Wikipedia:Reference desk/Science: Difference between revisions
→Why do we find baby animals cute?: ObGeorge |
→Visualizing point data: Thanks, more interactive? |
||
| Line 357: | Line 357: | ||
:For a free, [[open source]] option, [[octave programming language|Octave]] can do that. It has MATLAB style syntax, and uses [[gnuplot]] for a variety of visualization techniques. [[User:SemanticMantis|SemanticMantis]] ([[User talk:SemanticMantis|talk]]) 23:19, 10 February 2012 (UTC) |
:For a free, [[open source]] option, [[octave programming language|Octave]] can do that. It has MATLAB style syntax, and uses [[gnuplot]] for a variety of visualization techniques. [[User:SemanticMantis|SemanticMantis]] ([[User talk:SemanticMantis|talk]]) 23:19, 10 February 2012 (UTC) |
||
::Thanks. Octave might do the trick, although I was hoping for more of a 'prepackaged' solution - perhaps from the GIS field. I shall have a look! [[Special:Contributions/131.111.255.9|131.111.255.9]] ([[User talk:131.111.255.9|talk]]) 03:45, 11 February 2012 (UTC) |
|||
== Astronomy stuffs == |
== Astronomy stuffs == |
||
Revision as of 03:45, 11 February 2012
of the Wikipedia reference desk.
Main page: Help searching Wikipedia
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Wikipedia:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
February 6
Order of a mixture
Is there an article here in wk about why the order of elements of a mixture matters? (for example, hot oil + water, or acid + water or Nescafe + hot water). — Preceding unsigned comment added by 88.8.79.238 (talk) 00:17, 6 February 2012 (UTC)
- For acid and water mixture, see Sulphuric_acid#Reaction_with_water_and_dehydrating_property99.245.35.136 (talk) 00:29, 6 February 2012 (UTC)
- Yup. regarding the acids, our Sulfuric acid article explains why it matters:
- "Preparation of the diluted acid can also be dangerous due to the heat released in the dilution process. The concentrated acid is always added to water and not the other way around, to take advantage of the relatively high heat capacity of water. Addition of water to concentrated sulfuric acid leads to the dispersal of a sulfuric acid aerosol or worse, an explosion. Preparation of solutions greater than 6 M (35%) in concentration is most dangerous, as the heat produced may be sufficient to boil the diluted acid: efficient mechanical stirring and external cooling (such as an ice bath) are essential.
- As for mixing hot oil and water, if the oil is above 100 C, adding water is going to result in vaporisation, and ejection of hot oil. This is why it is extremely dangerous to try to put out a cooking-oil fire with water - you can end up with a fireball - see Chip_pan_fire#Fire_hazard. AndyTheGrump (talk) 00:34, 6 February 2012 (UTC)
- I've also noticed that you want to add cocoa powder to hot water, not the reverse, or it will stick to the bottom of the cup. StuRat (talk)
- For Nescafe + hot water, you've apparently never seen what happens when you add powdered substances to superheated microwaved water. The temperature of the water can exceed 100 degrees, and adding the powder can trigger a mess, and even dangerous, explosion. Same goes with hot cocoa powder. Dominus Vobisdu (talk) 01:36, 6 February 2012 (UTC)
- I just use hot tap water, no nuking here. StuRat (talk) 01:50, 6 February 2012 (UTC)
- Frankly I imagine that the issue is less about explosive water (which is pretty uncommon) but the fact that if you add the powder last it becomes very hard to mix in. I've done in backwards many times and what you end up with is a lot of powder floating on top, vigorously resisting making a mixed liquid. If you do it the other way, that doesn't happen. --Mr.98 (talk) 03:18, 6 February 2012 (UTC)
- Yes, because it's all stuck to the bottom. :-) I think adding a bit of hot water, then the cocoa powder, then the rest of the hot water, is the best approach. StuRat (talk) 05:05, 6 February 2012 (UTC)
- I concur with StuRat, but additionally stir the initial water + powder mix vigorously with the teaspoon (why do we never say cocoaspoon?) before topping up. This enables you to reverse the first two steps and add some water to the dry powder, which is sometimes more convenient, as you can have the cocoa jar put away again before starting to mess with the kettle, thus decluttering the work surface. It's all in the therbligs! {The poster formerly known as 87.81.230.195} 90.197.66.42 (talk) 12:46, 6 February 2012 (UTC)
- For what it's worth, I treat hot chocolate powder like cornflour: dry stuff goes in mug first, then add splash of water and stir thoroughly to make a thick paste. Then water down the paste, stirring constantly. No clumps at the bottom! Brammers (talk/c) 16:42, 6 February 2012 (UTC)
- For Americans who might be confused — Brammers is talking about cornstarch, which for some odd reason Brits call cornflour. --Trovatore (talk) 16:53, 6 February 2012 (UTC)
- For what it's worth, I treat hot chocolate powder like cornflour: dry stuff goes in mug first, then add splash of water and stir thoroughly to make a thick paste. Then water down the paste, stirring constantly. No clumps at the bottom! Brammers (talk/c) 16:42, 6 February 2012 (UTC)
- I concur with StuRat, but additionally stir the initial water + powder mix vigorously with the teaspoon (why do we never say cocoaspoon?) before topping up. This enables you to reverse the first two steps and add some water to the dry powder, which is sometimes more convenient, as you can have the cocoa jar put away again before starting to mess with the kettle, thus decluttering the work surface. It's all in the therbligs! {The poster formerly known as 87.81.230.195} 90.197.66.42 (talk) 12:46, 6 February 2012 (UTC)
- Yes, because it's all stuck to the bottom. :-) I think adding a bit of hot water, then the cocoa powder, then the rest of the hot water, is the best approach. StuRat (talk) 05:05, 6 February 2012 (UTC)
- The mnemonic I was taught: You're doin' watcha otter when you add the acid to the water. --Trovatore (talk) 16:45, 6 February 2012 (UTC)
- The mnemonic I remember is "don't do what I did that time many years ago when I added water to acid and the flask shattered". DMacks (talk) 17:33, 6 February 2012 (UTC)
- Well, good for you if you can remember for sure which one you did. In junior high school I had a similar experience (sodium thiosulfite solution plus acid --> cloud of yellow gas that sent everyone out of the room) but I thought I had done it right. Maybe I did add the acid to the water but just too much at once, or something. At this remove it's impossible to be sure. --Trovatore (talk) 19:05, 6 February 2012 (UTC)
- "May her rest be long and placid,
- she added water to the acid.
- Quite forgot what we had taught her,
- add the acid to the water"
- - I guess this worked as a mnemonic, as I last saw it thirty years ago, and reading this thread brought it all back. (this is my first wiki edit, so please feel free to correct any mistakes I have made with format) TrohannyEoin (talk) 10:31, 8 February 2012 (UTC)
- Well, good for you if you can remember for sure which one you did. In junior high school I had a similar experience (sodium thiosulfite solution plus acid --> cloud of yellow gas that sent everyone out of the room) but I thought I had done it right. Maybe I did add the acid to the water but just too much at once, or something. At this remove it's impossible to be sure. --Trovatore (talk) 19:05, 6 February 2012 (UTC)
- The mnemonic I remember is "don't do what I did that time many years ago when I added water to acid and the flask shattered". DMacks (talk) 17:33, 6 February 2012 (UTC)
Boiling points and tripple points for monatomic gasses
Does anybody know where I can find the (notional) boiling points (at standard pressure) and the triple point for monatomic gasses including H, O, N ? I assume these must be calculated/estimated values as they will not be stable at such low temperatures. Thanks Keit124.182.1.238 (talk) 02:50, 6 February 2012 (UTC)
- See our articles on hydrogen, oxygen, and nitrogen, under "physical properties".--Shantavira|feed me 09:01, 6 February 2012 (UTC)
I wish it was that easy, Shantivira. These Wikipedia articles, although describing the elements H, O, & N, actually give the properties for the molecular form, H2, O2, and N2. If you look up the NIST online database for H2, O2, and N2, you get the same values as given in the WP articles. Keit121.221.76.67 (talk) 12:28, 6 February 2012 (UTC)
- Then the only gases you're talking about are the noble gases Helium neon Argon Krypton Xenon and Radon. Dmcq (talk) 14:03, 6 February 2012 (UTC)
- No, definately not talking about the noble gases. Monatomic gasses such as H, O, and N are not stable at low temperatures (and pressures), but they CAN and DO never the less exist - that's why I need values for them. I could find all manner of properties for them, but not boiling and triple points. Keit124.178.157.111 (talk) 15:20, 6 February 2012 (UTC)
- Don't you see a problem with high temperature low pressure solid gas? Dmcq (talk) 16:10, 6 February 2012 (UTC)
- No, definately not talking about the noble gases. Monatomic gasses such as H, O, and N are not stable at low temperatures (and pressures), but they CAN and DO never the less exist - that's why I need values for them. I could find all manner of properties for them, but not boiling and triple points. Keit124.178.157.111 (talk) 15:20, 6 February 2012 (UTC)
- You are essentially asking a counter-factual. Monoatomic H, O, N can never form liquids or solids because long before they reach that state they will first form diatomic molecules. Hence it makes no sense to ask what their boiling points / triple point would be since they will never reach such a state without forming molecules. I don't see how such notional values could be useful. More to the point, I don't think there is anyway to usefully calculate such values. The interatomic forces between these monoatomic gases lead to pair-bonding, but you want to calculate how the interatomic forces lead to the formation of liquids and solids while somehow preventing pair-bonding. Whatever answer you get from such a calculation would depend strongly on the modifications you made in order to prevent the formation of pair bonds, and at that point it wouldn't be representative of any true behavior for the monoatomic gas. Dragons flight (talk) 18:36, 6 February 2012 (UTC)
- It seems valid to ask what the properties are, though maybe there are no published values. Once created, unstable forms can exist, it's just for a limited time - in some cases, years, in some cases, minutes, in some cases picoseconds. But they can exist. Monatomic gasses are extremely unstable at standard temperature (~298K) and pressure (~1 Bar), you can certainly find thermodynamic data (eg specific heat, enthalpy, etc) for them (eg Rose & Cooper, NIST, etc etc) - presumably these are calculated values. And the common forms H2, O2, N2 are quite unstable at 6000K, but you can find calculated properties for them at 6000K. Don't forget that gases unstable below certain temperatures can be made so by increasing pressure. Judging by the marked upward tilt of specific heat at low temperatures and 1 bar, the triple point and boiling point of monatomic oxygen must be close to standard temperature and pressure, that of H must be very low. Ratbone121.221.96.117 (talk) 01:22, 7 February 2012 (UTC)
- Where on NIST did you find thermodynamic data for monatomic gases? The boiling and triple points are statistical properties resulting from the interaction of the molecules. I don't see how you can expect to determine a statistical property caused by interaction if you neglect the most significant aspect of that interaction, the pair bonding. --140.180.7.220 (talk) 06:57, 7 February 2012 (UTC)
- Go to the NIST website http://webbook.nist.gov/chemistry/form-ser.html, key in, say "H" in the chemical formula filed, click the Search button, and up will come a page headed "Hydrogen atom" and showing an index of what they have. Choose "Gas Phase Thermochemistry Data" from this index - you'll then get a page with a table of key thermodynamic data, and the Shomate coefficients so you can calculate specfic heat etc at any temperature your heat desires, at standard pressure, including low temperatures where H is most unstable. If you prefer, you could also go to your local university's library and look up the NIST-JANAF tables. Or look at Rose & Cooper and similar standard texts. The 4th ed of NIST-JANAF has data at standard pressure for all monatomic gases down to 100K where H is extremely unstable. But none have the triple point, unfortunately. You are quite wrong in your comment about calculating boiling and triple points - you have missed a key part of my original question - but it is somewhat complex to explain - I will see if I can give a simplified explanation in a separate post. Keit121.221.73.82 (talk) 13:55, 7 February 2012 (UTC)
- If the reaction you are interested in takes orders of magnitude longer than the substances involved are stable for, then for all intents and purposes the reaction can't happen. That's why, for instance, you won't find any data about hadrons containing top quarks. At first glance, it makes sense to talk about top quark hadrons, but they would take longer to form than the top quark is stable for. That means they don't actually exist and there is no meaningful data, even theoretical, for them. The same is true for liquid monoatomic hydrogen, oxygen and nitrogen. --Tango (talk) 12:44, 7 February 2012 (UTC)
- "If the reaction ... takes...". I'm certainly aware of that, though I know nowt about quarks. The reactions I am interested in may or may not be noticeably affected by the presence of low concentrations of monatomic H, O, N, and perhaps C. I'm NOT interested in their liquid forms. I'm only interested in their gas forms, i.e., their behavior above the line joining the boiling/sublimation point to their triple point (which is a straight line if plotted on a reciprocal progression temperature scale). To do that I need to know (at least approximately) where these points are. Keit121.221.73.82 (talk) 14:43, 7 February 2012 (UTC)
- I meant "reaction" very generally - I was referring to the condensation/evaporation. I don't understand how you can not be interested in their liquid forms. You asked about their boiling points. Boiling is something liquids do. It doesn't make sense to talk about the boiling point of anything that isn't a liquid. --Tango (talk) 22:26, 7 February 2012 (UTC)
- "If the reaction ... takes...". I'm certainly aware of that, though I know nowt about quarks. The reactions I am interested in may or may not be noticeably affected by the presence of low concentrations of monatomic H, O, N, and perhaps C. I'm NOT interested in their liquid forms. I'm only interested in their gas forms, i.e., their behavior above the line joining the boiling/sublimation point to their triple point (which is a straight line if plotted on a reciprocal progression temperature scale). To do that I need to know (at least approximately) where these points are. Keit121.221.73.82 (talk) 14:43, 7 February 2012 (UTC)
- I've just realised that I have been writing "boiling point" where I should have written "critical point". I don't know why I did that - must be not enough sleep or too many parties. My question may make more sense now. My appologies. But those who understand gas phase reactions could probably have seen thru it anyway. Tango is probably still confused, as he now thinks it only makes sense if I am interested in the state at temperatures below the critical temperature, and pressures above the vapour pressure, which is a liquid. No I'm not interested in the liquid state. To see why, consider a broad analogy: the notional absolute zero temperature of metals. To calculate the electrical resistance of conductors, which is approx proportional to temperature, electrical engineers use a simple formula incorporating absolute zero
R2 = R1 (T2 + T0)/(T1 + T0) . T2 is the temperature of the conductor; T1 is the temperature (usually 20C) at which R1 is the resistance listed in tables.
- This formula assumes that the resistance is a straight line relationship and all metals have zero resistance at notional absolute zero (T0). They commonly use a value of -235C, whereas the thermodynamic absolute zero is -273.15C. They use -235 because it compensates for a number of factors that make the relationship depart from an exact straight line. It DOES NOT mean that electrical engineers are interested in what metals do at -235 or -273C, which mostly is not what this usefull practical formula predicts. They want to know the resistance at operating temperature, usually between 0 and 75C. In a broadly similar manner, I am not interested in liquids. But to work out the behavior of monatomic gasses at "low temperatures" (up to around 900K for oxygen) I would like to know what the NOTIONAL state transition temperatures are. Keit124.182.169.5 (talk) 01:38, 8 February 2012 (UTC)
- You shouldn't think of T0 in that equation as being absolute zero. That equation is a first order approximation and T0 is the constant you happen to get when you plot a straight line of best fit to the relevant data points. It's not entirely coincidental that it comes out as being fairly close to absolute zero, but that isn't a useful comparison. If you are working with first order approximations, you need to determine your constants based on data points near the point you are interested in. If you try and base them on points a long way from those that you are interested in, especially points that are physically meaningless, you will get massive errors. --Tango (talk) 12:31, 8 February 2012 (UTC)
- This formula assumes that the resistance is a straight line relationship and all metals have zero resistance at notional absolute zero (T0). They commonly use a value of -235C, whereas the thermodynamic absolute zero is -273.15C. They use -235 because it compensates for a number of factors that make the relationship depart from an exact straight line. It DOES NOT mean that electrical engineers are interested in what metals do at -235 or -273C, which mostly is not what this usefull practical formula predicts. They want to know the resistance at operating temperature, usually between 0 and 75C. In a broadly similar manner, I am not interested in liquids. But to work out the behavior of monatomic gasses at "low temperatures" (up to around 900K for oxygen) I would like to know what the NOTIONAL state transition temperatures are. Keit124.182.169.5 (talk) 01:38, 8 February 2012 (UTC)
- Tango, I think this is a case where you and I agree in principle, we just use different language. I know T0 is not TRUE absolute zero - I said so above. But to electrical engineers it IS a "notional" absolute zero - the notional/fictitous temperature where the resistance of any metal conductor is notionally zero, and there's nothing wrong in thinking that way - so long as you are aware that the thermodynamic absolute zero is different. We ARE all aware of the thermodynamic absolute zero. As far as errors are concerned, you are right in principle, however the accuracy with this simple formula, in common use, is most certainly good enough over the temperature range that electrical engineers are interested in. It is certainly NOT necessary to choose data points close to the desired point - the standard -235C for T0 and 20C for T1 work just fine.
- Now, I guess any WP fans out there, who might have an answer to my original question about notional triple and critical points, have gone elsewhere due to a) my use of "boiling point" where I meant "critical point", and b) the tangents introduced by chaps who want to say I haven't asked a valid question, without carefully reading and understanding the question, so perhaps we should leave it at that. It's been fun though, and hopefully has caused a few readers to think about things they don't normally think about. Keit121.221.165.218 (talk) 12:56, 8 February 2012 (UTC)
- There is something very wrong with thinking in that way - it's not true. The resistance of metals at -235C is not zero. That equation is only a good approximation in a fairly narrow range, as is generally the case with first order approximations. You misunderstand what I mean by "data point". -235C is not a data point, it's a constant derived from data points. The number -235C was determined by considering the actual resistances of various metals at various temperatures and drawing a line of best fit through them. Those actual resistances will have all been measured at temperatures near the temperatures of interest to users of the equation - they won't have measured resistances near -235C. Once you've got your line of best fit, you work out the equation of it and you find that there is a constant of -235C. That constant doesn't have any physical significance and would be completely different if you were going to come up with the similar equation for use in a different temperature range. --Tango (talk) 19:42, 8 February 2012 (UTC)
- Tango, everything you've said in your last post is true,and in complete agreement with what I said, except for your first and last sentences. (a) there is nothing wrong with thinking that way - almost everything in science & engineering is just a mathematical model, and not completely proven it's only necessary that such models give good results. (b) it hapens that the -235 value gives adequately accurate results over the temperature range engineers are normally interested in (typically 0 to 75C), but you'll find that if you try a quite different range, say 100 to 300 C, the notional absolute zero is not very different (about -233C in this case, as I recall). The true relationship is very close to a straight line. Yes, the formula I gave does not have any physical significance whatsoever - doesn't matter, it works. Same with thermodynamic properties of elements and chemicals - Engineers and others typically use the so-called "NASA" method, or the Shomate formula, or even the "3-lines" method. NASA and Shomate formulas look completely different, have no physical basis at all, but they curve fit over about a 4:1 temperature range to 4 place accuracy, up to 5000K (NASA) and 6000K (shomate). The three-lines method is slightly less accurate, but usually good to 3 places from 200K to 6000K. And absolute no physical basis or logical derivation. It just works. Enough said. Keit121.215.22.13 (talk) 22:31, 8 February 2012 (UTC)
- I never said first order approximations don't work - they are incredibly useful. That doesn't make -235C absolute zero in any meaningful way, though. It is just a constant that you get when you fit a straight line to some data points. It doesn't have any physical significance - nothing interesting happens at that temperature. You can't use the fact that such constants are useful as an argument for the idea of a critical point of monoatomic hydrogen making sense. It can't exist as a liquid, so it doesn't have a critical point. --Tango (talk) 01:08, 9 February 2012 (UTC)
- Tango, everything you've said in your last post is true,and in complete agreement with what I said, except for your first and last sentences. (a) there is nothing wrong with thinking that way - almost everything in science & engineering is just a mathematical model, and not completely proven it's only necessary that such models give good results. (b) it hapens that the -235 value gives adequately accurate results over the temperature range engineers are normally interested in (typically 0 to 75C), but you'll find that if you try a quite different range, say 100 to 300 C, the notional absolute zero is not very different (about -233C in this case, as I recall). The true relationship is very close to a straight line. Yes, the formula I gave does not have any physical significance whatsoever - doesn't matter, it works. Same with thermodynamic properties of elements and chemicals - Engineers and others typically use the so-called "NASA" method, or the Shomate formula, or even the "3-lines" method. NASA and Shomate formulas look completely different, have no physical basis at all, but they curve fit over about a 4:1 temperature range to 4 place accuracy, up to 5000K (NASA) and 6000K (shomate). The three-lines method is slightly less accurate, but usually good to 3 places from 200K to 6000K. And absolute no physical basis or logical derivation. It just works. Enough said. Keit121.215.22.13 (talk) 22:31, 8 February 2012 (UTC)
- At low pressures (typically <0.1 Bar) (or at a notional zero pressure - sorry Tango, I couldn't resist that one) gasses display three regions of specific heat: 1) a flat, temperature independent region at low temperatures where the specific heat comforms to the classical formula based on kinetic degrees of freedom. 2) A region at high temperatures where specific heat is higher than the classical kinetic degree of freedom, and slowly increases with temperature due to bond excitation and electronic effects, and (3) a transition at medium temperatures (typicallly around 800 to 2000K, depending on the gas) where specific heat relatively rapidly changes from (1) to (2). At higher pressures, Region 2 is for all intents and purposes unchanged. Region 3 hardly changes - for engineering purposes Region 3 can be regarded as pressure independent. But Region 1 IS pressure dependent. Increasingly with increasing pressure, specific heat INCREASES with DECREASING temperature. Why? Because at 1 Bar, it must intercept the triple-point / critical point line at the boiling point (which by convention is defined at 1 Bar), and this is at generally greater than the gas classical value. More signifantly, at critical pressure, the specific heat of a gas must INCREASE with falling temperature to intercept exactly the critical point, where the specific heat is INFINITE. In other words, gasses have, at real pressures, a temperature where the specific heat is a minimum - this temperature is in the range 500 to 2000 K depending on the gas. Below this temperature you get a range of negative slopes depending on the pressure-temperature location of the triple and critical points. Or, looking at it another way, if you know the low pressure curve of specific heat vs temperature, and you know the triple and critical points, you can with the right mapping, predict the specific heat vs temperature curve at any desired pressure.
- THIS TRIPLE & CRITICAL POINT DEFINING LOW TEMPERATURE BEHAVIOUR IS SEEN WITH MONATOMIC GASSES. For H the increase is negigible at 1 bar (might not be at higher pressures - I haven't seen any data), but for O the increase is very marked at 1 bar, the curce being very steep at 200K - the lowest temperature at which I have trustworthy data. Carbon gas (both C1 & C2) shows a distinct negative slope at 1 Bar in Region 1 too. Please explain that if you think such gases don't have notional critical points.
- I think this has gone on long enough under this Question Heading. We must have set a record for the longest discussion in the Science Desk. May I suggest that if Tango, or anyone else, wants to continue down this side path, then create a new question, headed "Thermodynamic behaviour of monatomic gasses" or some such, and ask "Can notional critical, boiling, and triple points be defined for monatomic gasses?" or whatever you think. I may then respond to that and we can allow the Wiki system to archive this question. Keit120.145.8.148 (talk) 15:36, 9 February 2012 (UTC)
Relife from pain
This question has been removed. Per the reference desk guidelines, the reference desk is not an appropriate place to request medical, legal or other professional advice, including any kind of medical diagnosis or prognosis, or treatment recommendations. For such advice, please see a qualified professional. If you don't believe this is such a request, please explain what you meant to ask, either here or on the talk page discussion (if a link has been provided). --Jayron32 04:48, 6 February 2012 (UTC)
The Ostrich & Disemboweling
The wikipedia article on the Ostrich mentions that they are capable of disemboweling people. Several other websites repeat this fact but without any citations. Are there any confirmed stories of ostrich disembowelment? --188.220.46.47 (talk) 14:56, 6 February 2012 (UTC)
- They're certainly capable of causing eye injuries to people, but I can't find any evidence of ostrich disembowelment in recent history. That doesn't mean it's not completely possible - cassowaries are apparently especially aggressive and do have sharp rear claws on their feet perfectly capable of disemboweling or other painful injuries. A cassowary did kill a boy in 1926 but no bowels were involved. §everal⇒|Times 17:23, 6 February 2012 (UTC)
- What about other predator disemboweling? --CGPGrey (talk) 17:34, 6 February 2012 (UTC)
- Does a locomotive count as a predator? Ostriches will attack all kinds of things but it's not clear that disembowelment is a frequent result. §everal⇒|Times 18:01, 6 February 2012 (UTC)
- What about other predator disemboweling? --CGPGrey (talk) 17:34, 6 February 2012 (UTC)
- Human disembowelment is a quite specific procedure of which very many animals are no doubt capable should they be so minded, but to categorise specific animals in this way seems not to be particularly useful or encyclopedic. It's not as though they routinely go around disembowelling people.--Shantavira|feed me 19:06, 6 February 2012 (UTC)
- I disagree that it is a "specific procedure." We are not talking about a precise and specific procedure. There are many ways that the abdomen could be cut open and some or all internal organs torn out. Edison (talk) 16:18, 7 February 2012 (UTC)
MAGNETIC GENERATORS - Worth using?
I REALLY don't care all that much about long-winded, techincally-termed details of how these things work, or are claimed to work. I'd just like to know in LAYMAN's TERMS: A. Do they work at all?; and B. Are they capable of substantially reducing electric bills or operating any electrical appliance (110 or 220V) independently with little "Supplied Electricity" [wired power companies] use? Much thanks. I don't trust them to refund ALL my money for their books. — Preceding unsigned comment added by Scizottstheb (talk • contribs) 16:53, 6 February 2012 (UTC)
- Well the first link I read, from "thefreeenergygenerator.org" talks about "perpetual motion" and doesn't explain the source of the energy, except they seem to confuse force with energy, so I suspect that the whole thing is a scam, unless, of course, they are selling a device to stop your meter from recording the real energy used, then they are illegal. The same site advertises running your car on water! Draw your own conclusions. (or read this) Dbfirs 17:24, 6 February 2012 (UTC)
- In layman's terms: no and no. §everal⇒|Times 17:28, 6 February 2012 (UTC)
- This saying comes to mind. Roger (talk) 09:01, 7 February 2012 (UTC)
Do not give these criminals your money. They are liars and thieves. There is no such thing as a free lunch. People have been scamming money out of people for literally centuries with crud like this. Steorn is another modern example. Liars and thieves. — Preceding unsigned comment added by 217.158.236.14 (talk) 14:47, 8 February 2012 (UTC)
Should I turn off the LCD before switching off the surge protector?
Thanks. — Preceding unsigned comment added by 66.108.223.179 (talk) 17:19, 6 February 2012 (UTC)
- I assume you mean an LCD computer monitor. If it's Energy Star compliant, the monitor will go into standby mode if not used for a few minutes, so there's no need to turn it off in either place. If the surge protector also powers other things you need to turn off, then it's OK to turn it off there first. I also do this with my non-Energy Star LCD monitor (stupid thing shuts off the screen except leaves the back-light on). Also, if the computer is on the same surge protector, then you do need to turn the computer off using the menu first. StuRat (talk) 19:35, 6 February 2012 (UTC)
Diesel hybrid car
Why don't they make hybrid diesel cars? Is it a technical thing? — Preceding unsigned comment added by 166.205.136.209 (talk) 20:08, 6 February 2012 (UTC)
- Diesel-electric transmission#Production-ready cars -- Finlay McWalterჷTalk 20:15, 6 February 2012 (UTC)
Electric transmission (using electric means to convert engine RPM and torque to road-appropriate speed and torque) should not be confused with Hybrid electric drive (using an electric motor for propulsion to assist an internal combustion engine). Electric transmision dates back at least to the 1920's but has never been popular as the conventional hydro-mechanical automatic transmission does the job just as well and is a lot lighter. Recent developments in better magnetic materials is changing this picture. Re hybrid diesel-electric propulsion, it will happen but there is less incentive than hybrid gasoline-electric because diesel engines are more expensive than gasoline engines, and the main advantage - fuel efficiency - is significantly discounted by the much greater efficiency of diesel engines at larger sizes. A diesel engine, more efficient that a gasoline engine at full throttle, is still more efficient at part thottle than is a gasoline engine. But in a hybrid vehicle, if you don't need high power at certain times during driving, the gasoline engine can be simply shut down. Keit121.221.96.117 (talk) 04:30, 7 February 2012 (UTC)
Any US cars with this feature?
I press a button on the dashboard. Later, I press it again, or another button, and the car tells me how many gallons of gas have been used between the two button presses. No, I don't care about the feature of seeing instantaneous calculated mileage, because slope snapshots are useless (to me) when I'm not constantly staying at the same mileage. 20.137.18.53 (talk) 20:45, 6 February 2012 (UTC)
- Some cars have trip computers, which have a button to start the "trip" and another to end it. However, the ones I've seen report total miles traveled and average MPG over the trip, from which you could calculate gallons of gasoline used, but don't actually report the gallons. StuRat (talk) 20:50, 6 February 2012 (UTC)
- A "gas used" readout was on some of the last years of Pontiacs. The dashboard display could select "gallons of gas" and it would keep adding up fuel as it was used. It could be reset to zero anytime, such as when the tank was filled, or when a trip began. The display could alternately show "miles remaining" "miles per gallon, or "% remaining before oil change needed." Edison (talk) 00:45, 7 February 2012 (UTC)
- Also bear in mind that a car fuel gauge is not a precision instrument.--Shantavira|feed me 10:14, 7 February 2012 (UTC)
- Yes, that's why I ask. :) 20.137.18.53 (talk) 15:35, 7 February 2012 (UTC)
- The General Motors gas used readout worked off the fuel pump, and consistently read about 5% less than what the gas station pump said the car had used, when it was filled each time. The needle on the fuel gauge was far less accurate, dropping slowly as the first few gallons were used, then dropping rapidly at the end. It has a sensor in the gas tank consisting of a float on a swivelling arm, which connects to a potentiometer. I've no idea if the inaccuracy came from a failure to allow for varying relationship between fuel level and arm angle, or the varying relationship between the change in fuel level and the varying cross-section of the fuel tank.With computers in the car's electronics, it would have been trivial to use a lookup correction table to make the dial (or digital display) accurately reflect the fuel remaining. Edison (talk) 16:15, 7 February 2012 (UTC)
- It's because between full and almost full the arm doesn't move at all. Between almost empty and empty, the same happens. (If you'll take Cracked for source, its here.) Grandiose (me, talk, contribs) 13:52, 8 February 2012 (UTC)
- The General Motors gas used readout worked off the fuel pump, and consistently read about 5% less than what the gas station pump said the car had used, when it was filled each time. The needle on the fuel gauge was far less accurate, dropping slowly as the first few gallons were used, then dropping rapidly at the end. It has a sensor in the gas tank consisting of a float on a swivelling arm, which connects to a potentiometer. I've no idea if the inaccuracy came from a failure to allow for varying relationship between fuel level and arm angle, or the varying relationship between the change in fuel level and the varying cross-section of the fuel tank.With computers in the car's electronics, it would have been trivial to use a lookup correction table to make the dial (or digital display) accurately reflect the fuel remaining. Edison (talk) 16:15, 7 February 2012 (UTC)
- What would be good for what I'm talking about would be a flowmeter on the line that carries the fuel from the fuel pump to the engine, because I want to know how much volume of gas has been consumed between time A and time B. This would not be frustrated by tank fill-ups between button presses. 20.137.18.53 (talk) 17:33, 7 February 2012 (UTC)
Saccharides
What is the most stable form of glucose in the crystaline, solid phase - open chain, furanose, pyranose or some other form? How does the chain length affect the stability of said form compared to the others? Plasmic Physics (talk) 21:52, 6 February 2012 (UTC)
- If you read Glucose you can answer much of your homework question yourself. If you can't find your answer, please look closely in the section titled "cyclic forms", especially the last paragraph. But the entire "Structure and nomenclature" section is good reading as well in this regard. You can also follow bluelinks from that article, especially to articles like furanose and pyranose, to find the other answers to your homework problems. --Jayron32 01:45, 7 February 2012 (UTC)
OK, so aldohexoses exist as the pyranose form in the crystaline solid phase. What about pentoses, heptoses, etc. What about ketoses?
P.S. It is not a homework question. Plasmic Physics (talk) 04:22, 7 February 2012 (UTC)
February 7
How big will sun be at first RGB in 5 billion years?
2008 studies show Earth will be swallow up because of the tidal interaction. Without considering loss of sun's mass/gravity to make it expand orbit, how big will sun be at first RGB in 5 billion years? is it roughly size of earth orbit little less than 1 AU or greatly than 1 AU. is it less than 200 solar radius or is is 250 solar radius? Does sun shrink between first and second RGB? My astronomy teacher display sun shrinks after first RGB then expands again at second RGB? How much will sun shrink between first and second RGB? about the size today or less than sun's size today. Becuase second RGB is 1.2 AU, or 260 solar radius. The article didn't mention the first RGB.--69.229.39.25 (talk) 00:37, 7 February 2012 (UTC)
Life form on Europa and Titan few billion years back in the history
I thought some scientist thought Europa was once filled with globes of liquid oceans and Titan might been habitatable at one time.[1] I wonder how they do it. Five billion years back in the history was sun bigger and brighter than now or was it smaller and fainter than now (I never paid attention in class, I just daydreamed). Europa and Titan is too small, I am surprised to hear Europa can handle off an atmosphere. How could Mars be blue and wet 4 billion years ago. Was sun bigger back then or was it smaller. Mars had alot of atmosphere back then was sun smaller, or was it bigger. Why did Mars lose its atmosphere?--69.229.39.25 (talk) 01:49, 7 February 2012 (UTC)
- 5 billion years ago the Sun did not even exist yet, let alone Jupiter and its moons.
- The presence or absence of an atmosphere has little to do with the size or mass of the body. Uranus is 63 times more voluminous than Earth, yet it has almost no atmosphere. Titan is 1/15th the size of Earth, yet its atmosphere is actually denser than Earth's. The critical factor is whether or not there is a magnetosphere to protect the atmosphere from solar wind.
- Atmosphere_of_Mars lists a few possible causes for Mar's current thin atmosphere.99.245.35.136 (talk) 02:47, 7 February 2012 (UTC)
- Venus has no magnetosphere, it can still hold atmosphere. I don't know if Titan and Pluto even have magnetosphere. When Pluto is closer to sun it actually have more magnetosphere, that is weird. Yes, Uranus has atmosphere.--69.229.39.25 (talk) 05:10, 7 February 2012 (UTC)
- Uranus has no atmosphere? It's a gas giant. All of it is atmosphere. --140.180.7.220 (talk) 05:16, 7 February 2012 (UTC)
- I don't think magnetosphere may entirely protect the atmosphere. Venus has no magnetic field, its atmosphere is 90xs stronger than Earth. Titan's gravity is like one-seventh that of Earth compare to moon which is one-sixth of earth. I thought the discussion is gravity holds the atmosphere, I forgot we talked about it before. I thought all the sources I found on Europa is reliable, could be the author is just biased about something, sometimes I am just not careful, they may just privode speculations to trick dummies.--69.229.39.25 (talk) 06:49, 7 February 2012 (UTC)
- The early sun was fainter, about 70% of modern. See faint young sun paradox. The presence or absence of an atmosphere is influenced by several factors: the mass of the planet/moon, the distance from the sun (affecting both surface temperature and solar wind), the composition of the atmosphere (heavier gases are more persistent than light gases), and the presence or absence of a magnetosphere. See atmospheric escape. Both Mars and Earth probably had a thicker atmosphere in the early solar system, but the Earth is much better at holding on to it due to its higher mass, so most of the Earth's atmosphere is still here while most of Mars' early atmosphere has been lost. Dragons flight (talk) 08:15, 7 February 2012 (UTC)
Special relativity
Does anyone know a good easy (but rigorous) introduction to special relativity? Money is tight (talk) 03:26, 7 February 2012 (UTC)
- Tipler's Modern Physics pulls no punches, but only covers elementary relativity. On the other hand, it does so in an application-centric way, rather than a purely theoretical derivation from first principles; so it's immediately useful to the experimentalist or engineer (as much as any relativity knowledge can be useful to the applied sciences). It reads at a level suitable for an advanced freshman or sophomore (university-level) physics student. It also covers many other topics besides relativity. Jackson's Electrodynamics also has a chapter where he derives Lorentz transforms, but it's more suitable for the advanced physics student. Personally, I think the best way to "rigorously" learn special relativity is to "rigorously" learn classical electrodynamics; after this foundation, special relativity is the obvious consequence. Relativistic gravitation, on the other hand, is a whole different subject (...that is, not special relativity); there's not a lot you can do to prepare for it, but it does help to have an advanced background in the analytic mathematical tools of classical physics. Nimur (talk) 05:11, 7 February 2012 (UTC)
- We do have an article Introduction to special relativity here. Graeme Bartlett (talk) 12:10, 7 February 2012 (UTC)
I learned it when I was in high school from Lillian Lieber's The Einstein Theory of Relativity, which I still think is wonderful if you can find a copy. Looie496 (talk) 18:49, 7 February 2012 (UTC)
- I was pretty impressed by Ray d'Inverno's Introducing Einstein's Relativity, although I didn't go far with it, since I don't know anything about Maxwell's equations. It seemed fairly rigorous, although it started with easier introductions (the initial proof of the Lorentz transformations was somewhat simple, then it covered the orthodox method, afaicr). Also the Schaum's guide, but that one leaves you to do most of the work (proving E=mc^2 is one of the exercises). IBE (talk) 22:05, 7 February 2012 (UTC)
Blue star-like object
On a walk tonight, I saw a "star" in the western sky; given that it was brighter than anything in Orion, visible behind me, given that it was visible at all even though it was the Las Vegas sky and slightly overcast in that direction, I'm guessing it was a planet. (I don't think it was Sirus, as it was opposite Orion in the sky.) My question is that I could see a blue light from the top of it. It wavered in and out, disappearing for a few minutes, and was never clearly distinguishable from the star, but it was visible for most of an hour. It and the star were stable in position for that hour, so it wasn't a plane or anything of the like. It was maybe 30 degrees up in the sky, well above the mountains, so it wasn't a land based light. Am I correct in assuming it was some sort of chromatic aberration of the atmosphere or something? That's the conclusion I'm left with. (Yes, I thought UFO; besides all the other problems with that conclusion, it was stable, and you'd think a bright flying object a few miles from Nellis would get intercepted in the time I watched it. Unless the Air Force had someone hover in place for over an hour... but I don't regard that as a serious possibility.)--24.120.231.24 (talk) 03:38, 7 February 2012 (UTC)
- Um, Venus? In tonight's Las Vegas sky, Venus would be to your west, pretty near the horizon, between Pisces and Aquarius. Someguy1221 (talk) 04:33, 7 February 2012 (UTC)
- Venus, Jupiter, and Mars are all visible in the night sky in the Northern Hemisphere right now, and all are as bright or brighter than Sirius (the bright star that Orion's Belt points at, and the brightest star in the sky). Planets are pretty easy to spot if you can find the ecliptic, which is roughly the plane of the solar system, and corresponds to the path the sun seems to follow as it goes through the sky. At night, the moon and planets also lie roughly along the ecliptic, so they're easy to spot, especially if you are spotting one of the three planets brighter than Sirius; Venus is currently quite bright, and obviously the brightest thing after the moon. --Jayron32 05:23, 7 February 2012 (UTC)
- Okay, thanks; but I wasn't really asking about the object itself. It seemed to be white, but the top of it sparkled with a bright blue light. That's why I thought it was an airplane at first glance, because that blue light isn't part of the natural hue of the night sky and it was so close to the other light (presumably Venus). I'm guessing the blue light was about a 2 magnitude by itself.--Prosfilaes (talk) 05:49, 7 February 2012 (UTC)
- Hot air balloon \ or weather balloon? \ Chinese lantern? SkyMachine (++) 07:11, 7 February 2012 (UTC)
- No, it was almost certainly Venus. The real question is why the top edge looked blue. Did the bottom edge look red? If so, that's a sign of chromatic aberration of your glasses and/or eyes. --140.180.7.220 (talk) 07:25, 7 February 2012 (UTC)
- I didn't notice a red bottom edge, though I wasn't looking. My glasses are pretty thick high-index plastic. I tried looking at Venus from different angles, and didn't notice the change in effects I usually see in chromatic aberration. It doesn't thrill me as an answer, but it does seem like the best fit.--Prosfilaes (talk) 13:11, 7 February 2012 (UTC)
Would a "warranty cutoff switch" exist?
You have heard/read of dealer cutoff switches - placed on cars purchased by customers with bad credit so that the dealer (or one in charge of the lien) can disable the car if the customer falls behind on payments (or start making annoying beeping sounds, disable the radio, etc. to entice the delinquent to pay up). Natch, they are triggered remotely, or even on a preset timer.
So, could "warranty cutoff switches" be placed in expensive equipment so that shortly after the warranty runs out, the timer running out causes the "switch" to foul up a critical component that is expensive to replace. (Perhaps the timer would choose a random time to toggle the switch in order to not create too many coincidences, but still take place shortly after the warranty ends.)
Why haven't I heard of such a switch? Wouldn't various manufacturers stand to make a killing on either expensive replacement parts, or just an upgrade to a newer product? I thought planned obsolescence would perhaps involve it, wouldn't you?
So on what products would there be "warranty cutoff switches" and yet, why haven't I heard of them on news and consumer reports? --70.179.174.101 (talk) 07:57, 7 February 2012 (UTC)
- "Why haven't I heard of such a switch"? Two possibilities: (A) they don't exist. Or (B) they don't want you to hear about them. Why do you think that we'd be able to say which alternative was the right one? AndyTheGrump (talk) 08:01, 7 February 2012 (UTC)
- You may be interested in Planned obsolescence 157.193.175.207 (talk) 08:23, 7 February 2012 (UTC)
- If this wasn't disclosed, and you own the item outright, I believe they could be charged with a crime. After all, they are intentionally damaging your property. This would be "willful destruction of property", would it not ? This assumes that you own the item, versus the car which you don't own until you complete payments. And, of course, once it got out that they did such a thing, nobody would ever buy from them again. StuRat (talk) 08:42, 7 February 2012 (UTC)
- It would be almost impossible to keep such a device secret long-term from the flapping lips of disgruntled workers, ex-employees, probing repairmen and techies, and the like. The manufacturer responsible would also gain a reputation for unreliability and poor quality if their "expensive equipment" repeatedly failed just after the warranty expired. Combine that progressive loss of face with their shattered reputation once they were inevitably outed, and why would any manufacturer with anything but the shortest possible timeframe in mind ever consider doing such a thing? As Stu says, no one would ever buy from them again, and their business would quickly fail through market forces, if not through government intervention. --jjron (talk) 10:01, 7 February 2012 (UTC)
- Are you sure you don't own the car until you complete the payments? If you have a loan secured to something, you still own it unless you default on the payments. Are car loans different from normal secured loans? --Tango (talk) 13:20, 7 February 2012 (UTC)
- The Quicken program stops working after a certain number of months, in that it will no longer download any data from banks or credit cards. You could, in principle, manually enter every transaction, but downloading is a major part of the functionality.This brute force way of getting you to buy the newer version is not present on most software, although vendors have historically cut off any tech support of old software, and have not provided free updates so the software works with newer operating systems. If a device has an internal clock, it is possible for it to shut down automatically after some "lifetime." This is common in carbon monoxide detectors,with a 5 year sensor life, as described in the instructions for this model: "Sensor Life Monitor: Internal clock starts once lithium battery is activated. Visual and audible signals notify you when sensor life has expired. " Edison (talk) 16:06, 7 February 2012 (UTC)
- Right, and there they have a justification for the action and notify the consumer, making it all legal. Another example is a toothbrush with bristles designed to dissolve over time, supposedly to let you know when it's germy and needs to be replaced. This is a rather iffy justification, as, of course, any toothbrush can be sterilized by soaking it in bleach or by several other methods. StuRat (talk) 20:30, 7 February 2012 (UTC)
- I don't know where you got that "germy" business from: a toothbrush's germ load will reach a steady-state level a few days after the first time you put it in your mouth. The reason to replace a toothbrush every few months is mechanical wear causing the bristles to lose their scrubbing power. --Carnildo (talk) 02:09, 8 February 2012 (UTC)
- I've seen ads specifically claiming the reason to replace a toothbrush frequently is "germs". The bristles seem capable of lasting for years, provided they aren't designed to wear out quickly. StuRat (talk) 03:09, 8 February 2012 (UTC)
- I'm no lawyer, but it seems to me there'd be some serious liability issues if the device were to be disabled at a particularly bad time, e.g. resulting in injury or death. Clarityfiend (talk) 21:57, 7 February 2012 (UTC)
- Presumably the dealer cut-off switch wouldn't disable the engine, as that would inevitably leave stalled cars on the freeway, creating a dangerous situation and making it difficult to recover the car. They might disable the starter, though, meaning it couldn't be restarted once stopped. This could still kill people, though, like if somebody stops the engine out in the middle of nowhere on a subzero night (say for a "snuggle" with their significant other). It could also leave cars stranded in places like gas stations, with some rather irate business owners suing them for the loss in business. Overall, it's just a terrible idea. StuRat (talk) 07:19, 8 February 2012 (UTC)
- Rather then random speculation which seems to be going on here, a simple search for 'dealer loan cut off switch' finds plenty of refs [2] [3] [4] most of which seem to link back to [5]. It seems clear they are real, but don't cut off the engine while the car is in motion and there is an option to extend the driving period in an emergency (although no description of how this works). Also the consumer signs and ticks a 5 page disclosure form the driver gets in car warnings for several days before the cut off is activated. It seems some people voluntarily sign up for these devices even when they aren't required to get a loan to ensure better loan terms. One of the companies making such devices has a website [6]. Of course stopping the car from working isn't unique to such scenarios, they're sometimes used for anti theft purposes. While immobilisers (which mean the car just never starts) may be more common, I believe there are some systems which allow the car to be driven for a while to be stopped later. We do have articles Kill switch and Anti-theft system which briefly describe these and in particularly mention their usage in Bait cars. Probably they are more common in Anti-hijack systems then anti theft. Nil Einne (talk) 08:33, 8 February 2012 (UTC)
- But the OP's question was never whether or not such devices existed in automobiles with the owner's consent. It's whether or not such devices are intentionally (but unknowningly to the consumer) placed in other expensive equipment in order to result in expensive repairs or replacement shortly after the warranty expires. --jjron (talk) 11:44, 8 February 2012 (UTC)
- I know that hence why I didn't reply until now (I regard it a silly question as with quite a number of questions of the OP). But it doesn't change the fact StuRat was in fact randomly speculating on the dealer cutoff switch in cars when a simple search finds info on such devices. Also my sourced answer does in fact provide a big hint to the OP's question. If these car loan devices require a 5 page disclosure form and have plenty of warnings and other such considerations, this speaks somewhat to the likelihood of someone putting in such a device without telling the consumer where it randomly disables without the consumer knowing why. (I didn't think this needed to be mentioned, since it should be obvious and is something others have already hinted at anyway.) Nil Einne (talk) 20:58, 8 February 2012 (UTC)
- I don't see how anything you found disagrees with anything I've said. I just didn't see the point in listing sources which say that cut-off switches don't stop the engine mid-drive, when common sense tells us as much (and especially since that wasn't even the OP's question). StuRat (talk) 23:14, 8 February 2012 (UTC)
- Philosophically, any car should have a "warranty cutoff switch", in the sense that the manufacturer should end the warranty at about the time when things usually start going wrong, so as to make the warranty sound attractive while not paying too much. But the "cutoff switch" might as well be the things that actually go wrong, of which there is no shortage. I've heard of a timing belt (as opposed to a timing chain) working quite effectively as such a "switch". I suppose a dishonest dealer might merely neglect to mention it requires a change, which indeed is consistent with such a story as I heard it, but can you prove that? And of course, it's technically your responsibility to RTFM, which (if true) is the beauty of the scheme. Wnt (talk) 20:50, 8 February 2012 (UTC)
- There is a related problem that some disreputable dealers will try to "run out the clock" on the warranty by returning cars either completely unfixed (for problems which occur infrequently) or with a temporary fix, knowing that by the time the consumer returns, the warranty will have expired. StuRat (talk) 23:14, 8 February 2012 (UTC)
science.chemistry
does ink and salt dissolve at the same rate and different temperature — Preceding unsigned comment added by 119.235.88.180 (talk) 11:20, 7 February 2012 (UTC)
- no. Ink has much larger molecules and may even be a suspension. Graeme Bartlett (talk) 12:22, 7 February 2012 (UTC)
- And ink molecules may be soluble in liquids in which salt is not soluble. But I think we've done enough of what sounds like your science homework for now. DMacks (talk) 15:25, 7 February 2012 (UTC)
- Did the OP mean to ask the same rate at different temperatures? This seems plausible to me, unless there's some sort of cap or floor on rate of dissolution. 216.197.66.61 (talk) 23:17, 7 February 2012 (UTC)
lines of field
I wanted to derive the equation (like, in Cartesian coordinate system) for electric field lines (for dipoles)and it turned out to be huge and not elegant at all. I just wanted to check if there's any online source to check it, or anybody here who knows the answer...-Irrational number (talk) 15:43, 7 February 2012 (UTC)
- ...please?--Irrational number (talk) 18:09, 7 February 2012 (UTC)
- Does Dipole#Field from an electric dipole help? I'm pretty sure the field around a dipole is never going to look elegant in any conventional co-ordinate system since it has an annoying asymmetry in it - this is why for some applications it's best to use [dipole co-ordinates]. Smurrayinchester 18:27, 7 February 2012 (UTC)
- My main reason to look for that was to be able to draw the lines of field for a dipole in "graph"(the program I mean), which was not successful... I had tried p orbital cross section before, so I guess I was underestimating the dipole?Is there a way for me to do that?--Irrational number (talk) 03:17, 8 February 2012 (UTC)
- If you're satisfied with generating a vector field, you could just do the vector addition of of fields generated by the positive and negative ends of the dipole. This also assumes you're happy to model the dipole as two point particles some distance apart. But one of the few things I clearly recall from my college electromagnetism course is that there is no elegant formula for for the field about a dipole, although there are elegant approximations to the field once you are significantly further from the dipole than its own length. Someguy1221 (talk) 07:27, 8 February 2012 (UTC)
- My main reason to look for that was to be able to draw the lines of field for a dipole in "graph"(the program I mean), which was not successful... I had tried p orbital cross section before, so I guess I was underestimating the dipole?Is there a way for me to do that?--Irrational number (talk) 03:17, 8 February 2012 (UTC)
- Does Dipole#Field from an electric dipole help? I'm pretty sure the field around a dipole is never going to look elegant in any conventional co-ordinate system since it has an annoying asymmetry in it - this is why for some applications it's best to use [dipole co-ordinates]. Smurrayinchester 18:27, 7 February 2012 (UTC)
Hawking radiation and Black hole
Having re-studied your exhaustive articles on "Black hole" and especially the segment on evaporation, cross checking the article on "Hawking radiation", having studied both the DVD on "Hawking's Universe" as well as the corresponding book, especially his "The Universe in a Nutshell", and having studied Kip Thorne's "Black Holes & Time Warps", I have noted a possible discrepancy between the sources and the published articles in Wikipedia: Stephen Hawking himself noted, especially in his book "The Universe in a Nutshell" that the discussed radiation just LOOKS as if the black hole would emit that radiation, but that this is just a picture where the mathematics happen to describe this specific behaviour of virtual particles near an event horizon, seemingly being radiated away by the black hole.
However, in physical reality, the black hole behaves "as usual" (or remaining "hairless"), especially not emitting any kind of radiation (or virtual particle). The consequence would be that a black hole would never evaporate, at least not via this kind of process.
My suggestion would be to expand your publications along those lines, provided you concur with the a.m findings. 87.184.34.215 (talk) 18:00, 7 February 2012 (UTC) edited for correction of main source 80.132.241.226 (talk) 20:14, 7 February 2012 (UTC)
- I am not aware of any currently-available observational techniques that could detect Hawking radiation, so whether any theory predicts its existence is moot: it's below the noise-floor of what we can currently measure using 2012 equipment. On the other hand, we can measure all sorts of other effects of black holes (rather, relativistically supermassive objects): among these, we have pulsar radiation due to accretion; we have several observational signatures associated with active galactic nuclei (usually postulated to be related to relativistic effects of the massive nucleus), and of course, we have gravitational lensing. In fact, the lack of direct observationm of Hawking radiation is still consistent with theoretical predictions: the theory predicts it should be a magnitude much smaller than these other effects. What this means: every time astronomers look at a distant active galactic nucleus or other massive deep-sky object - and don't see Hawking radiation - they are confirming that our theoretical understanding of general relativity is pretty good. Nimur (talk) 20:12, 7 February 2012 (UTC)
Thanks! As for an observational technique that can detect Hawking radiation, and considering that this radiation could be called a derivative of the Casimir effect, this is well known and established. I do not doubt the existence of virtual particle pairs nor their interaction with a black hole, one of the pair (as a very rare event), not finding a partner to annihilate, falling into the black hole. It is the consequence I'm looking at (please see below, since the second answer deals with this aspect).
- It does not really matter if the Hawking radiation comes from "inside" the black hole, or from "just outside". There are two alternative "simplified to my level" explanations for it - the "virtual particle" explanation, and the "uncertain speed of light" explanation. The fist one depends on virtual particle pairs, one escaping, and one dropping into the black hole. It's hard to understand how that would lead to evaporation, but it does. The reason is that the particle that drops into the hole has a negative total energy (i.e. the negative potential energy it has from materializing just inside the Schwarzschild radius is larger than its positive rest mass/energy). So, by falling into the black hole, it decreases the energy (and, amazingly, by just the energy of the escaping particle, so that the conservation of mass/energy is maintained). The second explanation is that particles in the black hole have to follow the uncertainty principle. Since their location is constrained by the Schwarzschild radius, their impulse has to be uncertain. But stuff falling into a black hole will travel (in the limit) at the speed of light. So, by applying the uncertainty principle, some of the particles will have a slightly higher speed, and can escape. Unsimplifying this to Hawing's or Thorne's level requires a good approximation of the TOE ;-) --Stephan Schulz (talk) 20:42, 7 February 2012 (UTC)
Thanks! However, the concept of negative energy in the realm of quantum electrodynamics is hardly supported, considering eg. the predicted vacuum energy in the order of some 10E113 J/m³. Besides this tremendous energy I have not found any scientific source (or explanation) for a negative energy (which should not be confused with postulates or even predictions). Not to mention classical physics, where either a frequency f or a mass m would need to be negative to maintain the formulae for E=m*c² and E=h*f. But even considering the virtual particle pair to come along with positive and negative energies, uncertainty does not predict more than a 50% probability for one of the particles to be positive or negative. Statistically, the odds should level out with time. So, if any (and looking at the magnitude of the effects), it might be that a black hole remains unaffected by this effect. One option to have a closer look at the process is the LHC. I remember people having been scared that (micro) black holes would be produced in the tests. The formation of such black holes was confirmed by CERN, but nevertheless not presenting any danger to earth. Are informations available on real formation(s) of such black hole(s) and their behaviour? (Still especially looking at some "evaporation".)
- The energy of one of the virtual particles is not negative because of "negative mass", but because of its potential energy. The potential energy is always negative if you are inside any gravity well. But at the Schwarzschild radius, its absolute value is the same as (the energy equivalent of) its rest mass. So if a virtual particle pair gets created on the event horizon, but one particle just inside, the other just outside the black hole, the inside particle has negative total energy simply because of its location. --Stephan Schulz (talk) 21:11, 8 February 2012 (UTC)
Thanks again! However, would this not imply that all matter crossing the event horizon would have negative potential energy, this in turn leading to an even accelerated "evaporation" of a black hole? Without further detailed calculations, and being aware that also the kinetic energy of virtual particles may be negative (as opposed to real matter), I'm quite sure that this problem cannot be solved in quantummechanics.
It may be the other way around, i.e. that real black holes don't exist. Count Iblis (talk) 23:41, 7 February 2012 (UTC)
Thanks - but I would not bet against them, as Stephen Hawking already HAS lost his bet against Kip Thorne on this issue. 87.184.58.106 (talk) 17:57, 8 February 2012 (UTC)
- You're reminding me of fuzzball (string theory), but I think that's something else again... Wnt (talk) 18:58, 8 February 2012 (UTC)
Thanks a lot! This reference (and some further information) might very well solve the problem (of mass being "radiated" away from a black hole), especially since supplementary strings apparently always add to the string ball, forming what is called a black hole without referring to some singularity. I recently started a brane description of black holes, so far being unaware that this has already been done. 80.132.232.112 (talk) 14:01, 9 February 2012 (UTC)
Clenbuterol, doping or food poisoning
If someone {like Alberto Contador eat food contaminated with Clenbuterol, would this Clenbuterol show up in a blood test? 212.170.181.95 (talk) 20:46, 7 February 2012 (UTC)
- Clenbuterol#Use as performance-enhancing drug mentions people testing positive in blood tests in that way, so I guess the answer is "yes". --Tango (talk) 22:36, 7 February 2012 (UTC)
- Perhaps the OP meant to ask, could such excuses be true? After all, they only allege their food had been poisoned. 216.197.66.61 (talk) 23:16, 7 February 2012 (UTC)
- The report on Contador did say that it could be true in theory, but they discounted this explanation because clenbuterol contaminated meat is rarely found in Europe. Count Iblis (talk) 23:34, 7 February 2012 (UTC)
- Perhaps the OP meant to ask, could such excuses be true? After all, they only allege their food had been poisoned. 216.197.66.61 (talk) 23:16, 7 February 2012 (UTC)
- What I thought about this case is that Contador indeed used Clenbuterol, however, he tried to cover it/eliminate it with another substance, which didn't work perfectly, therefore, thorough testing could still find some traces of it. 88.8.79.238 (talk) 23:50, 7 February 2012 (UTC)
- Looking around I found a site "Dopeology", which despite the corny name sounds like it makes an effort to get hard data. [7] It says that the typical dose for an athlete taking the drug is 50-100 micrograms/day (somewhat above the standard treatment dose listed in our article). It says the amount allowed to be in meat in the U.S. and Europe is 100 ng/kg (isn't that just a cheery thought?) and in Mexico and other countries it could be more. And the required detection threshold is 2 ng/ml in urine - but the most sensitive equipment can detect 5-10 pg/ml, and there is no lower threshold. Under these circumstances it is plausible that a person can eat a half kg of meat (50 ng), pee a standard liter of urine, and have a detectable 50 pg/ml of the drug in it. Now the slop for this is only a factor of 10, in which all the processes of metabolism, dilution over time, cleaner meat, more fluids consumed, etc. have to work. But certainly I can picture an innocent running afoul of the test. Wnt (talk) 19:10, 8 February 2012 (UTC)
does gelatin formation in chicken soup start during simmering?
Is there a way to speed it up? Or does it really have to incubate overnight in the fridge to taste better? 216.197.66.61 (talk) 23:05, 7 February 2012 (UTC)
- Gelatin forms when animal connective tissue (collagen and stuff like that) is heated to near boiling in a water solution. Gelatin takes time to form and disolve into the water, so it definately has to simmer for a while. The gelatin also does have to cool to form proper cross-linkages that give that unctuous mouthfeel associated with gelatin; that's why soups and stews and chilis always taste better after being refrigerated overnight and then reheated; the solution has to cool and rest for a while so the gelatin can "set up". Unfortunately, it's not a step that can be rushed or skipped, if gelatin formation is what you are going for. --Jayron32 06:00, 8 February 2012 (UTC)
Not eating before surgery
A family member of mine recently underwent a fairly modest surgery. He was asked not to eat for about a day before the surgery and wasn't fed in the hospital for that period. I've never understood why this is. Surely you want people as strong as possible for surgery, not weakened by a short fast? Thanks, 130.88.172.34 (talk) 23:42, 7 February 2012 (UTC)
- Under general anaesthesia a patient can vomit (or just leak) stomach contents, which they may then breathe (ref). -- Finlay McWalterჷTalk 23:47, 7 February 2012 (UTC)
That clears that up, thanks. 130.88.172.34 (talk) 23:48, 7 February 2012 (UTC)
- Relevant Wikipedia articles: Preoperative fasting, nil per os,pulmonary aspiration, aspiration pneumonia. --Carnildo (talk) 02:15, 8 February 2012 (UTC)
- It is a problem though, especially with diabetics, who really shouldn't fast. StuRat (talk) 02:49, 8 February 2012 (UTC)
- I believe that diabetics have the option of taking glucose tablets to maintain blood sugar while keeping their stomach contents relatively empty. Doctors should know of diabetes and be able to advise on what diabetics can do before going under general anesthesia. --Jayron32 05:58, 8 February 2012 (UTC)
- Yes, and that (along with insulin) should prevent a blood sugar crisis. However, eating nothing but sugar certainly isn't the best thing for a diabetic (or anyone else, for that matter). Diabetics should probably be monitored in a hospital setting during this fasting period, as the chances of a blood sugar crisis are increased by eating glucose tabs alone, since the patient won't be accustomed to the rapid BS spike and collapse they cause. StuRat (talk) 07:04, 8 February 2012 (UTC)
- We seem to be veering in to original research related to medical and surgical procedures. Here is an article by the American Diabetes Association on management of diabetic patients having surgery. It notes measures recommended with the variables of Type 1/Type 2/oral medications/insulin/major surgery/minor surgery. Edison (talk) 21:04, 8 February 2012 (UTC)
You can get all the nutrients you need via a saline drip. There are people whose bowels have been partially removed who permanently get all their nutrients from a saline drip. Some of them haven't tasted food for decades. Count Iblis (talk) 23:54, 8 February 2012 (UTC)
- That seems quite risky, and they are reluctant to do this if any other option exists. StuRat (talk) 00:31, 9 February 2012 (UTC)
- Surely such a drip would have contain a lot more than just salt, which is what "saline" implies. HiLo48 (talk) 20:39, 10 February 2012 (UTC)
- Not really. Most thing administered by IV are either given along with a saline drip or a dextrose drip, to dilute it. Administering undiluted IVs causes problems, and they can't dilute with water alone, as pure water would flow into cells and pop them. StuRat (talk) 00:06, 11 February 2012 (UTC)
February 8
Embryological Skeleto/muscular development
Hello. I've been looking to find out information on whether in a developing human embryo, does the skeleton/bones or the muscle develop first? My understanding is that both are produced simultaneously through differentiation of the mesoderm, but I have surprisingly been unable to find much on this. Any references (preferably online) that would shed light on this matter would be greatly appreciated. 114.77.39.141 (talk) 03:45, 8 February 2012 (UTC)
- Hmmm. Both can emerge from somites, in limb bud development, or from branchial arches. Chondrogenesis involves the condensation of precartilage into cartilaginous structures. Muscle patterning involves a more dynamic process involving founder cells/muscle precursor cells. See [8] for a useful reference. Wnt (talk) 06:26, 8 February 2012 (UTC)
- This reference [9] gives more about tendon development, the physical link between bone and muscle. It seems that bone, tendon, and muscle are independently specified during development from the somite stage onward; but there is cross-talk from bone to tendon and tendon to bone (at least, in the expansion of projections already present) and from muscle to tendon also. In a quick search of OMIM ("enthesis"; "tendon AND insertion") I didn't find any condition where muscles form independently of tendons (and thus perhaps bone), nor have I ever heard of it. Given Nature's fondness for cruel juvenile humor such as the imperforate anus I'm quite surprised really. The authors of the paper I cited remark on the absence of similar mutants in mice (at least for FGF-based mechanisms). But somites, bone, muscle, tendon - these are a large part of the overall vertebrate body plan, and so we should expect the situation to be complicated and well regulated. Wnt (talk) 14:18, 8 February 2012 (UTC)
OP here. Thanks for both responses; however I must admit I am lost by all the medical terminology. Especially with the second article, could someone direct me to where specifically it talks about the developmental stages of skeletons and muscle. 114.77.39.141 (talk) 11:09, 9 February 2012 (UTC)
- That paper focuses on more on tendons, which to me seem a crucial conceptual and physical link; Figure 3 illustrates that at mouse E12.5 the differentiating bone and muscle are separated by a band of tendon progenitors which are still relatively unorganized until E13.5. Note: The "E" notation for mice is less sophisticated than it looks - it just means the number of days since a vaginal plug (evidence of mating) is observed, with the first day called "0.5" because it is assumed they mated sometime during the night. Sometime around day 19-20 birth occurs, but baby mice are even less developed than baby humans (mouse eyes open at 13 days after birth vs. humans in the 6-month fetus; I think epithelial stratification [an equivalent of amphibian metamorphosis] occurs around 3 months in humans vs. E11.5 or so in mice). Wnt (talk) 08:19, 10 February 2012 (UTC)
Brownian motion as perpetual motion
Could Brownian motion be actually considered the perpetual motion in a closed system?--46.204.55.76 (talk) 05:37, 8 February 2012 (UTC)
- If you do, you might as well include planetary orbits, too. StuRat (talk) 05:41, 8 February 2012 (UTC)
- When it comes to energy, there ain't no such thing as a free lunch. See Maxwell's demon and Brownian ratchet. Red Act (talk) 05:46, 8 February 2012 (UTC)
- To make things a bit simpler: Perpetual motion is not an impossibility; there's lots of things in the universe which are essentially in perpetual motion; the molecules in the air around you are in perpetual motion. However you cannot use such motion to do work; any attempt to use a system in perpetual motion to do work will result in the system "winding down", and losing energy until it reaches some state of equilibrium and becomes unable to do more work. --Jayron32 05:50, 8 February 2012 (UTC)
- Except that's not really true. The molecules in the air, or things illustrating Brownian motion in general, still have continual energy inputs, e.g., energy inputs from the sun continually add energy to the molecules in the air in the atmosphere. It's not a closed system operating indefinitely, which is required for perpetual motion. --jjron (talk) 11:53, 8 February 2012 (UTC)
- My understanding of it, building on Jjron's comment: Real-life (as opposed to ideally-modeled) Brownian motion is usually something like the motion of very small particles of say, dust, as have molecules of gas (e.g. air) collide with them. The molecules are only colliding because they are themselves moving — they have kinetic energy in the form of heat. Every time they collide with the dust particle, they lose a little energy. If they were thermodynamically isolated from any additional temperature source, they would eventually expend all of their energy on colliding and it would stop moving. So it's not really perpetual — it runs down, like everything does, if there isn't a source of input energy (e.g. if it is in a closed system). In practice such systems probably never wind down to that stage, I speculate, because the amount of energy needed isn't much, and once you approach temperatures like absolute zero, all sorts of other weird things start to occur... --Mr.98 (talk) 12:51, 8 February 2012 (UTC)
- To be clear, a closed system is one which can exchange energy but not mass with its surroundings; this is in contrast to an isolated system, which can exchange neither energy nor mass. A thermodynamically closed system will exchange energy with its surroundings as long as the system and its surroundings are at different temperatures; if you have a sealed container with a hot beverage in it, heat (energy) will be conducted through the walls of the container until the container (and the coffee inside) reach the same temperature as their surroundings. A thermodynamically isolated system, on the other hand, can't exchange energy with its surroundings. All the parts of the isolated system will reach equilibrium with themselves, but the total amount of energy present and the effective temperature of that system will remain constant. In practice, it's impossible to achieve complete thermodynamic isolation, as energy can even be conveyed across hard vacuum by electromagnetic radiation.
- The closed system will 'wind down' only until it reaches equilibrium with its environment. (Note that this works both ways—a cold can of soda will also absorb energy from its surroundings until equilibrium is reached.) The isolated system, on the other hand, would – in principle – remain at a constant temperature forever.
- Mr. 98's statement about Brownian motion running down is mistaken. While a gas molecule that collides with a dust particle may itself lose some kinetic energy, that energy is transferred to the dust mote, which in turn can transfer energy to another gas molecule in the future. In an isolated system, the total energy (and temperature) of the gas-dust mixture will remain constant forever. (In a merely closed system, the gas-dust mixture will exchange energy with its surroundings until they're both at the same temperature, and then the system will stay at that temperature.) If you wait long enough, you'll find that the highest concentration of dust will be near the bottom of the box and very few dust particles will be near the top, but the dust will never settle completely; there will gradually be an equilibrium established between Brownian mixing and gravitationally-driven settling. (This is a form of sedimentation equilibrium.) TenOfAllTrades(talk) 13:50, 8 February 2012 (UTC)
- I was assuming that the energy would not be exchanged with 100% efficiency and some of it will be converted into a non-useful form, ergo the running down. Is this wrong? --Mr.98 (talk) 14:23, 8 February 2012 (UTC)
- Gas collisions are generally elastic collisions. What form would collisions due to heat energy be converted to? Heat? 216.197.66.61 (talk) 15:41, 8 February 2012 (UTC)
- I'd considered that, but wasn't sure. Good to be corrected! (Though the fourth paragraph of the elastic collision article does put somewhat of a limit on that if we are talking about molecules, no?) --Mr.98 (talk) 17:11, 8 February 2012 (UTC)
- Once you get into situations that are more complex than monatomic gases, then you have to account for energy finding its way into other modes—that is, into things like rotational and vibrational excitation of the molecules. The energy is always conserved, though, and the equipartition theorem even means that at equilibrium each mode stores essentially the same amount of energy. TenOfAllTrades(talk) 19:05, 8 February 2012 (UTC)
- I'd considered that, but wasn't sure. Good to be corrected! (Though the fourth paragraph of the elastic collision article does put somewhat of a limit on that if we are talking about molecules, no?) --Mr.98 (talk) 17:11, 8 February 2012 (UTC)
- Gas collisions are generally elastic collisions. What form would collisions due to heat energy be converted to? Heat? 216.197.66.61 (talk) 15:41, 8 February 2012 (UTC)
- I was assuming that the energy would not be exchanged with 100% efficiency and some of it will be converted into a non-useful form, ergo the running down. Is this wrong? --Mr.98 (talk) 14:23, 8 February 2012 (UTC)
Sun leaving main sequence between 1 and 5 billion years from now
How much bigger suppose the sun to get between 1 and 5 billion years from now. Our article said 10% luminosity in 1.1 Gyr, 3.5 Gyr is 40%. What is 10%, 20% mean by growing luminosity. Does sun get 10 times larger in size, every billion years, 20 times larger in size every billion years. Is sun's RGB 250xs current radius 5000 times more luminosity it means 5000 times brighter, or 2000 times brighter. If 5000 times brighter means habitable zone surface temperature everywhere in our solar system is too hot,even 100 AUs away. 2000 times brighter is habitable zone at Saturn system, Neptune system, or Pluto system? I am all messed up confused right now.--69.229.39.25 (talk) 06:06, 8 February 2012 (UTC)
- I notice that neither this nor your previous query further up the page have yet been answered. To be honest, I started to yesterday, but then gave up because I found both queries too confusing. Each seems to have several intertwined questions, which makes the task of answering more daunting. Also, both are written in English sufficiently imperfect as to be difficult to understand. You might want to think a bit about exactly what you want to know, and then write one or more simpler questions, clearly separated. In the meantime, Section 5 'Life cycle' within our article Sun includes a graph which you might find helpful, though the concept of effective temperature is not wholly straightforward.
- To address what I can: the Sun's radius will only increase to about 110% of its current value in 1 billion years from now, and to about 130% of today's value in 5 billion years from now. I'm sure that you can work out how much that will increase its surface area, which is the main cause of its gradually increasing luminosity during the main sequence part of its lifetime, during which its surface temperature will remain roughly the same. Such modest changes in size are of course trivial compared to the distance of the planets from the Sun – for example, the Earth orbits the Sun at a distance of about 214 times (21400% of) the Sun's current radius.
- When the Sun, now with a little less mass after 5 billion more years of fusion, leaves the main sequence and becomes a Red giant, it will indeed grow to a size comparable to the size of the Earth's orbit (and will for this reason will become more luminous), but its surface temperature will also drop (lessening the increase in luminosity a little bit). Since the changes in mass and so on associated with these processes will, as you have already learned, cause changes to the orbits of the planets, it's difficult to calculate exactly where everything will end up, and I'm not familiar with the latest theories. Where the Habitable zone will be and whether any terrestrial-type planets will be in it depends partly on how you interpret that term: consider also that some moons of Jupiter and Saturn, for example are now thought potentially life-bearing right now, for reasons independent of Solar heating.
- Hope this helps a little. {The poster formerly known as 87.81.230.195} 90.197.66.165 (talk) 00:32, 9 February 2012 (UTC)
nerves to size depiction chart required
I am trying to find a chart of the human body which depicts skin area size in proportion to the richness of nerve cells in that area. Thus, for example, eyes, lips, hands and feet are shown much larger than areas such as the forearms and thighs. I remember that genitals are not depicted at all, as the testes, for example, would be the size of cannonballs! Myles325a (talk) 10:58, 8 February 2012 (UTC)
- I know the picture you mean, although in the version I recall the genitals were depicted, which is what made it so striking! The closest I have been able to find so far is the cortical homunculus, but I realize that's not quite what you're looking for.--Shantavira|feed me 11:50, 8 February 2012 (UTC)
- As for the number of nerves in the genitals, it's not particularly high. Those nerves can trigger pleasure without being very numerous. More nerves are present where needed, such as the hands, for delicate work with tools, and the lips and tongue, for separating inedible things like pits and bone fragments from our food. StuRat (talk) 20:00, 8 February 2012 (UTC)
Op myles325a back live. Thanks guys. It's called a Motor / Sensory Homonculus. I needed it for an essay I'm writing. Oh, and StuRat is probably right about the genitals, leastways for MOST guys that is. My weiner is prehensile. Myles325a (talk) 02:47, 9 February 2012 (UTC)
- Well I've lived on this earth for over 50 years and I've never seen or heard of a penis that can wind itself round a branch of a tree and hold on... --TammyMoet (talk) 10:09, 9 February 2012 (UTC)
- Well, now you have: Hectocotylus. StuRat (talk) 05:56, 10 February 2012 (UTC)
Unusual fungus
I took this photo today at a park in Byron Bay, NSW, Australia. I'm wondering what type of fungus it is. http://tinypic.com/r/33ur9ft/5 180.181.84.225 (talk) 12:34, 8 February 2012 (UTC)
- In a very broad sense, this looks like a type of fungus called a Puffball. Beyond that, however, we'll have to wait for a proper mycologist to come and identify which type of puffball. --Jayron32 14:52, 8 February 2012 (UTC)
- I'm no mycologist, but it looks pretty similar to Lycoperdon perlatum. If you upload the shot to Mushroom Observer, you're likely to get a better ID. SmartSE (talk) 15:05, 8 February 2012 (UTC)
- It's clearly this type of mushroom, which is labeled as an amanita, species not specified. Looie496 (talk) 02:16, 9 February 2012 (UTC)
- Oh, wait, that's your picture, isn't it? Heh. Looie496 (talk) 02:19, 9 February 2012 (UTC)
- Do you mind?! I don't resemble that fungus in the slightest, 'cepting I'm white.Myles325a (talk) 02:51, 9 February 2012 (UTC)
about electrical plug
why in a electrical plug, the earthing terminal is more thicker and longer than other two terminals? — Preceding unsigned comment added by 125.19.211.130 (talk) 13:00, 8 February 2012 (UTC)
- So that it will fit in the socket. 180.181.84.225 (talk) 13:04, 8 February 2012 (UTC)
- ...in only one direction. --184.100.88.44 (talk) 03:07, 9 February 2012 (UTC)
- It longer because if an electrical lead is over tensioned and pulls away from the terminals, the last conductor you want to go open circuit is the earth. It is thicker, because one wants the earth to have the lowest residence and so a thicker and longer conductor gives you that.--Aspro (talk) 13:13, 8 February 2012 (UTC)

- Can I take it your are talking about this type of plug. Both ensure that on insertion into the socket the equipment is earthed before any current can flow and that the power circuit is disconnected before earth continuity is disconnected on extraction of said plug.--Aspro (talk) 13:34, 8 February 2012 (UTC)
- And on UK plug sockets, at least, the longer prong of the earth makes contact with a simple mechanism which withdraws covers from the live & neutral terminal holes. Absent the earth prong, the live and neutral remain covered and thus pretty much inaccessible. --Tagishsimon (talk) 13:37, 8 February 2012 (UTC)
- Think the OP is referring not to the UK but the old UK BS 546 standard which still in existence in many parts of the world that the US Empire din'nt reach. Perhaps he can come back and elucidate.--Aspro (talk) 14:20, 8 February 2012 (UTC)
- There could be all sorts of "mechanical" explanations for various modern plugs, but I think at the basic level Aspro has it right in saying that "one wants the earth to have the lowest residence and so a thicker and longer conductor gives you that." HiLo48 (talk) 20:35, 10 February 2012 (UTC)
Earth spin redux
sir, What will happen if the speed of rotation of earth about its own axis is doubled. The distance between the sun and earth will increase or decrease? — Preceding unsigned comment added by Tobyaickara (talk • contribs) 13:39, 8 February 2012 (UTC)
- See the answers where you asked this question previously. — Lomn 13:50, 8 February 2012 (UTC)
Violating the 2nd law of thermodynamics leading to violating the 1st law?
Is this true that if you can violate the 2nd law of thermodynamics, it will eventually lead to violating the 1st law?
Please tell me if this is a valid example.
You have a river flowing downstream with a turbine extracting energy from it that is 100% efficient. You then have some mechanism that brings the water downstream to the top of the river that is 100% efficient at moving that water to the top in a closed system. The turbine and the mechanism are both examples of violating the 2nd law of thermodynamics. Extracting energy from the river with the turbine is essentially extracting energy from nothing thus violating the 1st law.
Correct or incorrect? ScienceApe (talk) 16:34, 8 February 2012 (UTC)
- I think a properly implemented Maxwell's Demon should violate only the second, provided that the energy it extracts comes out of the hot or cold reservoirs it creates. Wnt (talk) 18:02, 8 February 2012 (UTC)
- Speaking of which, someone scroll down that article around where it mentions Atomoscience and tell me if your BSometer is in the red also. Wnt (talk) 18:06, 8 February 2012 (UTC)
- I don't think it is "BS" (the main researcher seems to be a reputable academic), I just think it is a very new field that isn't notable at this stage. Therefore, I have PRODed the article. --Tango (talk) 20:00, 8 February 2012 (UTC)
- Actually I meant the Maxwell's Demon article, which has a section "In 2005, Raizen and collaborators showed how to realize Maxwell's Demon for an ensemble of dilute gas-phase atoms or molecules. The new concept is a one-way wall for atoms or molecules that allows them move in one direction, but not go back. The operation of the one-way wall relies on an irreversible atomic and molecular process of absorption of a photon at a specific wavelength, followed by spontaneous emission to a different internal state. The irreversible process is coupled to a conservative force created by magnetic fields and/or light. Raizen and collaborators proposed to use the one-way wall in order to reduce the entropy of an ensemble of atoms." On a closer look I think this is legit, but just sounds like something unlikely. I think the "prod" is unfortunate, though you might have it on the merits. Wnt (talk) 20:40, 8 February 2012 (UTC)
- I don't think it is "BS" (the main researcher seems to be a reputable academic), I just think it is a very new field that isn't notable at this stage. Therefore, I have PRODed the article. --Tango (talk) 20:00, 8 February 2012 (UTC)
- In your scenario, the first law isn't being violated as long as the only thing the turbine powers is the pump. But if some of the turbine's power is used to power something else in addition to the pump, and the system magically didn't slow down to a stop, that would be a violation of the first law. Red Act (talk) 19:10, 8 February 2012 (UTC)
- The second law of thermodynamics is a statistical result about large systems of particles that are all behaving according to more fundamental laws of physics. As long as the particles are following the more fundamental laws, then they'll probably obey the second law of thermodynamics. In a hypothetical scenario where the second law is violated, either no laws of physics are violated and you're just proposing impossibly good luck, or something more fundamental is breaking. If the scenario is constructed by breaking conservation of energy, then the first law of thermodynamics is also being broken, but there are plenty of other ways you could decide to hypothetically break physics. Rckrone (talk) 04:02, 9 February 2012 (UTC)
the role of hormones
- What are the specific roles of hormones in spermatogenesis in a human male?
- How can the following processes give rise to genetic variation? -fertilization and -vegetative reproduction?
- Which type of organism will exhibit the greatest genetic variability and why? -Asexually reproducing organisms and Self-fertilizing organisms — Preceding unsigned comment added by 41.205.4.87 (talk) 18:03, 8 February 2012 (UTC)
- Pardon, but this sounds like a homework question, and if so people here prefer to have students work through them on their own, at least until they get stumped and have a question of their own to ask. I've taken the liberty of adding a few Wikilinks to your question above - tell us where they fall short. Wnt (talk) 18:09, 8 February 2012 (UTC)
asexual reproduction
one way of asexual reproduction is by vegetative propagation; this implies the offspring are gotten from the parent, according to me, there should be no genetic variation between the parent and the offspring and i am unable to find how that is possible. Any contribution will be of great help!! — Preceding unsigned comment added by 41.205.4.87 (talk) 18:35, 8 February 2012 (UTC)
- When a plant undergoes vegetative reproduction, you are correct, there is no significant genetic variation, and plants that depend on vegetative reproduction (e.g. strawberries and bamboo) often form clonal colonies. As for "how that is possible", we can get into what physiological traits allow for such reproduction if you like (e.g. not all plants can be grown from cuttings), but the basic idea is that, if it's possible for a certain species, then the vegetatively grown "individual" must be genetically identical to the parent, because there has been no introduction of new genetic material, and no chance for recombination. There are a few edge cases. Some plants (e.g. spider plants) naturally form chimeras, and a cutting my only have some of the genotypes present. If your question is more about evolution in a clonal organism, note that very few things solely reproduce in this manner, and usually there is some opportunity for sexual reproduction. Fun fact resulting from clonal reproduction: every granny smith or red delicious apple you've ever eaten has come from a single individual. The same genetic material is maintained by cuttings and grafting :) SemanticMantis (talk) 19:00, 8 February 2012 (UTC)
- (edit conflict) Vegetative reproduction, like many types of asexual reproduction, is basically a form of cloning, or just growth (for some plants). You're right that it doesn't add genetic variation. It's never the exclusive form of reproduction — it's something that happens under specific circumstances, often stressful ones where sexual reproduction would be riskier. There is usually (always?) some other form of reproduction that introduces in genetic variation, as your reasoning would lead you to conclude. Usually when we talk about vegetative reproduction is in the context of artificial reproduction, when humans are the ones doing the cloning, and have purposefully limited sexual reproduction (with seedless varieties being the ultimate extension of this — plants which cannot self-reproduce without being cloned or grafted). --Mr.98 (talk) 19:05, 8 February 2012 (UTC)
- Except that reasoning is backwards. The general wisdom, and observation, is that organisms capable of both sexual and asexual reproduction utilize the asexual variant unless they are under stress. This is certainly the case for hermaphroditic nematodes and some species of rotifer. Vertebrates capable of asexual reproduction typically do so only when there are no mates available, at least in the case of parthenogenic lizards. Someguy1221 (talk) 22:49, 8 February 2012 (UTC)
- Ah, you are right. How interesting. --Mr.98 (talk) 23:42, 8 February 2012 (UTC)
- Except that reasoning is backwards. The general wisdom, and observation, is that organisms capable of both sexual and asexual reproduction utilize the asexual variant unless they are under stress. This is certainly the case for hermaphroditic nematodes and some species of rotifer. Vertebrates capable of asexual reproduction typically do so only when there are no mates available, at least in the case of parthenogenic lizards. Someguy1221 (talk) 22:49, 8 February 2012 (UTC)
- And now that you mention rotifers, the Bdelloidea are certainly an edge case. All individuals are female, and they only reproduce partenogenetically. The closest they ever come to sex is incorporating random DNA when they are reanimated from a dessicated state o.O SemanticMantis (talk) 01:45, 9 February 2012 (UTC)
- That is some crazy sh*t. --Mr.98 (talk) 15:16, 9 February 2012 (UTC)
- And now that you mention rotifers, the Bdelloidea are certainly an edge case. All individuals are female, and they only reproduce partenogenetically. The closest they ever come to sex is incorporating random DNA when they are reanimated from a dessicated state o.O SemanticMantis (talk) 01:45, 9 February 2012 (UTC)
- Also, mutation may come into play here. Let's say one cell mutates in a way that allows it to better retain water than the others. This cell is more likely to survive and reproduce than the others during dry periods. Over time, more of the plant will be composed of such cells, and more of the new individuals that survive will be, as well. Combine this with organisms being able to select their own rate of mutation (by only having one copy of a gene or many copies, for example, or exposing the DNA to UV in sunlight), and you get a viable form of reproduction with the mutation rate fairly high. StuRat (talk) 19:53, 8 February 2012 (UTC)
- I really doubt individual cell mutation has too much to do with it in complex multicellular organisms. I think you overestimate how important any individual cell can be in a positive sense. --Mr.98 (talk) 23:44, 8 February 2012 (UTC)
Plutonium inventory
Is there a good estimate on how much plutonium was produced during the runtime of all nuclear power plants since the 1940s? --Stone (talk) 20:12, 8 February 2012 (UTC)
- Do you mean civilian, military, or both? Do you mean plutonium that was separated or are you including plutonium that remains in a form of waste? There are fairly nice numbers for worldwide stocks of military plutonium that was separated. There are some hand-wavy figures for plutonium content in wastes (which could be separated, but isn't). Civilian-generated plutonium is only separated in reprocessing plants, in some countries, and very careful inventories of those exist. Depending on what you're asking for specifically there are more or less better estimates, but all of it is complicated and with heavy qualifications. (e.g.) The ranges I have seen for separated plutonium are between 300-500 tonnes. I've no idea about unseparated, but it's quite large too. --Mr.98 (talk) 23:40, 8 February 2012 (UTC)
- Thanks! This is exactly what I was searching for! Perfect!--Stone (talk) 18:34, 9 February 2012 (UTC)
How long does ice in the Antarctic ice sheet last
Antarctic ice sheet article states that the ice sheets began 45-34 mya. Have they mostly covered Antartica continuously since this time? (Any evidence for major periods of decline?) Also how long does it take for ice to flow from the time it was originally deposited in the interior to the time it breaks up in the ocean. How old is the oldest ice to be found there? SkyMachine (++) 22:14, 8 February 2012 (UTC)
- I believe most of the ice only lasts for a small fraction of that period. That is, it flows to the sea and melts and is replaced by new ice. However, there may be spots where it is prevented from flowing to the sea, such as in a volcanic caldera, where the ice may be much older. StuRat (talk) 23:03, 8 February 2012 (UTC)
- Indeed, even the lowest portions of the oldest ice cores are believed to be less than 1 million years old. Someguy1221 (talk) 23:09, 8 February 2012 (UTC)
- Some of the oldest ice on the continent is believed to come from the Antarctic dry valleys, where claims of ice (usually mixed with soil) up to 8 million years in age have been made. Most of the ice on the continent is far younger though as the large ice sheets slowly flow towards the oceans and carry much of the oldest ice out to sea. The oldest reliably dated ice from ice cores on the ice sheet are about 800000 years old. There is speculation that some of the ice at the base of the ice sheet is older, perhaps 1.2 million, but basal ice tends to be highly disturbed by turbulent flow and/or basal melting, which makes it very difficult to date reliably. Dragons flight (talk) 21:05, 9 February 2012 (UTC)
Brain and learning
Is there a certain part of the brain responsible for human capability of learning? If so, does this part of the brain also affect other things? Thanks! 64.229.180.189 (talk) 23:47, 8 February 2012 (UTC)
- More than one part of the brain, since there's more than one type of learning. Learning how to walk, for example, is quite distinct from learning the capital of North Dakota. Perhaps the better question would be if there are parts of the brain which don't learn, that is, which operate on instinct alone. I believe so, such as the brain stem, which regulates breathing and heartbeats, neither of which needs to be learned. Portions related to the senses are a mixed bag, as the parts that process the images, smells, sounds, etc., initially don't require learning, but the associations with events, objects, dangers, etc., do. StuRat (talk) 00:17, 9 February 2012 (UTC)
- See long term potentiation for a major variety of learning; see the hippocampus for a major seat of memory. Wnt (talk) 15:00, 9 February 2012 (UTC)
February 9
Ionic gases
When an ionic compound is vaporised, does it form individual molecules or does it separate into its component ions? Whoop whoop pull up Bitching Betty | Averted crashes 01:24, 9 February 2012 (UTC)
- Stays as molecules, like water vapor. StuRat (talk) 01:26, 9 February 2012 (UTC)
- Water is covalently bonded. Whoop whoop pull up Bitching Betty | Averted crashes 03:30, 9 February 2012 (UTC)
- Just to correct Stu, in the solid phase, there is no such thing as an ionic molecule. Sodium chloride doesn't form molecules in the solid phase. In something like Ionic liquids, the situation is complex, but roughly speaking the individual ions are free moving, though they can group up in molecule-like structures. Ionic gases form ion pairs or complexes which behave kinda like molecules, near as I can find from a quick literature review. Such clusters are called "molecules", except that they are held together purely by the electrostatic interaction of the positive and negative ions. See this paper from 1951 which gives an overview. If heated enough, such an ionic gas may form a plasma as the individual ions are seperated. --Jayron32 04:44, 9 February 2012 (UTC)
- Did I say anything about the solid phase ? I just said that ionic particles stay bonded, just like water vapor (which is covalently bonded) does. And, of course, I was assuming conditions short of those needed to form a plasma. StuRat (talk) 04:53, 9 February 2012 (UTC)
- In sodium chloride gas, there are weakly bonded polar molecules. This gas consists of a mixture Na, NaCl, Na
2, Na
2Cl, Cl, NaCl
2 and a variety of other shortlived species. Upon collision, individual molecules always exchange partners. Plasmic Physics (talk) 01:01, 10 February 2012 (UTC)
- In sodium chloride gas, there are weakly bonded polar molecules. This gas consists of a mixture Na, NaCl, Na
The percentage of DNA sharing in Monozygotic twins
what is the "exact" percentage? (if known), and, could be variants? thanks. — Preceding unsigned comment added by 81.218.145.251 (talk) 03:43, 9 February 2012 (UTC)
- Almost 100%. At least a handful of mutations will inevitably occur prior to the splitting of the embryo. Even if the split occurred at the two cell stage, one would expect genetic variance of 1 part per billion (99.9999999% identicalness, if that's a word). In practice, copy number variation has been observed between "identical" twins, as well as variation in DNA methylation patterns. Someguy1221 (talk) 03:52, 9 February 2012 (UTC)
Could someone please draw up a schematic of an obvicopter?
Or link one if it already exists?
Some Californian Hispanic dude named "Juan Carlos" drops the term "obvicopters" occasionally - "Can't hear, obvicopters too loud," "...it is ##obvicopters," etc. Another user around him stated that their engine sound would sound slightly different from the helicopters'.
How would an obvicopter be built differently? Is it, perhaps, the mystery jet-run tilt-rotor craft seen in the film, I, Robot?
Apparently it's a concept helicopter that isn't in full production yet, but Google seems to prefer to keep it under wraps. Perhaps under government order to keep it classified...
Or it's not forthcoming for other reasons. Anyway, what could you dig up about this? --70.179.174.101 (talk) 11:47, 9 February 2012 (UTC)
- It seems fairly obvious you're referring to Meta:Foundation wiki feedback/Archive/2011#Talk:Staff, Meta:IRC office hours/Office hours 2011-02-11, [10] (connection between names apparently self disclosed on publicly logged IRC channels Meta:IRC office hours/Office hours 2012-01-20) so why don't you just ask user:killiondude rather then coming up with another random wacky conspiracy theory? Seems fairly likely it's a joke, although it wouldn't surprise me if the joke continues if you indicate you think it's otherwise as done so here. Nil Einne (talk) 14:48, 9 February 2012 (UTC)
- Guessing, based on Nil Einne's provided information: "Obvi-" looks to be "obvious". "-copter" is a throwaway. The statement is a jesting reply to a correction: "I can pretend I didn't hear that, so I can pretend I wasn't wrong / didn't make that mistake / have eleventy billion dollars." Should killiondude appear and clarify, the obvicopters will no doubt be flying low over my area. — Lomn 15:21, 9 February 2012 (UTC)
- It's probably derived somewhat from roflcopter, an undoubtedly hilarious internet meme of times gone by. - Cucumber Mike (talk) 16:09, 9 February 2012 (UTC)
- Guessing, based on Nil Einne's provided information: "Obvi-" looks to be "obvious". "-copter" is a throwaway. The statement is a jesting reply to a correction: "I can pretend I didn't hear that, so I can pretend I wasn't wrong / didn't make that mistake / have eleventy billion dollars." Should killiondude appear and clarify, the obvicopters will no doubt be flying low over my area. — Lomn 15:21, 9 February 2012 (UTC)
- Is his accent that thick or do you mean Ornithopter? (Juan's email address is also Ornithopterman@ ------.com). Sound-wise, if a helicopter goes thwhaat-thwaat-thwaat then a ornithopter goes more like whaap-whaap-whaap-whaap. The air flowing over the rotor blades of a heli is accelerated to much higher speeds, hence the sharper attack and decay of the beats. The engines should not (as far as I can make out) sound any different. Maybe he is just talking simply. --Aspro (talk) 21:11, 9 February 2012 (UTC)
I see the Reference Desk continues its celebrated tradition of indulging in trolls. It's just a meme that I did not initiate but did perpetuate. Killiondude (talk) 22:57, 9 February 2012 (UTC)
Do permanent magnets generate heat at all?
I had someone argue with me that if you use a permanent magnet to "launch" a projectile (in space), it would not generate any heat. I'm not entirely sure what the hell he was talking about, but I wanted to confirm if his claim was true or not. ScienceApe (talk) 13:31, 9 February 2012 (UTC)
- Magnets are not magical physics-violating devices, and byproduct heat results from all useful work, per the second law of thermodynamics. — Lomn 14:30, 9 February 2012 (UTC)
- Launching a projectile into space with a permanent magnet would be quite a feat, unless you live on an asteroid. But there should be some heat from eddy currents in any changing magnetic field. Wnt (talk) 14:58, 9 February 2012 (UTC)
- That's what I thought, thanks. ScienceApe (talk) 21:27, 9 February 2012 (UTC)
question about plutonium
I've seen pictures on the Internet of workers holding objects such as the plutonium buttons and working directly in front of weapons grade plutonium. How is this possible because during Chernobyl people would die from radiation exposure just from looking briefly at the plutonium rods. --208.83.61.116 (talk) 17:43, 9 February 2012 (UTC)
- Plutonium-238 decays by alpha decay. Alpha particles are blocked by very little material (see alpha decay#Toxicity), so it's pretty safe to handle (when it's in a big block; you wouldn't want to be breathing or drinking this stuff). Other radionuclides decay by gamma emission, which is very penetrative. Used nuclear fuel elements, such as one would find in an burning reactor, contain a variety of daughter products, of which plutonium is one of the lesser hazards. -- Finlay McWalterჷTalk 18:14, 9 February 2012 (UTC)
- You may find Chernobyl#Radioactive release useful. It says, among other things: "While the general population often perceives plutonium as a particularly dangerous nuclear fuel, its effects are almost eclipsed by those of its fission products." --Tango (talk) 18:47, 9 February 2012 (UTC)
- The fuel rods used uranium, not plutonium, although there was plutonium present as a consequence of the nuclear reactions. As others point out, irradiated fuel rods are dangerous to directly observe (through air - water of sufficient depth is a good shield) due to radiation created by decay products, not because of inherent danger posed by uranium or plutonium, which can be handled with due precaution. I believe Richard Rhodes described a piece of plutonium in The Making of the Atomic Bomb that when handled (with gloves) was warm, rather like a live rabbit. Short exposure, and avoidance of ingestion, is not particularly perilous. Acroterion (talk) 18:55, 9 February 2012 (UTC)
- I suspect that in almost all cases of the photos of people handling plutonium, the people are working with glove boxes. (E.g. This guy.) That means they're behind glass. I've never seen any where there wasn't a glovebox, anyway. or some kind of containment (e.g. even just a plastic bag, much less more complicated shielding devices).
- But in any event, the main exposure-based health hazard from plutonium is through inhalation. Most plutonium in the real world, if not in a glove box, is either coated in something or is not handled except with gloves and by people trained to handle it. It's not particularly radioactive in and of itself, compared with the sorts of things that are inside of a working nuclear reactor (where the problem are the fission products, which are highly radioactive). But still, people are on the whole pretty careful in how they handle plutonium, though if you aren't aware that these photos are generally taken in glove boxes, I could see you thinking people were being rather cavalier about it. --Mr.98 (talk) 01:42, 10 February 2012 (UTC)
- About Chernobyl, people did not fall over dead from looking at rods. 31 people died in the three months after the event - mainly from radiation from breathing the smoke. See also Acute radiation syndrome. 75.41.110.200 (talk) 05:06, 10 February 2012 (UTC)
- Though physical proximity to unshielded control rods does expose one to a lot of radiation, even without the added issue of inhaling radioactive particles. But yes, "looking at" states that poorly. --Mr.98 (talk) 13:58, 10 February 2012 (UTC)
Human hair
Does an adult's body need some DHL for living healthily? If I would cut a hair off completely(even under the skin without the root), how much time it will take for it to grow up beyoned the scalp? — Preceding unsigned comment added by 87.68.222.18 (talk) 21:29, 9 February 2012 (UTC)
- I'm guessing you don't mean the parcel company, but that's all Google, Wikipedia and Wiktionary can find with that acronym... --Tango (talk) 22:32, 9 February 2012 (UTC)
- Nothing that acronymfinder.com brings up seems appropriate, though I am intrigued by "Dynamic Head Loading". Anybody know what that is? Clarityfiend (talk) 22:43, 9 February 2012 (UTC)
- A pubmed search for "DHL nutrition" gives one, irrelevant hit (with DHL being part of the name of a cell line). Human hair grows by about 1.25 cm per month, see Human hair growth. --NorwegianBlue talk 23:16, 9 February 2012 (UTC)
- Is it possible the OP means Docosahexaenoic acid (DHA) and is remembering wrong? DHA seems to be a common supplement and is evidentally sometimes marketed to reduce hair loss, although it's not clear to me that OP was suggesting 'DHL' was needed for anything related to hair anyway. Nil Einne (talk) 23:37, 9 February 2012 (UTC)
- Or maybe they mean DHT (dihydrotestosterone) ? StuRat (talk) 05:48, 10 February 2012 (UTC)
- I thought of that but it's definitely not something many people should be taking even if they have it naturally present. Nil Einne (talk) 09:40, 10 February 2012 (UTC)
- But they didn't ask if they should take it, only if the human body needs it. StuRat (talk) 19:18, 10 February 2012 (UTC)
February 10
Meter definition
i read that the definition of metre is "The metre is the length of the path traveled by light in vacuum during a time interval of 1⁄299792458 of a second." Why not define metre as 1/300,000,000 of a second so that speed of light will be exactly 300,000,000m/s? MahAdik usap 01:05, 10 February 2012 (UTC)
- Because, as Metre#Meridional definition states, it was not defined in terms of the speed of light originally, but rather as "one ten-millionth of the length of the Earth's meridian along a quadrant". It's a little too late to go changing it now. Clarityfiend (talk) 01:13, 10 February 2012 (UTC)
- (ec) Because the meter (and the second, and the gram) already had a widely-implemented physical meaning prior to defining it in terms of the speed of light. Switching its size capriciously would create untold havoc and inconvenience for very little gain. Instead, the speed of light definition ties an existing value to a physical constant. — Lomn 01:14, 10 February 2012 (UTC)
- The key issue is that, while 300 000 000 m/s and 299 792 458 m/s only differ by less than one part in a thousand, when the definition of a meter was set to the 1⁄299792458 of a second definition in 1983, people were already using the meter, and were able to accurately measure distances with much greater accuracy than one part in a thousand. (Which isn't actually that hard, as the difference between the two meters is about 0.7 (current) mm - a difference that's visible to the naked eye.) While ponderously slow about it, the international metrology community is perfectly willing to redefine units (see New SI definitions) however, the key consideration in all such changes is that the old and new units must match up to within the error for even the most accurate measurements. That is, if something measured 10245.6±0.3 mm prior to the redefinition, it should measure 10245.6±0.3 mm after the definition. To do otherwise invites confusion as you'd constantly have to check the publication date of books, references, etc. to see if they were measuring things with the old meter or the new meter. -- 67.40.215.173 (talk) 17:03, 10 February 2012 (UTC)
BOMA
Crossword Clue : Anaconda Answer Given. : Boma.
Nowhere can I find any mention of, or reference to, the word BOMA in any capacity much less to connect with Anacona. Can you help me - I hate words I cannot explain. My, hopeful, thanks - J. Mumvonston (talk) 02:40, 10 February 2012 (UTC)
- Googling for those two terms finds many hits. The first two are "boma" in a Scrabble dictionary and a work by Richard Price (American anthropologist) about explorers in Suriname. Each one gives them as synonymous or a cross-breed or more specific type or something like that. DMacks (talk) 02:48, 10 February 2012 (UTC)
- The OED has it: bom or boma - "The name in Congo, W. Africa, of ‘a huge non-poisonous snake swallowing deer, etc.’ (see Merolla, Vocab.; Proyart; Cavazzi Congo, Matamba, & Angola; Magyar Süd-Afrika). Apparently carried by the Portuguese from Congo to Brazil (Roquete has bom bôma ‘serpent d'Angola et du Brésil’), and there applied to the largest boas, in which sense it appears in some English works. (The history has been traced for us by Dr. E. B. Tylor.)" (Oops, forgot to sign.)--Rallette (talk) 08:31, 10 February 2012 (UTC)
May I know how to distinguish colour coding for 5 bands described by this article from the same colour coding used by some commercial vendors such as [11].--Almuhammedi (talk) 08:35, 10 February 2012 (UTC)
- As far as I can tell, that coding schemes are completely compatible. The second one simply lists extra colors for the fifth band that aren't even used in military applications. (Unless the US military color-coding chart I found is simply incomplete.) Someguy1221 (talk) 10:09, 10 February 2012 (UTC)
Permission
Dear Sirs My colleague Rex Palmer and I are preparing a 5th edition of "Structure Determination by X-ray Crystallography". We would be grateful for permission to reproduce the top right-hand two Wikipedia figures that appear under "Penrose Tiling!. Yours sincerely Mark Ladd & pp Rex Palmer — Preceding unsigned comment added by Kramdaal (talk • contribs) 10:13, 10 February 2012 (UTC)
- The article in question is Penrose tiling. Do you mean the two pictures - File:Penrose Tiling (Rhombi).svg and File:RogerPenroseTileTAMU2010.jpg? Wikipedia doesn't own those pictures, and so can't grant you anything. The diagram is in the public domain, but the photo was uploaded by Solarflare100. That was Solarflare100's only contribution, so I know of no way to contact them. -- Finlay McWalterჷTalk 10:25, 10 February 2012 (UTC)
- However Wikipedia:Reusing Wikipedia content and Commons:Commons:Reusing content outside Wikimedia should provide you some idea how you may be able to reuse the content without further permission of the copyright holder. Bear in mind this is not intended to be legal advice and we cannot guarantee the copyright situation of either image is as suggested in the file description. Nil Einne (talk) 15:49, 10 February 2012 (UTC)
- You should probably discuss with your publisher about what licensing rules they have. The licenses on the two images in question are clearly marked: this one was released into the public domain by its copyright holder, and this one is licensed for free re-use under the Creative Commons "Attribution 3.0 Unported (CC BY 3.0)" license. Your publisher will probably know whether such content is acceptable for their uses. Nimur (talk) 19:19, 10 February 2012 (UTC)
UV light from sun
Our article on vitamin D says that humans only make vitamin D when the UV index is above 3. Assuming someone standing directly in the sun with no cloud cover, roughly what angle of the sun with the horizon is that equivalent to? And is there a website that you can stick your location or even just latitude in, and it tells you what hours and days the sun is above that angle? I assume such things must be possible, because our articles seem to talk about different places having strong enough sunlight for given percentages of the year. 86.163.211.160 (talk) 14:06, 10 February 2012 (UTC)
- You do know that you can get vitamin D from vitamin pills or foods and beverages supplemented with it, right ? Considering the damage UV causes to the skin, this is the safer approach. StuRat (talk) 19:13, 10 February 2012 (UTC)
- Apparently sunscreen or other sunlight denial has not proved itself as an unmixed blessing. (See PMID 8475009) It should be very surprising to me if humans truly suffer from an appropriate level of exposure to the Earth's sun. Wnt (talk) 20:45, 10 February 2012 (UTC)
- A couple questions about that study:
- 1) Did they eliminate vitamin D deficiency by comparing those with sunlight exposure with those taking vitamin D ?
- 2) People who get more sunlight exposure may also get more exercise (the outdoorsy type of people). Did they control for this variable ? StuRat (talk) 20:55, 10 February 2012 (UTC)
I'm not especially interested in what various unqualified people think about appropriate sources of vitamin D (this, and the dangers of sunlight, attracts a lot of crank views for some reason, and I don't really want to play that game). I'm really interested in being able to look at when there is strong enough sunlight to make vitamin D, in different parts of the world. I assume such a thing is achievable, and given the various interests it seem like something that would be a widget on a website somewhere. Is this so? 86.163.211.160 (talk) 23:06, 10 February 2012 (UTC)
- I won't offer an unqualified opinion on the Solar altitude, but will mention that air quality may also be an important factor: even on a cloud-free day, the degree of various pollutants in the air may, I suspect, greatly influence how much UV gets to the ground. {The poster formerly known as 87.81.230.195} 90.197.66.183 (talk) 00:48, 11 February 2012 (UTC)
Such a widget to compute the U index is undoubtably possible. Its really just the same as what experts have come up with for the photo-voltaic (solar panels) industry to predict power output of solar panels. But I doubt that it would be any practical value. Vitamin research is notoriously shakey, and requirements will vary from race to race and person to person. I've never seen it cited anywhere what area of skin is required to be exposed to maintain a given vitamin level at a given UV index, and that is surely a valid and important question. Keit120.145.158.234 (talk) 02:59, 11 February 2012 (UTC)
Let me first note that the UV index is defined as a measure of UV exposure at noon, so it is not a simple measure of UV exposure as a function of sun angle. With that being said, it seems to be difficult to give an authoritative answer to the question in terms. Calculating the UV index requires having a "radiative transfer model", a mathematical formula for the amount of UV light that reaches the ground at each frequency for a range of parameters, and it seems that each agency that calculates UV indices uses its own idiosyncratic transfer model. According to this paper, vitamin D production in the skin begins when the sun is around 20-30 degrees above the horizon. Looie496 (talk) 03:12, 11 February 2012 (UTC)
Hello. Can Hemoptysis result from flu, or flu-like conditions? (Before some zealot removes this question, please note that it is just a general medical question, and that I am not asking for a medical diagnosis, and don't want one, either. Thank you). Polisher of Cobwebs (talk) 18:59, 10 February 2012 (UTC)
- Any condition which causes a sore throat could cause blood to be coughed up from the esophagus. However, hemoptysis doesn't include that. It does include blood from the trachea, but if the patient has an infected trachea, that might then be classified as pneumonia. StuRat (talk) 19:10, 10 February 2012 (UTC)
Age and intelligence
At what age do humans reach the maximum capacity for learning ? (Note that this should be earlier than the age at which they attain the maximum amount of knowledge, since there is a substantial delay between obtaining the capacity to learn and absorbing all the information possible.) StuRat (talk) 19:22, 10 February 2012 (UTC)
- What makes you think there is a "maximum capacity for learning"? The brain is not a bucket. It is not a hard drive. It is a complicated living organ. Over time, various parts of it have increased ability (e.g. language sparks up in an incredible way when very young), and some parts decline over time, but I'm not sure how one correlates that with a singular notion of a "maximum capacity for learning." I don't know if the processes described on this page are correct or not, but it points out what a more nuanced analysis of age and learning might look at. --Mr.98 (talk) 20:04, 10 February 2012 (UTC)
Relativistic (polar) jet directed in one direction
It seems that the Messier 87's jet is going only in one direction while other jets as seen in Bipolar outflow seem to go in two opposite directions. The article on relativistic jets seems to indicate that jets from black holes can also go in two directions. What causes the jet to either go in one or two directions? ScienceApe (talk) 20:53, 10 February 2012 (UTC)
- That's explained in the article Relativistic_beaming (found via M87#Jet...) --Wrongfilter (talk) 20:59, 10 February 2012 (UTC)
- As a physicist, I would simply say "this behavior is obviously caused by an asymmetry in the physical system." Isn't it obvious? The interesting follow-up question is, of course: is this a fundamental asymmetry of the process (which has universal cosmological implications), or is it simply due to initial inhomogeneity (i.e., did the accreted dust-cloud have a net initial angular momentum)? Here's a nice web-page from UCLA, on cosmological anisotropy and inhomogeneity, and here's an online text from CalTech, specifically discussing velocity inhomogeneity in M87. As an engineer, I'd just say, "... there's probably a second jet we just can't see from Earth. Either way, the experts have chimed in with their opinions; the M87 radio jet is quite famous. Nimur (talk) 22:08, 10 February 2012 (UTC)
- Obvious? What's obvious about it? @_@ I hope that was a joke.. Not all of us are physicists, I'm just a layman. ScienceApe (talk) 23:57, 10 February 2012 (UTC)
- (It was a physicist joke, sorry if it was lost on the uninitiated). Nimur (talk) 00:26, 11 February 2012 (UTC)
- Obvious? What's obvious about it? @_@ I hope that was a joke.. Not all of us are physicists, I'm just a layman. ScienceApe (talk) 23:57, 10 February 2012 (UTC)
- As a physicist, I would simply say "this behavior is obviously caused by an asymmetry in the physical system." Isn't it obvious? The interesting follow-up question is, of course: is this a fundamental asymmetry of the process (which has universal cosmological implications), or is it simply due to initial inhomogeneity (i.e., did the accreted dust-cloud have a net initial angular momentum)? Here's a nice web-page from UCLA, on cosmological anisotropy and inhomogeneity, and here's an online text from CalTech, specifically discussing velocity inhomogeneity in M87. As an engineer, I'd just say, "... there's probably a second jet we just can't see from Earth. Either way, the experts have chimed in with their opinions; the M87 radio jet is quite famous. Nimur (talk) 22:08, 10 February 2012 (UTC)
Related question, if the single jet is just an optical illusion, and there's really two jets, is it possible for there to be just one jet? ScienceApe (talk) 00:02, 11 February 2012 (UTC)
- This is, essentially, the crux the links I posted. The answer is, "it's hotly debated, because the observational evidence is still fuzzy enough that either answer makes sense." We don't really know whether the other jet exists (and is invisible); or if it doesn't exist, and there's some interesting physics happening. Nimur (talk) 00:29, 11 February 2012 (UTC)
How much processing power would be needed to emulate a 4D visual cortex?
That would be as good in 4D space as a human visual cortex is in 3D space. How many rods and cones would 3D retinas need to get similar resolution and field of view? 1 trillion? (100 million3/2) How many kilograms would it weigh if built out of neurons (in 3D space)? (and presumably would need to be fed Matrix-style to run as the requisite 4D eyeball is not in existance). Since I read that even the current neuron-based paradigm is close to limits of intelligence (tradeoff of wiring thinness vs. transmission quickness and energy usage) then this number might be ridiculouly high or maybe impossible. On the other hand, how well would a current visual cortex do if rewired for 4D space? Not very well I suppose? Sagittarian Milky Way (talk) 22:35, 10 February 2012 (UTC)
I guess from teh way you worded it that you did not consider time as the 4th dimension, as is sometimes done. This question can be answered in two ways:-
1) The question is not a valid question because systems of greater than 3 dimensions are mathematical constructs useful in certain fields and do not represent any physical reality.
2) No increase in retina sensors and no increase in brain neurons would be necessary. This may surprise you, but there are good reasons as follows: We don't see like a televion camera - most of the information from our retinas is thrown away on the path to conscious vision. The brain selectively, depending on the merits of the scene and what you are looking for, "focusses" on only a limitted subset of visual information, and reconstructs what you consciously see or think you see. This is easily demonstrated with optical illusions. So if you get 4D eyes, the brain can still throw most of the info away and reconstruct. We don't actually see in full 3D, its more like enhanced 2D, as demonstrated by the long success of 2D representations in printed matter and movies, and perhaps the success of 3D enhancemnt to movies shot in 2D (eg Titanic). Also, human vison is essentially with 3 primary colours - red, green, and blue. Most birds insects have 4 primary colours - red, green, blue, and ultravioet, and dogs sort of 2 and a bit. An extra primary colour is potentially adding information just as an extra dimension would, but in fact its just a re-allocation of a portion of rods and cones or sensor cells to a different colour. But researches have in certain animals manipulated the genetic expression of retinal cells to add a primary colour. They fould that the standard brain adapted and coped just fine. So it should also cope with an extra dimension. As I recall, this was covered in a recent Scientific American article. Keit120.145.158.234 (talk) 02:35, 11 February 2012 (UTC)
Visualizing point data
Does there exist software which would allow me to view point clouds (at least XYZ, possibly XYZ+colour) and measure distances between points? e.g. so I can measure the approximate distance between two walls in a model. Thanks! 131.111.255.9 (talk) 22:44, 10 February 2012 (UTC)
- Certainly, pretty much any 3D CAD system will do that, although it's quite difficult to visualize 3D points. Some type of motion (rotation or Z-clipping as you move through the point field) or connections drawn between them (like constellations) can help. (Z-clipping is where the parts of the object closer to you are hidden so you can see the parts farther away.) Otherwise, the usual cues for telling which objects are closer and which are farther don't work with points. I suppose you could have them be little spheres or cubes, in a perspective view, so they do get larger as you get closer. You could also use color, where points farther away become darker and bluer colors, like distant mountains. StuRat (talk) 22:48, 10 February 2012 (UTC)
- For a free, open source option, Octave can do that. It has MATLAB style syntax, and uses gnuplot for a variety of visualization techniques. SemanticMantis (talk) 23:19, 10 February 2012 (UTC)
- Thanks. Octave might do the trick, although I was hoping for more of a 'prepackaged' solution - perhaps from the GIS field. I shall have a look! 131.111.255.9 (talk) 03:45, 11 February 2012 (UTC)
Astronomy stuffs
For number 18, the answer is ultra light. I want to know the relationship between temperature and wavelength range. And a brief description of wavelength for each of the answer.
For number 19, the answer is 4 times more. I don't really understand how they got it. So basically if what is the relationship between angular diameter and amount of energy it radiates and its wavelength. So if i was given 2 out of 3 i can figure out the last one. And don't ask me why i didn't ask my teacher. This is not part of my classes. Thanks.Pendragon5 (talk) 23:21, 10 February 2012 (UTC)
- Wien's displacement law will give you a relation between peak blackbody-wavelength and temperature. The Stefan–Boltzmann law will give you a relation between temperature and the rate of energy radiated (per area or per solid angle, depending on the formula you choose to use). By stringing these two equations together with a bit of algebra, you can calculate the total energy radiated, if you know the size of the objects. Whether these laws are a good approximation for your object will depend on how accurately you need to calculate: there are loads of empirical adjustment "fudge factors" - like "grey body radiation;" and my personal favorite astrophysical fudge factor - whether the radiant antenna temperature of the universe is 0 or 4 kelvins. (Properly, you should modify Stefan Boltzmann equation to account for this, but in practice it makes almost no difference when you are considering stellar radiation). And for the true astrophysical pedant, 4 kelvin isn't accurate enough; you should actually calculate the impedance-matching for every single emitted photon wavelength to the color temperature of the universe, lest you calculate a miniscule error on the radiated power that is far below any realistic noise-floor. (As an addendum, I'll just comment that our cosmic microwave background radiation article cites recent COBE measurements of the blackbody temperature as "2.725 K" with error-bars too small to mention. I still always use 4 K, and it's never interfered with any of my work so far). Nimur (talk) 00:49, 11 February 2012 (UTC)
- 2.8977685(51)×10−3 What is the (51) mean? And when i plug it in my calculator. For the temperature, do i put 8,500 or 8,500,000? Pendragon5 (talk) 01:06, 11 February 2012 (UTC)
- Alright never mind. Base off from the answer i got, it all makes sense now. Let me put it out here and can you tell me if i did it correctly ok? So let put 2.8977685 X 10^-3/8,500= 3 X 10^-7. Ultra violet range is from 1 X 10^-8 to 1 X 10^-7. So 3 X 10^-7 is in that range so that's why the answer is ultra violet right?Pendragon5 (talk) 01:20, 11 February 2012 (UTC)
- Almost, except 3x10^-7 is greater than 1x10^-7, so it isn't actually in that range. The first sentence of ultraviolet says it goes up to 4x10^-7, though, so it in the range, the range is just a little bigger than you thought. --Tango (talk) 01:27, 11 February 2012 (UTC)
- The (51) is a short way of saying +/- 0.0000051, ie. it's the uncertainty in the last two digits of the main number. K means Kelvin, not thousand. --Tango (talk) 01:22, 11 February 2012 (UTC)
- Oh wow pretty useless then. I'm looking for the rough estimate is good enough. On number 19 i'm still lost on how to do it. How am i going to calculate the energy of Star F and E then compare them? Thanks.Pendragon5 (talk) 01:31, 11 February 2012 (UTC)
- Alright never mind. Base off from the answer i got, it all makes sense now. Let me put it out here and can you tell me if i did it correctly ok? So let put 2.8977685 X 10^-3/8,500= 3 X 10^-7. Ultra violet range is from 1 X 10^-8 to 1 X 10^-7. So 3 X 10^-7 is in that range so that's why the answer is ultra violet right?Pendragon5 (talk) 01:20, 11 February 2012 (UTC)
- 2.8977685(51)×10−3 What is the (51) mean? And when i plug it in my calculator. For the temperature, do i put 8,500 or 8,500,000? Pendragon5 (talk) 01:06, 11 February 2012 (UTC)
Light
If for example humans evolve w/o the sense of sight, can we come up with theories about light? MahAdik usap 23:53, 10 February 2012 (UTC)
- I suppose so. After all, we are able to come up with theories to explain X-rays, and we can't see those. Sound is not as similar as X-rays, but still somewhat similar to light, so, once we explained that, we would have a head start towards explaining light. StuRat (talk) 00:00, 11 February 2012 (UTC)
- Can you think of a scenario where we will be curious about light? MahAdik usap 00:32, 11 February 2012 (UTC)
- It is a weird question but i suppose pretty interesting. We can't see anything then we must have developed some special kinds of functions that don't require the sense of sight to do all the things humans have accomplished until to this day. If we don't have any special function to interchange with the sense of light then i suppose we probably are still stay at same as cavemen. And by the way, you don't need to see or hear or sense something to understand it. What makes us special is our conscious and imagination, which are far more important than knowledge. Knowledge is already there, you just have to learn it. But to invent, motivate, something new... like a theory or new technology... A lot of people can learn but not many have rich imagination such as Albert Einstein. As smart as Einstein has said once: "Imagination is more important than knowledge", which is really true when i think about it.Pendragon5 (talk) 00:42, 11 February 2012 (UTC)
- And in human history, there are A LOT of predictions and theories through out human history about something that we don't even understand it yet but with our imagination we can come out with something that makes sense. I'm really astonished that people thousands years ago already have richer imagination than me. Imagination is something that has no limit and infinite of possibilities. And later on, when we are advanced enough then we can confirm it. Not all of them were correct though, just some of them. Predictions and theories came first then the confirmation comes later, it may take few years up to hundreds or thousands of years to confirm them.Pendragon5 (talk) 00:48, 11 February 2012 (UTC)
- (edit conflict) Einstein is what im thinking when i asked this question, i just thought that if we cannot percieve light, then we wont have much understanding about it, then we wont be able to formulate a good explanation for gravity, im just thinking that if its true, then maybe their some forces that we cant percieve right now that might help us explain the universe and i hope i am making sense MahAdik usap 01:06, 11 February 2012 (UTC)
- We better try to predict those forces now and let wait until we are advanced enough to find them out. But as always the first step is to predict and choose the correct direction.Pendragon5 (talk) 01:13, 11 February 2012 (UTC)
- (edit conflict) Einstein is what im thinking when i asked this question, i just thought that if we cannot percieve light, then we wont have much understanding about it, then we wont be able to formulate a good explanation for gravity, im just thinking that if its true, then maybe their some forces that we cant percieve right now that might help us explain the universe and i hope i am making sense MahAdik usap 01:06, 11 February 2012 (UTC)
- Can you think of a scenario where we will be curious about light? MahAdik usap 00:32, 11 February 2012 (UTC)
Average lifespan of the stars
What is an average lifespan for the stars in the universe. I also want to know the relationship between mass and lifespan. According to this 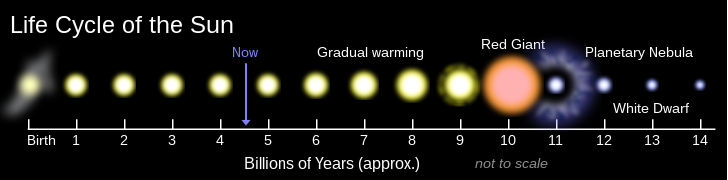 , our sun has 1 solar mass and its lifespan is about 10 billion. So if a star has 2 solar mass and so on or has less than .9 solar mass and so on. What are their expectancy lifespan?Pendragon5 (talk) 01:37, 11 February 2012 (UTC)
, our sun has 1 solar mass and its lifespan is about 10 billion. So if a star has 2 solar mass and so on or has less than .9 solar mass and so on. What are their expectancy lifespan?Pendragon5 (talk) 01:37, 11 February 2012 (UTC)
- Never mind, i think i have found an awesome data table of it.Pendragon5 (talk) 01:52, 11 February 2012 (UTC)
- Note that you would get a very different answer if you averaged by number of stars or by mass of stars. StuRat (talk) 01:57, 11 February 2012 (UTC)
Valence of argon in argon fluorohydride
What is the valence of argon in HArF (argon fluorohydride)? Until recently Wikipedia stated the maximum valence of argon to be 0, but R8R Gtrs tells me that it should be +2. Double sharp (talk) 02:07, 11 February 2012 (UTC)
Why do we find baby animals cute?
Has there ever been an attempt to explain why humans find baby animals cute, particularly babies of other mammals? HiLo48 (talk) 02:51, 11 February 2012 (UTC)
- I'm guessing there'll be some info at neoteny, though I don't know how much or how specific to your question. --Trovatore (talk) 02:52, 11 February 2012 (UTC)
- The evolutionary adaptation is that we think our babies are cute so we will take care of them. By transference, the baby-like features of other animals (whether juvenile or adult) trigger the same response. StuRat (talk) 03:30, 11 February 2012 (UTC)
- Yeah, I get the idea that it's good for humanity to find its own babies cute. But why the other animals? Personally, I reckon some human babies are pretty ugly, but every puppy is cute. As an Australian, I think about our native marsupial babies. Cute as all heck (I really must upload my baby wombat pics), but humans evolved on completely different continents. Why should we care? HiLo48 (talk) 03:38, 11 February 2012 (UTC)
- I just noticed that wombat sounds a lot like womp rat. Coincidence? --Trovatore (talk) 03:43, 11 February 2012 (UTC)
- Yeah, I get the idea that it's good for humanity to find its own babies cute. But why the other animals? Personally, I reckon some human babies are pretty ugly, but every puppy is cute. As an Australian, I think about our native marsupial babies. Cute as all heck (I really must upload my baby wombat pics), but humans evolved on completely different continents. Why should we care? HiLo48 (talk) 03:38, 11 February 2012 (UTC)
Magnetic liquid
Are there any magnetic liquids? Whoop whoop pull up Bitching Betty | Averted crashes 03:27, 11 February 2012 (UTC)
- There certainly are liquids which would be attracted by a magnet (just mix some oil with iron filings). But a liquid that acts as a magnet sounds difficult to achieve, because the north poles and south poles would bind to each other and cancel each other out. At best, I'd expect one that acts like a magnet when exposed to a magnetic or electric field or charge. StuRat (talk) 03:33, 11 February 2012 (UTC)

