Cirrhosis: Difference between revisions
→Common causes: link tweak |
|||
| Line 150: | Line 150: | ||
Other laboratory studies performed in newly diagnosed cirrhosis may include: |
Other laboratory studies performed in newly diagnosed cirrhosis may include: |
||
* Serology for [[hepatitis]] viruses, [[autoantibody|autoantibodies]] ([[Anti-nuclear antibody|ANA]], anti-smooth muscle, [[Anti-mitochondrial antibody|anti-mitochondria]], anti-LKM) |
* Serology for [[hepatitis]] viruses, [[autoantibody|autoantibodies]] ([[Anti-nuclear antibody|ANA]], anti-smooth muscle, [[Anti-mitochondrial antibody|anti-mitochondria]], anti-LKM) |
||
* [[Ferritin]]<ref>{{Cite journal|last=Tornai|first=David|last2=Antal-Szalmas|first2=Peter|last3=Tornai|first3=Tamas|last4=Papp|first4=Maria|last5=Tornai|first5=Istvan|last6=Sipeki|first6=Nora|last7=Janka|first7=Tamas|last8=Balogh|first8=Boglarka|last9=Vitalis|first9=Zsuzsanna|date=2021-03-02|title=Abnormal ferritin levels predict development of poor outcomes in cirrhotic outpatients: a cohort study|url=https://doi.org/10.1186/s12876-021-01669-w|journal=BMC Gastroenterology|volume=21|issue=1|pages=94|doi=10.1186/s12876-021-01669-w|issn=1471-230X|pmc=PMC7923668|pmid=33653274}}</ref><ref>{{Cite journal|last=Maiwall|first=Rakhi|last2=Kumar|first2=Suman|last3=Chaudhary|first3=A. K.|last4=Maras|first4=Jaswinder|last5=Wani|first5=Zeeshan|last6=Kumar|first6=Chandan|last7=Rastogi|first7=A.|last8=Bihari|first8=C.|last9=Vashisht|first9=Chitranshu|last10=Sarin|first10=S. K.|date=2014-07|title=Serum ferritin predicts early mortality in patients with decompensated cirrhosis|url=https://pubmed.ncbi.nlm.nih.gov/24681346|journal=Journal of Hepatology|volume=61|issue=1|pages=43–50|doi=10.1016/j.jhep.2014.03.027|issn=1600-0641|pmid=24681346}}</ref> and [[transferrin saturation]]: markers of iron overload as in hemochromatosis, [[copper]] and [[ceruloplasmin]]: markers of copper overload as in Wilson's disease |
|||
* [[Ferritin]] and [[transferrin saturation]]: markers of iron overload as in hemochromatosis, [[copper]] and [[ceruloplasmin]]: markers of copper overload as in Wilson's disease |
|||
* [[Immunoglobulin]] levels (IgG, IgM, IgA) – these immunoglobins are non-specific, but may help in distinguishing various causes |
* [[Immunoglobulin]] levels (IgG, IgM, IgA) – these immunoglobins are non-specific, but may help in distinguishing various causes |
||
* [[Cholesterol]] and [[glucose]] |
* [[Cholesterol]] and [[glucose]] |
||
* [[Alpha 1-antitrypsin]] |
* [[Alpha 1-antitrypsin]] |
||
Markers of inflammation and immune cell activation are usually elevated in cirrhotic patients: |
|||
* [[C-reactive protein]] (CRP)<ref name=":1">{{Cite journal|last=Papp|first=Maria|last2=Vitalis|first2=Zsuzsanna|last3=Altorjay|first3=Istvan|last4=Tornai|first4=Istvan|last5=Udvardy|first5=Miklos|last6=Harsfalvi|first6=Jolan|last7=Vida|first7=Andras|last8=Kappelmayer|first8=Janos|last9=Lakatos|first9=Peter L.|last10=Antal‐Szalmas|first10=Peter|date=2012|title=Acute phase proteins in the diagnosis and prediction of cirrhosis associated bacterial infections|url=https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1478-3231.2011.02689.x|journal=Liver International|language=en|volume=32|issue=4|pages=603–611|doi=10.1111/j.1478-3231.2011.02689.x|issn=1478-3231}}</ref> |
|||
* [[Procalcitonin]] (PCT)<ref name=":1" /> |
|||
* [[Presepsin]]<ref>{{Cite journal|last=Papp|first=Maria|last2=Tornai|first2=Tamas|last3=Vitalis|first3=Zsuzsanna|last4=Tornai|first4=Istvan|last5=Tornai|first5=David|last6=Dinya|first6=Tamas|last7=Sumegi|first7=Andrea|last8=Antal-Szalmas|first8=Peter|date=2016-11-07|title=Presepsin teardown - pitfalls of biomarkers in the diagnosis and prognosis of bacterial infection in cirrhosis|url=https://pubmed.ncbi.nlm.nih.gov/27895404|journal=World Journal of Gastroenterology|volume=22|issue=41|pages=9172–9185|doi=10.3748/wjg.v22.i41.9172|issn=2219-2840|pmc=5107598|pmid=27895404}}</ref> |
|||
* soluble [[CD14]]<ref name=":1" /> |
|||
* soluble [[CD163]]<ref name=":5">{{Cite journal|last=Tornai|first=Tamas|last2=Vitalis|first2=Zsuzsanna|last3=Sipeki|first3=Nora|last4=Dinya|first4=Tamas|last5=Tornai|first5=David|last6=Antal‐Szalmas|first6=Peter|last7=Karanyi|first7=Zsolt|last8=Tornai|first8=Istvan|last9=Papp|first9=Maria|date=2016|title=Macrophage activation marker, soluble CD163, is an independent predictor of short-term mortality in patients with cirrhosis and bacterial infection|url=https://onlinelibrary.wiley.com/doi/abs/10.1111/liv.13133|journal=Liver International|language=en|volume=36|issue=11|pages=1628–1638|doi=10.1111/liv.13133|issn=1478-3231}}</ref> |
|||
* soluble [[Mannose receptor|CD206]] (mannose receptor)<ref>{{Cite journal|last=Laursen|first=Tea L.|last2=Rødgaard-Hansen|first2=Sidsel|last3=Møller|first3=Holger J.|last4=Mortensen|first4=Christian|last5=Karlsen|first5=Stine|last6=Nielsen|first6=Dennis T.|last7=Frevert|first7=Susanne|last8=Clemmesen|first8=Jens O.|last9=Møller|first9=Søren|last10=Jensen|first10=Jørgen S.|last11=Bendtsen|first11=Flemming|date=2017-04|title=The soluble mannose receptor is released from the liver in cirrhotic patients, but is not associated with bacterial translocation|url=https://pubmed.ncbi.nlm.nih.gov/27706896|journal=Liver International: Official Journal of the International Association for the Study of the Liver|volume=37|issue=4|pages=569–575|doi=10.1111/liv.13262|issn=1478-3231|pmid=27706896}}</ref> |
|||
* soluble [[TREM1|TREM-1]]<ref name=":6">{{Cite journal|date=2021-09-01|title=Increased sTREM-1 levels identify cirrhotic patients with bacterial infection and predict their 90-day mortality|url=https://www.sciencedirect.com/science/article/pii/S2210740120303454|journal=Clinics and Research in Hepatology and Gastroenterology|language=en|volume=45|issue=5|pages=101579|doi=10.1016/j.clinre.2020.11.009|issn=2210-7401}}</ref> |
|||
===Imaging=== |
===Imaging=== |
||
| Line 267: | Line 276: | ||
===Infection=== |
===Infection=== |
||
Cirrhosis can cause immune system dysfunction, leading to [[infection]]. Signs and symptoms of infection may be nonspecific and are more difficult to recognize (for example, worsening encephalopathy but no fever). |
Cirrhosis can cause immune system dysfunction, leading to [[infection]]. Signs and symptoms of infection may be nonspecific and are more difficult to recognize (for example, worsening encephalopathy but no fever).<ref name=":7">{{Cite journal|last=Sipeki|first=Nora|last2=Antal-Szalmas|first2=Peter|last3=Lakatos|first3=Peter L.|last4=Papp|first4=Maria|date=2014-03-14|title=Immune dysfunction in cirrhosis|url=https://pubmed.ncbi.nlm.nih.gov/24627592|journal=World Journal of Gastroenterology|volume=20|issue=10|pages=2564–2577|doi=10.3748/wjg.v20.i10.2564|issn=2219-2840|pmc=3949265|pmid=24627592}}</ref> Moreover infections in cirrhosis are major triggers for other complications (ascites, variceal bleading, hepatic enecphalopathy, organ failures, death).<ref name=":7" /><ref name=":5" /><ref name=":6" /> |
||
===Hepatocellular carcinoma=== |
===Hepatocellular carcinoma=== |
||
Revision as of 10:31, 25 April 2021
| Cirrhosis | |
|---|---|
| Other names | Cirrhosis of the liver, hepatic cirrhosis |
 | |
| The abdomen of a person with cirrhosis showing massive fluid buildup and very visible veins | |
| Pronunciation | |
| Specialty | Gastroenterology, Hepatology |
| Symptoms | Tiredness, itchiness, swelling in the lower legs, jaundice, easily bruising, fluid build-up in the abdomen[1] |
| Complications | Spontaneous bacterial peritonitis, hepatic encephalopathy, dilated veins in the esophagus, liver cancer[1] |
| Usual onset | Over months or years[1] |
| Duration | Long term[1] |
| Causes | Alcoholic liver disease, hepatitis B, hepatitis C, non-alcoholic steatohepatitis[2][3][4] |
| Diagnostic method | Blood tests, medical imaging, liver biopsy[5][1] |
| Prevention | Vaccination (such as hepatitis B), avoiding alcohol[1] |
| Treatment | Depends on underlying cause[6] |
| Frequency | 2.8 million (2015)[7] |
| Deaths | 1.3 million (2015)[8] |
Cirrhosis, also known as liver cirrhosis or hepatic cirrhosis, and end-stage liver disease, is the impaired liver function caused by the formation of scar tissue known as fibrosis, due to damage caused by liver disease.[9] Damage causes tissue repair and subsequent formation of scar tissue, which over time can replace normal functioning tissue leading to the impaired liver function of cirrhosis.[9][10] The disease typically develops slowly over months or years.[1] Early symptoms may include tiredness, weakness, loss of appetite, unexplained weight loss, nausea and vomiting, and discomfort in the right upper quadrant of the abdomen.[2] As the disease worsens, symptoms may include itchiness, swelling in the lower legs, fluid build-up in the abdomen, jaundice, bruising easily, and the development of spider-like blood vessels in the skin.[2] The fluid build-up in the abdomen may become spontaneously infected.[1] More serious complications include hepatic encephalopathy, bleeding from dilated veins in the esophagus, stomach or intestines, and liver cancer.[11]
Cirrhosis is most commonly caused by alcoholic liver disease, non-alcoholic steatohepatitis (NASH) – (the progressive form of non-alcoholic fatty liver disease),[12] chronic hepatitis B, and chronic hepatitis C.[2][4] Heavy drinking over a number of years can cause alcoholic liver disease.[13] NASH has a number of causes, including obesity, high blood pressure, abnormal levels of cholesterol, type 2 diabetes, and metabolic syndrome.[3] Less common causes of cirrhosis include autoimmune hepatitis, primary biliary cholangitis and primary sclerosing cholangitis that disrupt bile duct function, genetic disorders such as Wilson's disease and hereditary hemochromatosis, and chronic heart failure with liver congestion.[2]
Diagnosis is based on blood tests, medical imaging, and liver biopsy.[5][1]
Hepatitis B vaccine can prevent hepatitis B and the development of cirrhosis, but there is no vaccination against hepatitis C.[1] There is no specific treatment for cirrhosis but many of the underlying causes may be treated by a number of medications that may slow or prevent worsening of the condition.[6] Avoiding alcohol is recommended in all cases.[1] Hepatitis B and C may be treatable with antiviral medications.[1] Autoimmune hepatitis may be treated with steroid medications.[1] Ursodiol may be useful if the disease is due to blockage of the bile ducts.[1] Other medications may be useful for complications such as abdominal or leg swelling, hepatic encephalopathy, and dilated esophageal veins.[1] If cirrhosis leads to liver failure a liver transplant may be an option.[3]
Cirrhosis affected about 2.8 million people and resulted in 1.3 million deaths in 2015.[7][8] Of these deaths, alcohol caused 348,000, hepatitis C caused 326,000, and hepatitis B caused 371,000.[8] In the United States, more men die of cirrhosis than women.[1] The first known description of the condition is by Hippocrates in the 5th century BCE.[14] The term cirrhosis was invented in 1819, from a Greek word for the yellowish color of a diseased liver.[15]
Signs and symptoms

Cirrhosis can take quite a long time to develop, and symptoms may be slow to emerge. Early symptoms may include tiredness, weakness, loss of appetite, unexplained weight loss, nausea and sickness, and discomfort in the upper right abdomen.[2] With declining liver function other signs and symptoms may develop such as cognitive impairments, confusion, memory loss, sleep disorders, and changes in personality. Further decline may result in a build-up of fluid in the lower legs and feet; severe bloating of the abdomen from a fluid build-up known as ascites; jaundice; severe itchy skin, and darkly colored urine.[2] Some of these symptoms may be secondary to subsequent portal hypertension – increased blood pressure in the blood supply to the liver.[16]
Liver dysfunction
The following features are as a direct consequence of liver cells not functioning.
- Spider angiomata or spider nevi are vascular lesions consisting of a central arteriole surrounded by many smaller vessels (hence the name "spider") and occur due to an increase in estradiol. One study found that spider angiomata occur in about 1/3 of cases.[17]
- Palmar erythema is a reddening of palms at the thenar and hypothenar eminences seen in about 23% of cirrhosis cases[18] as a result of increased estrogen.[19]
- Gynecomastia, or benign increase in breast size in men, is caused by increased estradiol and can occur in up to 2/3 of cases.[20] This is different from increase in breast fat in overweight people.[21] A swollen scrotum may also be evident.[22]
- Hypogonadism, a decrease in male sex hormones may manifest as impotence, infertility, loss of sexual drive, and testicular atrophy, and can result from primary gonadal injury or suppression of hypothalamic/pituitary function. Hypogonadism is associated with cirrhosis due to alcoholism or iron overload.[23]
- Liver size can be enlarged, normal, or shrunken in people with cirrhosis.
- Ascites, accumulation of fluid in the peritoneal cavity in the abdomen, gives rise to bulging flanks.[24]
- Jaundice, is yellow discoloration of the skin and mucous membranes notably of the white of the eyes due to increased levels of bilirubin which may also cause the urine to be dark colored.[24]
Portal hypertension
Liver cirrhosis increases resistance to blood flow and leads to higher pressure in the portal venous system, resulting in portal hypertension. Effects of portal hypertension include:
- An enlarged spleen is found in 35% to 50% of cases.[16]
- Esophageal varices result from collateral portal blood flow through vessels in the stomach and esophagus (a process called portacaval anastomosis). When these blood vessels become enlarged, they are called varices and are more likely to rupture.[24] Variceal rupture often leads to severe bleeding, which can prove fatal.
- Caput medusae are dilated paraumbilical collateral veins due to portal hypertension. Blood from the portal venous system may be shunted through the paraumbilical veins and ultimately to the abdominal wall veins, manifesting as a pattern that may resemble the head of Medusa.[24]
- Cruveilhier-Baumgarten bruit is a venous hum heard in the epigastric region (on examination by stethoscope) due to collateral connections forming between the portal system and the paraumbilical veins as a result of portal hypertension.
Other non-specific signs
Some signs that may be present but not specific include changes in the nails such as Muehrcke's lines, Terry's nails, and nail clubbing; hypertrophic osteoarthropathy, and Dupuytren's contracture.[25]
Advanced disease
As the disease progresses, complications may develop. In some people, these may be the first signs of the disease.
- Bruising and bleeding resulting from decreased production of clotting factors.
- Hepatic encephalopathy (HE) – occurs when ammonia and related substances build up in the blood and affect brain function when they are not cleared from the blood by the liver. This may result in neglect of personal appearance, unresponsiveness, forgetfulness, trouble concentrating, changes in sleep habits or psychosis. One classic physical exam findings is asterixis, bilateral asynchronous flapping of outstretched, dorsiflexed hands.[24] Fetor hepaticus is a musty breath odor resulting from increased dimethyl sulfide and is a feature of HE.[26]
- Sensitivity to medication caused by decreased metabolism of the active compounds.
- Acute kidney injury (particularly hepatorenal syndrome).[27]
- Cachexia associated with muscle wasting and weakness.[28]
Causes
Cirrhosis has many possible causes, and sometimes more than one cause may be present. History taking is of real importance in trying to determine the most likely cause.[5] Globally, 57% of cirrhosis is attributable to either hepatitis B (30%) or hepatitis C (27%).[29] Alcohol use disorder is another major cause, accounting for about 20-40% of the cases.[29][24]
Common causes
- Alcoholic liver disease (ALD). Alcoholic cirrhosis develops for 10–20% of individuals who drink heavily for a decade or more.[30] Alcohol seems to injure the liver by blocking the normal metabolism of protein, fats, and carbohydrates. This injury happens through the formation of acetaldehyde from alcohol which itself is reactive, but which also leads to the accumulation of other reactive products in the liver.[24] People with ALD may also have concurrent alcoholic hepatitis with fever, hepatomegaly, jaundice, and anorexia. AST and ALT blood levels are both elevated, but at less than 300 IU/liter, with an AST:ALT ratio > 2.0, a value rarely seen in other liver diseases.[16] In the United States, 40% of cirrhosis-related deaths are due to alcohol.[24]
- Non-alcoholic fatty liver disease (NAFLD). In NAFLD, fat builds up in the liver and eventually causes scar tissue. This type of disorder appears to be associated with obesity (40% of NAFLD cases) diabetes, protein malnutrition, coronary artery disease, and treatment with steroids. Though similar in signs to alcoholic liver disease, there is no history of notable alcohol use. Blood tests, and medical imaging are used to diagnose NAFLD and NASH and sometimes a liver biopsy is needed.[31]
- Chronic hepatitis C. Infection with the hepatitis C virus causes inflammation of the liver and a variable grade of damage to the organ. Over several decades, this inflammation and damage can lead to cirrhosis. Among patients with chronic hepatitis C, 20–30% will develop cirrhosis.[24][32] Cirrhosis caused by hepatitis C and alcoholic liver disease are the most common reasons for liver transplant.[33]
- Chronic hepatitis B. The hepatitis B virus causes liver inflammation and injury that over several decades can lead to cirrhosis. Hepatitis D is dependent on the presence of hepatitis B and accelerates cirrhosis in co-infection.[16]
Less common causes
- Primary biliary cholangitis (previously known as primary biliary cirrhosis). The bile ducts become damaged by an autoimmune process, leading to secondary liver damage. Patients may be asymptomatic or have fatigue, pruritus, and non-jaundice skin hyperpigmentation with hepatomegaly. There is prominent alkaline phosphatase elevation as well as elevations in cholesterol and bilirubin and usually positive anti-mitochondrial antibodies.
- Primary sclerosing cholangitis. PSC is a progressive cholestatic disorder presenting with pruritus, steatorrhea, fat-soluble vitamin deficiencies, and metabolic bone disease. There is a strong association with inflammatory bowel disease (IBD), especially ulcerative colitis.[24]
- Autoimmune hepatitis. This disease is caused by an attack of the liver by lymphocytes, causing inflammation and eventually scarring and cirrhosis. Findings include elevations in serum globulins, especially gamma globulins.[24]
- Hereditary hemochromatosis. Usually presents with a family history of cirrhosis, skin hyperpigmentation, diabetes mellitus, pseudogout, or cardiomyopathy, all due to signs of iron overload.[24][34]
- Wilson's disease. Autosomal recessive disorder characterized by low serum ceruloplasmin and increased hepatic copper content on liver biopsy and elevated 24-hour urine copper. May also have Kayser-Fleischer rings in the cornea and altered mental status.
- Indian childhood cirrhosis is a form of neonatal cholestasis characterized by deposition of copper in the liver.[35]
- Alpha 1-antitrypsin deficiency (A1AD). Autosomal recessive disorder of decreased levels of the enzyme alpha 1—antitrypsin.[24]
- Cardiac cirrhosis. Due to chronic right sided heart failure, which leads to liver congestion.[24]
- Galactosemia
- Glycogen storage disease type IV
- Cystic fibrosis[24]
- Hepatotoxic drugs or toxins, such as acetaminophen, methotrexate, or amiodarone
Pathophysiology
The liver plays a vital role in the synthesis of proteins (for example, albumin, clotting factors and complement), detoxification, and storage (for example, of vitamin A). In addition, it participates in the metabolism of lipids and carbohydrates.
Cirrhosis is often preceded by hepatitis and fatty liver (steatosis), independent of the cause. If the cause is removed at this stage, the changes are fully reversible.
The pathological hallmark of cirrhosis is the development of scar tissue that replaces normal tissue. This scar tissue blocks the portal flow of blood through the organ, raising the blood pressure and disturbing normal function. Research has shown the pivotal role of the stellate cell, that normally stores vitamin A, in the development of cirrhosis. Damage to the liver tissue from inflammation, leads to the activation of stellate cells, which increases fibrosis through the production of myofibroblasts, and obstructs hepatic blood flow.[36] In addition, stellate cells secrete TGF beta 1, which leads to a fibrotic response and proliferation of connective tissue. Furthermore, it secretes TIMP1 and TIMP2, naturally occurring inhibitors of matrix metalloproteinases, which prevents them from breaking down the fibrotic material in the extracellular matrix.[37][38]
As this cascade of processes continues, fibrous tissue bands (septa) separate hepatocyte nodules, which eventually replace the entire liver architecture, leading to decreased blood flow throughout. The spleen becomes congested, and enlarged resulting in its retention of platelets, which are needed for normal blood clotting. Portal hypertension is responsible for the most severe complications of cirrhosis.
Diagnosis
The gold standard for diagnosis of cirrhosis is a liver biopsy. This is usually carried out as a fine-needle approach, through the skin (percutaneous), or internal jugular vein (transjugular).[39] Endoscopic ultrasound (EUS)-guided liver biopsy, using the percutaneous or transjugular route has become a good alternative to use.[40][39] EUS can target liver areas that are widely separated,[41] and can deliver bi-lobar biopsies.[40] A biopsy is not necessary if the clinical, laboratory, and radiologic data suggests cirrhosis. Furthermore, there is a small but significant risk of complications from liver biopsy, and cirrhosis itself predisposes for complications caused by liver biopsy.[42]
| Score | Platelet count x109 | ALT/AST ratio | INR |
|---|---|---|---|
| 0 | >340 | >1.7 | <1.1 |
| 1 | 280-340 | 1.2-1.7 | 1.1-1.4 |
| 2 | 220-279 | 0.6-1.19 | >1.4 |
| 3 | 160–219 | <0.6 | ... |
| 4 | 100-159 | ... | ... |
| 5 | 40-99 | ... | ... |
| 6 | <40 | ... | ... |
The best predictors of cirrhosis are ascites, platelet count < 160,000/mm3, spider angiomata, and a Bonacini cirrhosis discriminant score greater than 7 (as the sum of scores for platelet count, ALT/AST ratio and INR as per table).[44]
Lab findings
The following findings are typical in cirrhosis:
- Thrombocytopenia – typically multifactorial. Due to alcoholic marrow suppression, sepsis, lack of folate, platelet sequestering in the spleen as well as decreased thrombopoietin.[16] However, this rarely results in a platelet count < 50 000/mL.[45]
- Aminotransferases – AST and ALT are moderately elevated, with AST > ALT. However, normal aminotransferase levels do not preclude cirrhosis.[16]
- Alkaline phosphatase – slightly elevated but less than 2–3 times the upper limit of normal.
- Gamma-glutamyl transferase – correlates with AP levels. Typically much higher in chronic liver disease from alcohol.[45]
- Bilirubin – levels normal when compensated but may elevate as cirrhosis progresses.
- Albumin – levels fall as the synthetic function of the liver declines with worsening cirrhosis, since albumin is exclusively synthesized in the liver
- Prothrombin time – increases, since the liver synthesizes clotting factors.
- Globulins – increased due to shunting of bacterial antigens away from the liver to lymphoid tissue.
- Serum sodium – hyponatremia due to inability to excrete free water resulting from high levels of ADH and aldosterone.
- Leukopenia and neutropenia – due to splenomegaly with splenic margination.
- Coagulation defects – the liver produces most of the coagulation factors and thus coagulopathy correlates with worsening liver disease.
- Glucagon – increased in cirrhosis[24]
- Vasoactive intestinal peptide – increased as blood is shunted in the intestinal system because of portal hypertension
- Vasodilators – increased (such as nitric oxide and carbon monoxide) reducing afterload with compensatory increase in cardiac output, mixed venous oxygen saturation[20]
- Renin – increased (as well as sodium retention in kidneys) secondary to fall in systemic vascular resistance[21]
FibroTest is a biomarker for fibrosis that may be used instead of a biopsy.[46]
Other laboratory studies performed in newly diagnosed cirrhosis may include:
- Serology for hepatitis viruses, autoantibodies (ANA, anti-smooth muscle, anti-mitochondria, anti-LKM)
- Ferritin[47][48] and transferrin saturation: markers of iron overload as in hemochromatosis, copper and ceruloplasmin: markers of copper overload as in Wilson's disease
- Immunoglobulin levels (IgG, IgM, IgA) – these immunoglobins are non-specific, but may help in distinguishing various causes
- Cholesterol and glucose
- Alpha 1-antitrypsin
Markers of inflammation and immune cell activation are usually elevated in cirrhotic patients:
- C-reactive protein (CRP)[49]
- Procalcitonin (PCT)[49]
- Presepsin[50]
- soluble CD14[49]
- soluble CD163[51]
- soluble CD206 (mannose receptor)[52]
- soluble TREM-1[53]
Imaging

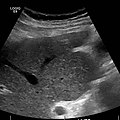
Ultrasound is routinely used in the evaluation of cirrhosis. It may show a small and nodular liver in advanced cirrhosis along with increased echogenicity with irregular appearing areas. Other liver findings suggestive of cirrhosis in imaging are an enlarged caudate lobe, widening of the fissures and enlargement of the spleen. An enlarged spleen, which normally measures less than 11–12 cm in adults, may suggest underlying portal hypertension. Ultrasound may also screen for hepatocellular carcinoma, and portal hypertension, by assessing flow in the hepatic vein. An increased portal vein pulsatility is an indicator of cirrhosis, but may also be caused by an increased right atrial pressure.[54] Portal vein pulsatility can be quantified by pulsatility indices (PI), where an index above a certain cutoff indicates pathology:
| Index | Calculation | Cutoff |
|---|---|---|
| Average-based | (Max – Min) / Average[54] | 0.5[54] |
| Max-relative | (Max – Min) / Max[55] | 0.5[55][56]–0.54[56] |
Cirrhosis is diagnosed with a variety of elastography techniques. Because a cirrhotic liver is generally stiffer than a healthy one, imaging the liver's stiffness can give diagnostic information about the location and severity of cirrhosis. Techniques used include transient elastography, acoustic radiation force impulse imaging, supersonic shear imaging and magnetic resonance elastography.[57] Vibration-controlled transient elastography and magnetic resonance elastography can give an indication of the stage of advanced fibrosis.[5] Compared to a biopsy, elastography can sample a much larger area and is painless. It shows a reasonable correlation with the severity of cirrhosis.[57] Other modalities have been introduced which are incorporated into ultrasonagraphy systems. These include point shear wave elastography using an added acoustic radiation force impulse imaging, or 2-dimensional shear wave elastography.[12]
Other scans performed in particular circumstances include CT of the abdomen, and magnetic resonance cholangiopancreatography – MRI of the pancreatic and bile ducts.
-
Liver cirrhosis with ascites
-
Liver cirrhosis as seen on a CT of the abdomen in transverse orientation
-
caudate lobe hypertrophy in ultrasound due to cirrhosis
-
Hepatofugal flow in portal vein in ultrasound
Endoscopy
Gastroscopy (endoscopic examination of the esophagus, stomach, and duodenum) is performed in cases of established cirrhosis. If esophageal varices are found, prophylactic local therapy may be applied such as sclerotherapy or banding, and beta blockers may be used.
Rarely are diseases of the bile ducts, such as primary sclerosing cholangitis, causes of cirrhosis. Imaging of the bile ducts, such as ERCP or MRCP (MRI of biliary tract and pancreas) may aid in the diagnosis.
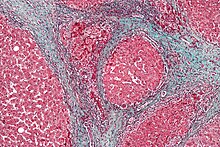
Pathology
Macroscopically, the liver is initially enlarged, but with the progression of the disease, it becomes smaller. Its surface is irregular, the consistency is firm, and if associated with steatosis the color is yellow. Depending on the size of the nodules, there are three macroscopic types: micronodular, macronodular, and mixed cirrhosis. In the micronodular form (Laennec's cirrhosis or portal cirrhosis), regenerating nodules are under 3 mm. In macronodular cirrhosis (post-necrotic cirrhosis), the nodules are larger than 3 mm. Mixed cirrhosis consists of nodules of different sizes.[58]
-
Micronodular cirrhosis, with diffuse areas of pallor.
-
Pale macronodules of cirrhosis.
-
Cirrhosis leading to hepatocellular carcinoma (autopsy specimen)
However, cirrhosis is defined by its pathological features on microscopy: (1) the presence of regenerating nodules of hepatocytes and (2) the presence of fibrosis, or the deposition of connective tissue between these nodules. The pattern of fibrosis seen can depend on the underlying insult that led to cirrhosis. Fibrosis can also proliferate even if the underlying process that caused it has resolved or ceased. The fibrosis in cirrhosis can lead to destruction of other normal tissues in the liver: including the sinusoids, the space of Disse, and other vascular structures, which leads to altered resistance to blood flow in the liver, and portal hypertension.[59]
-
No fibrosis, but mild zone 3 steatosis, in which collagen fibres (pink–red, arrow) are confined to portal tracts (P) (Van Gieson's stain)[60]
-
Histopathology of steatohepatitis with mild fibrosis in the form of fibrous expansion (Van Gieson's stain)[60]
-
Histopathology of steatohepatitis with moderate fibrosis, with thin fibrous bridges (Van Gieson's stain)[60]
-
Histopathology of steatohepatitis with established cirrhosis, with thick bands of fibrosis (Van Gieson's stain)[60]
-
Trichrome stain, showing cirrhosis as a nodular texture surrounded by fibrosis (wherein collagen is stained blue).
As cirrhosis can be caused by many different entities which injure the liver in different ways, cause-specific abnormalities may be seen. For example, in chronic hepatitis B, there is infiltration of the liver parenchyma with lymphocytes.[59] In congestive hepatopathy there are erythrocytes and a greater amount of fibrosis in the tissue surrounding the hepatic veins.[61] In primary biliary cholangitis, there is fibrosis around the bile duct, the presence of granulomas and pooling of bile.[62] Lastly in alcoholic cirrhosis, there is infiltration of the liver with neutrophils.[59]
Grading
The severity of cirrhosis is commonly classified with the Child–Pugh score. This scoring system uses bilirubin, albumin, INR, the presence and severity of ascites, and encephalopathy to classify patients into class A, B, or C. Class A has a favourable prognosis, while class C is at high risk of death. This system was devised in 1964 by Child and Turcotte, and modified in 1973 by Pugh and others.[63]
A later introduction, the Model for End-Stage Liver Disease (MELD) score, uses three laboratory values (total bilirubin, creatinine, and INR) and is primarily used to determine the allocation of liver transplants.[64]
MELD-Plus is an even later risk score to assess severity of chronic liver disease. The score includes nine variables as effective predictors for 90-day mortality after a discharge from a cirrhosis-related admission. The variables include all Model for End-Stage Liver Disease (MELD)'s components, as well as sodium, albumin, total cholesterol, white blood cell count, age, and length of stay. MELD-Plus was created as a result of a collaboration between Massachusetts General Hospital and IBM.[65]
The hepatic venous pressure gradient, (difference in venous pressure between afferent and efferent blood to the liver) also determines the severity of cirrhosis, although it is hard to measure. A value of 16 mm or more means a greatly increased risk of death.[66]
Prevention
Key prevention strategies for cirrhosis are population-wide interventions to reduce alcohol intake (through pricing strategies, public health campaigns, and personal counseling), programs to reduce the transmission of viral hepatitis, and screening of relatives of people with hereditary liver diseases.[citation needed]
Little is known about factors affecting cirrhosis risk and progression. However, many studies have provided increasing evidence for the protective effects of coffee consumption against the progression of liver disease. These effects are more noticeable in liver disease that is associated with alcohol use disorder. Coffee has antioxidant and antifibrotic effects. Caffeine may not be the important component; polyphenols may be more important. Drinking two or more cups of coffee a day is associated with improvements in the liver enzymes ALT, AST, and GGTP. Even in those with liver disease, coffee consumption can lower fibrosis and cirrhosis.[67]
Treatment
Generally, liver damage from cirrhosis cannot be reversed, but treatment can stop or delay further progression and reduce complications. A healthy diet is encouraged, as cirrhosis may be an energy-consuming process. Close follow-up is often necessary. Antibiotics are prescribed for infections, and various medications can help with itching. Laxatives, such as lactulose, decrease the risk of constipation; their role in preventing encephalopathy is limited.
Alcoholic cirrhosis caused by alcohol use disorder is treated by abstaining from alcohol. Treatment for hepatitis-related cirrhosis involves medications used to treat the different types of hepatitis, such as interferon for viral hepatitis and corticosteroids for autoimmune hepatitis.
Cirrhosis caused by Wilson's disease, is treated by removing the copper which builds up in organs.[5] This is carried out using chelation therapy such as penicillamine. When the cause is an iron overload then the iron is removed using a chelation agent such as deferoxamine.
Preventing further liver damage
Regardless of the underlying cause of cirrhosis, consumption of alcohol, as well as other potentially damaging substances, are discouraged. There is no evidence that supports the avoidance or dose reduction of paracetamol in people with compensated cirrhosis; it is thus considered a safe analgesic for said individuals.[68]
Vaccination of susceptible patients should be considered for hepatitis A and hepatitis B. Treating the cause of cirrhosis prevents further damage; for example, giving oral antivirals such as entecavir and tenofovir where cirrhosis is due to hepatitis B prevents progression of cirrhosis. Similarly, control of weight and diabetes prevents deterioration in cirrhosis due to non-alcoholic fatty liver disease.
Transplantation
If complications cannot be controlled or when the liver ceases functioning, liver transplantation is necessary. Survival from liver transplantation has been improving over the 1990s, and the five-year survival rate is now around 80%. The survival rate depends largely on the severity of disease and other medical risk factors in the recipient.[69] In the United States, the MELD score is used to prioritize patients for transplantation.[70] Transplantation necessitates the use of immune suppressants (ciclosporin or tacrolimus).
Decompensated cirrhosis
Manifestations of decompensation in cirrhosis include gastrointestinal bleeding, hepatic encephalopathy (HE), jaundice or ascites. In patients with previously stable cirrhosis, decompensation may occur due to various causes, such as constipation, infection (of any source), increased alcohol intake, medication, bleeding from esophageal varices or dehydration. It may take the form of any of the complications of cirrhosis listed below.
People with decompensated cirrhosis generally require admission to a hospital, with close monitoring of the fluid balance, mental status, and emphasis on adequate nutrition and medical treatment – often with diuretics, antibiotics, laxatives or enemas, thiamine and occasionally steroids, acetylcysteine and pentoxifylline.[71] Administration of saline is avoided, as it would add to the already high total body sodium content that typically occurs in cirrhosis. Life expectancy without liver transplant is low, at most 3 years.
Palliative care
Palliative care is specialized medical care that focuses on providing patients with relief from the symptoms, pain, and stress of a serious illness, such as cirrhosis. The goal of palliative care is to improve quality of life for both the patient and the patient's family and it is appropriate at any stage and for any type of cirrhosis.[72]
Especially in the later stages, people with cirrhosis experience significant symptoms such as abdominal swelling, itching, leg edema, and chronic abdominal pain which would be amenable for treatment through palliative care.[73] Because the disease is not curable without a transplant, palliative care can also help with discussions regarding the person's wishes concerning health care power of attorney, Do Not Resuscitate decisions and life support, and potentially hospice.[73] Despite proven benefit, people with cirrhosis are rarely referred to palliative care.[74]
Complications
Ascites
Salt restriction is often necessary, as cirrhosis leads to accumulation of salt (sodium retention). Diuretics may be necessary to suppress ascites. Diuretic options for inpatient treatment include aldosterone antagonists (spironolactone) and loop diuretics. Aldosterone antagonists are preferred for people who can take oral medications and are not in need of an urgent volume reduction. Loop diuretics can be added as additional therapy.[75]
Where salt restriction and the use of diuretics are ineffective then paracentesis may be the preferred option.[76] This procedure requires the insertion of a plastic tube into the peritoneal cavity. Human serum albumin solution is usually given to prevent complications from the rapid volume reduction. In addition to being more rapid than diuretics, 4–5 liters of paracentesis is more successful in comparison to diuretic therapy.[75]
Esophageal variceal bleeding
For portal hypertension, nonselective beta blockers such as propranolol or nadolol are commonly used to lower blood pressure over the portal system. In severe complications from portal hypertension, transjugular intrahepatic portosystemic shunting (TIPS) is occasionally indicated to relieve pressure on the portal vein. As this shunting can worsen hepatic encephalopathy, it is reserved for those patients at low risk of encephalopathy. TIPS is generally regarded only as a bridge to liver transplantation[77] or as a palliative measure.[citation needed]
Hepatic encephalopathy
Hepatic encephalopathy is a potential complication of cirrhosis that may lead to functional neuronal impairment, ranging from mild confusion to coma.[78] Common first line treatment may include lactulose, or the antibiotic rifaximin.[78] Protein uptake is encouraged to at least match general recommendations for cirrhosis.[79] A low protein diet may be recommended for short term use in severe cases with gastrointestinal bleeding.[79]
Hepatorenal syndrome
Hepatorenal syndrome is a serious complication of end-stage cirrhosis when kidney damage is also involved.[80]
Spontaneous bacterial peritonitis
People with ascites due to cirrhosis are at risk of spontaneous bacterial peritonitis.
Portal hypertensive gastropathy
Portal hypertensive gastropathy refers to changes in the mucosa of the stomach in people with portal hypertension, and is associated with cirrhosis severity.[81]
Infection
Cirrhosis can cause immune system dysfunction, leading to infection. Signs and symptoms of infection may be nonspecific and are more difficult to recognize (for example, worsening encephalopathy but no fever).[82] Moreover infections in cirrhosis are major triggers for other complications (ascites, variceal bleading, hepatic enecphalopathy, organ failures, death).[82][51][53]
Hepatocellular carcinoma
Hepatocellular carcinoma is the most common primary liver cancer, and the most common cause of death in people with cirrhosis.[83] Screening using an MRI scan can detect this cancer and is often carried out for early signs which has been shown to improve outcomes.[5][84]
Epidemiology


Each year, approximately one million deaths are due to complications of cirrhosis, making cirrhosis the 11th most common cause of death globally.[86] Cirrhosis and chronic liver disease were the tenth leading cause of death for men and the twelfth for women in the United States in 2001, killing about 27,000 people each year.[87]
The cause of cirrhosis can vary; alcohol and non-alcoholic fatty liver disease are main causes in western and industrialized countries, whereas viral hepatitis is the predominant cause in low and middle income countries.[86] Cirrhosis is more common in men than in women.[88] The cost of cirrhosis in terms of human suffering, hospital costs, and lost productivity is high.
Globally, age-standardized disability-adjusted life year (DALY) rates have decreased from 1990 to 2017, with the values going from 656.4 years per 100,000 people to 510.7 years per 100,000 people.[89] In males DALY rates have decreased from 903.1 years per 100,000 population in 1990, to 719.3 years per 100,000 population in 2017; in females the DALY rates have decreased from 415.5 years per 100,000 population in 1990, to 307.6 years per 100,000 population in 2017.[89] However, globally the total number of DALYs have increased by 10.9 million from 1990 to 2017, reaching the value of 41.4 million DALYs.[89]
Etymology
The word "cirrhosis" is a neologism derived from Greek: κίρρωσις; kirrhos κιρρός, meaning "yellowish, tawny" (the orange-yellow colour of the diseased liver) and the suffix -osis, i.e. "condition" in medical terminology.[90][91][92] While the clinical entity was known before, René Laennec gave it this name in an 1819 paper.[15]
References
- ^ a b c d e f g h i j k l m n o p "Cirrhosis". National Institute of Diabetes and Digestive and Kidney Diseases. April 23, 2014. Archived from the original on 9 June 2015. Retrieved 19 May 2015.
- ^ a b c d e f g "Symptoms & Causes of Cirrhosis | NIDDK". National Institute of Diabetes and Digestive and Kidney Diseases. Retrieved 8 February 2021.
- ^ a b c "Definition & Facts of NAFLD & NASH | NIDDK". National Institute of Diabetes and Digestive and Kidney Diseases. Retrieved 9 March 2021.
- ^ a b GBD 2013 Mortality and Causes of Death, Collaborators (17 December 2014). "Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013". Lancet. 385 (9963): 117–71. doi:10.1016/S0140-6736(14)61682-2. PMC 4340604. PMID 25530442.
{{cite journal}}:|first1=has generic name (help)CS1 maint: numeric names: authors list (link) - ^ a b c d e f Ferri, Fred F. (2019). Ferri's clinical advisor 2019 : 5 books in 1. Philadelphia, PA. pp. 337–339. ISBN 9780323530422.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ a b "Treatment for Cirrhosis | NIDDK". National Institute of Diabetes and Digestive and Kidney Diseases. Retrieved 6 March 2021.
- ^ a b GBD 2015 Disease and Injury Incidence and Prevalence, Collaborators. (8 October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMC 5055577. PMID 27733282.
{{cite journal}}:|first1=has generic name (help)CS1 maint: numeric names: authors list (link) - ^ a b c GBD 2015 Mortality and Causes of Death, Collaborators. (8 October 2016). "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1459–1544. doi:10.1016/s0140-6736(16)31012-1. PMC 5388903. PMID 27733281.
{{cite journal}}:|first1=has generic name (help)CS1 maint: numeric names: authors list (link) - ^ a b "Cirrhosis". nhs.uk. 29 June 2020. Retrieved 8 February 2021.
- ^ Sherlock's diseases of the liver and biliary system. Dooley, James (James S.),, Lok, Anna S. F.,, Garcia-Tsao, Guadalupe,, Pinzani, Massimo (Thirteenth ed.). Hoboken, NJ. p. 82. ISBN 978-1-119-23756-3. OCLC 1019837000.
{{cite book}}: CS1 maint: others (link) - ^ "Definition & Facts for Cirrhosis | NIDDK". National Institute of Diabetes and Digestive and Kidney Diseases.
- ^ a b Castera L, Friedrich-Rust M, Loomba R (April 2019). "Noninvasive Assessment of Liver Disease in Patients With Nonalcoholic Fatty Liver Disease". Gastroenterology. 156 (5): 1264–1281.e4. doi:10.1053/j.gastro.2018.12.036. PMC 7505052. PMID 30660725.
- ^ "Alcoholic liver disease: MedlinePlus Medical Encyclopedia". medlineplus.gov.
- ^ Brower, Steven T. (2012). Elective general surgery : an evidence-based approach. New York: McGraw-Hill Medical. p. 36. ISBN 9781607951094. Archived from the original on 2017-09-08.
- ^ a b Roguin A (2006). "Rene Theophile Hyacinthe Laënnec (1781–1826): The Man Behind the Stethoscope". Clinical Medicine & Research. 4 (3): 230–5. doi:10.3121/cmr.4.3.230. PMC 1570491. PMID 17048358.
- ^ a b c d e f Friedman LS (2014). Current medical diagnosis and treatment 2014. [S.l.]: Mcgraw-Hill. pp. Chapter 16. Liver, Biliary Tract, & Pancreas Disorders. ISBN 978-0071806336.
- ^ Li CP, Lee FY, Hwang SJ, et al. (1999). "Spider angiomas in patients with liver cirrhosis: role of alcoholism and impaired liver function". Scand. J. Gastroenterol. 34 (5): 520–3. doi:10.1080/003655299750026272. PMID 10423070.
- ^ "Palmar erythema | DermNet NZ". dermnetnz.org.
- ^ William, James (2005). Andrews' Diseases of the Skin: Clinical Dermatology. Saunders. ISBN 978-0-7216-2921-6.
- ^ a b Slater, Joseph S. Esherick, Daniel S. Clark, Evan D. (2012-12-18). Current practice guidelines in primary care 2013. New York: McGraw-Hill Medical. pp. Chapter 3: Disease Management. ISBN 978-0071797504.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b Van Thiel, DH; Gavaler, JS; Schade, RR (February 1985). "Liver disease and the hypothalamic pituitary gonadal axis". Seminars in Liver Disease. 5 (1): 35–45. doi:10.1055/s-2008-1041756. PMID 3983651.
- ^ "Symptoms of cirrhosis". nhs.uk. 3 October 2018. Retrieved 22 March 2021.
- ^ van Thiel, DH; Gavaler, JS; Spero, JA; Egler, KM; Wright, C; Sanghvi, AT; Hasiba, U; Lewis, JH (Jan–Feb 1981). "Patterns of hypothalamic-pituitary-gonadal dysfunction in men with liver disease due to differing etiologies". Hepatology. 1 (1): 39–46. doi:10.1002/hep.1840010107. PMID 6793494. S2CID 43547817.
- ^ a b c d e f g h i j k l m n o p Longo, Dan L.; et al., eds. (2012). Harrison's principles of internal medicine (18th ed.). New York: McGraw-Hill. pp. Chapter 308. Cirrhosis and Its Complications. ISBN 9780071748896.
- ^ Suurmond, D. (2009). Color Atlas and Synopsis of Clinical Dermatology: Common and Serious Diseases. McGraw-Hill. pp. Section 33: Disorders of the nail apparatus. ISBN 978-0071793025.
- ^ Tangerman, A; Meuwese-Arends, MT; Jansen, JB (Feb 19, 1994). "Cause and composition of foetor hepaticus". Lancet. 343 (8895): 483. doi:10.1016/s0140-6736(94)92729-4. PMID 7905979. S2CID 10538949.
- ^ Longo et al. Harrison's Principles of Internal Medicine, 18th ed., p.2295
- ^ Plauth, Mathias; Schütz, Elke-Tatjana (2002). "Cachexia in liver cirrhosis". International Journal of Cardiology. 85 (1): 83–87. doi:10.1016/s0167-5273(02)00236-x. ISSN 0167-5273. PMID 12163212.
- ^ a b Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP (October 2006). "The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide". J. Hepatol. 45 (4): 529–38. doi:10.1016/j.jhep.2006.05.013. PMID 16879891.
- ^ Alcohol-Induced Liver Disease; "Alcohol Related Liver Disease and Alcohol Damage – ALF". Archived from the original on 2012-01-12. Retrieved 2012-01-25.
- ^ "Diagnosis of NAFLD & NASH | NIDDK". National Institute of Diabetes and Digestive and Kidney Diseases. Retrieved 9 March 2021.
- ^ Huang H; et al. (2004). "AASLD Abstract (Pp. 162A–266A)". Hepatology. 40: 230A. doi:10.1002/hep.1840400503.
- ^ Longo, Dan L.; et al., eds. (2011). Harrison's principles of internal medicine (18th ed.). New York: McGraw-Hill. p. Liver Transplantation. ISBN 9780071748896.
- ^ Edwards, CQ; Kushner, JP (Jun 3, 1993). "Screening for hemochromatosis". The New England Journal of Medicine. 328 (22): 1616–20. doi:10.1056/NEJM199306033282208. PMID 8110209.
- ^ Tanner, MS (May 1998). "Role of copper in Indian childhood cirrhosis". The American Journal of Clinical Nutrition. 67 (5 Suppl): 1074S–1081S. doi:10.1093/ajcn/67.5.1074S. PMID 9587155.
- ^ Hammer, edited by Stephen J. McPhee, Gary D. (2010). Pathophysiology of disease : an introduction to clinical medicine (6th ed.). New York: McGraw-Hill Medical. pp. Chapter 14: Liver Disease. Cirrhosis. ISBN 978-0071621670.
{{cite book}}:|first=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ Iredale JP (2003). "Cirrhosis: new research provides a basis for rational and targeted treatments". BMJ. 327 (7407): 143–7. doi:10.1136/bmj.327.7407.143. PMC 1126509. PMID 12869458. Archived from the original on 2004-10-29.
- ^ Puche, JE; Saiman, Y; Friedman, SL (Oct 1, 2013). "Hepatic stellate cells and liver fibrosis". Comprehensive Physiology. 3 (4): 1473–92. doi:10.1002/cphy.c120035. PMID 24265236.
- ^ a b McCarty, TR; Bazarbashi, AN; Njei, B (September 2020). "Endoscopic Ultrasound-Guided, Percutaneous, and Transjugular Liver Biopsy: A Comparative Systematic Review and Meta-Analysis". Clinical endoscopy. 53 (5): 583–593. doi:10.5946/ce.2019.211. PMID 33027584.
- ^ a b Mok, SRS; Diehl, DL (31 January 2019). "The Role of EUS in Liver Biopsy". Current gastroenterology reports. 21 (2): 6. doi:10.1007/s11894-019-0675-8. PMID 30706151.
- ^ Diehl, DL (April 2019). "Endoscopic Ultrasound-guided Liver Biopsy". Gastrointestinal endoscopy clinics of North America. 29 (2): 173–186. doi:10.1016/j.giec.2018.11.002. PMC 6383155. PMID 30846147.
- ^ Grant, A; Neuberger J (1999). "Guidelines on the use of liver biopsy in clinical practice". Gut. 45 (Suppl 4): 1–11. doi:10.1136/gut.45.2008.iv1. PMC 1766696. PMID 10485854. Archived from the original on 2007-06-30.
The main cause of mortality after percutaneous liver biopsy is intraperitoneal haemorrhage as shown in a retrospective Italian study of 68,000 percutaneous liver biopsies, in which all six patients who died did so from intraperitoneal haemorrhage. Three of these patients had had a laparotomy, and all had either cirrhosis or malignant disease, both of which are risk factors for bleeding.
- ^ Gudowska, Monika; Gruszewska, Ewa; Panasiuk, Anatol; Cylwik, Bogdan; Świderska, Magdalena; Flisiak, Robert; Szmitkowski, Maciej; Chrostek, Lech (2016). "Selected Noninvasive Markers in Diagnosing Liver Diseases". Laboratory Medicine. 47 (1): 67–72. doi:10.1093/labmed/lmv015. ISSN 0007-5027. PMID 26715612.
- ^ Udell, JA; Wang, CS; Tinmouth, J; FitzGerald, JM; Ayas, NT; Simel, DL; Schulzer, M; Mak, E; Yoshida, EM (Feb 22, 2012). "Does this patient with liver disease have cirrhosis?". JAMA: The Journal of the American Medical Association. 307 (8): 832–42. doi:10.1001/jama.2012.186. PMID 22357834.
- ^ a b Maddrey, edited by Eugene R. Schiff, Michael F. Sorrell & Willis C. (1999). Schiff's diseases of the liver (11th ed. / edited by Eugene R. Schiff, Willis C. Maddrey, Michael F. Sorrell. ed.). Chichester, West Sussex, UK: John Wiley & Sons. pp. Evaluation of the Liver A: Laboratory Test. ISBN 978-0-470-65468-2.
{{cite book}}:|first=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ Halfon P, Munteanu M, Poynard T (2008). "FibroTest-ActiTest as a non-invasive marker of liver fibrosis". Gastroenterol Clin Biol. 32 (6): 22–39. doi:10.1016/S0399-8320(08)73991-5. PMID 18973844.
- ^ Tornai, David; Antal-Szalmas, Peter; Tornai, Tamas; Papp, Maria; Tornai, Istvan; Sipeki, Nora; Janka, Tamas; Balogh, Boglarka; Vitalis, Zsuzsanna (2021-03-02). "Abnormal ferritin levels predict development of poor outcomes in cirrhotic outpatients: a cohort study". BMC Gastroenterology. 21 (1): 94. doi:10.1186/s12876-021-01669-w. ISSN 1471-230X. PMC 7923668. PMID 33653274.
{{cite journal}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ Maiwall, Rakhi; Kumar, Suman; Chaudhary, A. K.; Maras, Jaswinder; Wani, Zeeshan; Kumar, Chandan; Rastogi, A.; Bihari, C.; Vashisht, Chitranshu; Sarin, S. K. (2014-07). "Serum ferritin predicts early mortality in patients with decompensated cirrhosis". Journal of Hepatology. 61 (1): 43–50. doi:10.1016/j.jhep.2014.03.027. ISSN 1600-0641. PMID 24681346.
{{cite journal}}: Check date values in:|date=(help) - ^ a b c Papp, Maria; Vitalis, Zsuzsanna; Altorjay, Istvan; Tornai, Istvan; Udvardy, Miklos; Harsfalvi, Jolan; Vida, Andras; Kappelmayer, Janos; Lakatos, Peter L.; Antal‐Szalmas, Peter (2012). "Acute phase proteins in the diagnosis and prediction of cirrhosis associated bacterial infections". Liver International. 32 (4): 603–611. doi:10.1111/j.1478-3231.2011.02689.x. ISSN 1478-3231.
- ^ Papp, Maria; Tornai, Tamas; Vitalis, Zsuzsanna; Tornai, Istvan; Tornai, David; Dinya, Tamas; Sumegi, Andrea; Antal-Szalmas, Peter (2016-11-07). "Presepsin teardown - pitfalls of biomarkers in the diagnosis and prognosis of bacterial infection in cirrhosis". World Journal of Gastroenterology. 22 (41): 9172–9185. doi:10.3748/wjg.v22.i41.9172. ISSN 2219-2840. PMC 5107598. PMID 27895404.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Tornai, Tamas; Vitalis, Zsuzsanna; Sipeki, Nora; Dinya, Tamas; Tornai, David; Antal‐Szalmas, Peter; Karanyi, Zsolt; Tornai, Istvan; Papp, Maria (2016). "Macrophage activation marker, soluble CD163, is an independent predictor of short-term mortality in patients with cirrhosis and bacterial infection". Liver International. 36 (11): 1628–1638. doi:10.1111/liv.13133. ISSN 1478-3231.
- ^ Laursen, Tea L.; Rødgaard-Hansen, Sidsel; Møller, Holger J.; Mortensen, Christian; Karlsen, Stine; Nielsen, Dennis T.; Frevert, Susanne; Clemmesen, Jens O.; Møller, Søren; Jensen, Jørgen S.; Bendtsen, Flemming (2017-04). "The soluble mannose receptor is released from the liver in cirrhotic patients, but is not associated with bacterial translocation". Liver International: Official Journal of the International Association for the Study of the Liver. 37 (4): 569–575. doi:10.1111/liv.13262. ISSN 1478-3231. PMID 27706896.
{{cite journal}}: Check date values in:|date=(help) - ^ a b "Increased sTREM-1 levels identify cirrhotic patients with bacterial infection and predict their 90-day mortality". Clinics and Research in Hepatology and Gastroenterology. 45 (5): 101579. 2021-09-01. doi:10.1016/j.clinre.2020.11.009. ISSN 2210-7401.
- ^ a b c Iranpour, Pooya; Lall, Chandana; Houshyar, Roozbeh; Helmy, Mohammad; Yang, Albert; Choi, Joon-Il; Ward, Garrett; Goodwin, Scott C (2016). "Altered Doppler flow patterns in cirrhosis patients: an overview". Ultrasonography. 35 (1): 3–12. doi:10.14366/usg.15020. ISSN 2288-5919. PMC 4701371. PMID 26169079.
- ^ a b Goncalvesova, E.; Varga, I.; Tavacova, M.; Lesny, P. (2013). "Changes of portal vein flow in heart failure patients with liver congestion". European Heart Journal. 34 (suppl 1): P627. doi:10.1093/eurheartj/eht307.P627. ISSN 0195-668X.
- ^ a b Page 367 in: Henryk Dancygier (2009). Clinical Hepatology: Principles and Practice of Hepatobiliary Diseases. Vol. 1. Springer Science & Business Media. ISBN 9783540938422.
- ^ a b >Foucher J, Chanteloup E, Vergniol J, et al. (2006). "Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study". Gut. 55 (3): 403–8. doi:10.1136/gut.2005.069153. PMC 1856085. PMID 16020491.
- ^ Bashar Sharma; Savio John. (2020). Hepatic Cirrhosis. StatPearls.
{{cite book}}:|website=ignored (help)CS1 maint: multiple names: authors list (link) Last Update: June 3, 2019. - ^ a b c Brenner, David; Richard A. Rippe (2003). "Pathogenesis of Hepatic Fibrosis". In Tadataka Yamada (ed.). Textbook of Gastroenterology. Vol. 2 (4th ed.). Lippincott Williams & Wilkins. ISBN 978-0-7817-2861-4.
- ^ a b c d Boyd, Alexander; Cain, Owen; Chauhan, Abhishek; Webb, Gwilym James (2020). "Medical liver biopsy: background, indications, procedure and histopathology". Frontline Gastroenterology. 11 (1): 40–47. doi:10.1136/flgastro-2018-101139. ISSN 2041-4137. PMC 6914302. PMID 31885839.
-"This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license" - ^ Giallourakis CC, Rosenberg PM, Friedman LS (November 2002). "The liver in heart failure". Clin Liver Dis. 6 (4): 947–67, viii–ix. doi:10.1016/S1089-3261(02)00056-9. PMID 12516201.
- ^ Heathcote EJ (November 2003). "Primary biliary cirrhosis: historical perspective". Clin Liver Dis. 7 (4): 735–40. doi:10.1016/S1089-3261(03)00098-9. PMID 14594128.
- ^ Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R (1973). "Transection of the oesophagus for bleeding oesophageal varices". Br J Surg. 60 (8): 646–9. doi:10.1002/bjs.1800600817. PMID 4541913. S2CID 382636.
- ^ Peng, Ying; Qi, Xingshun; Guo, Xiaozhong (2016). "Child-Pugh Versus MELD Score for the Assessment of Prognosis in Liver Cirrhosis: A Systematic Review and Meta-Analysis of Observational Studies". Medicine. 95 (8): e2877. doi:10.1097/MD.0000000000002877. ISSN 1536-5964. PMC 4779019. PMID 26937922.
- ^ Kartoun, Uri; Corey, Kathleen E; Simon, Tracey G; Zheng, Hui; Aggarwal, Rahul; Ng, Kenney; Shaw, Stanley Y (2017). "The MELD-Plus: A generalizable prediction risk score in cirrhosis". PLOS ONE. 12 (10): e0186301. Bibcode:2017PLoSO..1286301K. doi:10.1371/journal.pone.0186301. PMC 5656314. PMID 29069090.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Patch D, Armonis A, Sabin C, et al. (1999). "Single portal pressure measurement predicts survival in cirrhotic patients with recent bleeding". Gut. 44 (2): 264–9. doi:10.1136/gut.44.2.264. PMC 1727391. PMID 9895388. Archived from the original on 2008-05-28.
- ^ Wadhawan, M; Anand, AC (March 2016). "Coffee and Liver Disease". Journal of clinical and experimental hepatology. 6 (1): 40–6. doi:10.1016/j.jceh.2016.02.003. PMC 4862107. PMID 27194895.
- ^ Schweighardt, Anne E.; Juba, Katherine M. (2018). "A Systematic Review of the Evidence Behind Use of Reduced Doses of Acetaminophen in Chronic Liver Disease". Journal of Pain & Palliative Care Pharmacotherapy. 32 (4): 226–239. doi:10.1080/15360288.2019.1611692. ISSN 1536-0539. PMID 31206302. S2CID 190535151.
- ^ "E-medicine liver transplant outlook and survival rates". Emedicinehealth.com. 2009-06-09. Archived from the original on 2009-07-14. Retrieved 2009-09-06.
- ^ Kamath PS, Kim WR (March 2007). "The model for end-stage liver disease (MELD)". Hepatology. 45 (3): 797–805. doi:10.1002/hep.21563. PMID 17326206. S2CID 10440305.
- ^ Chavez-Tapia, NC; Barrientos-Gutierrez, T; Tellez-Avila, FI; Soares-Weiser, K; Uribe, M (8 September 2010). "Antibiotic prophylaxis for cirrhotic patients with upper gastrointestinal bleeding". The Cochrane Database of Systematic Reviews (9): CD002907. doi:10.1002/14651858.CD002907.pub2. PMC 7138054. PMID 20824832.
- ^ Ferrell, B; Connor, SR; Cordes, A; Dahlin, CM; Fine, PG; Hutton, N; Leenay, M; Lentz, J; Person, JL; Meier, DE; Zuroski, K; National Consensus Project for Quality Palliative Care Task Force, Members (Jun 2007). "The national agenda for quality palliative care: the National Consensus Project and the National Quality Forum". Journal of Pain and Symptom Management. 33 (6): 737–44. doi:10.1016/j.jpainsymman.2007.02.024. PMID 17531914.
- ^ a b Sanchez, W; Talwalkar, JA (Mar 2006). "Palliative care for patients with end-stage liver disease ineligible for liver transplantation". Gastroenterology Clinics of North America. 35 (1): 201–19. doi:10.1016/j.gtc.2005.12.007. PMID 16530121.
- ^ Poonja, Z; Brisebois, A; van Zanten, SV; Tandon, P; Meeberg, G; Karvellas, CJ (Apr 2014). "Patients with cirrhosis and denied liver transplants rarely receive adequate palliative care or appropriate management". Clinical Gastroenterology and Hepatology. 12 (4): 692–8. doi:10.1016/j.cgh.2013.08.027. PMID 23978345.
- ^ a b Moore KP, Aithal GP (October 2006). "Guidelines on the management of ascites in cirrhosis". Gut. 55 Suppl 6 (Suppl 6): vi1–12. doi:10.1136/gut.2006.099580. PMC 1860002. PMID 16966752.
- ^ Piano, S; Tonon, M; Angeli, P (February 2018). "Management of ascites and hepatorenal syndrome". Hepatology international. 12 (Suppl 1): 122–134. doi:10.1007/s12072-017-9815-0. PMID 28836115.
- ^ Sellers CM, Nezami N, Schilsky ML, Kim HS (April 2019). "Transjugular intrahepatic portosystemic shunt as a bridge to liver transplant: Current state and future directions". Transplant Rev (Orlando). 33 (2): 64–71. doi:10.1016/j.trre.2018.10.004. PMID 30477811.
- ^ a b Patidar, Kavish R.; Bajaj, Jasmohan S. (2015). "Covert and Overt Hepatic Encephalopathy: Diagnosis and Management". Clinical Gastroenterology and Hepatology. 13 (12): 2048–2061. doi:10.1016/j.cgh.2015.06.039. ISSN 1542-7714. PMC 4618040. PMID 26164219.
- ^ a b Merli, Manuela; Berzigotti, Annalisa; Zelber-Sagi, Shira; Dasarathy, Srinivasan; Montagnese, Sara; Genton, Laurence; Plauth, Mathias; Parés, Albert (2019). "EASL Clinical Practice Guidelines on nutrition in chronic liver disease". Journal of Hepatology. 70 (1): 172–193. doi:10.1016/j.jhep.2018.06.024. ISSN 0168-8278. PMC 6657019. PMID 30144956.
- ^ Francoz C, Durand F, Kahn JA, Genyk YS, Nadim MK (May 2019). "Hepatorenal Syndrome". Clin J Am Soc Nephrol. 14 (5): 774–781. doi:10.2215/CJN.12451018. PMC 6500947. PMID 30996046.
- ^ Kim MY, Choi H, Baik SK, et al. (April 2010). "Portal Hypertensive Gastropathy: Correlation with Portal Hypertension and Prognosis in Cirrhosis". Dig Dis Sci. 55 (12): 3561–7. doi:10.1007/s10620-010-1221-6. PMID 20407828. S2CID 24332780.
- ^ a b Sipeki, Nora; Antal-Szalmas, Peter; Lakatos, Peter L.; Papp, Maria (2014-03-14). "Immune dysfunction in cirrhosis". World Journal of Gastroenterology. 20 (10): 2564–2577. doi:10.3748/wjg.v20.i10.2564. ISSN 2219-2840. PMC 3949265. PMID 24627592.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Forner A, Llovet JM, Bruix J (2012). "Hepatocellular carcinoma". The Lancet. 379 (9822): 1245–1255. doi:10.1016/S0140-6736(11)61347-0. PMID 22353262.
- ^ Singal AG, Pillai A, Tiro J (2014). "Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis". PLOS Med. 11 (4): e1001624. doi:10.1371/journal.pmed.1001624. PMC 3972088. PMID 24691105.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "WHO Disease and injury country estimates". World Health Organization. 2009. Archived from the original on 2009-11-11. Retrieved Nov 11, 2009.
- ^ a b Asrani, Sumeet K.; Devarbhavi, Harshad; Eaton, John; Kamath, Patrick S. (2019). "Burden of liver diseases in the world". Journal of Hepatology. 70 (1): 151–171. doi:10.1016/j.jhep.2018.09.014. ISSN 1600-0641. PMID 30266282.
- ^ Anderson RN, Smith BL (2003). "Deaths: leading causes for 2001". National Vital Statistics Reports. 52 (9): 1–85. PMID 14626726.
- ^ Tamparo, Carol (2011). Fifth Edition: Diseases of the Human Body. Philadelphia, PA: F. A. Davis Company. p. 422. ISBN 978-0-8036-2505-1.
- ^ a b c GBD 2017 Cirrhosis Collaborators (2020). "The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017". The Lancet. Gastroenterology & Hepatology. 5 (3): 245–266. doi:10.1016/S2468-1253(19)30349-8. ISSN 2468-1253. PMC 7026710. PMID 31981519.
{{cite journal}}:|last=has generic name (help)CS1 maint: numeric names: authors list (link) - ^ κιρρός. Liddell, Henry George; Scott, Robert; A Greek–English Lexicon at the Perseus Project.
- ^ Harper, Douglas. "cirrhosis". Online Etymology Dictionary.
- ^ Harper, Douglas. "-osis". Online Etymology Dictionary.
External links
- Cirrhosis of the Liver at the National Digestive Diseases Information Clearinghouse (NDDIC). NIH Publication No. 04-1134, December 2003.
- "Cirrhosis". MedlinePlus. U.S. National Library of Medicine.







![No fibrosis, but mild zone 3 steatosis, in which collagen fibres (pink–red, arrow) are confined to portal tracts (P) (Van Gieson's stain)[60]](http://upload.wikimedia.org/wikipedia/commons/thumb/2/23/Histopathology_of_mild_zone_3_steatosis_without_fibrosis_%28van_Gieson%29.jpg/120px-Histopathology_of_mild_zone_3_steatosis_without_fibrosis_%28van_Gieson%29.jpg)
![Histopathology of steatohepatitis with mild fibrosis in the form of fibrous expansion (Van Gieson's stain)[60]](http://upload.wikimedia.org/wikipedia/commons/thumb/a/af/Histopathology_of_steatohepatitis_with_mild_fibrosis_in_the_form_of_fibrous_expansion_%28van_Gieson%29.jpg/120px-Histopathology_of_steatohepatitis_with_mild_fibrosis_in_the_form_of_fibrous_expansion_%28van_Gieson%29.jpg)
![Histopathology of steatohepatitis with moderate fibrosis, with thin fibrous bridges (Van Gieson's stain)[60]](http://upload.wikimedia.org/wikipedia/commons/thumb/d/da/Histopathology_of_steatohepatitis_with_moderate_fibrosis%2C_with_thin_fibrous_bridges_%28van_Gieson%29.jpg/120px-Histopathology_of_steatohepatitis_with_moderate_fibrosis%2C_with_thin_fibrous_bridges_%28van_Gieson%29.jpg)
![Histopathology of steatohepatitis with established cirrhosis, with thick bands of fibrosis (Van Gieson's stain)[60]](http://upload.wikimedia.org/wikipedia/commons/thumb/0/0d/Histopathology_of_steatohepatitis_with_established_cirrhosis%2C_with_thick_bands_of_fibrosis_%28van_Gieson%29.jpg/120px-Histopathology_of_steatohepatitis_with_established_cirrhosis%2C_with_thick_bands_of_fibrosis_%28van_Gieson%29.jpg)
