Wikipedia:Reference desk/Science: Difference between revisions
| Line 464: | Line 464: | ||
==[[Higgs boson]]== |
==[[Higgs boson]]== |
||
in this context what is the [[Golden Channel]]? [[User:Kittybrewster|Kittybrewster ]] [[User_talk:Kittybrewster|<font color="0000FF">☎</font>]] 09:08, 4 July 2012 (UTC) |
in this context what is the [[Golden Channel]]? [[User:Kittybrewster|Kittybrewster ]] [[User_talk:Kittybrewster|<font color="0000FF">☎</font>]] 09:08, 4 July 2012 (UTC) |
||
::It's a fine stream of sub-atomic particles. That's why it's often called the "Golden Showers". [[User:Myles325a|Myles325a]] ([[User talk:Myles325a|talk]]) 09:16, 4 July 2012 (UTC) |
|||
Revision as of 09:16, 4 July 2012
of the Wikipedia reference desk.
Main page: Help searching Wikipedia
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Wikipedia:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
June 29
Rat poison
I have a pretty severe rat infestation in my house. The rats seem to mostly live in my roof and just come down to eat food. I've tried using warfarin based poisons to kill them. I placed them inside the roof through the manhole. The baits get eaten completely, but the rats don't die. The amount of poison I have placed up there has been up to 1kg of 0.105% warfarin. Essentially all of it was gone within a week but the rats were not. It's possible that the rats are warfarin resistant, but there's no other options at the local shops here. I'm thinking of taking a cereal and potassium cyanide and mixing together with honey to bind it and placing that in the roof. I don't have any pets or children. Would this be an effective mixture for erradicating my rats? 112.215.36.172 (talk) 04:42, 29 June 2012 (UTC)
- Try calcium phosphide, it isn't so much a poison really, but it works, unless the calcium phosphide is past it's best before date. On contact with acid, like stomach acid, it produces diphosphane, which is spontaneously combustable. Essentially, it turns a rat crispy on the inside. Plasmic Physics (talk) 05:27, 29 June 2012 (UTC)
- Can I mix that with cereal and honey as I was going to do with the cyanide? 112.215.36.177 (talk) 06:11, 29 June 2012 (UTC)
- Dealing with mice, I've found glue traps to be effective, although not at all humane (they rip their faces off trying to escape). If they make rat-sized glue boards, I'd try those. StuRat (talk) 05:32, 29 June 2012 (UTC)
- Okay, why would you *want* to do something like that in the first place? Use another method. That sounds sadistic and wrong. (talk) 01:40, 2 July 2012 (UTC)
- Rat glue is available here, but I don't really know how to dispose of a live rat glued to a plate. I checked the instructions and their silence on the matter is telling. 112.215.36.177 (talk) 06:11, 29 June 2012 (UTC)
- I'm guessing a high-tech euthanasia device is the preferred solution to that (*ahem*) sticky situation. Evanh2008 (talk|contribs) 06:16, 29 June 2012 (UTC)
- For mice, I used one of those grabber thingies to get hold of the edge of the glue board, then put it in the sink and drowned it (had to hold it under with the grabby thing). If the rat is too big for that, drag it into a trash bag, take it outside, and crush it with a cinder block. Then toss it in the trash, or bury it if dead animals aren't allowed in the trash. Or, you could just let it die and rot in the attic, the smell will likely mostly go up and out. StuRat (talk) 06:22, 29 June 2012 (UTC)
- What if the bait in the rat glue is also poison? Then it will die and I can just throw it away. Would that work? I'm also having trouble imagining how I would get a plate with a rat down from in the roof. I don't have an attic, just a crawl space so no lighting and very little room to move. The man hole is only about 1.5sqr feet, no fixed stairs or ladder and the drop is about 3m to the floor.112.215.36.177 (talk)
- You could also shoot it on the glue board, perhaps a BB gun would work. Once you have it in the garbage bag (you might want to double bag it), just tie it shut and drop it down. Note that with poison they often die in really bad places, like in the walls, where they smell up the entire house. Or, worse yet, they can stagger into your living area, bleeding profusely. StuRat (talk) 06:41, 29 June 2012 (UTC)
- This word count on the posts so far shows infestation by rodents:
- rat : 16
- man: 3
- DriveByWire (talk) 15:03, 29 June 2012 (UTC)
- This word count on the posts so far shows infestation by rodents:
Do you have any access to sodium azide-based pesticides? In sufficient quantity, the water-dissolved form should kill essentially anything. But given that, there may be restrictions on its use in your area, due to the potential for environmental contamination. If you are legally permitted to use it, simply spraying it on the glued rat should kill it, of course being very careful not to get it on yourself. Someguy1221 (talk) 06:29, 29 June 2012 (UTC)
- I don't know of any sodium azide containing products that I have at my disposal. There's litterally piles of cyandide at the gold mine where I work, so I thought that would be the obvious choice. I also don't know where I would get the calcium phosphide mentioned above, but it sounds like it would allow me to not bother getting the corpses out of the roof, which is an advantage. I suppose the most important question is whether mixing any of these things with honey is going to liberate a toxic gas and kill me, or will it remain solid until the rat digests it. 112.215.36.177 (talk) 06:47, 29 June 2012 (UTC)
This is why we have professionals.--Shantavira|feed me 11:47, 29 June 2012 (UTC)
- The dangers of amateur poison warfarin' make one sigh an' hide. DriveByWire (talk) 15:15, 29 June 2012 (UTC)
Let me point out that the OP geolocates to Jakarta, Indonesia. I doubt that many people here are qualified to give advice on getting rid of rats in a place like that, especially not knowing the setting of the house in question. And in any case I certainly wouldn't give advice to anybody who starts talking about using cyanide. Looie496 (talk) 15:54, 29 June 2012 (UTC)
- I would be interested to know which mining company would allow people to walk off site with cyanide in what looks like South Sulawesi. We live in a rat-rich tropical environment and our house sometimes has guests in the roof spaces. We don't do anything to control them other than figure out how they get up there and cut any tree branches etc they use, allow cats into the house and allow any rat snakes we see to stick around. It's pretty effective. Sean.hoyland - talk 17:15, 29 June 2012 (UTC)
- I'm on holiday in Bali right now, but I live in Australia. Unfortunately, if rats are next door, which I'm pretty certain they are, then they will just walk accross the ground to my house. Mining companies that use cyanide for gold leaching do not keep it very secure as a general statement about the industry. Obviously I'm not going to say who I work for, but it's literally sprayed around the place in heap leaches. I don't know why me saying that I thought cyanide might work would stop people from giving me advice. Wikipedia lists it as a rodenticide, and if it really is a terrible idea, shouldn't you want to advise against it? 112.215.36.174 (talk) 03:41, 30 June 2012 (UTC)
Cheese with Polonium 210 Count Iblis (talk) 18:15, 29 June 2012 (UTC)
- Yeah, right. Moon rocks are expensive. 112.215.36.172 (talk) 03:25, 1 July 2012 (UTC)
- My experience has been that cheese is some sort of miracle rodent bait only in cartoons. Peanut butter is much more effective in real life. Evanh2008 (talk|contribs) 01:35, 30 June 2012 (UTC)
- A rat trap with peanut butter, yes; or with a bit of bacon or some other meat firmly tied on to the trigger. ←Baseball Bugs What's up, Doc? carrots→ 04:49, 30 June 2012 (UTC)
Using cyanide doesn't seem like such a good idea. The rats will taste just a little bit of the food and notice they become sick. And if one of them dies shortly after having eaten from a new food source, the others might be smart enough to avoid eating from that same food source. That's the reason for using slowly-acting poisons like warfarin. Icek (talk) 09:06, 30 June 2012 (UTC)
I don't like all this poison stuff. If you end up killing yourself with it, it looks bad for humanity versus ratkind in the global intelligence ranking. Not to mention that you'll have dead rats in your walls, getting ... juicy. Why not use a good old fashioned rat trap? Or a glue trap - apparently they still work for rats, and it has the added benefit of often providing you the live rat for ... wherever your imagination takes you. Or countless other mechanical options. Wnt (talk) 18:14, 30 June 2012 (UTC)
- That may be valid, but did you consider the ramifications for humanity's global intelligence ranking if I were to kill myself with rat glue? Generally, I'm much better with chemicals than I am with anything mechanical. 112.215.36.172 (talk) 01:52, 1 July 2012 (UTC)
- Glue boards have another advantage, in that most rodents start squeaking when they get trapped, providing their own alarm. That allows you to dispose of them before they die and start to rot. StuRat (talk) 20:09, 30 June 2012 (UTC)
Resolution
Combining several of the above solutions with some externally sourced ingenuity, I've decided how I will proceed.
Calcium phosphide and sodium azide were determined to be too difficult to procure. Rat glue was rejected due to my squeamish side not wanting to handle live rats who are angry enough to have literally ripped their own faces off. This suggestion from a previous reference desk question was rejected on the basis of unreasonable cost. Allowing cats and snakes into my roof was considered, but not accepted since the goal is to reduce the number of pests in my house. As a request made under the Freedom of Information Act revealed, attempts to acquire polonium laced cheese resulted in my name appearing on a suspected terrorist watch list. The original idea of cyanide was determined to be so 1940's.
The ideal toxin and route of administration was determined to be remotely actuated direct subcutaneous plumbum injection. In the end, much greater weight was given to the level of satisfaction achieved over actual probability that it will result in my house not having lots of rats in it. The chosen method is as follows:
- Clear the house of human occupants by fabricating a story about a sale at Guess.
- Place a suitable backstop against the door from my kitchen to the dining room.
- Make a mound of peanut butter laced with bacon bits on a paper plate.
- Set up a hide ~5 meters away with my gamo at the ready.
- Wait.
- Aim for the fat one.
- ?????????
- Profit.
Thanks for your help everyone.
- Your proposed solution is self-contradictory because injured rats will die and rot in the walls (and feed other rats and pests!) like poison would. I recommend rat traps plus nitrogen asphyxiation. 75.166.192.187 (talk) 19:50, 2 July 2012 (UTC)
Borrow a cat. --Dweller (talk) 14:54, 4 July 2012 (UTC)
- Slaughtering rats is unlikely to solve your problem; they reproduce very rapidly and can quickly make up losses. If you do manage to kill every female (or every male), then their cousins from next door will move in. They will just keep coming, like in Space invaders. You need to deny them access to food (including waste food and anything remotely edible), by keeping everything in containers that can't be gnawed into and can't be smashed by knocking over. This will motivate the rats to spend their time in your neighbour's house instead of yours. You also need to deny them access to your house entirely; this is hard as they can get through very small holes. They are most likely gaining entry by ascending your downspouts, going under your eaves and then descending to the larder. Block any small holes with screwed-up chicken-wire (which can't be gnawed), packed in too tightly to be pushed out. Many cats are too useless to sort out a rat, but plenty of fresh cat-urine deters rats from loitering on the premises. --catslash (talk) 20:53, 4 July 2012 (UTC)
- I've seen lion droppings for sale on the internet to deter all kinds of animals... might work on rodents. --93.96.36.99 (talk) 05:50, 5 July 2012 (UTC)
- Slaughtering rats is unlikely to solve your problem; they reproduce very rapidly and can quickly make up losses. If you do manage to kill every female (or every male), then their cousins from next door will move in. They will just keep coming, like in Space invaders. You need to deny them access to food (including waste food and anything remotely edible), by keeping everything in containers that can't be gnawed into and can't be smashed by knocking over. This will motivate the rats to spend their time in your neighbour's house instead of yours. You also need to deny them access to your house entirely; this is hard as they can get through very small holes. They are most likely gaining entry by ascending your downspouts, going under your eaves and then descending to the larder. Block any small holes with screwed-up chicken-wire (which can't be gnawed), packed in too tightly to be pushed out. Many cats are too useless to sort out a rat, but plenty of fresh cat-urine deters rats from loitering on the premises. --catslash (talk) 20:53, 4 July 2012 (UTC)
iodine reacting with titanium
A few months back I posted this
"I have a titanium mug/cup. After drinking some milk I forgot to rinse it out and it sat and became gross. I decided to add some tincture of iodine along with some water to help sanitize the cup. After adding this in letting it sit for about a minute it changed color and started a foul smell that made my nose sting. Is it possible it reacted with titanium to release some sort of harmful gas or substance? — Preceding unsigned comment added by 64.38.198.61 (talk) 00:08, 4 March 2012 (UTC)
The elemental iodine (I2) is probably reacting with the titanium in the same way it reacts with aluminium. The product of that reaction would be titanium iodide, which is a very soluble salt, but it would also generate heat which volatilises some of the remaining elemental iodine. That stuff's not very nice to breath in. 203.27.72.5 (talk) 00:55, 4 March 2012 (UTC)
According to the titanium alloy article, titanium is usually alloyed with aluminium anyway. The reaction between iodine and aluminium is very spontaneous and can result in iodine vapor. 203.27.72.5 (talk) 04:28, 4 March 2012 (UTC)
The thing is I didn't see any actual cloud of vapor rise from the cup I just smelled it is that normal for this type of reaction? — Preceding unsigned comment added by 64.38.197.212 (talk) 12:04, 4 March 2012 (UTC)
If there had been enough vapor for you to see it in the air it would have been at a lethal concentration. Tincture of iodine only contains a few percent of iodine and not all of it is elemental either. I can imagine it would have been enough to smell but not enough to see by several orders of magnitude. 203.27.72.5 (talk) 20:14, 4 March 2012 (UTC)"
I haven't used this cup since then but I liked it a lot and it was expensive so im wondering is it safe to drink out of it?--64.38.226.89 (talk) 05:04, 29 June 2012 (UTC)
- Without giving medical advice, I can point out that the product of the reaction, titanium tetraiodide would be in low concentrations and is highly reactive with water, so a few good rinses should remove it all. Titanium metal in and of itself is nontoxic. Handschuh-talk to me 09:37, 29 June 2012 (UTC)
Glucosamine and tendonitis
Where can I find scientific studies on the effect of the supplement glucocamine on tendonitis?
Just to clarify I am not asking for medical advice. — Preceding unsigned comment added by 99.146.124.35 (talk) 20:43, 29 June 2012 (UTC)
- Our glucosamine article contains a long and well-referenced section on health effects; that would be a good place to start. Looie496 (talk) 20:52, 29 June 2012 (UTC)
June 30
given that gold foil averages 2 atoms thick...
... how many atoms, very approximately, would be in a square centimetre of 22k foil?
thanks Adambrowne666 (talk) 04:46, 30 June 2012 (UTC)
- What's the source for that "given"? ←Baseball Bugs What's up, Doc? carrots→ 04:48, 30 June 2012 (UTC)
It's two atoms thick so,
Assuming the impurities are the same atomic mass of gold and they occupy the same size space in the lattice;
112.215.36.174 (talk) 05:45, 30 June 2012 (UTC)
that's fantastic, thank you .174; the formula will look very impressive in my artist's statement. Bugs, I must admit, I can't remember where i read that factoid, and am now finding estimates on the net ranging from 2 to 300....Adambrowne666 (talk) 07:29, 30 June 2012 (UTC)
- Just to let you know, the assumption that the impurities are similar to gold in atomic mass and size in the lattice is wrong, but shouldn't make a big difference. It's almost certainly copper which is 3.1x lighter in terms of atomic mass, but the small proportion of it in the gold makes it more or less negliglible. Also, I didn't calculate this in the easiest way; it is much simpler if you do it like this:
I just found the first way a bit more logical as it showed the volume of the individual atom and worked out the total from there. 112.215.36.174 (talk) 07:49, 30 June 2012 (UTC)
- 300 would be the absolute thinnest gold leaf you'll find, I think. I have a hard time imagining going thinner without breaking it, unless you're using electroplating, but then it's not foil. Someguy1221 (talk) 07:46, 30 June 2012 (UTC)
- According to gold, 1 gram of Au can be beaten into 1sqm of gold leaf. That corresponds to 110x thicker than my calcs above i.e. ~220 atoms thick. 112.215.36.174 (talk) 07:59, 30 June 2012 (UTC)
- Gold foil a few atoms thick is used in science labs. I think you are right that you wouldn't be able to use it for art without breaking it. --Tango (talk) 08:01, 30 June 2012 (UTC)
- You can also work it out by taking the atomic radius, multipling by 2 to get the diameter and dividing 1cm into it. Square that to get the number of atoms of 1 thickness in 1cm^2 and then multiply by 2 to get it for 2 atoms thickness or whatever. I got 2.4x10^15 atoms for 2 atoms thick using a radius of 144pm. 112.215.36.174 (talk) 08:36, 30 June 2012 (UTC)
- It can be fewer. Gold foils down to 0.02 µm (2x10-8m or 20nm) are supplied by this company. At this thickness the foil is transparent. The atomic diameter of gold is 2.88x10-10 m so its thickness is about 70 atoms. Its density is 19.32 kg m-3. DriveByWire (talk) 12:25, 30 June 2012 (UTC)
- That makes an unstated assumption about the orientation of the crystal. The distance between the layers is only 2.03x10-10m, so there could be up to 98.5 atoms of thickness in 0.02µm if you assume optimum packing. 112.215.36.172 (talk) 00:05, 1 July 2012 (UTC)
- It can be fewer. Gold foils down to 0.02 µm (2x10-8m or 20nm) are supplied by this company. At this thickness the foil is transparent. The atomic diameter of gold is 2.88x10-10 m so its thickness is about 70 atoms. Its density is 19.32 kg m-3. DriveByWire (talk) 12:25, 30 June 2012 (UTC)
- You can also work it out by taking the atomic radius, multipling by 2 to get the diameter and dividing 1cm into it. Square that to get the number of atoms of 1 thickness in 1cm^2 and then multiply by 2 to get it for 2 atoms thickness or whatever. I got 2.4x10^15 atoms for 2 atoms thick using a radius of 144pm. 112.215.36.174 (talk) 08:36, 30 June 2012 (UTC)
science
is students perusing engineering from computer science branch can participate in u.p.s.c exams or they can give i.e.s exams? as computer science is not a core branch so to give such exams what should be the plan of action for such students?Divyajharia (talk) 07:40, 30 June 2012 (UTC)
- Your question isn't very clear. Is "u.p.s.c" the Indian Union Public Service Commission? And is "i.e.s" the Indian Engineering Services? You probably have a tutor that can help you easier than we can. If you want our help, you'll need to ask your question more clearly. What are you studying? Where are you studying? What exams do you want to take? Why do you want to take them? --Tango (talk) 08:08, 30 June 2012 (UTC)
What is this machine
What might this machine be for? Roadsworth88 (talk) 17:38, 30 June 2012 (UTC)
- A (1940's era ?) device for measuring the flow rate (of water ?) between Croydon North (UK Parliament constituency) and Mitcham and Morden (UK Parliament constituency), presumably. StuRat (talk) 17:51, 30 June 2012 (UTC)
- Indeed, the river Wandle flows just south of a sewage treatment plant in Beddington Park, so it's important to be able to tell if things are threatening to back up, and drop a trouble ticket if they do. 75.166.192.187 (talk) 17:55, 30 June 2012 (UTC)
- More modern I think. Seems to show flow rate of water (or sewage?) and the total quantity (calculated by integrating the flow rate. Integrator round the back!)--78.150.226.117 (talk) 17:59, 30 June 2012 (UTC)
- Dial on right looks like pressure meter.--78.150.226.117 (talk) 18:01, 30 June 2012 (UTC)
- Agreed. It must have something to do with the Wandle Trust. 75.166.192.187 (talk) 18:02, 30 June 2012 (UTC)
- <nitpick> It's almost certainly not measuring flow between Croydon North (UK Parliament constituency) and Mitcham and Morden (UK Parliament constituency), since Parliamentary constituencies of the UK parliament are almost never used for any purpose other than electing MPs and local councillors. It's much more likely that the areas are the County Borough of Croydon and the Municipal Borough of Mitcham. Having Mitcham mentioned as a separate entity, as opposed to a part of the London Borough of Merton, would place the machine's date as between 1915 and 1965. </nitpick> - Cucumber Mike (talk) 11:06, 1 July 2012 (UTC)
- Unless the Metropolitan Water Board and their successor, Thames Water, have their own area boundaries. Alansplodge (talk) 16:56, 1 July 2012 (UTC)
- <nitpick> It's almost certainly not measuring flow between Croydon North (UK Parliament constituency) and Mitcham and Morden (UK Parliament constituency), since Parliamentary constituencies of the UK parliament are almost never used for any purpose other than electing MPs and local councillors. It's much more likely that the areas are the County Borough of Croydon and the Municipal Borough of Mitcham. Having Mitcham mentioned as a separate entity, as opposed to a part of the London Borough of Merton, would place the machine's date as between 1915 and 1965. </nitpick> - Cucumber Mike (talk) 11:06, 1 July 2012 (UTC)
How to fix scrolling/tiling interference
I recently purchased a Panasonic Plasma TV 720 DPI 600 Hertz. After hooking it up to my digital low-def cable service I got a low hum in the audio and lines of lighter and darker interference traveling in horizontal bands up the screen taking maybe 5-7 seconds to go from the bottom of the screen to the top. No interference originated from the DVD signal, only from the cable signal when routed directly or indirectly into the TV through the AV (red/white/yellow) input. The RF (co-ax) input was clear. I had the cable guy visit. Depending on which of the three cable boxes we used the problem was more or less pronounced. We tried analog TV's, and the problem disappeared. I finally took the Panasonic TV back and traded it in for a Samsung plasma 720 dpi which is getting the same exact video problem, but now through both its RF and AV inputs. I have scheduled the cable guy back out. Is there any way to identify this type of rolling interference and what causes it? Can I assume it is an interaction between the signal source and the TV, i.e., interference on the cable line which shows up only on a certain type of set? Will ferrite cores potentially correct this sort of problem? Will it help to call 911? μηδείς (talk) 19:27, 30 June 2012 (UTC)
- Assuming you tried different red/white/yellow cables, from the tests which have been done so far, it sounds like the interference comes from the cable boxes, apparently a defect in their design. Assuming they don't have another model of cable box for you to try, this leaves the following options:
- 1) Use an HDMI cable to connect to the cable box, if it has an HDMI output. Same with S-Video, etc.
- 2) Use the RF (co-ax) input. Picture won't be as clear, though.
- 3) Switch cable companies (or switch to satellite) and hope new one has a better cable box model.
- 4) Stick with analog TVs. If your digital TV has an option to lower resolution below 720, that might be worth a try, too. StuRat (talk) 20:00, 30 June 2012 (UTC)
The Panasonic which we took back had a perfect picture with the RF cable and craptastic with the RCA. The Samsung picture has weak interference through both lines. I don't see the ultimate problem being with the boxes but with a voltage on the line itself from the pole. Our neighbours all have the same cable company but not the interference we are getting.
The cable company doesn't want to give us a box with an hdmi connection unless we pay for hidef, although I intend to have the tech let us try out a box to see if it fixes the problem. Sattellite does not offer the sports broadcasts we want. μηδείς (talk) 21:27, 30 June 2012 (UTC)
- If the HDMI test is clear, then you might be able to convince them to give you that box for free, if their tech tells them that their non-HDMI box just isn't working and you threaten to find another cable company due to their failure to uphold their end of the contract (provide a clear pic). StuRat (talk) 21:39, 30 June 2012 (UTC)
- At this point what I really want to know is what sort of interference we are having and what typically causes it and what can fix it, like can a ferrite core fix visual interference. Again, the interference is perfectly horizontal bands of lightening or darkening of the picture of varying widths from fractions of an inch to a few inches which travel in regular waves up the screen from bottom to top over a period of about five to 10 seconds, maintaining their spacing and speed as they rise. The article on ferrite core is not very helpful and I don't know where to begin to look under interference. μηδείς (talk) 21:27, 30 June 2012 (UTC)
- What you have described is often caused by what techs call an "earth loop", which causes a small amount of AC power to get into the signal cables. The subject of earth loops is complex, especially for unqualified people, however powering the TV from separate wall outlet to the rest of your interworking equipment is a possible cause in some houses. To fixed earth loop problems you need a good knowedgable electronics tech.
- Ferrite cores are designed to fix radio frequency interference, such as may be caused by motorised applicances. This means that ferrite cores will NOT address your problem.
- As the cable TV tech tried different boxes, faulty boxes are unlikely. I would investigate further before changing cable companies.
- There is a (very) small possibility that the power voltage is low at your house, Electronic equipment (including TV's and set-top boxes) have internal voltage regulator circuits. If the power voltage goes lower that these regulators can cope with, the result is often hum in audio and rolling dark/light bars in pictures. You can tell if voltage is low as it casues light from incadescent globes to be yellowish, but some people don't notice it. In any case many people no longer have incandescent globes. If you have a multimeter and knbow how to use it, check your power voltage at the wall outlet - this is easy to do and will rule out this being the cause. If voltage is less than 90% of nominal, call the power company. It is quite common for low power voltage from the power company to apparenetly affect only one house in a street. This is because your neighbours have different brands/models of equipment, which may be more tolerant, and/or they may have their TV's etc on a different phase.
- Otherwise, of if voltage is normal, call a TV serviceman - not because your TV is suspected faulty, but because, in general, TV service techs are smarter that cable company techs, and are more likely to deduce what the cause is. Keit60.230.195.130 (talk) 04:26, 1 July 2012 (UTC)
Yes, I am fairly certain you have identified the problem. Ground_loop_(electricity) One question. The TV and (cable box and VCR/DVD) are plugged into separate sockets on the same two-socket outlet. Will plugging all the devices into one power surge strip possibly help? Or is the voltage possibly flowing out the cable rf cord rather than between two electric cords? μηδείς (talk) 20:55, 1 July 2012 (UTC)
- If ALL devices in the system (TV, cable box, DVD player, etc) are powered from the 2 outlets of the same two-socket outlet, electrically that's about as good as it can be, and using additional power strip(s) in order to use only one of the 2 outlets is quite unlikely to help. The Wikipedia article has a good point - if you have an antenna system connected somewhere for free-to-air reception, then, if it is earthed/grounded, that can contribute an earth loop issue. This is quite unlikely to be a problem in a stand-alone dwelling, but if you live in a duplex or block of flats, sharing a common antenna, it is possible. If you have an antenna connected, unplug it and see if cable reception improves. Don't forget the supply voltage check. While that is a low probability issue, it is easy to check. Keit121.221.222.136 (talk) 01:36, 2 July 2012 (UTC)
July 1
PC to PC communication using laser torch
what will be the transmitting and receiving circuit diagrams for 'pc to pc data transfer using laser torch?' — Preceding unsigned comment added by Rosemail9876 (talk • contribs) 06:02, 1 July 2012 (UTC)
- Here are circuit diagrams for a voice communicator that in principle could be modified to carry low-speed data. Another way to transfer data between PCs is physically as a writeable CD or DVD, which also involve lasers. DriveByWire (talk) 00:32, 2 July 2012 (UTC)
- And there are free space laser communication systems to connect computer networks together. They are superceded by wireless ethernet mostly. But may have the advantage of a lack of signal spillover. The cuircuit could be similar to a fibreoptic transceiver, but with a much higher power laser, and to get the best you would have to cope with frequency shifts and temporary drop outs (when birds flew through the path) Graeme Bartlett (talk) 03:03, 2 July 2012 (UTC)
- You may be interested in RONJA.--Srleffler (talk) 01:28, 3 July 2012 (UTC)
- For electricity (and in general): wireless energy transfer. ~AH1 (discuss!) 18:25, 3 July 2012 (UTC)
wiggly line in molecule diagram
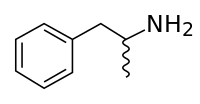
Ya got yer benzene ring with –CH2-CH-NH2 ... and what's that wiggly thing? —Tamfang (talk) 06:09, 1 July 2012 (UTC)
- It indicates that the bond is not in the plane of the page (or monitor screen) i.e. it projects either towards you or away from you, but it doesn't indicate which way specifically, so it's probably a mixture of the two isomers. 112.215.36.172 (talk) 07:31, 1 July 2012 (UTC)
- Actually, these would not constitute two isomers. Whether it's going into or coming out of the page makes no difference to the structure, as the single bonds may rotate freely. 112.215.36.172 (talk) 07:53, 1 July 2012 (UTC)
- They can rotate, but they can't swap positions. The carbon attached to the squiggly methyl group is bound to three distinct species, so the molecule is almost necessarily chiral. See also Amphetamine#Chemical_properties. Someguy1221 (talk) 08:13, 1 July 2012 (UTC)
- Yeah, you're right. It's definitely chiral. 101.170.42.168 (talk) 18:09, 1 July 2012 (UTC)
- They can rotate, but they can't swap positions. The carbon attached to the squiggly methyl group is bound to three distinct species, so the molecule is almost necessarily chiral. See also Amphetamine#Chemical_properties. Someguy1221 (talk) 08:13, 1 July 2012 (UTC)
- The "Skeletal formula" article has lots of details about the various conventions used in these types of diagrams. The specific structure the OP mentions is even the exact one used to illustrate the wavy-line meaning for stereochemistry:) DMacks (talk) 15:44, 2 July 2012 (UTC)
- Actually, these would not constitute two isomers. Whether it's going into or coming out of the page makes no difference to the structure, as the single bonds may rotate freely. 112.215.36.172 (talk) 07:53, 1 July 2012 (UTC)
question about foreskin retraction
Hi,I find a photograph series of foreskin retraction ,which is http://commons.wikimedia.org/wiki/File:A_Foreskin_Retraction_Series.JPG I want to ask if the process of the foreskin retraction is automatic in the above picture when the penis expands to erection ,or foreskin pulled back after the glans by the hands? Thanks!!! Pdh2441 (talk) 08:23, 1 July 2012 (UTC)pdh2441(talk), June 26,2012
- I don't think "retraction" is the right description, as the penis lengthens and extends beyond the foreskin during an erection. StuRat (talk) 08:57, 1 July 2012 (UTC)
- From our article: "There is considerable variation in the degree to which the foreskin retracts during erection; in some adults the foreskin remains covering the glans until retracted by sexual activity." For the specific picture you linked to, I haven't a clue. Probably no one will be able to answer that question, short of tracking the subject down and asking him. Evanh2008 (talk|contribs) 09:04, 1 July 2012 (UTC)
- I could be wrong, but it looks to me like the erection level of the penis hasn't significantly changed in most of the photo implying it was retracted manually. Nil Einne (talk) 17:52, 2 July 2012 (UTC)
- Note that foreskins aren't unique to humans, and many other animals would lack the ability to retract the foreskin manually, therefore it must be accomplished automatically. StuRat (talk) 09:09, 1 July 2012 (UTC)
- ....or not at all.--Shantavira|feed me 14:33, 1 July 2012 (UTC)
- Or as the above ref suggests incidentally by sexual activity. Nil Einne (talk) 17:52, 2 July 2012 (UTC)
- ....or not at all.--Shantavira|feed me 14:33, 1 July 2012 (UTC)
Does the Abyssinian Highland Zebu exist?
I came across the article Abyssinian Highland Zebu and went to expand it a little but I failed to find much on it. The only two places I have found it referenced is http://dad.fao.org/cgi-bin/EfabisWeb.cgi?sid=-1%2Creportsreport8a_50001170 which is the reference for the article, and http://www.ajol.info/index.php/ajb/art%20icle/viewFile/58020/46385 which is a research paper. I checked out the other two names given on the reference site, but none of those gave results for it either. — Preceding unsigned comment added by Manofgun (talk • contribs) 13:21, 1 July 2012 (UTC)
- From what I gather, the Abyssinian Highland Zebu is also known as the Ethiopian Highland Zebu or the Abyssinian Shorthorned Zebu. The International Livestock Research Institute has a page on this breed (or group of breeds): [1]. - Lindert (talk) 14:51, 1 July 2012 (UTC)
- So if the Abyssinian highland zebu is basically the abyssinian shorthorned zebu, should the highland zebu page be deleted? Manofgun (talk) 23:22, 1 July 2012 (UTC)
probablity of ejection in the photoelectric effect
I wanted to ask that if an electron absorbs a photon of energy 'e' which is greater than the work function, will the electron surely be ejected? Or is there some probability that the energy will just be re emitted?
P.S.- Our teacher at high school said there was no surety of ejection in the photoelectric effect, but i am not so sure on this one.
- I would reformulate the problem this way. The work function on average has a certain value for a sample of material; but the magnitude of the work function for any individual electron follows a distribution. So, you can statistically encounter some electrons who would not be ejected by the same energy photon, and conceivably you could trace that to a defect or nonuniformity in the material's lattice structure.
- On a completely different tack, raman scattering is the complex interaction of a photon with a material; it is rarely covered in high school physics classes because it's such a strange (and weak) effect, but it is real. It will often be explained as a "non-linear optics" phenomenon. If you treat the photoelectric effect as a special case of Raman scattering, you normally have an elastic collision, but occasionally have an inelastic scattering, yielding a photon with an unexpected wavelength, and an electron with an unexpected energy. The formal mathematics of this process are described in our article. Nimur (talk) 15:57, 1 July 2012 (UTC)
- Not to be confused with ramen scattering, which is when I try to break up my ramen noodles before putting them in the pot, and pieces go flying across the kitchen. :-) StuRat (talk) 17:25, 1 July 2012 (UTC)
about universe
is there any power that runs the whole universe.if it is there then what is it — Preceding unsigned comment added by Ram manohar 01 (talk • contribs) 16:26, 1 July 2012 (UTC)
- The power that runs the universe is pizza. Looie496 (talk) 16:34, 1 July 2012 (UTC)
- Please try to at least let someone give a helpful answer to a question before you start making inane comments. --Tango (talk) 18:16, 1 July 2012 (UTC)
- The energy released by the Big Bang, I suppose. This created atoms which have enough nuclear potential energy to power stars, once ignited, and generated enough gravitational potential energy to ignite the stars, too. It also created some heat/background radiation, but, being rather evenly distributed, that's not much of a power source. StuRat (talk) 17:17, 1 July 2012 (UTC)
- What do you mean by "power that runs the whole universe"? If you mean a higher power (ie. some kind of god or gods or spiritual force or whatever), then that's not within the realms of science and we can't help you on this desk. If you "power" as in energy, then the energy that runs the universe is just energy. If you mean some kind of underlying explanation for why things happen the way they do, then that's that physics is all about - trying to come up with that explanation. --Tango (talk) 18:16, 1 July 2012 (UTC)
- If you're asking what powers the expansion of the universe, that's dark energy. I suppose dark energy per unit time would be dark power, but I don't think anyone ever uses that term in that sense. 101.170.42.168 (talk) 18:32, 1 July 2012 (UTC)
- Dark energy causes the expansion to accelerate. Most of the existing rate of expansion is just momentum imparted by the big bang, though (at least, I think it is most, it's definitely a large proportion). --Tango (talk) 19:19, 1 July 2012 (UTC)
- Ok, our article Metric expansion of space says it is roughly half and half. --Tango (talk) 19:21, 1 July 2012 (UTC)
- Actually, all of it is currently powered by dark energy. Momentum is just that, and continues in the absence of any power being supplied. Power is the rate of energy being supplied, and momentum makes no contribution to that. 101.170.42.157 (talk) 19:24, 1 July 2012 (UTC)
- Ok, then, half the expansion is unpowered. --Tango (talk) 19:35, 1 July 2012 (UTC)
- That's the spirit. 101.170.42.157 (talk) 19:39, 1 July 2012 (UTC)
- Ok, then, half the expansion is unpowered. --Tango (talk) 19:35, 1 July 2012 (UTC)
- Actually, all of it is currently powered by dark energy. Momentum is just that, and continues in the absence of any power being supplied. Power is the rate of energy being supplied, and momentum makes no contribution to that. 101.170.42.157 (talk) 19:24, 1 July 2012 (UTC)
- It makes no sense to talk about "dark power"--that's a fundamental misunderstanding of the nature of dark energy. According to the best evidence available, dark energy is distributed uniformly across the universe with an energy density that does not change with time. Its effect on the universe's evolution depends solely on the value of this density. This energy, if that's what it really is, cannot be transferred or transported like energy through a heat pump; thus, "dark power" is a meaningless term. --140.180.5.169 (talk) —Preceding undated comment added 02:08, 2 July 2012 (UTC)
- Ok, our article Metric expansion of space says it is roughly half and half. --Tango (talk) 19:21, 1 July 2012 (UTC)
- Dark energy causes the expansion to accelerate. Most of the existing rate of expansion is just momentum imparted by the big bang, though (at least, I think it is most, it's definitely a large proportion). --Tango (talk) 19:19, 1 July 2012 (UTC)
- If you're asking what powers the expansion of the universe, that's dark energy. I suppose dark energy per unit time would be dark power, but I don't think anyone ever uses that term in that sense. 101.170.42.168 (talk) 18:32, 1 July 2012 (UTC)
No Drinking on Dialysis
Hello. Why are patients on dialysis not permitted to drink? I understand why they feel thirsty when the unit extracts the blood for cleaning. But what can go wrong if they just have a sip? Thanks in advance. --Mayfare (talk) 16:47, 1 July 2012 (UTC)
- We cannot give medical advice. Please consult a quilified medical practitioner. Roger (talk) 16:51, 1 July 2012 (UTC)
- Medical advice is telling them what they should do to improve their health, not explaining medical clinic rules. StuRat (talk) 17:12, 1 July 2012 (UTC)
- I don't believe there is a universal ban on drinking fluids while on hemodialysis. However, some clinics might want to ban eating and drinking, as it interferes with their ability to measure how much weight was taken off the patient by the dialysis, which is an important metric in determining how the patient's treatment is going. They could, of course, weigh anything the patient consumes and figure that into the calculation, but that would mean extra work for them. A secondary consideration is that drinks can be spilled, possibly damaging valuable medical equipment. (Of course, patients in hospital rooms consume fluids, but here the assumption is that a patient can go a few hours without a drink.) When my Dad was on dialysis, we found that some clinics/nurses were absolute Nazis about the rules, while others strived to accommodate the patients. StuRat (talk) 17:12, 1 July 2012 (UTC)
A successful dialysis was not seen until well after WW2. Was one of the "absolute Nazi" clinicians Dr. Josef Mengele? DriveByWire (talk) 00:14, 2 July 2012 (UTC)
- Not only can spillage damage machines but, if what you say is correct in the OP's case, spillage would throw off the measurements of weight loss. It would be difficult and likely impossible to measure what a person spilled versus what they drank. Dismas|(talk) 19:14, 1 July 2012 (UTC)
- But if you have kidney failure, you can't drink much anyway, so why would you want to drink while on dialysis? Count Iblis (talk) 19:27, 1 July 2012 (UTC)
- Sure they can drink, as long as the dialysis removes the water. Also, those with high output renal failure need dialysis to remove the waste, but can still pee out the water on their own. In any case, during dialysis, as the water is removed from their body, they naturally become thirsty, especially if the clinic overdoes it and dehydrates them. StuRat (talk) 21:00, 1 July 2012 (UTC)
- I understand that many (most?) hemodialysis patients are placed on very strict fluid control regimes. This paper, for instance, states:
- Advice on the restriction of fluid intake is a cornerstone of management of patients with kidney failure. Doctors ‘prescribe’ fluid restrictions on ward rounds for patients with acute renal failure. Nurses on the dialysis unit, backed up by dieticians and psychologists, exhort patients to drink less, telling those with large inter‐dialytic weight gains that they are putting themselves at risk of heart failure and death.
- though it goes on to argue that sodium control is more effective, "Look after the salt intake, and the water will look after itself." A web search suggests that the standard fluid intake limit is about 1 liter per day. I recall how surprised I was when I first heard this. A dialysis nurse I knew was complaining about about a patient, saying "Mr. X lived it up this weekend, drinking as much as he wanted to, but he sure paid for it today." I assumed she meant that he was drinking alcohol until she clarified that it was water. -- ToE 23:09, 1 July 2012 (UTC)
- I understand that many (most?) hemodialysis patients are placed on very strict fluid control regimes. This paper, for instance, states:
- Most likely, they don't want patients to eat or drink in case something goes wrong that requires emergency surgery. Surgeons don't want to perform operations if you've had anything to eat or drink in the last 8 hours, due to chances of you vomiting while under anesthesia (and subsequently choking to death on it). — The Hand That Feeds You:Bite 17:25, 2 July 2012 (UTC)
- Hemodialysis isn't likely to make surgery necessary. The most common immediate issues it causes are low blood pressure, dehydration, and problems from inappropriate medications/quantities administered intravenously during the session, none of which are addressed by surgery. An infection could require surgery (lancing the infected area and draining it), but an infection wouldn't set in until well after the session ended. StuRat (talk) 18:27, 2 July 2012 (UTC)
- Another concern with food and drink is that they can introduce bacteria into the area they want to keep sterile. StuRat (talk) 18:27, 2 July 2012 (UTC)
What level of weight loss causes stretch marks?
Well title is pretty self explanatory, what's the rate of kilos lost I should limit myself in order to avoid blemishes?Bastard Soap (talk) 18:45, 1 July 2012 (UTC)
- This question sounds like a request for medical advice. 101.170.42.166 (talk) 19:09, 1 July 2012 (UTC)
- This is strongly dependent on age. Younger people have more elastic skin than older people, so they can lose weight more rapidly without getting stretch marks. But even with age taken into account, skin elasticity is still pretty variable. Lots of women do fancy things in order to maintain it; I have no opinion about whether any of them are effective. Looie496 (talk) 19:01, 1 July 2012 (UTC)
- I'm a bit confused. Rapid weight gain causes stretch marks, while rapid weight loss can cause sagging skin. StuRat (talk) 21:02, 1 July 2012 (UTC)
- That's what I thought, but our article mentions both weight gain and weight loss as causes. --Tango (talk) 22:16, 1 July 2012 (UTC)
- Perhaps the unsupported sagging skin stretches under it's own weight, causing stretch marks. StuRat (talk) 02:18, 2 July 2012 (UTC)
- I think weight gain causes the stretch marks while weight loss makes the stretch marks visible. As for what you should do, that does come down to medical advice, it would depend on too many personal factors, such as your skin type, your current weight, your weight history, etc... The best you could get here are personal opinions and anecdotes which might not apply to your situation and could just as likely make your case worse instead of better. Vespine (talk) 22:43, 1 July 2012 (UTC)
Some ranges would be nice,for example most 20 year olds can lose easily 1kg per week,30year olds 1kg per two weeks. Some numbers and statistics to get an idea. I'm not aiming for an extreeme weight loss mind you,I'm probably safe, just want to make sure and possibly warn people who are dieting too harshly. You're a bit too loose on the medical question thing, every question concerning biology is a medical question. You should only refrain from providing information which can be dangerous. You can only stop me from getting stretch marks here you cannot cause harm as I won't change my program unless I find it will cause stretch marksBastard Soap (talk) 23:16, 1 July 2012 (UTC)
- It's not just about giving "dangerous" information, it's about accurate information. If you're just asking about general opinions on weight loss and stretch marks, that information is easily available a mere google search away. Vespine (talk) 00:23, 2 July 2012 (UTC)
I'm looking for numbers to get a general idea. If there is no accurate information, information with a known level on inaccuracy is the second best thing.And no, I'm not here for opinions, I'm here for the science on this, however little of it there might be. Science really seems to drop the ball on fitness stuff. Bastard Soap (talk) 04:50, 2 July 2012 (UTC)
- I doubt there would be a study, as it would be unethical to have people lose weight at variable rates, to see which ones suffer ill effects. (Noting that losing weight quickly enough to cause stretch marks may well cause other medical problems, too.) StuRat (talk) 18:21, 2 July 2012 (UTC)
An animal study wouldn't be too unethical. So we know nothing concrete about it? I'd expect some ammount of knowledge to have arisen from trial and error aloneBastard Soap (talk) 20:26, 2 July 2012 (UTC)
- I'm not sure if an animal study would be much use, as animal skin is quite a bit different (thickness, hair cover, etc.) and many other animals are designed to rapidly gain and lose weight, like those which hibernate. StuRat (talk) 20:29, 2 July 2012 (UTC)
- So, what you get, instead, is people self-reporting the conditions under which they got stretch marks, which doesn't qualify as "scientific research", but is still better than nothing. StuRat (talk) 20:31, 2 July 2012 (UTC)
Solar electrolysis
Can a solar panel be used to perform electrolysis, splitting water into hydrogen and oxygen? 64.229.5.242 (talk) 23:03, 1 July 2012 (UTC)
- Directly, or indirectly? Plasmic Physics (talk) 23:15, 1 July 2012 (UTC)
- Apparently yes, either way, according to my chemistry textbook. (The ones used for direct water splitting may not be the ordinary silicon-with-trace-amounts-of-phosphorus-and-boron kind, though...) Whoop whoop pull up Bitching Betty | Averted crashes 23:52, 1 July 2012 (UTC)
Yes. A solar panel or any other source of DC (direct current) can electrolyse water provided it overcomes the water cell potential nominally 1.23 V. Electrolysis of water (see article) works much faster when a water-soluble electrolyte e.g. NaCl (salt) is added to raise the conductivity of the water. DriveByWire (talk) 23:49, 1 July 2012 (UTC)
- But that would cause the production of sodium hydroxide and chlorine gas instead of oxygen gas, although it would produce hydrogen gas either way. Whoop whoop pull up Bitching Betty | Averted crashes 23:52, 1 July 2012 (UTC)
- Photoelectrolysis. Zing! ~AH1 (discuss!) 18:04, 3 July 2012 (UTC)
July 2
strange respirator
I saw this strange respirator/face shield combo. It looks like it may be disposable and it doesn't look like forms a very tight seal around the face. It also looks like it would be prone to fogging up quickly. It is apparently used by the CDC. Does anyone have any more information about this or how it works? Here is the picture- http://upload.wikimedia.org/wikipedia/commons/8/86/Influenza_virus_research.jpg — Preceding unsigned comment added by Wrk678 (talk • contribs)
- Looks like a very low-end device to me, just with a fan to blow air from the room into the face mask. Obviously not what you would want with a deadly microbe in the room (note the exposed ears), but perhaps enough if the chemical reaction you're working on may create a slight volume of irritating gas. StuRat (talk) 02:06, 2 July 2012 (UTC)
- There could easily be a filter in there somewhere. The lack of a tight seal around the face is a problem, though. I agree it's probably designed as a kind of wearable fume cupboard rather than as an infection control measure. --Tango (talk) 11:32, 2 July 2012 (UTC)
- Looks like they rely on the positive pressure created by the fan to blow air out from the edges of the face mask, preventing air around the mask from seeping in. Judging from the location of the air intake, you'd better be careful not to pass gas while using it. :-) StuRat (talk) 18:12, 2 July 2012 (UTC)
- BSL-3 isn't for instant-death-on-contact toxins and isn't usually designed for 100% body coverage. For this particular material being handled (influenza culture, according to the image-description), inhalable mists and perhaps small splashes are probably the hazards of interest, so physical barriers don't need to block from other directions as much, and positive air-flow from "away from the front work-area" (PAPR) prevents chemicals from getting to the breathing area. As long as there is positive air-flow, fogging is not any more of a problem than in other full-face breathing masks (diving, etc.) because the humid exhalation is constantly blown away out of the face-mask. But safety-equipment vendors will be happy to sell you all sorts of anti-fog sprays and tout all sorts of special coatings and such on the non-disposable models. DMacks (talk) 22:03, 2 July 2012 (UTC)
- Presumably they are working with an ordinary flu strain, already out in the public, so the risk both to the researchers and public, should it get out, is fairly minimal. StuRat (talk) 18:19, 3 July 2012 (UTC)

- Influenza A is listed as BSL-2. I'll embed the image here (makes it easier to access the WP page for the image and its links) and include a key part of the file-description in the caption to help us decide how "ordinary" it is vs requiring any enhanced protection per the descriptions of BSL-3. And this is all with hindsight information about the agent and its effects. Comparing the image info to the timeline of Spanish flu research, it doesn't seem like it was fully understood at the time. Handling a not-fully-characterized agent that had killed 3% of the world population seems like it would merit a cautious approach. DMacks (talk) 23:19, 3 July 2012 (UTC)
Can you survive without drinking fluids?
When I was checking my diet, I was surprised to learn how much water is present in the food I consume each day. At first I actually forgot to enter the 4 litres of water I drink each day and I got a warning that my fluid intake was too low. However it was still more than 1 liter (e.g. the 300 grams of brown rice I ate becomes 800 grams after cooking so this alone yields half a liter of water). So, I was wondering if the water that is present in bread, cooked rice, vegetables etc. etc. would be enough for the body if it is the recommended minimum amount of 1.5 litres. Or could it be that the more you eat, the more you need to drink to survive? Count Iblis (talk) 01:06, 2 July 2012 (UTC)
- Koalas don't drink water if their leafy diet is moist enough, which is most of the time. But I don't think humans could easily do it eating a normal diet, like the stuff you list. That's stuff you are already eating and it doesn't give you enough water, so doubling the amount of food you eat just to get enough water would not be practical. But I expect you could easily survive without actually drinking water if you changed your diet to include more moist fruit, like eat a watermellon and a few oranges every day or something. Vespine (talk) 01:18, 2 July 2012 (UTC)
- I suspect (and please nobody try this - just musing) that one could survive indefinitely in terms of fluids at least by eating a couple of large watermelons per day. --Kurt Shaped Box (talk) 01:22, 2 July 2012 (UTC)
- It wouldn't be a very balanced diet, but you could probably keep from dying of dehydration, at any rate. StuRat (talk) 02:13, 2 July 2012 (UTC)
- You'd think so, as watermelons can be comprised of upwards of 90% water and are rich in electrolytes. In reality though, watermelons also have a diuretic effect, making them impractical for maintaining hydration long-term. That level of consumption would also probably mean you were getting the vast majority of your calories from sugars, which would grow problematic quickly with regard to body weight, blood sugar levels and metabolic homeostasis in general. You could also end up turning your skin yellow-orange from carotenosis (watermelons are high in beta carotene), though this is an otherwise harmless condition. All of that said, for most in good health it would be possible to survive without hydration via pure water, provided you had a varied and tightly regimented diet. Snow (talk) 02:47, 2 July 2012 (UTC)
- This idea of watermelons being a diuretic is surprising, considering the considerable quantities of mellon consumed in hot countries such as Australia and Saudi Arabia. The fact is, as they are almost entirely water, for every 1 kg of mellon you eat, that's 1 litre of water you don't need to drink. My wife is on chemotherapy for her cancer. Consuming sufficient fluid is key to maximising the ratio of good effects of chemo (shrinking tumours) to bad effects (side effects) by flushing the chemo metabolites out. A local cancer support group advocates watermellons as a duretic, so she bought some. But she found she didn't like them, so I ate them (one half a good sized melon per day for a week). They certainly had no durectic effect on me, especially considering they are 95% water anyway. We asked her (very experienced) doctor - he stated that the idea of watermellons being a duretic was utter nonsense. I did a search of the internet - there are naturapathy and home remedy web sites that claim watermellons are a duretic, but the only "trustworthy" i.e., referenced article I could find was a report in a US newspaper published a few years ago. Ratbone58.169.255.232 (talk) 03:27, 2 July 2012 (UTC)
- I understand a healthy skepticism with regard to homeopathic remedies, certainly, but the idea of a watermelon being a diuretic actually makes sense, if only because of the glucose - mind you, in a healthy person with an average diet, glucose is a small factor, but when you're consuming that much sugar, it can inhibit the efficiency of a loop of henle from using osmotic gradients to reclaim water, which then instead is passed with the more dilute urine. That's without even factoring in the possibility of diabetes, which, with an all-watermelon diet, is gonna become a possibility! Notice also that while watermelons do posses electrolytes, they aren't present in as high a ratio as you usually get with other fruit, or even other melons, so the urine is going to be further dilute if you're entire diet consists of watermelon. So is it the same type of diuretic as, say, caffeine? Nah, but if you eat nothing but, you're gonna be getting pretty familiar with the nearest urinals. As to why it's so popular in hot countries, the answer is the same as why iced teas, chilled soda, cool beer, ice cream, and snow cones are all popular in those circumstances, despite being likely to dehydrate you in the long run - it's a refreshing, thirst-quenching, energy-packed treat. And short term it does hydrate you more. It's spread was also assisted benefited by way of historical economics too, since it was a cheap and efficient means of resupplying a labour force with calories and short-term hydration. Hence the melon's association with slave labour. In fact, in this way it would have had the same effect as beer did in earlier epochs of slave-labour - gives a burst of energy for the working hours and then leaves the worker drained when it comes time for rest - in the case of beer because of the alcohol content. In the case of watermelon though, it's because glucose is a simple sugar - meaning that it is assimilated into useful energy quite efficiently by the body and this does not maintain blood sugar and energy levels well over the long-haul. But yes, for your wife's needs there are definitely better options, I'm sure. But as to the issue of the proposed watermelon diet - I wouldn't recommend it for that purpose either! Snow (talk) 05:01, 2 July 2012 (UTC)
- This idea of watermelons being a diuretic is surprising, considering the considerable quantities of mellon consumed in hot countries such as Australia and Saudi Arabia. The fact is, as they are almost entirely water, for every 1 kg of mellon you eat, that's 1 litre of water you don't need to drink. My wife is on chemotherapy for her cancer. Consuming sufficient fluid is key to maximising the ratio of good effects of chemo (shrinking tumours) to bad effects (side effects) by flushing the chemo metabolites out. A local cancer support group advocates watermellons as a duretic, so she bought some. But she found she didn't like them, so I ate them (one half a good sized melon per day for a week). They certainly had no durectic effect on me, especially considering they are 95% water anyway. We asked her (very experienced) doctor - he stated that the idea of watermellons being a duretic was utter nonsense. I did a search of the internet - there are naturapathy and home remedy web sites that claim watermellons are a duretic, but the only "trustworthy" i.e., referenced article I could find was a report in a US newspaper published a few years ago. Ratbone58.169.255.232 (talk) 03:27, 2 July 2012 (UTC)
- You'd think so, as watermelons can be comprised of upwards of 90% water and are rich in electrolytes. In reality though, watermelons also have a diuretic effect, making them impractical for maintaining hydration long-term. That level of consumption would also probably mean you were getting the vast majority of your calories from sugars, which would grow problematic quickly with regard to body weight, blood sugar levels and metabolic homeostasis in general. You could also end up turning your skin yellow-orange from carotenosis (watermelons are high in beta carotene), though this is an otherwise harmless condition. All of that said, for most in good health it would be possible to survive without hydration via pure water, provided you had a varied and tightly regimented diet. Snow (talk) 02:47, 2 July 2012 (UTC)
- How much water you need to drink depends on whether you look short term or long term. The amount of fluid is not dependent on how much food you eat (because the large intestine recovers vitually all water used in digestion). In the very short term, how much you need to drink is dependent on ambient temperature and humidity - particularly if ambient temperature is above body temperature (~36 C). This is because at high temperature, the body cannot control core temperature by radiation and conduction and must resort to sweating. If the ambient is near to or above 36 C, then no heat is lost via radiation and conduction - sweating is the only way, and rises dramatically. I have worked oudoors in remote parts of Australia. I usually need only drink ~~300 ml of water per day when working indoors and up to 35 C or so, but when working in 45 C I drink several litres per day, and at 50 C I need to drink continuously. You can only keep this up short term. Longer term it appears that urinary tract problems (infections, kidney stones) rise in probability unless your kidneys get a good flush - the rule of thumb is that if your pee stream looks clear, you are drinking adequately, if yellowish, you are not. The common "medical" advice to drink x litres a day (I notice that x varies quite a bit from reference to reference) is nonsense - what matters is how much you pee. This rule means peeing about 500 mL per day (average weight person), and up to 750 mL per day if physically working hard) If you are cold, you don't sweat, so you only need to consume (drink plus water in food) enough to pee about 500 ml per day. But if you located in an ambient of 45 C, you will sweat roughly 3 litres/day (average sized person, light work), so you need to drink 3 + 0.5 = 3.5 litres per day. If working physically hard at 45 C, it will be Y + 0.75 l/day, where Y may be very much larger than 3 l/day. Ratbone60.230.225.247 (talk) 02:25, 2 July 2012 (UTC)
[Discussion on body temperature typo removed and typo corrected] Ratbone124.182.182.130 (talk) 02:12, 3 July 2012 (UTC)
Yes, you can survive without drinking as do thousands of hospital patients being hydrated by IV drip. DriveByWire (talk) 16:04, 2 July 2012 (UTC)
Thanks for the answers! So, if it doesn't depend on how much you eat, you should be able to get the necessary amounts of water from eating relatively dry stuff alone like bread and rice, as I'm close to 1 liter from such sources alone (I also get something from fruits and vegetables, bringing the total over 1 liter). However, I do feel I need to drink a lot of water, though. Count Iblis (talk) 16:20, 3 July 2012 (UTC)
- It does matter how much and what you eat:
- 1) Excess fluid is needed for the digestion process. The amount of fluid your body can add to your digestive tract is limited, so you either need to eat moist food or drink fluids.
- 2) Your body must maintain a balance between water and salt and other solutes. Thus, when you consume more of those items, you need to consume more water, to dilute them. StuRat (talk) 16:37, 3 July 2012 (UTC)
- You might also be interested in metabolic water - 300 grams of rice (dry weight) gets turned into ~ 150 ml of water. IIRC this is an important source of water for desert animals like kangaroo rats. SmartSE (talk) 17:56, 3 July 2012 (UTC)
- This brings to mind a recent TV report in Canada claiming that the eight glasses a day guideline is actually a myth, and nobody can find the source of this claim. Most food is likely to contain water, though if you're trying to drink more than 1.8 litres of water at once, beware fatal water intoxication.
- From fluid balance:
- The common misconception that everyone should drink two litres (68 ounces, or about eight 8-oz glasses) of water per day is not supported by scientific research. Various reviews of all the scientific literature on the topic performed in 2002 and 2008 could not find any solid scientific evidence that recommended drinking eight glasses of water per day. ~AH1 (discuss!) 18:00, 3 July 2012 (UTC)
Suppositories - and the people who eat them
Just something based on a silly conversation I had today, and granted, there may not really be a good answer for this (but here goes)... Does anyone know if it's actually common for people who've been prescribed suppositories by their doctor to return a couple of days later and complain that the things are really difficult to swallow? Or is it more of an apocryphal tale? --Kurt Shaped Box (talk) 01:13, 2 July 2012 (UTC)
- I suspect so, since there are plenty of oral laxatives, too, an inserting any med into the rectum seems very odd. So, those unfamiliar with them who don't read the label might very well swallow them. StuRat (talk) 02:11, 2 July 2012 (UTC)
- On a quick look, this supports the idea that patients do sometimes do nonstandard things with medication designed to be delivered this way; whereas this one, perhaps surprisingly, dismisses as probably apocryphal the whole idea. I strongly suspect that a more structured search on PubMed or whatever would find more articles confirming that the problem is real. For some reason the BMJ Christmas edition springs to mind here ... Best wishes DBaK (talk) 07:49, 2 July 2012 (UTC)
- On a related note - anyone got a reference for the 'I eat a tampon every month but it has no effect on the duration of my period' woman? Or the 'both my legs and one of my arms hurt when I touch them' guy (who actually has a broken finger)? I do suspect that these are urban legends. --Kurt Shaped Box (talk) 23:00, 2 July 2012 (UTC)
- The former sounds like a "blonde joke". The latter was in Henny Youngman's catalog of one-liners. As regards eating those things, the article implies that it would be harmless, albeit probably not very effective. ←Baseball Bugs What's up, Doc? carrots→ 23:25, 3 July 2012 (UTC)
- It occurs to me, Bugs, the suppository would still get where it needed to go. Someguy1221 (talk) 02:01, 4 July 2012 (UTC)
- The former sounds like a "blonde joke". The latter was in Henny Youngman's catalog of one-liners. As regards eating those things, the article implies that it would be harmless, albeit probably not very effective. ←Baseball Bugs What's up, Doc? carrots→ 23:25, 3 July 2012 (UTC)
- On a related note - anyone got a reference for the 'I eat a tampon every month but it has no effect on the duration of my period' woman? Or the 'both my legs and one of my arms hurt when I touch them' guy (who actually has a broken finger)? I do suspect that these are urban legends. --Kurt Shaped Box (talk) 23:00, 2 July 2012 (UTC)
Turkey Turquoise
Added section header Rojomoke (talk) 06:39, 2 July 2012 (UTC)
Many big E-seller sites clients when describing their merchandise are confusing the terms for colors with the synonymns for minerals, such as silver, tibetan silver, or turquoise. I frequently see the term "turkey turquoise" which is not covered in wikipedia, except for word origin for turquoise. Please clarify the term "turkey turqoise" as it seems to include black and white, as well as many other color stones. Buyers have no hardness testing standards to check the main characteristic, while being subject to the technical correctness of being a turquoise color. If you can get E-seller sites to accept standard definitions it would be most helpful.
Thank you, 75.186.36.70 (talk) 06:20, 2 July 2012 (UTC)
- http://www.internetstones.com/turquoise-gemstone-jewelry-gallery.html has a mention of it. I see Ebay has lots of listings with turquoise in quotations like 'ranch fur' which is artificial fur. They may have many artificial types of 'turquoise' as well.--Canoe1967 (talk) 17:53, 2 July 2012 (UTC)
Restricted FTL Without Causality Violation?
Ok, I've been brainstorming ideas for a story setting featuring faster than the speed of light interstellar travel, and trying to avoid 'easy' causality violations as a result. I'm wondering if this is possible.
The key trick I'm considering is this: in this setting, the FTL technology itself is hopelessly out of the realm of humans, but rather consists of automated ships flying fixed routes, that people merely piggyback on. So the question is this: can there been a configuration of fixed routes, and intervals, and speeds for FTL, that will allow FTL to take place, but which will avoid creating observable causality paradoxes (at least, without substantial effort)?
Some thoughts I'm considering: 1. How about setting the speeds of the Ships such that a 'round trip' will always arrive after one has set off? Is this possible, or will this lead to a contradiction?
2. How about changing the network shape so that certain causality violations cannot happen? I'm not sure how, but maybe a network of routes without any loops in it would work?
3. How about having it so that all star systems the Ships go to are *stationary* relative to each other?
Any other ideas? Would these suggestions work?--Fangz (talk) 11:45, 2 July 2012 (UTC)
- Even if FTL travel were possible, it would not allow for traveling backwards in time for two reasons, first that the experienced time would be imaginary, not negative, second that even if someone traveling FTL would experience negative time flow, that would make that person younger, but he/she would still arrive at his/her destination after takeoff. That in itself creates a contradiction, because if someone becomes younger, he/she will never have departed in the first place. The problem with describing this scenario is not that the technology is not available, but rather it is fundamentally impossible and therefore cannot be described in terms of relativistic physics. It's like asking what would be the circumference of a square circle. If you still want to write such a story, you'll have to make up your own physics, so asking at the science desk may not be of much help. - Lindert (talk) 12:45, 2 July 2012 (UTC)
- Well, the issue isn't so much 'can FTL be done with current physics'. The question is 'can FTL be sealed into a black box so as not to imply various large and disruptive effects on the rest of the story'. Assuming as much of relativity etc is preserved as possible, the ideal is that we have a situation where (ignoring how this is done), people can go to some distant star and back again in a reasonable time, without logically implying that they can make a phone call to themselves in the past, etc. --Fangz (talk) 13:15, 2 July 2012 (UTC)
See here for a detailed discussion. Count Iblis (talk) 16:50, 2 July 2012 (UTC)
- It's actually not that hard to invent a way of doing FTL without violating causality. The easiest way, I think, is to assume there is a preferred reference frame (an "ether"), and only permit travel that goes forward in time in the preferred reference frame. That way it will still be possible to appear to go backward in time, but it will not be possible to do a loop that brings you back to the starting point earlier than you left. Looie496 (talk) 17:37, 2 July 2012 (UTC)
- I suggest using the many worlds hypothesis. Then, when you go back in time, you appear in another universe, identical to our own up to the time when you arrived. You are then free to mess that universe up as bad as you want to, since it won't affect ours. You can even return to our own universe, but not at a point earlier than when you left. You could then use this other universe as a simulation of what will happen in our own, given certain actions on your part. StuRat (talk) 18:01, 2 July 2012 (UTC)
Your biological clock can travel faster than light in vitrified cryonic suspension. However, the clocks everywhere else will be tens of thousands of years in the future. 75.166.192.187 (talk) 19:18, 2 July 2012 (UTC)
- If the technology was available, I'd make a wormhole, put one end on Earth, the other on a spaceprobe, send it on a time dilated 10 year round trip journey, and plan to walk through it when it arrives 50 years later. When 50 years older me walks through the stargate only 10 years later I'd ask him what my children's names are and then not name them those names. Sagittarian Milky Way (talk) 20:09, 2 July 2012 (UTC)
- I like the idea of wormholes with the many worlds hypothesis. That way, I can ask my kind friends from the future to please work out the tech, then send me back a gram of gold as proof it works, and because this one moment in time corresponds to a bazillion possible futures, it'll add up to a massive pile of loot. The problem is, trying to calibrate it so that the incomprehensibly large number of possible followers of this missive in possible futures don't end up sending so much gold that it collapses into a black hole around the Earth, at which point it wouldn't be gold any more. But I suppose I won't get gold back from the possible universes where the Earth collapsed, only the ones where it didn't, which means that the pile should be manageably small, only a few cubic miles... hmmm, but then the others get the chance to send their share after all... maybe there's a happy medium where there's, like, a 10-10-10 chance that the Earth doesn't collapse into a gold-fueled black hole, reducing the amount of gold received to what just barely allows that chance that somebody survives? ;) Wnt (talk) 20:41, 2 July 2012 (UTC)
Making a primitive bathtub
Let's say the world runs out of oil, natural gas, coal and all that and we're all back to 15th century living. I was wondering if there would be any way to construct a functional bathtub so that I could at least enjoy that creature comfort. The heat source would either be directly from a fire underneath, or by taking rocks out of a fire and placing them in the tub, probably with a loose buffer between the rocks and the bottom of the tub. Does anyone have the slightest clue how you might fashion a tub like this? I suppose the simplest solution would be to drag a bathtub out of a derelict house and use that. But is it possible to create a tightly-joined wooden tub, for instance, that won't be horrendously leaky? Thanks. Vranak (talk) 18:15, 2 July 2012 (UTC)
- Carve one out of a tree trunk, sand it, and seal it with oil (animal grease will work). This would work better with the "hot rocks dropped into the water" method. Flip it over to drain it, or add a drain and plug. (Of course, during the 15th century they could make elaborate ceramic bathtubs, but you had to be rich to afford one.) StuRat (talk) 18:34, 2 July 2012 (UTC)
- I hate to state the obvious, but in the actual "pre-technological" past, nobody used bathtubs that had active flames or heat sources as part of them, for the fairly obvious reason that there are real risks of burning or scalding associated with that. It's much easier to heat the water first and then add it to the tub, mixed with as much cold water as you need to keep things at a desired, non-cooking temperature. The same as we usually do now, frankly — we just have elaborate ways of moving the hot and cold water from tanks that you can't see. You can make basins out of wood, ceramics, or metal with technology that is well prior to the 15th century (there are evidence of this technology back to the Ancient world, it isn't a new thing), and can heat water similarly. --Mr.98 (talk) 19:06, 2 July 2012 (UTC)
- Whoo there! If the world runs out of oil, natural gas and coal then you're going to compound the problem by burning what combustibles are left. If you look at Jenny Agutter here [2]. She seem very happy in a naturally formed, solar heated Billabong (there are better images of her than this but they are a wee bit too revealing). What more creature comforts do you need than that? --Aspro (talk) 19:10, 2 July 2012 (UTC)
- If you want to have a proper bath, I'd forget about 15th-century technology, and go back a lot further - the Romans had a better approach. And yes, the water was heated by 'active flames', along with the rest of the building. AndyTheGrump (talk) 19:42, 2 July 2012 (UTC)
- I'm assuming they want to make this tub themself, not using the skills of metal smelters or a Roman engineering team. StuRat (talk) 19:57, 2 July 2012 (UTC)
- By active flames, I just meant, nobody sits in an individual bathtub with a fire underneath it. For water with a very large volume, maybe you could get away with it. But it would be hard to control to temperature, easy to get burnt. There's a reason that for small basins, people have nearly always heated the water separately and then mixed it with cold water to get the right temperature. --Mr.98 (talk) 21:43, 2 July 2012 (UTC)
- Japanese Goemon bath (named after Ishikawa Goemon) is a kind of bathtub you described. A metal bathtub made of iron, with a fire underneath it. --Kusunose 04:09, 4 July 2012 (UTC)
- By active flames, I just meant, nobody sits in an individual bathtub with a fire underneath it. For water with a very large volume, maybe you could get away with it. But it would be hard to control to temperature, easy to get burnt. There's a reason that for small basins, people have nearly always heated the water separately and then mixed it with cold water to get the right temperature. --Mr.98 (talk) 21:43, 2 July 2012 (UTC)
- Running out of fossil fuels doesn't mean going back to 15th century technology, but going forward to 20th or 21st century tech. Try a solar hot water heater, for example. Wnt (talk) 20:25, 2 July 2012 (UTC)
- Or just using wood, which is renewable. --Mr.98 (talk) 21:08, 2 July 2012 (UTC)
Thanks everyone, very interesting answers so far. Vranak (talk) 00:42, 3 July 2012 (UTC)
- Wood is a pretty good thermal insulator, so externallly heating a wooden tub of water is not going to work. Do what is a Japanese tradition. Use your wood fire (which can be the fire you use to cook with) to heat rocks. When rocks are hot, place in wooden tub of water, or natural pool of water. Rocks will heat the water very effectively but are too hot to step or sit directly upon, so place a piece of wood on top of the rocks. Ratbone124.182.182.130 (talk) 02:18, 3 July 2012 (UTC)
Traditional Japanese bathtubs are made of wood. See Furo. --Kusunose 04:25, 4 July 2012 (UTC)
Local robot SETI
The question on the cost of interstellar colonization veered into local robot SETI, after taking a turn into statistics education:
- Hypothetically, if machines are going to colonize the galaxy, then I wonder which planets / moons they would prefer? In terms of building durable, long-lasting technology there would seem to be some real advantages to avoiding both weather and biological life. Robots, at least the simple ones that humans have been able to make, tend to operate best is simple and predictable environments. A robot colonist first arriving in the solar system might well think that the Moon or Mars is a more inviting first stop than a place like Earth. Engineers motivated by "moral" concerns might even tell the robots to always avoid planets (or even whole systems) with biological life. Dragons flight (talk) 23:57, 30 June 2012 (UTC)
- Position preferences are things that life has, but I'm not sure if machines would have strong position preferences. 75.166.192.187 (talk) 01:31, 1 July 2012 (UTC)
- Large robots might prefer asteroid belts or planetary rings, as those provide lots of materials, sunlight (for solar power), and less gravity/atmospheric resistance to fight against (especially if they want to leave orbit). Smaller bots would have insufficient shielding to protect their electronics, control systems, etc., from radiation damage and micro-meteors. StuRat (talk) 02:26, 2 July 2012 (UTC)
- That is why Asteroid 2010 SO16 should be explored.
Is there a better target for local robot SETI exploration than Asteroid 2010 SO16? 75.166.192.187 (talk) 19:15, 2 July 2012 (UTC)
- Well, if it's just a single robot which lacks the ability to move to other asteroids, moons, and planets, you might want it to explore a large object, like the Moon or Mars, since it will soon explore all of any one asteroid. However, if it can move on to others, or has the ability to replicate itself and send it's copies on, this no longer applies. Also note that Asteroid 2010 SO16 is only interesting because of it's unusual orbit, not because it shows any promise of extraterrestrial intelligence. When searching our solar system for life, the best bet is planets and moons large enough to have an atmosphere and/or oceans. StuRat (talk) 19:47, 2 July 2012 (UTC)
- This may be too speculative, but wouldn't a single large robot want to keep hidden from nuclear capable biological organisms? Wouldn't it attempt to communicate by placing artifacts in impossibly unnatural orbits just out of reach of biological civilizations without global cooperation, or at least some semblance of it? 70.91.171.54 (talk) 02:50, 3 July 2012 (UTC)
The smart thing to do for a machine civilization is to first colonize their own solar system. Then, instead of attepting to directly move on to another solar system, they could attempt to detect signals from another machine civilization. There is then the possibility of traveling directly to another machine civilization by uploading the software and the design of the machines to the other civilization, or to download a copy of another civilization and implement that civilization here.
This way, a civilization can jump from one place to another place millions of lightyears away. The entire output of the star is available for transmitting the data, so an astronomical high data rate can be achieved over long distances. Count Iblis (talk) 16:14, 3 July 2012 (UTC)
- I think nonbiological machine civilizations would be more likely to get along with each other than life-based civilizations, and they would also want to keep life around, but at a distance, because they would admire the Darwinian nanomachines of life. But they would also see life as dangerous and unpredictable, I would think. I doubt a machine civilization would want to stop life from colonizing other planets in the same or different solar system, but it seems very unlikely that they would take any steps to help unless the life made some really concrete progress on reducing nuclear weapons. If the machines did not feel vulnerable, then they might want to help in the same way a gardner might want to make cuttings of a favorite tree for a different garden. 75.166.192.187 (talk) 22:47, 3 July 2012 (UTC)
Energy in a mass of fluid
Im guessing the components of energy in a mass of fluid are kinetic energy, potential energy and internal energy. If so, what is the source of each of the components of these energies? 176.250.196.132 (talk) 19:18, 2 July 2012 (UTC)
- Kinetic: motion of molecules, from weather, heat, life and seismic disturbances; potential: gravitation and binding energy of nucleons; internal: heat, turbulence, laminar flow. 75.166.192.187 (talk) 19:26, 2 July 2012 (UTC)
- Heat being the same as kinetic motion of molecules, added to molecular vibration and rotation, of course. Ratbone124.182.182.130 (talk) 02:22, 3 July 2012 (UTC)
- There is of course also turbulence kinetic energy. ~AH1 (discuss!) 17:47, 3 July 2012 (UTC)
- Heat being the same as kinetic motion of molecules, added to molecular vibration and rotation, of course. Ratbone124.182.182.130 (talk) 02:22, 3 July 2012 (UTC)
Please help me
- This question has been removed as it may be a request for medical advice. Wikipedia does not give medical advice because there is no guarantee that our advice would be accurate or relate to you and your symptoms. We simply cannot be an alternative to visiting the appropriate health professional, so we implore you to try them instead. If this is not a request for medical advice, please explain what you meant to ask, either here or at the talk page discussion (if a link was provided).
Why isn't solid combustion a popular topic?
There's talk of engines that run on biodiesel, ethanol, vegetable oil, algae oil, restaurant deep fryer crap, grease, crud so viscuous it needs to be heated to flow, why not very fine powders like sawdust? Sagittarian Milky Way (talk) 20:44, 2 July 2012 (UTC)
- Feeding into into the combustion chamber is more difficult, but not impossible. The extra weight and complexity involved in using something like Archimedes' screw to feed it in makes it not a very good choice for a vehicle, but it might work for heating a building (say the saw mill where the sawdust is generated). StuRat (talk) 21:48, 2 July 2012 (UTC)
- To expand on that, a powdered fuel might require larger diameter injection tubes, would have to be air-pumped through the engine, it might clog the airtight valves around the piston, and you'd need to find a lubricant for the piston that didn't gunk up with the powder. A sawmill might find it easier to burn off excess dust in a steam-powered generator designed to handle it without exploding. Someguy1221 (talk) 22:03, 2 July 2012 (UTC)
- Gasoline is chemically stable at high temperature, burns at a predictable rate, and does not burn at all in the absence of oxygen. On the other hand, sawdust decomposes to charcoal at high temperatures and has a huge surface-area-to-volume ratio, meaning that it burns explosively when spread around the air at the right density. Sawdust particles are irregularly shaped, meaning there's going to be large amounts of air trapped inside, so combustion rate is difficult to control. --140.180.5.169 (talk) 23:26, 2 July 2012 (UTC)
- Technically, nothing burns in the absence of oxygen. But yes, you're right about sawdust not burning in a manner conducive to automobile engines. In fact, I'm not sure there's any solid fuel that would work well in an internal combustion engine. As I see it, the easiest way to get an automobile running on solid fuel would be to introduce coal-fired steam engines, but that brings with it a whole host of other problems. Evanh2008 (talk|contribs) 02:29, 3 July 2012 (UTC)
- The above post by 140.180.5.169 is a little misleading, as hydrocarbon fuels (eg gasoline, diesel) also decompose at high temperatures - if temperature is high enough, what you get is a mix of monatomic hydrogen and carbon.
- A perhaps more precise answer to the OP's question is that actual combustion can only occur when the fuel is in gaseous form. For fuels like gasoline and diesel, all that is required to get from the liquid to the gaseous form is vaporisation. Combustion then occurs at a very predictable and controlled rate and proceeds to virtual completion (ie exhaust is almost entirely water vapour and carbon dioxide. Any solid fuel, like wood, that cannot be vapourised or sublimated (http://en.wikipedia.org/wiki/Sublimation_(phase_transition)) must first be pyrolysed, i.e., chemically (driven by heat) separated into gas components, which can be burnt. This takes too much time in an engine, is too variable, and gnerally won't be a complete conversion. As well as the injection mechanical issues mentioned by StuRat, pyrolysation of wood results in an unburnable component, ash, as well as the gas components. It is quite simple to remove accumulating ash periodically, or by conveyor belt etc from a furnace at atmospheric pressure. However, removing ash from an internal combustion engine is likely to be difficult, and will cause extremely rapid wear. Which leads to how wood, swadust, and wood flour HAS been succesfully used to run an IC engine:-
- Producer Gas method (http://en.wikipedia.org/wiki/Producer_gas)- wood is burnt in oxygen starved conditions in a vessel at atmospheric pressure. The resulting fumes, which are carbon & hydrogen rich, are sucked into a conventional piston engine. Every few 10's of miles you add more wood and dump the ash. Many early IC engines were designed to run on producer gas. During World War 2, gasoline use in Australia was restricted to military, police, and emergency services. Ordinary citizens purchased producer units, usually installed on trailers, to run their cars. It was very succesfull, though the engine power output was quite a bit less than with petrol. Producer units are simple low tech - many folk made their own. You may enjoy http://www.lowtechmagazine.com/2010/01/wood-gas-cars.html
- Ratbone124.182.182.130 (talk) 02:51, 3 July 2012 (UTC)
- Apparently there are designs for gunpowder engines. Mythbusters tried it on a 2006 show but it didn't work.Sjö (talk) 06:07, 3 July 2012 (UTC)
Genes coding for brain stuff
What percentage of the human genome codes for brain related stuff, and is this number typical for mammals? 65.92.7.168 (talk) 20:52, 2 July 2012 (UTC)
- According to the Allen Institute for Brain Science, 82% of human genes are expressed in the brain. It should be noted that most of these genes will also be expressed elsewhere in the body. A similar number is found in monkeys, in this paper, although you have to dig through their methods a bit to figure that out. Furthermore, that papers shows that less than 10% of genes in the brain are even unique to it. I'm guessing the inspiration for this question is that you are wondering whether smarter animal means more genes that work in the brain, but that is unlikely to be true. What's far more likely, and what researchers are actually looking for, are changes in those genes, that are already expressed in the brain. Someguy1221 (talk) 21:15, 2 July 2012 (UTC)
- Yes, strange as it seems, it appears that one gene can do different (apparently unrelated) things, in different parts of the body. The FOXP2 gene comes to mind. StuRat (talk) 22:36, 2 July 2012 (UTC)
- And that doesn't even take into account alternative splicing. A single "gene" could potentially encode thousands of distinct proteins. Most studies of the transcriptomes or proteomes of specific tissues do not distinguish between the different isoforms, unless alternative splicing was the precise focus of the study, which is rare. So when a paper says that 90% of the genes expressed in the brain are also expressed in the liver, we can't say for certain the same isoforms are expressed in both tissues. It's possible reanalysis of existing deep sequencing done on cDNA could reveal the answer in this specific context. Someguy1221 (talk) 00:46, 3 July 2012 (UTC)
- There's not actually a lot of difference between a brain cell, and a skin cell, or for that matter any cell. They all have nucleus, mitochondria, membrane, etc, etc. That's why >82% of genome expression is common. What is more important for brain functionality is the interconnection of neurons. On a macro scale it is genetically coded, but on the micro level it isn't. In fact there is nowhere near enough data capacity in our DNA to specify the interconnectivity. Connections are made during experience-driven development with a high degree of randomness - no 2 persons (even identical twins) have their brains wired up even approximately identically. Ratbone124.182.182.130 (talk) 03:07, 3 July 2012 (UTC)
Perfect, thanks for the answers. 65.92.7.168 (talk) 04:01, 4 July 2012 (UTC)
Salt water fuel
I read recently there was a man that used a certain type of radio wave, put it over salt water, and it combusted. He thinks that it is possible that one day this method could be used in the future. I realized one problem with it could be the fact that it takes more power to make the radio work than it produces, but what byproduct would this method of combustion make? 64.229.5.242 (talk) 21:29, 2 July 2012 (UTC)
- Generally these schemes and scams are some sort of electrolysis or related process, which converts water into hydrogen and oxygen, which burn to give water. See Stanley Meyer's water fuel cell for one of the popular forms of this nonsense. DMacks (talk) 21:38, 2 July 2012 (UTC)
- I would like to answer your question about how harmless the by-products are but it is a commercial secret. However, I can let you know privately if you invest in the development of this amazing high return –guaranteed!!!-- opportunity of a lifetime. You will miss the boat if you waste time asking more questions – just send your money to me- then lay back and dream about how you will spend that fortune that awaits you. --Aspro (talk) 21:42, 2 July 2012 (UTC)
- And you're right that you'd need to put more energy in than you get out. An exception is using substances which already have a lot of chemical potential energy. Such substances tend to be flammable and/or explosive or at least give off heat spontaneously. Salt water doesn't. StuRat (talk) 21:44, 2 July 2012 (UTC)
This one specifically is the invention of John Kanzius. It has been discussed previously on the science desk, here and here. Basically, what you saw is real. Passing radio waves through saltwater at the appropriate frequencies does liberate a flammable gas, which combusts spontaneously during the experiment. But you are also correct that this is absolutely not a way to generate energy. When the combustion occurs, all you will have done is reconstitute what was originally in the salt water, and so you could not have released any net energy from the water. A use might still be found for it, but it certainly won't be energy production. Someguy1221 (talk) 21:50, 2 July 2012 (UTC)
- That mysterious gas is just hydrogen, no? Evanh2008 (talk|contribs) 22:27, 2 July 2012 (UTC)
- And some pure oxygen, presumably, which makes it burn better than hydrogen in air. StuRat (talk) 22:33, 2 July 2012 (UTC)
- If you're electrolysing salt water, though, aren't you going to be getting chlorine and unpleasant sodium-oxygen-hydrogen compounds in the place of oxygen? Evanh2008 (talk|contribs) 22:39, 2 July 2012 (UTC)
- I'm assuming that the salt merely acts as a catalyst for the hydrolysis of water, and does not itself react. StuRat (talk) 22:44, 2 July 2012 (UTC)
July 3
Succulent plant ID and advice please
Hello everyone. Awhile back I bought a dumpy little succulent plant from a street vendor and to my surprise it has done really well at home. I have turned its tray many times, but have ended up with a sprawling beast somewhat reminiscent of a grasping hand. [[3]] [[4]]There are now also plantlets budding at the base of the plant. I would like to know what kind of succulent this is, and what I can/should do to ensure its safe further growth. Can these be propagated via cuttings, for example? Thank you. The Masked Booby (talk) 00:34, 3 July 2012 (UTC)
- I believe that's Crassula marnieriana (the picture on our page certainly doesn't look much like your plant, but cf. the image here, for instance). Deor (talk) 02:18, 3 July 2012 (UTC)
- My theory is, "if it ain't broke, don't fix it". However, it will soon outgrow that pot, so you might want to transplant it into a larger pot. I'd take the entire root ball, including those other plants, if the roots are intertwined, and plop it all into the larger pot, so your plant is now centered, and fill in the empty space with more of the same soil you are using. StuRat (talk) 06:27, 3 July 2012 (UTC)
- Crassulas generally do well in spaces where their roots are slightly confined, giving them too much space may cause watering problems and root rot. They come from areas with a hot sunny climate so the best you could do for your plant is get it into some sunlight (the sempervivums in the pot would also benefit). If they get enough sun they will get nice pink edges to their leaves and even flower. Crassulas generally are tough plants that can be propagated easily. See here for more good info and pictures Richard Avery (talk) 08:00, 3 July 2012 (UTC)
- How does extra space cause watering problems and root rot ? StuRat (talk) 16:43, 3 July 2012 (UTC)
- Because when the soil is watered the plant does not take it all up sufficiently quickly and there is a much greater risk that the roots will saturate and rot. this is a succulent that has developed to live in harsh and arid surroundings and will not like to be mollycoddled with lots of new soil. My experience has shown that people tend to kill plants with "kindness" not so much with neglect, especially crassulas who love neglect - but love sunshine even more. Richard Avery (talk) 19:04, 3 July 2012 (UTC)
- Why can't you put it in a larger pot, but continue to water it a much as before ? StuRat (talk) 04:22, 4 July 2012 (UTC)
- Yes, you can. If you are an experienced plant owner then that's fine, but I was just giving a little warning about a common problem among less experienced plant carers. Richard Avery (talk) 07:01, 4 July 2012 (UTC)
- Why can't you put it in a larger pot, but continue to water it a much as before ? StuRat (talk) 04:22, 4 July 2012 (UTC)
Why fungi was differ of green plant?
Why fungi was differ of green plant? Can you give me main points thanks. — Preceding unsigned comment added by 175.137.136.85 (talk) 00:46, 3 July 2012 (UTC)
- Please see fungus and plant. They are completely different kinds of organisms, although they are commonly and mistakenly believed to be closely related. If you still have questions after reading those, feel free to come back and ask them. Someguy1221 (talk) 00:48, 3 July 2012 (UTC)
- It's enough to read fungus, which covers the differences specifically. Looie496 (talk) 01:02, 3 July 2012 (UTC)
- Just go to any party, where it's simple to tell the diff between a fun guy and the common wallflower. StuRat (talk) 06:30, 3 July 2012 (UTC)
- You may also be interested in mycorrhiza, hypha and actinomycetes. ~AH1 (discuss!) 17:43, 3 July 2012 (UTC)
ID Please (Cixiidae)
I suppose this is a Cixiidae. Could you able to suggest the species or genus, please. Thanks, Jee Jkadavoor (talk) 08:10, 3 July 2012 (UTC)
- You'll have more success if you tell people where you took it. In this case I can see in the description it is from Kadavoor, Kerala, India. If no one here can help, then try WT:INSECT or http://www.whatsthatbug.com SmartSE (talk) 17:50, 3 July 2012 (UTC)
Does swimming make you lose muscle mass?
I've been hearing by a lot of very sure sounding people that if you're a body builder and you start swimming your muscle mass will shrink. From my medical knowledge I believe that only if you do very intense cardio you will lose muscle mass and swimming will not reduce muscle unless you do very intense long periods of it. Am I wrong? Bastard Soap (talk) 11:37, 3 July 2012 (UTC)
- The answer depends on a number of factors that are not presupposed in your question. I'm presuming, for examples, that by "lose" you mean that your overall mass will decrease, but not so much as a result of the swimming itself, but rather from the redirection of your priorities in working out (that is, that you could maintain your current mass or even improve it if you stuck to your current routine). In this respect, yes, it may be that you would lose some overall mass depending on how much you re-prioritized your exercise. That being said, as a body builder you probably are already quite familiar with the concept that you reach a point of diminishing returns with repetitive motions, so supplementing your workout with swimming might not cause you to lose overall mass at all, or plausibly to put more on, since it is a workout that, almost more than any other, works a broad array of muscles. But your mileage will vary considerably with the amount of time you decide to put in, needless to say. Also note that there is difference between overall mass and the characteristic bulk of a bodybuilder's physique. In any event, if you are going to start doing any form of intensive cardio after a long period of being committed to anaerobic exercise you should take things slowly at first and ideally consult a doctor -- for the obvious safety reasons and because he'll also likely be able to provide you assistance in meeting your training goals. Let me know if this is more basic than you intended and I'll go into further detail. Snow (talk) 13:19, 3 July 2012 (UTC)
- As long as you are getting adequate nutrition, I can't see why intense cardio would cause a reduction in muscle mass, all else being equal (as Snow Rise says, if you are doing cardio instead of other exercise, then it might, but that's just because of a reduction in the other exercise). --Tango (talk) 15:08, 3 July 2012 (UTC)
- Agreed. Specifically, if you were concentrating on a small number of muscles in your previous workout (bodybuilders with a massive chest and tiny legs come to mind. ), and then switch to swimming, you will lose those few large muscles and get more of an even muscle tone. StuRat (talk) 16:51, 3 July 2012 (UTC)
Let's say you keep your training program with weights and add swimming at a rate that isn't too intensive, it will only help you build muscle right? Snow Rise if you have anything more to add feel free Bastard Soap (talk) 17:51, 3 July 2012 (UTC)
- That's correct; as far as I know, there is no major metabolic reason why swimming, or any cardio, would cause you to retain less mass, if it were just added on top of your existing routine. With regard to bodybuilding (or any kind of strength training), there are two different kinds of muscle tissue growth at work: muscular hyperplasia (the increase in the number of cells in the relevant tissues) and muscular hypertrophy (the increase in the size of the cells in those same tissues, since, unlike many other forms of tissue, this can be made quite variable through exercise). The interaction between these two different types of muscle development, and other factors, are also probably known to you already through the concept of "bulk vs. tone"; in general, the extra mass you gain from swimming will tend towards the "toned" end of the spectrum, owing to the fact that you will be "recruiting" (as exercise physiologists say) a larger number of muscles per motion, but stressing most of them far less (again, per motion) towards top exertion and because certain types of muscle respond differently in terms of growth to different exercise. Bodybuilders often exploit this formula to tailor their workouts by either doing a higher number of reps with lower weight for tone or a lower number of reps with higher weight for bulk and the same principles will apply here, only, since the level of resistance is determined only by the water and your muscles leveraging off of each other and the rest of your body (as opposed to free weights where you can change the resistance), the overall trend will be towards the muscle added to be "toned". This can be controlled somewhat by choosing your swimming motions and is also variable depending upon how fast as opposed to how long you choose to swim, your nutrition, and your unique metabolism -- but again, the overall trend will be toward toned muscle in the new mass. Note that there are other potential benefits to adding muscle in this fashion, including increased muscle stamina, flexibility and even strength; bodybuilders are typically strong, of course, but not always as strong as they look, since they tend to prioritize hypertrophic exercises that promote bulk vs. those that provide strength (this is why the fields of bodybuilder and weight-lifter are not automatically interchangeable). This type of exercise also promotes strength by making sure that the inter-dependencies between muscles are strengthened as well, such that they grow together and there's less occurrence of a "weak link" (an underdeveloped muscle group which inhibits peak performance/efficiency of another in a specific movement by not being able to pull it's own weight, so to speak ;). There's also the fact that incorporating more muscles means you will be developing a broader base of muscle tissue types. Hope that helps - though, not to sound like a broken record, but all radical changes to your exercise routine should be broached with a physician, even if you think of yourself as being in good health! Snow (talk) 21:00, 3 July 2012 (UTC)
- These Olympic medallists are primarily swimmers. Most guys would envy their muscle mass. HiLo48 (talk) 22:53, 3 July 2012 (UTC)
- As Tango wrote above, you have to make sure you eat enough when you do a lot of cardio exercise. E.g. I exercise 35 to 40 minute per day, I do quite heavy cardio exercise, and I really need to eat about 3500 Kcal/day just to keep my weight of about 59 Kg stable. A more extreme example is Michael Phelps' diet. Count Iblis (talk) 01:06, 4 July 2012 (UTC)
addmision
Hello.Good evening.I want to register in your university but I am a muslim woman.Can I accept with your university? Thank you of your consideration. — Preceding unsigned comment added by 199.127.102.196 (talk) 16:34, 3 July 2012 (UTC)
- Yes. Just about any college or university in the West will be open to people of all religions and genders. The only case of religious exclusion I can think of is a seminary, where people train to be Christian or Jewish missionaries. There may still be a tiny number of single-sex education universities, but those are more likely to be female only, in any case. Depending on how conservative your religious beliefs are, you might prefer an all-female college. StuRat (talk) 16:53, 3 July 2012 (UTC)
- Messages such as this mean that a person tried to Google for a university, found Wikipedia as the first link and followed it thinking it is the web site of the university, followed the "Information" link that appears and thereby got taken to the Ref Desks, and asked a question thinking she was asking it of an official representative of the university. It is useless to answer them. Looie496 (talk) 17:38, 3 July 2012 (UTC)
- (edit conflict) Are you asking about Wikiversity? If so, then of course you can join - anyone can. It's not a real university though... SmartSE (talk) 17:39, 3 July 2012 (UTC)
- [Edit conflict x 2]Although Wikipedia is not a university, we do have a sister project over at Wikiversity. For higher education schools in the Miami area, try Universities and colleges in Florida. ~AH1 (discuss!) 17:40, 3 July 2012 (UTC)
This question is positive feedback for us! Count Iblis (talk) 17:57, 3 July 2012 (UTC)
- And who wouldn't want to learn at the feet of such sage and knowledgeable personages? :) Snow (talk) 04:36, 4 July 2012 (UTC)
- Just a word of advice, if you (the original poster) are planning on applying to a Western university: do not, under any circumstances, mention religion in a conversation with a university representative. This isn't because Muslims are not welcome (discrimination based on religion is illegal), or that the university will necessarily be biased; it's because religion is simply not an appropriate topic. In both Canada and the US, for example, it is outright illegal for employers to ask candidates about their religion. I don't know if the same applies to admissions interviews, but there's certainly a strong cultural aversion against it. --140.180.5.169 (talk) 02:41, 4 July 2012 (UTC)
- At least in some Western countries, however, while there is no discrimination on religous grounds (Moslem women are welcomed at the Australian university I (an Australian male) went to, there are some minor practical issues that you probably should be aware of. Generally, Australian universities make it a condition of graduation that you hold an Australian Senior First Aid certificate, or an equivalent. Since most undergraduates do not have it, classes are run on campus, and this involves practicing on each other in a mixed sex class (bandaging, placing inert buddy in coma position, feeling for pulse at wrist, neck & ankle, feeling ribs for breathing, etc). This is all very ho-hum boring for westerners, but moslems may be uncomfortable. Usually, the university is happy to run an all-female class for first aid or accept a first aid certificate from your home country. I did engineering - this involves getting assignments to be completed in groups of 4 to 6, generally for a week or 2. The assignments are designed so that there is too much work for one person, so the group must coordinate and cooperate. Naturally, your predominantly Australian male class mates will want to hold assigment coordination meetings, and joint work ssesions, at places convenient to the greatest number - this may be in a University library room booked for the purpose, someone's house, or even, sometimes, a nearby coffee house. So you need to be comfortable around males. When I was at uni, a couple of moslem girls brought chaperones along - that's cool, we understood. When I've visited moslem friends, their female folk did not come into the same room, per their custom. Westerners, especially Australians, don't work that way - all of both sexes will expect to be introduced, and any may offer you food and drink. None of this should distress you - the predominant reaction of western male (& female) students will be to adjust to your needs and look after you, not distress or embarras you. In some moslem countries (eg Iran), if you are offered sweats to eat, it means "please eat and then leave". Again, Westerners are different. If they offer you extra food, it means they like you, you are not required to eat it if you don't want to, but please don't leave yet. If you drive a motor vehicle (almost a necessity in Australia unless you live in a campus college or live nearby), and you are veiled, the police may require you to show your face for identification purposes - this is the only circumstance where you would be required to do so. If you come from a couple of countries, in particular Iran and Afganistan, when you return home after completing your degree, your experience may be misunderstood. Ratbone124.178.52.55 (talk) 03:38, 4 July 2012 (UTC)
- If I was offered sweats to eat, I'd certainly take the hint and leave. Offer me sweets, on the other hand, and I'd never leave. :-) StuRat (talk) 04:31, 4 July 2012 (UTC)
- Yeah, we Ozzies will bend over and spread 'em for anyone who comes here. My advice to Moslems is "You are in someone else's country. Try to behave like them, or go home. You would expect us to do the same if we visited YOUR local toilet (sorry, nation)". Why should we change so that backward, violent and primitive customs can be accommodated? No sir, not me.... (But I wouldn't mind some of those sweats tho...) Myles325a (talk) 09:14, 4 July 2012 (UTC)
July 4
Pollination
How pollen get to the stigma? — Preceding unsigned comment added by 175.137.136.34 (talk) 00:39, 4 July 2012 (UTC)
- Please read the article pollination. It should answer your question pretty thoroughly. Evanh2008 (talk|contribs) 00:43, 4 July 2012 (UTC)
Artificial Insemination for "Lonesome George"?
The recent death of the last Pinta Island tortoise, Lonesome George, resulted in the extinction of this sub-species of Galapagos tortoises [here]. Attempts to mate him with related species did not work, as Lonesome was not really interested. I am wondering why Artificial Insemination was not used, as it is in just about any species which have a commercial angle for humans.
Then again, I am not really sure how AI works (Same acronmy as Artificial Intelligence!). Is it just a case of masturbating the animal with electricity? If so, can I buy such a device? Maybe with tortoises, it would just take too long. Would they let him look at some piccies of really horny she-tortoises while they did it?
I've heard that men in comas have "donated" sperm via this procedure. It gives the term "manual work" a whole new dimension. Seriously, though, how DOES AI work, and could it have been used in Lonesome George's case? Coz if it could, we could all have these lovely creatures home as pets - there would be zillions of 'em! Myles325a (talk) 09:04, 4 July 2012 (UTC)
in this context what is the Golden Channel? Kittybrewster ☎ 09:08, 4 July 2012 (UTC)
- It's a fine stream of sub-atomic particles. That's why it's often called the "Golden Showers". Myles325a (talk) 09:16, 4 July 2012 (UTC)







![{\displaystyle {\sqrt[{3}]{\frac {1}{6.022\times 10^{22}}}}=2.55\times 10^{-8}cm}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b2195fb22bf320aafbf3b1537b590b465c896a4f)





![{\displaystyle {\sqrt[{3}]{6.022\times 10^{22}}}=3.92\times 10^{7}atoms}](https://wikimedia.org/api/rest_v1/media/math/render/svg/0c009e96bc3ef2dbb08ef649a59170e042905549)

