪt, -koʊ-/ KAL-kə-PY-ryte, -koh-) is a copper iron sulfide mineral and the most abundant copper ore mineral. It has the chemical formula CuFeS2 and crystallizes in the tetragonal system. It has a brassy to golden yellow color and a hardness of 3.5 to 4 on the Mohs scale. Its streak is diagnostic as green-tinged black.
On exposure to air, chalcopyrite tarnishes to a variety of oxides, hydroxides, and sulfates. Associated copper minerals include the sulfides bornite (Cu5FeS4), chalcocite (Cu2S), covellite (CuS), digenite (Cu9S5); carbonates such as malachite and azurite, and rarely oxides such as cuprite (Cu2O). It is rarely found in association with native copper. Chalcopyrite is a conductor of electricity.
Copper can be extracted from chalcopyrite ore using various methods. The two predominant methods are pyrometallurgy and hydrometallurgy, the former being the most commercially viable. (Full article...) -
Image 21
Magnetite is a mineral and one of the main iron ores, with the chemical formula Fe2+Fe3+2O4. It is one of the oxides of iron, and is ferrimagnetic; it is attracted to a magnet and can be magnetized to become a permanent magnet itself. With the exception of extremely rare native iron deposits, it is the most magnetic of all the naturally occurring minerals on Earth. Naturally magnetized pieces of magnetite, called lodestone, will attract small pieces of iron, which is how ancient peoples first discovered the property of magnetism.
Magnetite is black or brownish-black with a metallic luster, has a Mohs hardness of 5–6 and leaves a black streak. Small grains of magnetite are very common in igneous and metamorphic rocks.
The chemical IUPAC name is iron(II,III) oxide and the common chemical name is ferrous-ferric oxide. (Full article...) -
Image 22Galena with minor pyrite
Galena, also called lead glance, is the natural mineral form of lead(II) sulfide (PbS). It is the most important ore of lead and an important source of silver.
Galena is one of the most abundant and widely distributed sulfide minerals. It crystallizes in the cubic crystal system often showing octahedral forms. It is often associated with the minerals sphalerite, calcite and fluorite. (Full article...) -
Image 23
Apatite is a group of phosphate minerals, usually hydroxyapatite, fluorapatite and chlorapatite, with high concentrations of OH−, F− and Cl− ion, respectively, in the crystal. The formula of the admixture of the three most common endmembers is written as Ca10(PO4)6(OH,F,Cl)2, and the crystal unit cell formulae of the individual minerals are written as Ca10(PO4)6(OH)2, Ca10(PO4)6F2 and Ca10(PO4)6Cl2.
The mineral was named apatite by the German geologist Abraham Gottlob Werner in 1786, although the specific mineral he had described was reclassified as fluorapatite in 1860 by the German mineralogist Karl Friedrich August Rammelsberg. Apatite is often mistaken for other minerals. This tendency is reflected in the mineral's name, which is derived from the Greek word ἀπατάω (apatáō), which means to deceive. (Full article...) -
Image 24A sample of andesite (dark groundmass) with amygdaloidal vesicles filled with zeolite. Diameter of view is 8 cm.
Andesite (/ˈændəzaɪt/) is a volcanic rock of intermediate composition. In a general sense, it is the intermediate type between silica-poor basalt and silica-rich rhyolite. It is fine-grained (aphanitic) to porphyritic in texture, and is composed predominantly of sodium-rich plagioclase plus pyroxene or hornblende.
Andesite is the extrusive equivalent of plutonic diorite. Characteristic of subduction zones, andesite represents the dominant rock type in island arcs. The average composition of the continental crust is andesitic. Along with basalts, andesites are a component of the Martian crust.
The name andesite is derived from the Andes mountain range, where this rock type is found in abundance. It was first applied by Christian Leopold von Buch in 1826. (Full article...) -
Image 25

A crystalline solid: atomic resolution image of strontium titanate. Brighter spots are columns of strontium atoms and darker ones are titanium-oxygen columns.
Crystallography is the branch of science devoted to the study of molecular and crystalline structure and properties. The word crystallography is derived from the Ancient Greek word κρύσταλλος (krústallos; "clear ice, rock-crystal"), and γράφειν (gráphein; "to write"). In July 2012, the United Nations recognised the importance of the science of crystallography by proclaiming 2014 the International Year of Crystallography.
Crystallography is a broad topic, and many of its subareas, such as X-ray crystallography, are themselves important scientific topics. Crystallography ranges from the fundamentals of crystal structure to the mathematics of crystal geometry, including those that are not periodic or quasicrystals. At the atomic scale it can involve the use of X-ray diffraction to produce experimental data that the tools of X-ray crystallography can convert into detailed positions of atoms, and sometimes electron density. At larger scales it includes experimental tools such as orientational imaging to examine the relative orientations at the grain boundary in materials. Crystallography plays a key role in many areas of biology, chemistry, and physics, as well new developments in these fields. (Full article...)
Selected mineralogist
-
Image 1Hatten Schuyler Yoder, Jr., (March 20, 1921 – August 2, 2003) was a geophysicist and experimental petrologist who conducted pioneering work on minerals under high pressure and temperature. He was noted for his study of silicates and igneous rocks. (Full article...)
-
Image 2
Albrecht Schrauf (14 December 1837, Vienna – 29 November 1897, Vienna) was an Austrian mineralogist and crystallographer. (Full article...) -
Image 3
Karl Georg von Raumer (9 April 1783 – 2 June 1865) was a German geologist and educator. (Full article...) -
Image 4

Karl Friedrich August Rammelsberg (1 April 1813 – 28 December 1899) was a German mineralogist from Berlin, Prussia. (Full article...) -
Image 5

Robert Miller Hazen (born November 1, 1948) is an American mineralogist and astrobiologist. He is a research scientist at the Carnegie Institution of Washington's Geophysical Laboratory and Clarence Robinson Professor of Earth Science at George Mason University, in the United States. Hazen is the Executive Director of the Deep Carbon Observatory. (Full article...) -
Image 6James Freeman Dana (born in Amherst, New Hampshire, 23 September 1793; died in New York City, 14 April 1827) was an American chemist. (Full article...)
-
Image 7

George Gibbs (January 7, 1776 – August 6, 1833) was an American mineralogist and mineral collector. The mineral gibbsite is named after him. (Full article...) -
Image 8
Georgius Agricola (/əˈɡrɪkələ/; born Georg Bauer; 24 March 1494 – 21 November 1555) was a German Humanist scholar, mineralogist and metallurgist. Born in the small town of Glauchau, in the Electorate of Saxony of the Holy Roman Empire, he was broadly educated, but took a particular interest in the mining and refining of metals. He was the first to drop the Arabic definite article al-, exclusively writing chymia and chymista in describing activity that we today would characterize as chemical or alchemical, giving chemistry its modern name. For his groundbreaking work De Natura Fossilium published in 1546, he is generally referred to as the father of mineralogy and the founder of geology as a scientific discipline.
He is well known for his pioneering work De re metallica libri XII, that was published in 1556, one year after his death. This 12-volume work is a comprehensive and systematic study, classification and methodical guide on all available factual and practical aspects, that are of concern for mining, the mining sciences and metallurgy, investigated and researched in its natural environment by means of direct observation. Unrivalled in its complexity and accuracy, it served as the standard reference work for two centuries. Agricola stated in the preface, that he will exclude "all those things which I have not myself seen, or have not read or heard of". He continued, "That which I have neither seen, nor carefully considered after reading or hearing of, I have not written about." (Full article...) -
Image 9Edward Sydney Simpson (11 March 1875 – 30 August 1939) was an Australian mineralogist and geochemist.
Simpson was born in Woollahra, New South Wales to an Irish father and English mother. He was educated at Sydney Grammar School and the University of Sydney where he graduated B.E. with honours, in 1895 and D.Sc. in 1919. (Full article...) -
Image 10Ralph Early Grim (February 25, 1902 – August 19, 1989) was an American geologist and scientist, often referred to as the "Father of Mineralogy" because he made many discoveries during his investigations of clay materials. He was one of the most outstanding mineralogists of his time and was well-known throughout the world in the field of clay science and technology. Grim's career spanned over 60 years and he received many honors and awards in the field of mineralogy. Some of the textbooks he wrote have been standard university textbooks in mineralogy for many years. (Full article...)
-
Image 11

Alfred Des Cloizeaux
Alfred Louis Olivier Legrand Des Cloizeaux (17 October 1817 – 6 May 1897) was a French mineralogist.
Des Cloizeaux was born at Beauvais, in the department of Oise. He studied with Jean-Baptiste Biot at the Collège de France. He became professor of mineralogy at the École Normale Supérieure and afterwards at the Muséum National d'Histoire Naturelle in Paris. He studied the geysers of Iceland, and wrote also on the classification of some of the eruptive rocks. (Full article...) -
Image 12Hans Morten Thrane Esmark (21 August 1801 – 24 April 1882) was a Norwegian priest and mineralogist. He is most noted for first locating the mineral thorite. (Full article...)
-
Image 13

Charles-Louis de la Vallée Poussin
Charles-Louis-Joseph-Xavier de la Vallée Poussin (6 April 1827, Namur – 15 March 1903, Brussels) was a Belgian geologist and mineralogist. His son was the mathematician Charles Jean de la Vallée Poussin. (Full article...) -
Image 14
(Karl) Heinrich/Harry (Ferdinand) Rosenbusch (24 June 1836 – 20 January 1914) was a German petrographer.
Harry Rosenbusch was born in Einbeck. He taught at Heidelberg University (1877–1908), where he founded the Mineralogisches-geologisches Institut. He died, aged 77, in Heidelberg. (Full article...) -
Image 15

Robert Jameson
Robert Jameson FRS FRSE (11 July 1774 – 19 April 1854) was a Scottish naturalist and mineralogist.
As Regius Professor of Natural History at the University of Edinburgh for fifty years, developing his predecessor John Walker's concepts based on mineralogy into geological theories of Neptunism which held sway into the 1830s. Jameson is notable for his advanced scholarship, and his museum collection. The minerals and fossils collection of the Museum of Edinburgh University became one of the largest in Europe during Jameson's long tenure at the university. (Full article...) -
Image 16

Ours-Pierre-Armand Petit-Dufrénoy (5 September 1792 – 20 March 1857) was a French geologist and mineralogist. (Full article...) -
Image 17Sarah Mawe (1767-1846) was an English mineralogist of the 19th century, appointed to serve Queen Victoria in that capacity from 1837 to 1840. (Full article...)
-
Image 18
Johann Nepomuk von Fuchs (15 May 1774 – 5 March 1856) was a German chemist and mineralogist, and royal Bavarian privy councillor. (Full article...) -
Image 19

George R. Rossman is an American mineralogist and the Professor of Mineralogy at the California Institute of Technology. (Full article...) -
Image 20Henri Hureau de Sénarmont (6 September 1808 – 30 June 1862) was a French mineralogist and physicist.
He was born in Broué, Eure-et-Loir. From 1822 to 1826, he studied at the École Polytechnique in Paris, then furthered his education at the École des Mines. During the course of his career, he became engineer-in-chief of mines, and professor of mineralogy and director of studies at the École des Mines in Paris. (Full article...) -
Image 21

Ernest-François Mallard (4 February 1833 – 6 July 1894) was a French mineralogist and a member of the French Academy of Sciences. He is also notable for his work with Henri Louis Le Chatelier in combustion as applied to mining safety. (Full article...) -
Image 22Leopold Heinrich Fischer (19 December 1817, Fribourg-en-Brisgau – 2 February 1886, Fribourg-en-Brisgau) was a German zoologist and mineralogist. (Full article...)
-
Image 23

André Laugier (1770–1832)
André Laugier (1 August 1770, in Lisieux – 19 April 1832, in Paris) was a French chemist, pharmacist and mineralogist. He was a cousin to famed chemist Antoine François Fourcroy and the father of astronomer Paul Auguste Ernest Laugier (1812–1872).
He received his education in his hometown of Lisieux, and during the French Revolution, was tasked with collecting church bells in Bretagne in order for them to be melted down for the production of cannons. In 1794 he was employed as head of the gunpowder and saltpeter works at the Comite de salut public. In 1797 he received his master's degree in pharmacy, and subsequently taught classes in chemistry and pharmacy at the military training schools in Toulon and Lille. (Full article...) -
Image 24João da Silva Barbosa or João da Silva Feijó, (1760 in Rio de Janeiro – 1824), was a naturalist, mineralogist and Portuguese soldier, born in Brazil.
João da Silva Feijó studied philosophy and mathematics at the University of Coimbra and adopted the name Feijó in homage to Benito Jerónimo Feijoo, Spanish philosopher, who at the time was respected among natural history students. (Full article...) -
Image 25Philip James Yorke (13 October 1799 – 14 December 1874) was a British Army officer, scientist and Fellow of the Royal Society. Yorke was a founder of the Chemical Society in the United Kingdom. (Full article...)
Related portals
Get involved
For editor resources and to collaborate with other editors on improving Wikipedia's Minerals-related articles, see WikiProject Rocks and minerals.
General images
-
Image 1Black andradite, an end-member of the orthosilicate garnet group. (from Mineral)
-
Image 2Sphalerite crystal partially encased in calcite from the Devonian Milwaukee Formation of Wisconsin (from Mineral)
-
Image 3An example of elbaite, a species of tourmaline, with distinctive colour banding. (from Mineral)
-
Image 4Perfect basal cleavage as seen in biotite (black), and good cleavage seen in the matrix (pink orthoclase). (from Mineral)
-
Image 7Schist is a metamorphic rock characterized by an abundance of platy minerals. In this example, the rock has prominent sillimanite porphyroblasts as large as 3 cm (1.2 in). (from Mineral)
-
Image 8Epidote often has a distinctive pistachio-green colour. (from Mineral)
-
Image 10Mohs Scale versus Absolute Hardness (from Mineral)
-
Image 11Red cinnabar (HgS), a mercury ore, on dolomite. (from Mineral)
-
Image 15Diamond is the hardest natural material, and has a Mohs hardness of 10. (from Mineral)
-
Image 16Asbestiform tremolite, part of the amphibole group in the inosilicate subclass (from Mineral)
-
Image 17When minerals react, the products will sometimes assume the shape of the reagent; the product mineral is termed a pseudomorph of (or after) the reagent. Illustrated here is a pseudomorph of kaolinite after orthoclase. Here, the pseudomorph preserved the Carlsbad twinning common in orthoclase. (from Mineral)
-
Image 18Pink cubic halite (NaCl; halide class) crystals on a nahcolite matrix (NaHCO3; a carbonate, and mineral form of sodium bicarbonate, used as baking soda). (from Mineral)
-
Image 19Muscovite, a mineral species in the mica group, within the phyllosilicate subclass (from Mineral)
-
Image 20Gypsum desert rose (from Mineral)
-
Image 21Hübnerite, the manganese-rich end-member of the wolframite series, with minor quartz in the background (from Mineral)
-
Image 24Native gold. Rare specimen of stout crystals growing off of a central stalk, size 3.7 x 1.1 x 0.4 cm, from Venezuela. (from Mineral)
-
Image 25Mohs hardness kit, containing one specimen of each mineral on the ten-point hardness scale (from Mohs scale)
Did you know ...?
- ... that the mineral shigaite (hexagonal crystal pictured) is named for the Japanese Prefecture where it was discovered in 1985?
- ... that naturally-occurring quasicrystals can be found in the rare minerals khatyrkite and cupalite?
- ... that the existence of the mineral frankdicksonite was predicted before its discovery?
- ... that seifertite, one of the densest polymorphs of silica, is named after Friedrich Seifert and has only been found in meteorites?
Subcategories
Topics
| Overview | ||
|---|---|---|
| Common minerals | ||
Ore minerals, mineral mixtures and ore deposits | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ores |
| ||||||||
| Deposit types | |||||||||
| Borates | |||||
|---|---|---|---|---|---|
| Carbonates | |||||
| Oxides |
| ||||
| Phosphates | |||||
| Silicates | |||||
| Sulfides | |||||
| Other |
| ||||
| Crystalline | |||||||
|---|---|---|---|---|---|---|---|
| Cryptocrystalline | |||||||
| Amorphous | |||||||
| Miscellaneous | |||||||
| Notable varieties |
| ||||||
| Oxide minerals |
| ||||
|---|---|---|---|---|---|
| Silicate minerals | |||||
| Other | |||||
Gemmological classifications by E. Ya. Kievlenko (1980), updated | |||||||||
| Jewelry stones |
| ||||||||
| Jewelry-Industrial stones |
| ||||||||
| Industrial stones |
| ||||||||
Mineral identification | |
|---|---|
| "Special cases" ("native elements and organic minerals") |
|
|---|---|
| "Sulfides and oxides" |
|
| "Evaporites and similars" |
|
| "Mineral structures with tetrahedral units" (sulfate anion, phosphate anion, silicon, etc.) |
|
Associated Wikimedia
The following Wikimedia Foundation sister projects provide more on this subject:
-
Commons
Free media repository -
Wikibooks
Free textbooks and manuals -
Wikidata
Free knowledge base -
Wikinews
Free-content news -
Wikiquote
Collection of quotations -
Wikisource
Free-content library -
Wikiversity
Free learning tools -
Wiktionary
Dictionary and thesaurus
References
- Manually maintained portal pages from May 2019
- All manually maintained portal pages
- Portals with triaged subpages from May 2019
- All portals with triaged subpages
- Portals with named maintainer
- Automated article-slideshow portals with 31–40 articles in article list
- Automated article-slideshow portals with 201–500 articles in article list
- Portals needing placement of incoming links