Influenza
| Influenza | |
|---|---|
| Specialty | Family medicine, pulmonology, infectious diseases, emergency medicine |
| Influenza (flu) |
|---|
 |
Influenza, commonly referred to as the flu, is an infectious disease caused by RNA viruses of the family Orthomyxoviridae (the influenza viruses), that affects birds and mammals. The name influenza comes from the Italian influenza, meaning "influence" (Template:Lang-la). The most common symptoms of the disease are chills, fever, sore throat, muscle pains, severe headache, coughing, weakness and general discomfort.[1] Fever and coughs are the most frequent symptoms. In more serious cases, influenza causes pneumonia, which can be fatal, particularly for the young and the elderly. Although it is often confused with other influenza-like illnesses, especially the common cold, influenza is a much more severe disease than the common cold and is caused by a different type of virus.[2] Influenza may produce nausea and vomiting, particularly in children,[1] but these symptoms are more common in the unrelated gastroenteritis, which is sometimes called "stomach flu" or "24-hour flu".[3]
Typically, influenza is transmitted through the air by coughs or sneezes, creating aerosols containing the virus. Influenza can also be transmitted by bird droppings, saliva, nasal secretions, feces and blood. Infection can also occur through contact with these body fluids or through contact with contaminated surfaces. Airborne aerosols have been thought to cause most infections, although which means of transmission is most important is not absolutely clear. Influenza viruses can be inactivated by sunlight, disinfectants and detergents.[4][5] As the virus can be inactivated by soap, frequent hand washing reduces the risk of infection.
Influenza spreads around the world in seasonal epidemics, resulting in the deaths of hundreds of thousands annually — millions in pandemic years. Three influenza pandemics occurred in the 20th century and killed tens of millions of people, with each of these pandemics being caused by the appearance of a new strain of the virus in humans. Often, these new strains appear when an existing flu virus spreads to humans from other animal species, or when an existing human strain picks up new genes from a virus that usually infects birds or pigs. An avian strain named H5N1 raised the concern of a new influenza pandemic, after it emerged in Asia in the 1990s, but it has not evolved to a form that spreads easily between people.[6] In April 2009 a novel flu strain evolved that combined genes from human, pig, and bird flu, initially dubbed "swine flu", emerged in Mexico, the United States, and several other nations. WHO officially declared the outbreak to be a "pandemic" on June 11, 2009.
Vaccinations against influenza are usually given to people in developed countries [7] and to farmed poultry.[8] The most common human vaccine is the trivalent influenza vaccine (TIV) that contains purified and inactivated material from three viral strains. Typically, this vaccine includes material from two influenza A virus subtypes and one influenza B virus strain.[9] The TIV carries no risk of transmitting the disease, and it has very low reactivity. A vaccine formulated for one year may be ineffective in the following year, since the influenza virus evolves rapidly, and new strains quickly replace the older ones. Antiviral drugs can be used to treat influenza, with neuraminidase inhibitors being particularly effective.
Classification
Types of influenza virus

In virus classification influenza viruses are RNA viruses that make up three of the five genera of the family Orthomyxoviridae:[10]
These viruses are only distantly related to the human parainfluenza viruses, which are RNA viruses belonging to the paramyxovirus family that are a common cause of respiratory infections in children such as croup,[11] but can also cause a disease similar to influenza in adults.[12]
Influenzavirus A
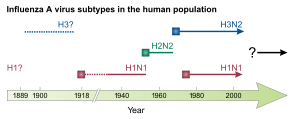
This genus has one species, influenza A virus. Wild aquatic birds are the natural hosts for a large variety of influenza A. Occasionally, viruses are transmitted to other species and may then cause devastating outbreaks in domestic poultry or give rise to human influenza pandemics.[13] The type A viruses are the most virulent human pathogens among the three influenza types and cause the most severe disease. The influenza A virus can be subdivided into different serotypes based on the antibody response to these viruses.[14] The serotypes that have been confirmed in humans, ordered by the number of known human pandemic deaths, are:
- H1N1, which caused Spanish flu in 1918, and the 2009 flu pandemic
- H2N2, which caused Asian Flu in 1957
- H3N2, which caused Hong Kong Flu in 1968
- H5N1, a current pandemic threat
- H7N7, which has unusual zoonotic potential[15]
- H1N2, endemic in humans and pigs
- H9N2
- H7N2
- H7N3
- H10N7
Influenzavirus B

This genus has one species, influenza B virus. Influenza B almost exclusively infects humans[14] and is less common than influenza A. The only other animals known to be susceptible to influenza B infection are the seal[16] and the ferret.[17] This type of influenza mutates at a rate 2–3 times lower than type A[18] and consequently is less genetically diverse, with only one influenza B serotype.[14] As a result of this lack of antigenic diversity, a degree of immunity to influenza B is usually acquired at an early age. However, influenza B mutates enough that lasting immunity is not possible.[19] This reduced rate of antigenic change, combined with its limited host range (inhibiting cross species antigenic shift), ensures that pandemics of influenza B do not occur.[20]
Influenzavirus C
This genus has one species, influenza C virus, which infects humans, dogs and pigs, sometimes causing both severe illness and local epidemics.[21][22] However, influenza C is less common than the other types and usually only causes mild disease in children.[23][24]
Structure, properties, and subtype nomenclature
Influenzaviruses A, B and C are very similar in overall structure.[25] The virus particle is 80–120 nanometres in diameter and usually roughly spherical, although filamentous forms can occur.[26][27] These filamentous forms are more common in influenza C, which can form cordlike structures up to 500 micrometres long on the surfaces of infected cells.[28] However, despite these varied shapes, the viral particles of all influenza viruses are similar in composition.[28] These are made of a viral envelope containing two main types of glycoproteins, wrapped around a central core. The central core contains the viral RNA genome and other viral proteins that package and protect this RNA.[27] Unusually for a virus, its genome is not a single piece of nucleic acid; instead, it contains seven or eight pieces of segmented negative-sense RNA, each piece of RNA contains either one or two genes.[28] For example, the influenza A genome contains 11 genes on eight pieces of RNA, encoding for 11 proteins: hemagglutinin (HA), neuraminidase (NA), nucleoprotein (NP), M1, M2, NS1, NS2(NEP), PA, PB1, PB1-F2 and PB2.[29]
Hemagglutinin (HA) and neuraminidase (NA) are the two large glycoproteins on the outside of the viral particles. HA is a lectin that mediates binding of the virus to target cells and entry of the viral genome into the target cell, while NA is involved in the release of progeny virus from infected cells, by cleaving sugars that bind the mature viral particles.[30] Thus, these proteins are targets for antiviral drugs.[31] Furthermore, they are antigens to which antibodies can be raised. Influenza A viruses are classified into subtypes based on antibody responses to HA and NA. These different types of HA and NA form the basis of the H and N distinctions in, for example, H5N1.[32] There are 16 H and 9 N subtypes known, but only H 1, 2 and 3, and N 1 and 2 are commonly found in humans.[33]
Replication

Viruses can only replicate in living cells.[34] Influenza infection and replication is a multi-step process: firstly the virus has to bind to and enter the cell, then deliver its genome to a site where it can produce new copies of viral proteins and RNA, assemble these components into new viral particles and finally exit the host cell.[28]
Influenza viruses bind through hemagglutinin onto sialic acid sugars on the surfaces of epithelial cells; typically in the nose, throat and lungs of mammals and intestines of birds (Stage 1 in infection figure).[35] After the hemagglutinin is cleaved by a protease, the cell imports the virus by endocytosis.[36]
Once inside the cell, the acidic conditions in the endosome cause two events to happen: first part of the hemagglutinin protein fuses the viral envelope with the vacuole's membrane, then the M2 ion channel allows protons to move through the viral envelope and acidify the core of the virus, which causes the core to dissemble and release the viral RNA and core proteins.[28] The viral RNA (vRNA) molecules, accessory proteins and RNA-dependent RNA polymerase are then released into the cytoplasm (Stage 2).[37] The M2 ion channel is blocked by amantadine drugs, preventing infection.[38]
These core proteins and vRNA form a complex that is transported into the cell nucleus, where the RNA-dependent RNA polymerase begins transcribing complementary positive-sense vRNA (Steps 3a and b).[39] The vRNA is either exported into the cytoplasm and translated (step 4), or remains in the nucleus. Newly synthesised viral proteins are either secreted through the Golgi apparatus onto the cell surface (in the case of neuraminidase and hemagglutinin, step 5b) or transported back into the nucleus to bind vRNA and form new viral genome particles (step 5a). Other viral proteins have multiple actions in the host cell, including degrading cellular mRNA and using the released nucleotides for vRNA synthesis and also inhibiting translation of host-cell mRNAs.[40]
Negative-sense vRNAs that form the genomes of future viruses, RNA-dependent RNA polymerase, and other viral proteins are assembled into a virion. Hemagglutinin and neuraminidase molecules cluster into a bulge in the cell membrane. The vRNA and viral core proteins leave the nucleus and enter this membrane protrusion (step 6). The mature virus buds off from the cell in a sphere of host phospholipid membrane, acquiring hemagglutinin and neuraminidase with this membrane coat (step 7).[41] As before, the viruses adhere to the cell through hemagglutinin; the mature viruses detach once their neuraminidase has cleaved sialic acid residues from the host cell.[35] Drugs that inhibit neuraminidase, such as oseltamivir, therefore prevent the release of new infectious viruses and halt viral replication.[31] After the release of new influenza viruses, the host cell dies.
Because of the absence of RNA proofreading enzymes, the RNA-dependent RNA polymerase that copies the viral genome makes an error roughly every 10 thousand nucleotides, which is the approximate length of the influenza vRNA. Hence, the majority of newly manufactured influenza viruses are mutants, this causes "antigenic drift", which is a slow change in the antigens on the viral surface over time.[42] The separation of the genome into eight separate segments of vRNA allows mixing or reassortment of vRNAs if more than one type of influenza virus infects a single cell. The resulting rapid change in viral genetics produces antigenic shifts, which are sudden changes from one antigen to another. These sudden large changes allow the virus to infect new host species and quickly overcome protective immunity.[32] This is important in the emergence of pandemics, as discussed below in the section on Epidemiology.
Signs and symptoms

Symptoms of influenza can start quite suddenly one to two days after infection. Usually the first symptoms are chills or a chilly sensation, but fever is also common early in the infection, with body temperatures ranging from 38-39 °C (approximately 100-103 °F).[45] Many people are so ill that they are confined to bed for several days, with aches and pains throughout their bodies, which are worse in their backs and legs.[1] Symptoms of influenza may include:
- Body aches, especially joints and throat
- Extreme coldness and fever
- Fatigue
- Headache
- Irritated watering eyes
- Reddened eyes, skin (especially face), mouth, throat and nose
- In children, gastrointestinal symptoms such as diarrhoea and abdominal pain,[46][47] (may be severe in children with influenza B)[48]
It can be difficult to distinguish between the common cold and influenza in the early stages of these infections,[2] but a flu can be identified by a high fever with a sudden onset and extreme fatigue. Diarrhoea is not normally a symptom of influenza in adults,[44] although it has been seen in some human cases of the H5N1 "bird flu"[49] and can be a symptom in children.[46] The symptoms most reliably seen in influenza are shown in the table to the right.[44]
| Symptom: | sensitivity | specificity |
|---|---|---|
| Fever | 68-86% | 25-73% |
| Cough | 84-98% | 7-29% |
| Nasal congestion | 68–91% | 19–41% |
|
Notes to table:
| ||
Since anti-viral drugs are effective in treating influenza if given early (see treatment section, below), it can be important to identify cases early. Of the symptoms listed above, the combinations of fever with cough, sore throat and/or nasal conjestion can improve diagnostic accuracy.[50] Two decision analysis studies[51][52] suggest that during local outbreaks of influenza, the prevalence will be over 70%,[52] and thus patients with any of these combinations of symptoms may be treated with neuraminidase inhibitors without testing. Even in the absence of a local outbreak, treatment may be justified in the elderly during the influenza season as long as the prevalence is over 15%.[52]
The available laboratory tests for influenza continue to improve. The United States Centers for Disease Control and Prevention (CDC) maintains an up-to-date summary of available laboratory tests.[53] According to the CDC, rapid diagnostic tests have a sensitivity of 70–75% and specificity of 90–95% when compared with viral culture. These tests may be especially useful during the influenza season (prevalence=25%) but in the absence of a local outbreak, or peri-influenza season (prevalence=10%[52]).
Mechanism
Transmission

People who contract influenza are most infective between the second and third days after infection and infectivity lasts for around ten days.[54] Children are much more infectious than adults and shed virus from just before they develop symptoms until two weeks after infection.[54][55] The transmission of influenza can be modeled mathematically, which helps predict how the virus will spread in a population.[56]
Influenza can be spread in three main ways: by direct transmission when an infected person sneezes mucus into the eyes, nose or mouth of another person; through people inhaling the aerosols produced by infected people coughing, sneezing and spitting; and through hand-to-mouth transmission from either contaminated surfaces or direct personal contact, such as a hand-shake.[57][58] The relative importance of these three modes of transmission is unclear, and they may all contribute to the spread of the virus.[59][60] In the airborne route, the droplets that are small enough for people to inhale are 0.5 to 5 µm in diameter and inhaling just one droplet might be enough to cause an infection.[57] Although a single sneeze releases up to 40,000 droplets,[61] most of these droplets are quite large and will quickly settle out of the air.[57] How long influenza survives in airborne droplets seems to be influenced by the levels of humidity and UV radiation: with low humidity and a lack of sunlight in winter probably aiding its survival.[57]
As the influenza virus can persist outside of the body, it can also be transmitted by contaminated surfaces such as banknotes,[62] doorknobs, light switches and other household items.[1] The length of time the virus will persist on a surface varies, with the virus surviving for one to two days on hard, non-porous surfaces such as plastic or metal, for about fifteen minutes from dry paper tissues, and only five minutes on skin.[63] However, if the virus is present in mucus, this can protect it for longer periods.[57] Avian influenza viruses can survive indefinitely when frozen.[64] They are inactivated by heating to 56 °C (133 °F) for a minimum of 60 minutes, as well as by acids (at pH <2).[64]
Pathophysiology

The mechanisms by which influenza infection causes symptoms in humans have been studied intensively. Consequently, knowing which genes are carried by a particular strain can help predict how well it will infect humans and how severe this infection will be (that is, predict the strain's pathophysiology).[22][65]
For instance, part of the process that allows influenza viruses to invade cells is the cleavage of the viral hemagglutinin protein by any one of several human proteases.[36] In mild and avirulent viruses, the structure of the hemagglutinin means that it can only be cleaved by proteases found in the throat and lungs, so these viruses cannot infect other tissues. However, in highly virulent strains, such as H5N1, the hemagglutinin can be cleaved by a wide variety of proteases, allowing the virus to spread throughout the body.[65]
The viral hemagglutinin protein is responsible for determining both which species a strain can infect and where in the human respiratory tract a strain of influenza will bind.[66] Strains that are easily transmitted between people have hemagglutinin proteins that bind to receptors in the upper part of the respiratory tract, such as in the nose, throat and mouth. In contrast, the highly-lethal H5N1 strain binds to receptors that are mostly found deep in the lungs.[67] This difference in the site of infection may be part of the reason why the H5N1 strain causes severe viral pneumonia in the lungs, but is not easily transmitted by people coughing and sneezing.[68][69]
Common symptoms of the flu such as fever, headaches, and fatigue are the result of the huge amounts of proinflammatory cytokines and chemokines (such as interferon or tumor necrosis factor) produced from influenza-infected cells.[2][70] In contrast to the rhinovirus that causes the common cold, influenza does cause tissue damage, so symptoms are not entirely due to the inflammatory response.[71] This massive immune response might produce a life-threatening cytokine storm. This effect has been proposed to be the cause of the unusual lethality of both the H5N1 avian influenza,[72] and the 1918 pandemic strain.[73][74] However, another possibility is that these large amounts of cytokines are just a result of the massive levels of viral replication produced by these strains, and the immune response does not itself contribute to the disease.[75]
Prevention
Vaccination

Vaccination against influenza with an influenza vaccine is often recommended for high-risk groups, such as children and the elderly, or in people who have asthma, diabetes, or heart disease. Influenza vaccines can be produced in several ways; the most common method is to grow the virus in fertilized hen eggs. After purification, the virus is inactivated (for example, by treatment with detergent) to produce an inactivated-virus vaccine. Alternatively, the virus can be grown in eggs until it loses virulence and the avirulent virus given as a live vaccine.[32] The effectiveness of these influenza vaccines are variable. Due to the high mutation rate of the virus, a particular influenza vaccine usually confers protection for no more than a few years. Every year, the World Health Organization predicts which strains of the virus are most likely to be circulating in the next year, allowing pharmaceutical companies to develop vaccines that will provide the best immunity against these strains.[76] Vaccines have also been developed to protect poultry from avian influenza. These vaccines can be effective against multiple strains and are used either as part of a preventative strategy, or combined with culling in attempts to eradicate outbreaks.[77]
It is possible to get vaccinated and still get influenza. The vaccine is reformulated each season for a few specific flu strains but cannot possibly include all the strains actively infecting people in the world for that season. It takes about six months for the manufacturers to formulate and produce the millions of doses required to deal with the seasonal epidemics; occasionally, a new or overlooked strain becomes prominent during that time and infects people although they have been vaccinated (as by the H3N2 Fujian flu in the 2003–2004 flu season).[78] It is also possible to get infected just before vaccination and get sick with the very strain that the vaccine is supposed to prevent, as the vaccine takes about two weeks to become effective.[79]
The 2006–2007 season was the first in which the CDC had recommended that children younger than 59 months receive the annual influenza vaccine.[80] Vaccines can cause the immune system to react as if the body were actually being infected, and general infection symptoms (many cold and flu symptoms are just general infection symptoms) can appear, though these symptoms are usually not as severe or long-lasting as influenza. The most dangerous side-effect is a severe allergic reaction to either the virus material itself or residues from the hen eggs used to grow the influenza; however, these reactions are extremely rare.[81]
Infection control
Good personal health and hygiene habits, like hand washing, avoiding spitting, and covering the nose and mouth when sneezing or coughing, are reasonably effective in reducing influenza transmission.[82] In particular, hand-washing with soap and water, or with alcohol-based hand rubs, is very effective at inactivating influenza viruses.[83] These simple personal hygiene precautions are recommended as the main way of reducing infections during pandemics.[82] Although face masks might help prevent transmission when caring for the sick, [84][85] there is mixed evidence on beneficial effects in the community.[82][86]
Since influenza spreads through both aerosols and contact with contaminated surfaces, surface sanitizing may help prevent some infections.[87] Alcohol is an effective sanitizer against influenza viruses, while quaternary ammonium compounds can be used with alcohol so that the sanitizing effect lasts for longer.[88] In hospitals, quaternary ammonium compounds and bleach are used to sanitize rooms or equipment that have been occupied by patients with influenza symptoms.[88] At home, this can be done effectively with a diluted chlorine bleach.[89]
During past pandemics, closing schools, churches and theaters slowed the spread of the virus but did not have a large effect on the overall death rate.[90][91] It is uncertain if reducing public gatherings, by for example closing schools and workplaces, will reduce transmission since people with influenza may just be moved from one area to another; such measures would also be difficult to enforce and might be unpopular.[82] When small numbers of people are infected, isolating the sick might reduce the risk of transmission.[82]
Treatment
People with the flu are advised to get plenty of rest, drink plenty of liquids, avoid using alcohol and tobacco and, if necessary, take medications such as paracetamol (acetaminophen) to relieve the fever and muscle aches associated with the flu. Children and teenagers with flu symptoms (particularly fever) should avoid taking aspirin during an influenza infection (especially influenza type B), because doing so can lead to Reye's syndrome, a rare but potentially fatal disease of the liver.[92] Since influenza is caused by a virus, antibiotics have no effect on the infection; unless prescribed for secondary infections such as bacterial pneumonia. Antiviral medication can be effective, but some strains of influenza can show resistance to the standard antiviral drugs.[93]
The two classes of antiviral drugs used against influenza are neuraminidase inhibitors and M2 protein inhibitors (adamantane derivatives). Neuraminidase inhibitors are currently preferred for flu virus infections since they are less toxic and more effective.[75] The CDC recommended against using M2 inhibitors during the 2005–06 influenza season due to high levels of drug resistance.[94] As pregnant women seem to be more severely affected than the general population by the 2009 H1N1 influenza virus, prompt treatment with anti-influenza drugs has been recommended.[95]
Neuraminidase inhibitors
Antiviral drugs such as oseltamivir (trade name Tamiflu) and zanamivir (trade name Relenza) are neuraminidase inhibitors that are designed to halt the spread of the virus in the body.[96] These drugs are often effective against both influenza A and B.[97] The Cochrane Collaboration reviewed these drugs and concluded that they reduce symptoms and complications.[98] Different strains of influenza viruses have differing degrees of resistance against these antivirals, and it is impossible to predict what degree of resistance a future pandemic strain might have.[99]
M2 inhibitors (adamantanes)
The antiviral drugs amantadine and rimantadine block a viral ion channel (M2 protein) and prevent the virus from infecting cells.[38] These drugs are sometimes effective against influenza A if given early in the infection but are always ineffective against influenza B because B viruses do not possess M2 molecules.[97] Measured resistance to amantadine and rimantadine in American isolates of H3N2 has increased to 91% in 2005.[100] This high level of resistance may be due to the easy availability of amantadines as part of over-the-counter cold remedies in countries such as China and Russia,[101] and their use to prevent outbreaks of influenza in farmed poultry.[102][103]
Prognosis
Influenza's effects are much more severe and last longer than those of the common cold. Most people will recover completely in about one to two weeks, but others will develop life-threatening complications (such as pneumonia). Influenza, however, can be deadly, especially for the weak, old, or chronically ill.[32] People with a weak immune system, such as people with advanced HIV infection or transplant patients (whose immune systems are medically suppressed to prevent transplant organ rejection), suffer from particularly severe disease.[104] Other high-risk groups include pregnant women and young children.[105]
The flu can worsen chronic health problems. People with emphysema, chronic bronchitis or asthma may experience shortness of breath while they have the flu, and influenza may cause worsening of coronary heart disease or congestive heart failure.[106] Smoking is another risk factor associated with more serious disease and increased mortality from influenza.[107]
According to the World Health Organization: "Every winter, tens of millions of people get the flu. Most are only ill and out of work for a week, yet the elderly are at a higher risk of death from the illness. We know the worldwide death toll exceeds a few hundred thousand people a year, but even in developed countries the numbers are uncertain, because medical authorities don't usually verify who actually died of influenza and who died of a flu-like illness."[108] Even healthy people can be affected, and serious problems from influenza can happen at any age. People over 50 years old, very young children and people of any age with chronic medical conditions are more likely to get complications from influenza, such as pneumonia, bronchitis, sinus, and ear infections.[79]
In some cases, an autoimmune response to an influenza infection may contribute to the development of Guillain-Barré syndrome.[109] However, as many other infections can increase the risk of this disease, influenza may only be an important cause during epidemics.[109][110] This syndrome can also be a rare side-effect of influenza vaccines, with an incidence of about one case per million vaccinations.[111]
Epidemiology
Seasonal variations
Influenza reaches peak prevalence in winter, and because the Northern and Southern Hemispheres have winter at different times of the year, there are actually two different flu seasons each year. This is why the World Health Organization (assisted by the National Influenza Centers) makes recommendations for two different vaccine formulations every year; one for the Northern, and one for the Southern Hemisphere.[76]
It is not clear why outbreaks of the flu occur seasonally rather than uniformly throughout the year. One possible explanation is that, because people are indoors more often during the winter, they are in close contact more often, and this promotes transmission from person to person. Another is that cold temperatures lead to drier air, which may dehydrate mucus, preventing the body from effectively expelling virus particles. The virus may also survive longer on exposed surfaces (doorknobs, countertops, etc.) in colder temperatures. Increased travel due to the Northern Hemisphere winter holiday season may also play a role.[112] A contributing factor is that aerosol transmission of the virus is highest in cold environments (less than 5 °C) with low relative humidity.[113] However, seasonal changes in infection rates also occur in tropical regions, and in some countries these peaks of infection are seen mainly during the rainy season.[114] Seasonal changes in contact rates from school terms, which are a major factor in other childhood diseases such as measles and pertussis, may also play a role in the flu. A combination of these small seasonal effects may be amplified by dynamical resonance with the endogenous disease cycles.[115] H5N1 exhibits seasonality in both humans and birds.[116]
An alternative hypothesis to explain seasonality in influenza infections is an effect of vitamin D levels on immunity to the virus.[117] This idea was first proposed by Robert Edgar Hope-Simpson in 1965.[118] He proposed that the cause of influenza epidemics during winter may be connected to seasonal fluctuations of vitamin D, which is produced in the skin under the influence of solar (or artificial) UV radiation. This could explain why influenza occurs mostly in winter and during the tropical rainy season, when people stay indoors, away from the sun, and their vitamin D levels fall.
Epidemic and pandemic spread

As influenza is caused by a variety of species and strains of viruses, in any given year some strains can die out while others create epidemics, while yet another strain can cause a pandemic. Typically, in a year's normal two flu seasons (one per hemisphere), there are between three and five million cases of severe illness and up to 500,000 deaths worldwide, which by some definitions is a yearly influenza epidemic.[119] Although the incidence of influenza can vary widely between years, approximately 36,000 deaths and more than 200,000 hospitalizations are directly associated with influenza every year in the United States.[120][121] Roughly three times per century, a pandemic occurs, which infects a large proportion of the world's population and can kill tens of millions of people (see history section). Indeed, one study estimated that if a strain with similar virulence to the 1918 influenza emerged today, it could kill between 50 and 80 million people.[122]
New influenza viruses are constantly evolving by mutation or by reassortment.[14] Mutations can cause small changes in the hemagglutinin and neuraminidase antigens on the surface of the virus. This is called antigenic drift, which slowly creates an increasing variety of strains until one evolves that can infect people who are immune to the pre-existing strains. This new variant then replaces the older strains as it rapidly sweeps through the human population—often causing an epidemic.[123] However, since the strains produced by drift will still be reasonably similar to the older strains, some people will still be immune to them. In contrast, when influenza viruses reassort, they acquire completely new antigens—for example by reassortment between avian strains and human strains; this is called antigenic shift. If a human influenza virus is produced that has entirely new antigens, everybody will be susceptible, and the novel influenza will spread uncontrollably, causing a pandemic.[124] In contrast to this model of pandemics based on antigenic drift and shift, an alternative approach has been proposed where the periodic pandemics are produced by interactions of a fixed set of viral strains with a human population with a constantly changing set of immunities to different viral strains.[125]
History
Etymology
The word Influenza comes from the Italian language and refers to the cause of the disease; initially, this ascribed illness to unfavorable astrological influences.[126] Changes in medical thought led to its modification to influenza del freddo, meaning "influence of the cold". The word influenza was first used in English in 1743 when it was adopted, with an anglicized pronunciation, during an outbreak of the disease in Europe.[127] Archaic terms for influenza include epidemic catarrh, grippe (from the French), sweating sickness, and Spanish fever (particularly for the 1918 pandemic strain).[128]
Pandemics
The symptoms of human influenza were clearly described by Hippocrates roughly 2,400 years ago.[130][131] Since then, the virus has caused numerous pandemics. Historical data on influenza are difficult to interpret, because the symptoms can be similar to those of other diseases, such as diphtheria, pneumonic plague, typhoid fever, dengue, or typhus. The first convincing record of an influenza pandemic was of an outbreak in 1580, which began in Russia and spread to Europe via Africa. In Rome, over 8,000 people were killed, and several Spanish cities were almost wiped out. Pandemics continued sporadically throughout the 17th and 18th centuries, with the pandemic of 1830–1833 being particularly widespread; it infected approximately a quarter of the people exposed.[132]
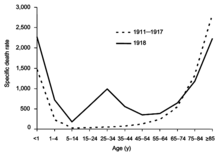
The most famous and lethal outbreak was the 1918 flu pandemic (Spanish flu pandemic) (type A influenza, H1N1 subtype), which lasted from 1918 to 1919. It is not known exactly how many it killed, but estimates range from 20 to 100 million people.[133][134] This pandemic has been described as "the greatest medical holocaust in history" and may have killed as many people as the Black Death.[132] This huge death toll was caused by an extremely high infection rate of up to 50% and the extreme severity of the symptoms, suspected to be caused by cytokine storms.[134] Indeed, symptoms in 1918 were so unusual that initially influenza was misdiagnosed as dengue, cholera, or typhoid. One observer wrote, "One of the most striking of the complications was hemorrhage from mucous membranes, especially from the nose, stomach, and intestine. Bleeding from the ears and petechial hemorrhages in the skin also occurred."[133] The majority of deaths were from bacterial pneumonia, a secondary infection caused by influenza, but the virus also killed people directly, causing massive hemorrhages and edema in the lung.[129]
The 1918 flu pandemic (Spanish flu pandemic) was truly global, spreading even to the Arctic and remote Pacific islands. The unusually severe disease killed between 2 and 20% of those infected, as opposed to the more usual flu epidemic mortality rate of 0.1%.[129][133] Another unusual feature of this pandemic was that it mostly killed young adults, with 99% of pandemic influenza deaths occurring in people under 65, and more than half in young adults 20 to 40 years old.[135] This is unusual since influenza is normally most deadly to the very young (under age 2) and the very old (over age 70). The total mortality of the 1918–1919 pandemic is not known, but it is estimated that 2.5% to 5% of the world's population was killed. As many as 25 million may have been killed in the first 25 weeks; in contrast, HIV/AIDS has killed 25 million in its first 25 years.[133]
Later flu pandemics were not so devastating. They included the 1957 Asian Flu (type A, H2N2 strain) and the 1968 Hong Kong Flu (type A, H3N2 strain), but even these smaller outbreaks killed millions of people. In later pandemics antibiotics were available to control secondary infections and this may have helped reduce mortality compared to the Spanish Flu of 1918.[129]
| Name of pandemic | Date | Deaths | Subtype involved | Pandemic Severity Index |
|---|---|---|---|---|
| Asiatic (Russian) Flu | 1889–1890 | 1 million | possibly H2N2 | NA |
| 1918 flu pandemic (Spanish flu) | 1918–1920 | 20 to 100 million | H1N1 | 5 |
| Asian Flu | 1957–1958 | 1 to 1.5 million | H2N2 | 2 |
| Hong Kong Flu | 1968–1969 | 0.75 to 1 million | H3N2 | 2 |
| 2009 flu pandemic | 2009–Present | NA | H1N1 | NA |
The first influenza virus to be isolated was from poultry, when in 1901 the agent causing a disease called "fowl plague" was passed through Chamberland filters, which have pores that are too small for bacteria to pass through.[136] The etiological cause of influenza, the Orthomyxoviridae family of viruses, was first discovered in pigs by Richard Shope in 1931.[137] This discovery was shortly followed by the isolation of the virus from humans by a group headed by Patrick Laidlaw at the Medical Research Council of the United Kingdom in 1933.[138] However, it was not until Wendell Stanley first crystallized tobacco mosaic virus in 1935 that the non-cellular nature of viruses was appreciated.
The first significant step towards preventing influenza was the development in 1944 of a killed-virus vaccine for influenza by Thomas Francis, Jr.. This built on work by Australian Frank Macfarlane Burnet, who showed that the virus lost virulence when it was cultured in fertilized hen's eggs.[140] Application of this observation by Francis allowed his group of researchers at the University of Michigan to develop the first influenza vaccine, with support from the U.S. Army.[141] The Army was deeply involved in this research due to its experience of influenza in World War I, when thousands of troops were killed by the virus in a matter of months.[133] In comparison to vaccines, the development of anti-influenza drugs has been slower, with amantadine being licensed in 1966 and, almost thirty years later, the next class of drugs (the neuraminidase inhibitors) being developed.[33]
Society and culture
Influenza produces direct costs due to lost productivity and associated medical treatment, as well as indirect costs of preventative measures. In the United States, influenza is responsible for a total cost of over $10 billion per year, while it has been estimated that a future pandemic could cause hundreds of billions of dollars in direct and indirect costs.[142] However, the economic impacts of past pandemics have not been intensively studied, and some authors have suggested that the Spanish influenza actually had a positive long-term effect on per-capita income growth, despite a large reduction in the working population and severe short-term depressive effects.[143] Other studies have attempted to predict the costs of a pandemic as serious as the 1918 Spanish flu on the U.S. economy, where 30% of all workers became ill, and 2.5% were killed. A 30% sickness rate and a three-week length of illness would decrease the gross domestic product by 5%. Additional costs would come from medical treatment of 18 million to 45 million people, and total economic costs would be approximately $700 billion.[144]
Preventative costs are also high. Governments worldwide have spent billions of U.S. dollars preparing and planning for a potential H5N1 avian influenza pandemic, with costs associated with purchasing drugs and vaccines as well as developing disaster drills and strategies for improved border controls.[145] On 1 November 2005, United States President George W. Bush unveiled the National Strategy to Safeguard Against the Danger of Pandemic Influenza[146] backed by a request to Congress for $7.1 billion to begin implementing the plan.[147] Internationally, on 18 January 2006, donor nations pledged US$2 billion to combat bird flu at the two-day International Pledging Conference on Avian and Human Influenza held in China.[148]
Research

Research on influenza includes studies on molecular virology, how the virus produces disease (pathogenesis), host immune responses, viral genomics, and how the virus spreads (epidemiology). These studies help in developing influenza countermeasures; for example, a better understanding of the body's immune system response helps vaccine development, and a detailed picture of how influenza invades cells aids the development of antiviral drugs. One important basic research program is the Influenza Genome Sequencing Project, which is creating a library of influenza sequences; this library should help clarify which factors make one strain more lethal than another, which genes most affect immunogenicity, and how the virus evolves over time.[149]
Research into new vaccines is particularly important, as current vaccines are very slow and expensive to produce and must be reformulated every year. The sequencing of the influenza genome and recombinant DNA technology may accelerate the generation of new vaccine strains by allowing scientists to substitute new antigens into a previously developed vaccine strain.[150] New technologies are also being developed to grow viruses in cell culture, which promises higher yields, less cost, better quality and surge capacity.[151] Research on a universal influenza A vaccine, targeted against the external domain of the transmembrane viral M2 protein (M2e), is being done at the University of Ghent by Walter Fiers, Xavier Saelens and their team[152][153][154] and has now successfully concluded Phase I clinical trials.
A number of biologics, therapeutic vaccines and immunobiologics are also being investigated for treatment of infection caused by viruses. Therapeutic biologics are designed to activate the immune response to virus or antigens. Typically, biologics do not target metabolic pathways like anti-viral drugs, but stimulate immune cells such as lymphocytes, macrophages, and/or antigen presenting cells, in an effort to drive an immune response towards a cytotoxic effect against the virus. Infuenza models, such as murine influenza, are convenient models to test the effects of prophylactic and therapeutic biologics. For example, Lymphocyte T-Cell Immune Modulator inhibits viral growth in the murine model of influenza.[155]
Infection in other animals
 |
Influenza infects many animal species, and transfer of viral strains between species can occur. Birds are thought to be the main animal reservoirs of influenza viruses.[156] Sixteen forms of hemagglutinin and nine forms of neuraminidase have been identified. All known subtypes (HxNy) are found in birds, but many subtypes are endemic in humans, dogs, horses, and pigs; populations of camels, ferrets, cats, seals, mink, and whales also show evidence of prior infection or exposure to influenza.[19] Variants of flu virus are sometimes named according to the species the strain is endemic in or adapted to. The main variants named using this convention are: Bird Flu, Human Flu, Swine Flu, Horse Flu and Dog Flu. (Cat flu generally refers to Feline viral rhinotracheitis or Feline calicivirus and not infection from an influenza virus.) In pigs, horses and dogs, influenza symptoms are similar to humans, with cough, fever and loss of appetite.[19] The frequency of animal diseases are not as well-studied as human infection, but an outbreak of influenza in harbour seals caused approximately 500 seal deaths off the New England coast in 1979–1980.[157] On the other hand, outbreaks in pigs are common and do not cause severe mortality.[19]
Bird flu
Flu symptoms in birds are variable and can be unspecific.[158] The symptoms following infection with low-pathogenicity avian influenza may be as mild as ruffled feathers, a small reduction in egg production, or weight loss combined with minor respiratory disease.[159] Since these mild symptoms can make diagnosis in the field difficult, tracking the spread of avian influenza requires laboratory testing of samples from infected birds. Some strains such as Asian H9N2 are highly virulent to poultry and may cause more extreme symptoms and significant mortality.[160] In its most highly pathogenic form, influenza in chickens and turkeys produces a sudden appearance of severe symptoms and almost 100% mortality within two days.[161] As the virus spreads rapidly in the crowded conditions seen in the intensive farming of chickens and turkeys, these outbreaks can cause large economic losses to poultry farmers.
An avian-adapted, highly pathogenic strain of H5N1 (called HPAI A(H5N1), for "highly pathogenic avian influenza virus of type A of subtype H5N1") causes H5N1 flu, commonly known as "avian influenza" or simply "bird flu", and is endemic in many bird populations, especially in Southeast Asia. This Asian lineage strain of HPAI A(H5N1) is spreading globally. It is epizootic (an epidemic in non-humans) and panzootic (a disease affecting animals of many species, especially over a wide area), killing tens of millions of birds and spurring the culling of hundreds of millions of other birds in an attempt to control its spread. Most references in the media to "bird flu" and most references to H5N1 are about this specific strain.[162][163]
At present, HPAI A(H5N1) is an avian disease, and there is no evidence suggesting efficient human-to-human transmission of HPAI A(H5N1). In almost all cases, those infected have had extensive physical contact with infected birds.[164] In the future, H5N1 may mutate or reassort into a strain capable of efficient human-to-human transmission. The exact changes that are required for this to happen are not well understood.[165] However, due to the high lethality and virulence of H5N1, its endemic presence, and its large and increasing biological host reservoir, the H5N1 virus was the world's pandemic threat in the 2006–07 flu season, and billions of dollars are being raised and spent researching H5N1 and preparing for a potential influenza pandemic.[145]
Swine flu

In pigs swine influenza produces fever, lethargy, sneezing, coughing, difficulty breathing and decreased appetite.[166] In some cases the infection can cause abortion. Although mortality is usually low, the virus can produce weight loss and poor growth, causing economic loss to farmers.[166] Infected pigs can lose up to 12 pounds of body weight over a 3 to 4 week period.[166] Direct transmission of an influenza virus from pigs to humans is occasionally possible (this is called zoonotic swine flu). In all, 50 human cases are known to have occurred since the virus was identified in the mid-20th century, which have resulted in six deaths.[167]
In 2009 a swine-origin H1N1 virus strain commonly referred to as "swine flu" caused the 2009 flu pandemic, but there is no evidence that it is endemic to pigs (i.e. actually a swine flu) or of transmission from pigs to people, instead the virus is spreading from person to person.[168][169] This strain is a reassortment of several strains of H1N1 that are usually found separately, in humans, birds, and pigs.[170]
See also
- Information concerning flu research can be found at
- Early Influenza Research
- Center for Biologics Evaluation and Research
- Cytokine storm
- H5N1 clinical trials
- H5N1 genetic structure
- ICEID
- Influenza Genome Sequencing Project
- Influenza research
- International Partnership on Avian and Pandemic Influenza
- National Influenza Centers
- Pandemic Preparedness and Response Act
References
- ^ a b c d "Influenza: Viral Infections: Merck Manual Home Edition". www.merck.com. Retrieved 2008-03-15.
- ^ a b c Eccles, R (2005). "Understanding the symptoms of the common cold and influenza". Lancet Infect Dis. 5 (11): 718–25. doi:10.1016/S1473-3099(05)70270-X. PMID 16253889.
- ^ Seasonal Flu vs. Stomach Flu by Kristina Duda, R.N.; accessed 12 March 2007 (Website: "About, Inc., A part of The New York Times Company")
- ^ Suarez, D (2003). "The effect of various disinfectants on detection of avian influenza virus by real time RT-PCR". Avian Dis. 47 (3 Suppl): 1091–5. doi:10.1637/0005-2086-47.s3.1091. PMID 14575118.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Avian Influenza (Bird Flu): Implications for Human Disease. Physical characteristics of influenza A viruses. UMN CIDRAP.
- ^ "Avian influenza ("bird flu") fact sheet". WHO. 2006. Retrieved 2006-10-20.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ WHO position paper: influenza vaccines WHO weekly Epidemiological Record 19 August 2005, vol. 80, 33, pp. 277–288.
- ^ Villegas, P (1998). "Viral diseases of the respiratory system". Poult Sci. 77 (8): 1143–5. PMID 9706079.
- ^ Horwood F, Macfarlane J (2002). "Pneumococcal and influenza vaccination: current situation and future prospects". Thorax. 57 (Suppl 2): II24–II30. PMC 1766003. PMID 12364707.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Kawaoka Y (editor). (2006). Influenza Virology: Current Topics. Caister Academic Press. ISBN 978-1-904455-06-6.
{{cite book}}:|author=has generic name (help) - ^ Vainionpää R, Hyypiä T (1994). "Biology of parainfluenza viruses". Clin. Microbiol. Rev. 7 (2): 265–75. PMC 358320. PMID 8055470.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Hall CB (2001). "Respiratory syncytial virus and parainfluenza virus". N. Engl. J. Med. 344 (25): 1917–28. PMID 11419430.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Klenk; et al. (2008). "Avian Influenza: Molecular Mechanisms of Pathogenesis and Host Range". Animal Viruses: Molecular Biology. Caister Academic Press. ISBN 978-1-904455-22-6.
{{cite book}}: Explicit use of et al. in:|author=(help); External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - ^ a b c d Hay, A (2001). "The evolution of human influenza viruses". Philos Trans R Soc Lond B Biol Sci. 356 (1416): 1861–70. doi:10.1098/rstb.2001.0999. PMID 11779385.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Fouchier, R (2004). "Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome". Proc Natl Acad Sci U S A. 101 (5): 1356–61. doi:10.1073/pnas.0308352100. PMID 14745020.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Osterhaus, A (2000). "Influenza B virus in seals". Science. 288 (5468): 1051–3. doi:10.1126/science.288.5468.1051. PMID 10807575.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Jakeman KJ, Tisdale M, Russell S, Leone A, Sweet C (1994). "Efficacy of 2'-deoxy-2'-fluororibosides against influenza A and B viruses in ferrets". Antimicrob. Agents Chemother. 38 (8): 1864–7. PMC 284652. PMID 7986023.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Nobusawa, E (2006). "Comparison of the mutation rates of human influenza A and B viruses". J Virol. 80 (7): 3675–8. doi:10.1128/JVI.80.7.3675-3678.2006. PMID 16537638.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ a b c d R, Webster (1992). "Evolution and ecology of influenza A viruses". Microbiol Rev. 56 (1): 152–79. PMC 372859. PMID 1579108.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Zambon, M (1999). "Epidemiology and pathogenesis of influenza". J Antimicrob Chemother. 44 Suppl B: 3–9. doi:10.1093/jac/44.suppl_2.3. PMID 10877456.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Matsuzaki, Y (2002). "Antigenic and genetic characterization of influenza C viruses which caused two outbreaks in Yamagata City, Japan, in 1996 and 1998". J Clin Microbiol. 40 (2): 422–9. doi:10.1128/JCM.40.2.422-429.2002. PMC 153379. PMID 11825952.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Taubenberger JK, Morens DM (2008). "The pathology of influenza virus infections". Annu Rev Pathol. 3: 499–522. doi:10.1146/annurev.pathmechdis.3.121806.154316. PMC 2504709. PMID 18039138.
- ^ Matsuzaki, Y (2006). "Clinical features of influenza C virus infection in children". J Infect Dis. 193 (9): 1229–35. doi:10.1086/502973. PMID 16586359.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Katagiri, S (1983). "An outbreak of type C influenza in a children's home". J Infect Dis. 148 (1): 51–6. PMID 6309999.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ International Committee on Taxonomy of Viruses descriptions of: Orthomyxoviridae, Influenzavirus B and Influenzavirus C
- ^ International Committee on Taxonomy of Viruses. "The Universal Virus Database, version 4: Influenza A".
- ^ a b Lamb RA, Choppin PW (1983). "The gene structure and replication of influenza virus". Annu. Rev. Biochem. 52: 467–506. doi:10.1146/annurev.bi.52.070183.002343. PMID 6351727.
- ^ a b c d e Bouvier NM, Palese P (2008). "The biology of influenza viruses". Vaccine. 26 Suppl 4: D49–53. PMID 19230160.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Ghedin, E (2005). "Large-scale sequencing of human influenza reveals the dynamic nature of viral genome evolution". Nature. 437 (7062): 1162–6. doi:10.1038/nature04239. PMID 16208317.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Suzuki, Y (2005). "Sialobiology of influenza: molecular mechanism of host range variation of influenza viruses". Biol Pharm Bull. 28 (3): 399–408. doi:10.1248/bpb.28.399. PMID 15744059.
- ^ a b Wilson, J (2003). "Recent strategies in the search for new anti-influenza therapies". Curr Drug Targets. 4 (5): 389–408. doi:10.2174/1389450033491019. PMID 12816348.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ a b c d e Hilleman, M (2002). "Realities and enigmas of human viral influenza: pathogenesis, epidemiology and control". Vaccine. 20 (25–26): 3068–87. doi:10.1016/S0264-410X(02)00254-2. PMID 12163258.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Lynch JP, Walsh EE (2007). "Influenza: evolving strategies in treatment and prevention". Semin Respir Crit Care Med. 28 (2): 144–58. doi:10.1055/s-2007-976487. PMID 17458769.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Smith AE, Helenius A (2004). "How viruses enter animal cells". Science. 304 (5668): 237–42. doi:10.1126/science.1094823. PMID 15073366.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Wagner, R (2002). "Functional balance between haemagglutinin and neuraminidase in influenza virus infections". Rev Med Virol. 12 (3): 159–66. doi:10.1002/rmv.352. PMID 11987141.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ a b Steinhauer DA (1999). "Role of hemagglutinin cleavage for the pathogenicity of influenza virus". Virology. 258 (1): 1–20. doi:10.1006/viro.1999.9716. PMID 10329563.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Lakadamyali, M (2003). "Visualizing infection of individual influenza viruses". Proc Natl Acad Sci U S A. 100 (16): 9280–5. doi:10.1073/pnas.0832269100. PMID 12883000.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ a b Pinto LH, Lamb RA (2006). "The M2 proton channels of influenza A and B viruses". J. Biol. Chem. 281 (14): 8997–9000. doi:10.1074/jbc.R500020200. PMID 16407184.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: unflagged free DOI (link) - ^ Cros, J (2003). "Trafficking of viral genomic RNA into and out of the nucleus: influenza, Thogoto and Borna disease viruses". Virus Res. 95 (1–2): 3–12. doi:10.1016/S0168-1702(03)00159-X. PMID 12921991.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Kash, J (2006). "Hijacking of the host-cell response and translational control during influenza virus infection". Virus Res. 119 (1): 111–20. doi:10.1016/j.virusres.2005.10.013. PMID 16630668.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Nayak, D (2004). "Assembly and budding of influenza virus". Virus Res. 106 (2): 147–65. doi:10.1016/j.virusres.2004.08.012. PMID 15567494.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Drake, J (1993). "Rates of spontaneous mutation among RNA viruses". Proc Natl Acad Sci USA. 90 (9): 4171–5. doi:10.1073/pnas.90.9.4171. PMID 8387212.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Centers for Disease Control and Prevention > Influenza Symptoms Page last updated November 16, 2007. Retrieved April 28, 2009
- ^ a b c d Call S, Vollenweider M, Hornung C, Simel D, McKinney W (2005). "Does this patient have influenza?". JAMA. 293 (8): 987–97. doi:10.1001/jama.293.8.987. PMID 15728170.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Suzuki E, Ichihara K, Johnson AM (2007). "Natural course of fever during influenza virus infection in children". Clin Pediatr (Phila). 46 (1): 76–9. doi:10.1177/0009922806289588. PMID 17164515.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Richards S (2005). "Flu blues". Nurs Stand. 20 (8): 26–7. PMID 16295596.
- ^ Heikkinen T (2006). "Influenza in children". Acta Paediatr. 95 (7): 778–84. doi:10.1080/08035250600612272. PMID 16801171.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Kerr AA, McQuillin J, Downham MA, Gardner PS (1975). "Gastric 'flu influenza B causing abdominal symptoms in children". Lancet. 1 (7902): 291–5. doi:10.1016/S0140-6736(75)91205-2. PMID 46444.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hui DS (2008). "Review of clinical symptoms and spectrum in humans with influenza A/H5N1 infection". Respirology. 13 Suppl 1: S10–3. doi:10.1111/j.1440-1843.2008.01247.x. PMID 18366521.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Monto A, Gravenstein S, Elliott M, Colopy M, Schweinle J (2000). "Clinical signs and symptoms predicting influenza infection". Arch Intern Med. 160 (21): 3243–7. doi:10.1001/archinte.160.21.3243. PMID 11088084.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Smith K, Roberts M (2002). "Cost-effectiveness of newer treatment strategies for influenza". Am J Med. 113 (4): 300–7. doi:10.1016/S0002-9343(02)01222-6. PMID 12361816.
- ^ a b c d Rothberg M, Bellantonio S, Rose D (2003). "Management of influenza in adults older than 65 years of age: cost-effectiveness of rapid testing and antiviral therapy". Ann Intern Med. 139 (5 Pt 1): 321–9. PMID 12965940.
{{cite journal}}: Unknown parameter|day=ignored (help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Centers for Disease Control and Prevention. Lab Diagnosis of Influenza. Accessed on 1 May 2009
- ^ a b Carrat F, Luong J, Lao H, Sallé A, Lajaunie C, Wackernagel H (2006). "A 'small-world-like' model for comparing interventions aimed at preventing and controlling influenza pandemics". BMC Med. 4: 26. doi:10.1186/1741-7015-4-26. PMC 1626479. PMID 17059593.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Mitamura K, Sugaya N (2006). "[Diagnosis and Treatment of influenza—clinical investigation on viral shedding in children with influenza]". Uirusu. 56 (1): 109–16. doi:10.2222/jsv.56.109. PMID 17038819.
- ^ Grassly NC, Fraser C (2008). "Mathematical models of infectious disease transmission". Nat. Rev. Microbiol. 6 (6): 477–87. PMID 18533288.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c d e Weber TP, Stilianakis NI (2008). "Inactivation of influenza A viruses in the environment and modes of transmission: a critical review". J. Infect. 57 (5): 361–73. doi:10.1016/j.jinf.2008.08.013. PMID 18848358.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Hall CB (2007). "The spread of influenza and other respiratory viruses: complexities and conjectures". Clin. Infect. Dis. 45 (3): 353–9. doi:10.1086/519433. PMID 17599315.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Tellier R (2006). "Review of aerosol transmission of influenza A virus". Emerging Infect. Dis. 12 (11): 1657–62. PMID 17283614.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Brankston G, Gitterman L, Hirji Z, Lemieux C, Gardam M (2007). "Transmission of influenza A in human beings". Lancet Infect Dis. 7 (4): 257–65. doi:10.1016/S1473-3099(07)70029-4. PMID 17376383.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Cole E, Cook C (1998). "Characterization of infectious aerosols in health care facilities: an aid to effective engineering controls and preventive strategies". Am J Infect Control. 26 (4): 453–64. doi:10.1016/S0196-6553(98)70046-X. PMID 9721404.
- ^ Thomas Y, Vogel G, Wunderli W; et al. (2008). "Survival of influenza virus on banknotes". Appl. Environ. Microbiol. 74 (10): 3002–7. doi:10.1128/AEM.00076-08. PMC 2394922. PMID 18359825.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Bean B, Moore BM, Sterner B, Peterson LR, Gerding DN, Balfour HH (1982). "Survival of influenza viruses on environmental surfaces". J. Infect. Dis. 146 (1): 47–51. PMID 6282993.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b "Influenza Factsheet" (PDF). Center for Food Security and Public Health, Iowa State University. p. 7
- ^ a b Korteweg C, Gu J (2008). "Pathology, molecular biology, and pathogenesis of avian influenza A (H5N1) infection in humans". Am. J. Pathol. 172 (5): 1155–70. doi:10.2353/ajpath.2008.070791. PMC 2329826. PMID 18403604.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Nicholls JM, Chan RW, Russell RJ, Air GM, Peiris JS (2008). "Evolving complexities of influenza virus and its receptors". Trends Microbiol. 16 (4): 149–57. doi:10.1016/j.tim.2008.01.008. PMID 18375125.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ van Riel D, Munster VJ, de Wit E; et al. (2006). "H5N1 Virus Attachment to Lower Respiratory Tract". Science. 312 (5772): 399. doi:10.1126/science.112554800. PMID 16556800.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Shinya K, Ebina M, Yamada S, Ono M, Kasai N, Kawaoka Y (2006). "Avian flu: influenza virus receptors in the human airway". Nature. 440 (7083): 435–6. doi:10.1038/440435a. PMID 16554799.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ van Riel D, Munster VJ, de Wit E; et al. (2007). "Human and avian influenza viruses target different cells in the lower respiratory tract of humans and other mammals". Am. J. Pathol. 171 (4): 1215–23. doi:10.2353/ajpath.2007.070248. PMC 1988871. PMID 17717141.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Schmitz N, Kurrer M, Bachmann M, Kopf M (2005). "Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection". J Virol. 79 (10): 6441–8. doi:10.1128/JVI.79.10.6441-6448.2005. PMID 15858027.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Winther B, Gwaltney J, Mygind N, Hendley J (1998). "Viral-induced rhinitis". Am J Rhinol. 12 (1): 17–20. doi:10.2500/105065898782102954. PMID 9513654.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cheung CY, Poon LL, Lau AS; et al. (2002). "Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease?". Lancet. 360 (9348): 1831–7. PMID 12480361.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Kobasa D, Jones SM, Shinya K; et al. (2007). "Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus". Nature. 445 (7125): 319–23. doi:10.1038/nature05495. PMID 17230189.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Kash JC, Tumpey TM, Proll SC; et al. (2006). "Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus". Nature. 443 (7111): 578–81. doi:10.1038/nature05181. PMC 2615558. PMID 17006449.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Beigel J, Bray M (2008). "Current and future antiviral therapy of severe seasonal and avian influenza". Antiviral Res. 78 (1): 91–102. doi:10.1016/j.antiviral.2008.01.003. PMC 2346583. PMID 18328578.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Recommended composition of influenza virus vaccines for use in the 2006–2007 influenza season WHO report 2006-02-14. Accessed 19 October 2006.
- ^ Capua, I (2006). "The challenge of avian influenza to the veterinary community". Avian Pathol. 35 (3): 189–205. doi:10.1080/03079450600717174. PMID 16753610.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Holmes, E (2005). "Whole-genome analysis of human influenza A virus reveals multiple persistent lineages and reassortment among recent H3N2 viruses". PLoS Biol. 3 (9): e300. doi:10.1371/journal.pbio.0030300. PMID 16026181.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: unflagged free DOI (link) - ^ a b Key Facts about Influenza (Flu) Vaccine CDC publication. Published 17 October 2006. Accessed 18 Oct 2006.
- ^ Prevention and Control of Influenza: Recommendations of the Advisory Committee on Immunization Practices (ACIP) CDC report (MMWR 2006 Jul 28;55(RR10):1–42) accessed 19 Oct 2006.
- ^ Questions & Answers: Flu Shot CDC publication updated Jul 24, 2006. Accessed 19 Oct 06.
- ^ a b c d e Aledort JE, Lurie N, Wasserman J, Bozzette SA (2007). "Non-pharmaceutical public health interventions for pandemic influenza: an evaluation of the evidence base". BMC Public Health. 7: 208. doi:10.1186/1471-2458-7-208. PMC 2040158. PMID 17697389.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Grayson ML, Melvani S, Druce J; et al. (2009). "Efficacy of soap and water and alcohol-based hand-rub preparations against live H1N1 influenza virus on the hands of human volunteers". Clin. Infect. Dis. 48 (3): 285–91. doi:10.1086/595845. PMID 19115974.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ MacIntyre CR, Cauchemez S, Dwyer DE; et al. (2009). "Face mask use and control of respiratory virus transmission in households". Emerging Infect. Dis. 15 (2): 233–41. doi:10.3201/eid1502.081167. PMC 2662657. PMID 19193267.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Bridges CB, Kuehnert MJ, Hall CB (2003). "Transmission of influenza: implications for control in health care settings". Clin. Infect. Dis. 37 (8): 1094–101. doi:10.1086/378292. PMID 14523774.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Interim Guidance for the Use of Masks to Control Influenza Transmission Coordinating Center for Infectious Diseases (CCID) August 8, 2005
- ^ Hota B (2004). "Contamination, disinfection, and cross-colonization: are hospital surfaces reservoirs for nosocomial infection?". Clin Infect Dis. 39 (8): 1182–9. doi:10.1086/424667. PMID 15486843.
- ^ a b McDonnell G, Russell A (1999). "Antiseptics and disinfectants: activity, action, and resistance". Clin Microbiol Rev. 12 (1): 147–79. PMID 9880479.
{{cite journal}}: Unknown parameter|day=ignored (help); Unknown parameter|month=ignored (help) - ^ "Chlorine Bleach: Helping to Manage the Flu Risk". Water Quality & Health Council. April 2009. Retrieved 2009-05-12.
- ^ Hatchett RJ, Mecher CE, Lipsitch M (2007). "Public health interventions and epidemic intensity during the 1918 influenza pandemic". Proc Natl Acad Sci U S A. 104 (18): 7582–7587. doi:10.1073/pnas.0610941104. PMID 17416679.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bootsma MC, Ferguson NM (2007). "The effect of public health measures on the 1918 influenza pandemic in U.S. cities". Proc Natl Acad Sci U S A. 104 (18): 7588–7593. doi:10.1073/pnas.0611071104. PMID 17416677.
- ^ Glasgow, J (2001). "Reye syndrome — insights on causation and prognosis". Arch Dis Child. 85 (5): 351–3. doi:10.1136/adc.85.5.351. PMID 11668090.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Hurt AC, Ho HT, Barr I (2006). "Resistance to anti-influenza drugs: adamantanes and neuraminidase inhibitors". Expert Rev Anti Infect Ther. 4 (5): 795–805. doi:10.1586/14787210.4.5.795. PMID 17140356.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Centers for Disease Control and Prevention. CDC Recommends against the Use of Amantadine and Rimantadine for the Treatment or Prophylaxis of Influenza in the United States during the 2005–06 Influenza Season. 14 January 2006. Retrieved on 2007-01-01
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/S0140-6736(09)61304-0, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/S0140-6736(09)61304-0instead. - ^ Moscona, A (2005). "Neuraminidase inhibitors for influenza". N Engl J Med. 353 (13): 1363–73. doi:10.1056/NEJMra050740. PMID 16192481.
- ^ a b Stephenson, I (1999). "Chemotherapeutic control of influenza". J Antimicrob Chemother. 44 (1): 6–10. doi:10.1093/jac/44.1.6. PMID 10459804.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Jefferson, T (2006). "Neuraminidase inhibitors for preventing and treating influenza in healthy adults". Cochrane Database Syst Rev. 3: CD001265. doi:10.1002/14651858.CD001265.pub2. PMID 16855962.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Webster, Robert G. (2006). "H5N1 Influenza — Continuing Evolution and Spread". N Engl J Med. 355 (21): 2174–77. doi:10.1056/NEJMp068205. PMID 16192481.
- ^ "High levels of adamantane resistance among influenza A (H3N2) viruses and interim guidelines for use of antiviral agents — United States, 2005–06 influenza season". MMWR Morb Mortal Wkly Rep. 55 (2): 44–6. 2006. PMID 16424859.
- ^ Bright RA, Medina MJ, Xu X; et al. (2005). "Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: a cause for concern" (PDF). Lancet. 366 (9492): 1175–81. doi:10.1016/S0140-6736(05)67338-2. PMID 16198766.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Ilyushina NA, Govorkova EA, Webster RG (2005). "Detection of amantadine-resistant variants among avian influenza viruses isolated in North America and Asia" (PDF). Virology. 341 (1): 102–6. doi:10.1016/j.virol.2005.07.003. PMID 16081121.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Parry J (2005). "Use of antiviral drug in poultry is blamed for drug resistant strains of avian flu". BMJ. 331 (7507): 10. doi:10.1136/bmj.331.7507.10. PMC 558527. PMID 15994677.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Hayden FG (1997). "Prevention and treatment of influenza in immunocompromised patients". Am. J. Med. 102 (3A): 55–60, discussion 75–6. PMID 10868144.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Whitley RJ, Monto AS. (2006). "Prevention and treatment of influenza in high-risk groups: children, pregnant women, immunocompromised hosts, and nursing home residents". J Infect Dis. 194 S2: S133-8. PMID 17163386.
- ^ Angelo SJ, Marshall PS, Chrissoheris MP, Chaves AM (2004). "Clinical characteristics associated with poor outcome in patients acutely infected with Influenza A". Conn Med. 68 (4): 199–205. PMID 15095826.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Murin S, Bilello K (2005). "Respiratory tract infections: another reason not to smoke". Cleve Clin J Med. 72 (10): 916–20. doi:10.3949/ccjm.72.10.916. PMID 16231688.
- ^ Peter M. Sandman and Jody Lanard "Bird Flu: Communicating the Risk" 2005 Perspectives in Health Magazine Vol. 10 issue 2.
- ^ a b Sivadon-Tardy V, Orlikowski D, Porcher R; et al. (2009). "Guillain-Barré syndrome and influenza virus infection". Clin. Infect. Dis. 48 (1): 48–56. doi:10.1086/594124. PMID 19025491.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Jacobs BC, Rothbarth PH, van der Meché FG; et al. (1998). "The spectrum of antecedent infections in Guillain-Barré syndrome: a case-control study". Neurology. 51 (4): 1110–5. PMID 9781538.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Vellozzi C, Burwen DR, Dobardzic A, Ball R, Walton K, Haber P (2009). "Safety of trivalent inactivated influenza vaccines in adults: Background for pandemic influenza vaccine safety monitoring". Vaccine. 27 (15): 2114–2120. doi:10.1016/j.vaccine.2009.01.125. PMID 19356614.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Weather and the Flu Season NPR Day to Day, 17 December 2003. Accessed, 19 October 2006
- ^ Lowen AC, Mubareka S, Steel J, Palese P (2007). "Influenza virus transmission is dependent on relative humidity and temperature". PLoS Pathog. 3 (10): 1470–6. doi:10.1371/journal.ppat.0030151. PMC 2034399. PMID 17953482.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Shek LP, Lee BW. "Epidemiology and seasonality of respiratory tract virus infections in the tropics." Paediatr Respir Rev. 2003 Jun;4(2):105–11. PMID 12758047
- ^ Dushoff J, Plotkin JB, Levin SA, Earn DJ. "Dynamical resonance can account for seasonality of influenza epidemics." Proc Natl Acad Sci U S A. 30 November 2004;101(48):16915–6. PMID 15557003
- ^ WHO Confirmed Human Cases of H5N1 Data published by WHO Epidemic and Pandemic Alert and Response (EPR). Accessed 24 Oct. 2006
- ^ Cannell, J (2006). "Epidemic influenza and vitamin D". Epidemiol Infect. 134 (6): 1129–40. doi:10.1017/S0950268806007175. PMID 16959053.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ HOPE-SIMPSON, R. "The nature of herpes zoster: a long-term study and a new hypothesis". Proc R Soc Med. 58: 9–20. PMID 14267505.
- ^ Influenza WHO Fact sheet No. 211 revised March 2003. Accessed 22 October 2006
- ^ Thompson, W (2003). "Mortality associated with influenza and respiratory syncytial virus in the United States". JAMA. 289 (2): 179–86. doi:10.1001/jama.289.2.179. PMID 12517228.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Thompson, W (2004). "Influenza-associated hospitalizations in the United States". JAMA. 292 (11): 1333–40. doi:10.1001/jama.292.11.1333. PMID 15367555.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Murray CJ, Lopez AD, Chin B, Feehan D, Hill KH (2006). "Estimation of potential global pandemic influenza mortality on the basis of vital registry data from the 1918-20 pandemic: a quantitative analysis". Lancet. 368 (9554): 2211–8. doi:10.1016/S0140-6736(06)69895-4. PMID 17189032.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Wolf, Yuri I (2006). "Long intervals of stasis punctuated by bursts of positive selection in the seasonal evolution of influenza A virus". Biol Direct. 1 (1): 34. doi:10.1186/1745-6150-1-34. PMID 17067369.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Parrish, C (2005). "The origins of new pandemic viruses: the acquisition of new host ranges by canine parvovirus and influenza A viruses". Annual Rev Microbiol. 59: 553–86. doi:10.1146/annurev.micro.59.030804.121059. PMID 16153179.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Recker M, Pybus OG, Nee S, Gupta S (2007). "The generation of influenza outbreaks by a network of host immune responses against a limited set of antigenic types". Proc Natl Acad Sci U S A. 104 (18): 7711–7716. doi:10.1073/pnas.0702154104. PMID 17460037.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Influenza, The Oxford English Dictionary, second edition.
- ^ Harper, D. "Influenza". Etymonlin.
- ^ Smith, P. "Archaic Medical Terms". Retrieved 2006-10-23.
- ^ a b c d Taubenberger, J (2006). "1918 Influenza: the mother of all pandemics". Emerg Infect Dis. 12 (1): 15–22. PMID 16494711.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) Cite error: The named reference "Taubenberger" was defined multiple times with different content (see the help page). - ^ Martin, P (2006). "2,500-year evolution of the term epidemic". Emerg Infect Dis. 12 (6). PMID 16707055.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Hippocrates (400 BCE). "Of the Epidemics". Retrieved 2006-10-18.
{{cite web}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c Potter CW (2001). "A History of Influenza". Journal of Applied Microbiology. 91 (4): 572–579. doi:10.1046/j.1365-2672.2001.01492.x. PMID 11576290.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c d e Knobler S, Mack A, Mahmoud A, Lemon S (ed.). "1: The Story of Influenza". The Threat of Pandemic Influenza: Are We Ready? Workshop Summary (2005). Washington, D.C.: The National Academies Press. pp. 60–61.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help)CS1 maint: multiple names: editors list (link) - ^ a b Patterson, KD (1991). "The geography and mortality of the 1918 influenza pandemic". Bull Hist Med. 65 (1): 4–21. PMID 2021692.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Simonsen, L (1998). "Pandemic versus epidemic influenza mortality: a pattern of changing age distribution". J Infect Dis. 178 (1): 53–60. PMID 9652423.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Heinen PP (15 September 2003). "Swine influenza: a zoonosis". Veterinary Sciences Tomorrow. ISSN 1569-0830.
- ^ Shimizu, K (1997). "History of influenza epidemics and discovery of influenza virus". Nippon Rinsho. 55 (10): 2505–201. PMID 9360364.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Smith, W (1933). "A virus obtained from influenza patients". Lancet. 2: 66–68. doi:10.1016/S0140-6736(00)78541-2.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Palese P (2004). "Influenza: old and new threats". Nat. Med. 10 (12 Suppl): S82–7. doi:10.1038/nm1141. PMID 15577936.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Sir Frank Macfarlane Burnet: Biography The Nobel Foundation. Accessed 22 Oct 06
- ^ Kendall, H (2006). "Vaccine Innovation: Lessons from World War II" ([dead link] – Scholar search). Journal of Public Health Policy. 27 (1): 38–57. doi:10.1057/palgrave.jphp.3200064.
{{cite journal}}: External link in|format= - ^ Statement from President George W. Bush on Influenza Accessed 26 Oct 2006
- ^ Brainerd, E. and M. Siegler (2003), “The Economic Effects of the 1918 Influenza Epidemic”, CEPR Discussion Paper, no. 3791.
- ^ Poland G (2006). "Vaccines against avian influenza—a race against time". N Engl J Med. 354 (13): 1411–3. doi:10.1056/NEJMe068047. PMID 16571885.
- ^ a b Rosenthal, E. and Bradsher, K. Is Business Ready for a Flu Pandemic? The New York Times 16-03-2006 Accessed 17-04-2006
- ^ National Strategy for Pandemic Influenza Whitehouse.gov Accessed 26 Oct 2006.
- ^ Bush Outlines $7 Billion Pandemic Flu Preparedness Plan State.gov. Accessed 26 Oct 2006
- ^ Donor Nations Pledge $1.85 Billion to Combat Bird Flu Newswire Accessed 26 Oct 2006.
- ^ Influenza A Virus Genome Project at The Institute of Genomic Research. Accessed 19 Oct 06
- ^ Subbarao K, Katz J. "Influenza vaccines generated by reverse genetics". Curr Top Microbiol Immunol. 283: 313–42. PMID 15298174.
- ^ Bardiya N, Bae J (2005). "Influenza vaccines: recent advances in production technologies". Appl Microbiol Biotechnol. 67 (3): 299–305. doi:10.1007/s00253-004-1874-1. PMID 15660212.
- ^ Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou WM, Fiers W (1999). "A universal influenza A vaccine based on the extracellular domain of the M2 protein". Nat. Med. 5 (10): 1157–63. doi:10.1038/13484. PMID 10502819.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Fiers W, Neirynck S, Deroo T, Saelens X, Jou WM (2001). "Soluble recombinant influenza vaccines". Philos. Trans. R. Soc. Lond., B, Biol. Sci. 356 (1416): 1961–3. doi:10.1098/rstb.2001.0980. PMC 1088575. PMID 11779398.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Fiers W, De Filette M, Birkett A, Neirynck S, Min Jou W (2004). "A "universal" human influenza A vaccine". Virus Res. 103 (1–2): 173–6. doi:10.1016/j.virusres.2004.02.030. PMID 15163506.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "[Lymphocyte T-Cell Immunomodulator: Review of the ImmunoPharmacology of a new Veterinary Biologic]" (PDF). Journal of Applied Research in Veterinary Medicine. 6 (2): 61–68. 2008. Retrieved 2009-04-26.
{{cite journal}}: Unknown parameter|quotes=ignored (help) - ^ Gorman O, Bean W, Kawaoka Y, Webster R (1990). "Evolution of the nucleoprotein gene of influenza A virus". J Virol. 64 (4): 1487–97. PMC 249282. PMID 2319644.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hinshaw V, Bean W, Webster R, Rehg J, Fiorelli P, Early G, Geraci J, St Aubin D (1984). "Are seals frequently infected with avian influenza viruses?". J Virol. 51 (3): 863–5. PMC 255856. PMID 6471169.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Elbers A, Koch G, Bouma A (2005). "Performance of clinical signs in poultry for the detection of outbreaks during the avian influenza A (H7N7) epidemic in The Netherlands in 2003". Avian Pathol. 34 (3): 181–7. doi:10.1080/03079450500096497. PMID 16191700.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Capua I, Mutinelli F. "Low pathogenicity (LPAI) and highly pathogenic (HPAI) avian influenza in turkeys and chicken." In: Capua I, Mutinelli F. (eds.), A Colour Atlas and Text on Avian Influenza, Papi Editore, Bologna, 2001, pp. 13–20
- ^ Bano S, Naeem K, Malik S (2003). "Evaluation of pathogenic potential of avian influenza virus serotype H9N2 in chickens". Avian Dis. 47 (3 Suppl): 817–22. doi:10.1637/0005-2086-47.s3.817. PMID 14575070.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Swayne D, Suarez D (2000). "Highly pathogenic avian influenza". Rev Sci Tech. 19 (2): 463–82. PMID 10935274.
- ^ Li K, Guan Y, Wang J, Smith G, Xu K, Duan L, Rahardjo A, Puthavathana P, Buranathai C, Nguyen T, Estoepangestie A, Chaisingh A, Auewarakul P, Long H, Hanh N, Webby R, Poon L, Chen H, Shortridge K, Yuen K, Webster R, Peiris J (2004). "Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia". Nature. 430 (6996): 209–13. doi:10.1038/nature02746. PMID 15241415.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Li KS, Guan Y, Wang J, Smith GJ, Xu KM, Duan L, Rahardjo AP, Puthavathana P, Buranathai C, Nguyen TD, Estoepangestie AT, Chaisingh A, Auewarakul P, Long HT, Hanh NT, Webby RJ, Poon LL, Chen H, Shortridge KF, Yuen KY, Webster RG, Peiris JS. "The Threat of Pandemic Influenza: Are We Ready?" Workshop Summary The National Academies Press (2005) "Today's Pandemic Threat: Genesis of a Highly Pathogenic and Potentially Pandemic H5N1 Influenza Virus in Eastern Asia", pages 116–130
- ^ Liu J (2006). "Avian influenza—a pandemic waiting to happen?" (PDF). J Microbiol Immunol Infect. 39 (1): 4–10. PMID 16440117.
- ^ Salomon R, Webster RG (2009). "The influenza virus enigma". Cell. 136 (3): 402–10. doi:10.1016/j.cell.2009.01.029. PMID 19203576.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c Kothalawala H, Toussaint MJ, Gruys E (2006). "An overview of swine influenza". Vet Q. 28 (2): 46–53. PMID 16841566.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Myers KP, Olsen CW, Gray GC (2007). "Cases of swine influenza in humans: a review of the literature". Clin. Infect. Dis. 44 (8): 1084–8. doi:10.1086/512813. PMC 1973337. PMID 17366454.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Maria Zampaglione (April 29, 2009). "Press Release: A/H1N1 influenza like human illness in Mexico and the USA: OIE statement". World Organisation for Animal Health. Retrieved April 29, 2009.
- ^ "W.H.O. Gives Swine Flu a Less Loaded, More Scientific Name - NYTimes.com".
- ^ "Virus's Tangled Genes Straddle Continents, Raising a Mystery About Its Origins - NYTimes.com".
Further reading
|
General
History
Microbiology
|
Pathogenesis
Epidemiology
Treatment and prevention
Research
|
External links
- Swine and Seasonal Flu, Institute for Good Medicine at the Pennsylvania Medical Society
- Info on influenza at CDC
- Summary of the disease at the NYTimes.
- 10 Genes, Furiously Evolving NYTimes May 4, 2009
- Outbreak Alerts United States based communicable disease notification website.
- BioHealthBase Bioinformatics Resource Center Database of influenza sequences and related information.
- Influenza (Mayo Clinic)
- Fact Sheet Overview of influenza at World Health Organization
- The Multinational Influenza Seasonal Mortality Study (MISMS) Fogarty International Center
- Health encyclopedia entry at NHS Direct
- Orthomyxoviridae The Universal Virus Database of the International Committee on Taxonomy of Viruses
- Influenza Virus Resource from the NCBI
- European Influenza Surveillance Scheme
- Flu Trends – flu activity across the U.S.
- Cold and flu advice (NHS Direct)
- Online video discussing influenza outbreaks and spread of other infectious diseases (Vega Science Trust)
