Histamine
This article needs additional citations for verification. (January 2008) |

| |

| |
| Names | |
|---|---|
| IUPAC name
2-(1H-imidazol-4-yl)ethanamine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.092 |
| KEGG | |
| MeSH | Histamine |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H9N3 | |
| Molar mass | 111.145 |
| Melting point | 83.5 °C (182.3 °F) |
| Boiling point | 209.5 °C (409.1 °F) |
| Easily soluble in cold water, hot water[1] | |
| Solubility | Easily soluble in methanol. Very slightly soluble in diethyl ether.[1] Easily soluble in ethanol. |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Histamine is an organic nitrogen compound involved in local immune responses as well as regulating physiological function in the gut and acting as a neurotransmitter.[2] Histamines trigger the inflammatory response. As part of an immune response to foreign pathogens, histamine is produced by basophils and by mast cells found in nearby connective tissues. Histamine increases the permeability of the capillaries to white blood cells and some proteins, to allow them to engage pathogens in the infected tissues.[3]
Properties
Histamine forms colorless hygroscopic crystals that melt at 84°C, and are easily dissolved in water or ethanol, but not in ether. In aqueous solution histamine exists in two tautomeric forms, Nπ-H-histamine and Nτ-H-histamine.
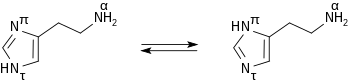
Histamine has two basic centres, namely the aliphatic amino group and whichever nitrogen atom of the imidazole ring does not already have a proton. Under physiological conditions, the aliphatic amino group (having a pKa around 9.4) will be protonated, whereas the second nitrogen of the imidazole ring (pKa ≈ 5.8) will not be protonated.[4] Thus, histamine is normally protonated to a singly charged cation.
Synthesis and metabolism
Histamine is derived from the decarboxylation of the amino acid histidine, a reaction catalyzed by the enzyme L-histidine decarboxylase. It is a hydrophilic vasoactive amine.

Once formed, histamine is either stored or rapidly inactivated by its primary degradative enzymes, histamine-N-methyltransferase or diamine oxidase. In the central nervous system, histamine released into the synapses is primarily broken down by histamine-N-methyltransferase, while in other tissues both enzymes may play a role. Several other enzymes, including MAO-B and ALDH2, further process the immediate metabolites of histamine for excretion or recycling.
Bacteria also are capable of producing histamine using histidine decarboxylase enzymes unrelated to those found in animals. A non-infectious form of foodborne disease, scombroid poisoning, is due to histamine production by bacteria in spoiled food, particularly fish. Fermented foods and beverages naturally contain small quantities of histamine due to a similar conversion performed by fermenting bacteria or yeasts. Sake contains histamine in the 20–40 mg/L range; wines contain it in the 2–10 mg/L range.[5]
Storage and release

Most histamine in the body is generated in granules in mast cells or in white blood cells called basophils. Mast cells are especially numerous at sites of potential injury - the nose, mouth, and feet, internal body surfaces, and blood vessels. Non-mast cell histamine is found in several tissues, including the brain, where it functions as a neurotransmitter. Another important site of histamine storage and release is the enterochromaffin-like (ECL) cell of the stomach.
The most important pathophysiologic mechanism of mast cell and basophil histamine release is immunologic. These cells, if sensitized by IgE antibodies attached to their membranes, degranulate when exposed to the appropriate antigen. Certain amines and alkaloids, including such drugs as morphine, and curare alkaloids, can displace histamine in granules and cause its release. Antibiotics like polymyxin are also found to stimulate histamine release.
Histamine release occurs when allergens bind to mast-cell-bound IgE antibodies. Reduction of IgE overproduction may lower the likelihood of allergens finding sufficient free IgE to trigger a mast-cell-release of histamine.
Mechanism of action
Histamine exerts its actions by combining with specific cellular histamine receptors. The four histamine receptors that have been discovered in humans are designated H1 through H4, and are all G protein-coupled receptors (GPCR). Histamine receptors in insects, like Drosophila melanogaster, are histamine-gated chloride channels that function in inhibition of neurons.[6] Histamine-gated chloride channels are implicated in neurotransmission of peripheral sensory information in insects, especially in photoreception/vision. Two receptors subtypes have been identified in Drosophila, HClA and HClB.[7] There are no known GPCRs for histamine in insects.
| Type | Location | Function |
| H1 histamine receptor | Found on smooth muscle, endothelium, and central nervous system tissue | Causes, bronchoconstriction, bronchial smooth muscle contraction, vasodilation, separation of endothelial cells (responsible for hives), and pain and itching due to insect stings; the primary receptors involved in allergic rhinitis symptoms and motion sickness; sleep regulation. |
| H2 histamine receptor | Located on parietal cells and vascular smooth muscle cells | Primarily involved in vasodilation. Also stimulate gastric acid secretion |
| H3 histamine receptor | Found on central nervous system and to a lesser extent peripheral nervous system tissue | Decreased neurotransmitter release: histamine, acetylcholine, norepinephrine, serotonin |
| H4 histamine receptor | Found primarily in the basophils and in the bone marrow. It is also found on thymus, small intestine, spleen, and colon. | Plays a role in chemotaxis. |
Effects on nasal mucous membrane
Increased vascular permeability causes fluid to escape from capillaries into the tissues, which leads to the classic symptoms of an allergic reaction: a runny nose and watery eyes. Allergens can bind to IgE-loaded mast cells in the nasal cavity's mucous membranes. This can lead to three clinical responses:[8]
- sneezing due to histamine-associated sensory neural stimulation;
- hyper-secretion from glandular tissue; and
- nasal congestion due to vascular engorgement associated with vasodilation and increased capillary permeability.
Roles in the body
Sleep regulation
Histamine is released as a neurotransmitter. The cell bodies of histaminergics, the neurons which release histamine, are found in the posterior hypothalamus, in various tuberomammillary nuclei. From here, these neurons project throughout the brain, to the cortex through the medial forebrain bundle. Histaminergic action is known to modulate sleep. Classically, antihistamines (H1 histamine receptor antagonists) produce sleep. Likewise, destruction of histamine releasing neurons, or inhibition of histamine synthesis leads to an inability to maintain vigilance. Finally, H3 receptor antagonists increase wakefulness.
It has been shown that histaminergic cells have the most wakefulness-related firing pattern of any neuronal type thus far recorded. They fire rapidly during waking, fire more slowly during periods of relaxation/tiredness and completely stop firing during REM and NREM (non-REM) sleep. Histaminergic cells can be recorded firing just before an animal shows signs of waking.
Suppressive effects
While histamine has stimulatory effects upon neurons, it also has suppressive ones that protect against the susceptibility to convulsion, drug sensitization, denervation supersensitivity, ischemic lesions and stress.[9] It has also been suggested that histamine controls the mechanisms by which memories and learning are forgotten.[10]
Erection and sexual function
Libido loss and erectile failure can occur following histamine (H2) antagonists such as cimetidine and ranitidine.[11] The injection of histamine into the corpus cavernosum in men with psychogenic impotence produces full or partial erections in 74% of them.[12] It has been suggested that H2 antagonists may cause sexual difficulties by reducing the uptake[clarification needed] of testosterone.[11]
Schizophrenia
Metabolites of histamine are increased in the cerebrospinal fluid of people with schizophrenia, while the efficiency of H(1) receptor binding sites is decreased. Many atypical antipsychotic medications have the effect of increasing histamine turnover[clarification needed].[13]
Disorders
As an integral part of the immune system, histamine may be involved in immune system disorders and allergies.
History
The properties of histamine, then called β-iminazolylethylamine, were first described in 1910 by the British scientists Henry H. Dale and P.P. Laidlaw.[14]
"H substance" or "substance H" are occasionally used in medical literature for histamine or a hypothetical histamine-like diffusible substance released in allergic reactions of skin and in the responses of tissue to inflammation.[citation needed]
See also
References
- ^ a b http://www.sciencelab.com/msds.php?msdsId=9924264
- ^ Marieb, E. (2001). Human anatomy & physiology. San Francisco: Benjamin Cummings. p. 414. ISBN 0-8053-4989-8.
- ^ Di Giuseppe, M.; et al. (2003). Nelson Biology 12. Toronto: Thomson Canada Ltd. p. 473. ISBN 0-17-625987-2.
{{cite book}}: Explicit use of et al. in:|author=(help) - ^ Paiva, T. B.; Tominaga, M.; Paiva, A. C. M. (1970). "Ionization of histamine, N-acetylhistamine, and their iodinated derivatives". Journal of Medicinal Chemistry. 13 (4): 689–692. doi:10.1021/jm00298a025. PMID 5452432.
- ^ http://astrobiology.berkeley.edu/PDFs_articles/WineAnalysisAnalChem.pdf
- ^ Hardie RC 1989 A histamine-activated chloride channel involved in neurotransmission at a photoreceptor synapse. Nature
- ^ Pantazis et al. 2008 Distinct Roles for Two Histamine Receptors (hclA and hclB) at the Drosophila Photoreceptor Synapse. Journal of Neuroscience
- ^ Monroe, E., Daly, A., and Shalhoub, R. 1997. "Appraisal of the validity of histamine-induced wheal and flare to predict the clinical efficacy of antihistamines". Journal of Allergy and Clinical Immunology 99(2): S789-806.
- ^ Yanai, K; Tashiro, M (2007). "The physiological and pathophysiological roles of neuronal histamine: an insight from human positron emission tomography studies". Pharmacology & therapeutics. 113 (1): 1–15. doi:10.1016/j.pharmthera.2006.06.008. PMID 16890992.
- ^ Alvarez, EO (2009). "The role of histamine on cognition". Behavioural brain research. 199 (2): 183–9. doi:10.1016/j.bbr.2008.12.010. PMID 19126417.
- ^ a b White, JM; Rumbold, GR (1988). "Behavioural effects of histamine and its antagonists: a review". Psychopharmacology. 95 (1): 1–14. PMID 3133686.
- ^ Cará, AM; Lopes-Martins, RA; Antunes, E; Nahoum, CR; De Nucci, G (1995). "The role of histamine in human penile erection". British journal of urology. 75 (2): 220–4. doi:10.1111/j.1464-410X.1995.tb07315.x. PMID 7850330.
- ^ Ito, C (2004). "The role of the central histaminergic system on schizophrenia". Drug news & perspectives. 17 (6): 383–7. doi:10.1358/dnp.2004.17.6.829029. PMID 15334189.
- ^ Dale HH, Laidlaw PP (1910). "The physiological action of β-iminazolylethylamine" (PDF). J. Physiol. (Lond.). 41 (5): 318–44. PMC 1512903. PMID 16993030.
{{cite journal}}: Unknown parameter|month=ignored (help)
External links
