Histamine

| |

| |
| Names | |
|---|---|
| IUPAC name
2-(1H-imidazol-4-yl)ethanamine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.092 |
| KEGG | |
| MeSH | Histamine |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H9N3 | |
| Molar mass | 111.145 |
| Melting point | 83.5 °C (182.3 °F) |
| Boiling point | 209.5 °C (409.1 °F) |
| Easily soluble in cold water, hot water[1] | |
| Solubility | Easily soluble in methanol. Very slightly soluble in diethyl ether.[1] Easily soluble in ethanol. |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Histamine is an organic nitrogen compound involved in local immune responses as well as regulating physiological function in the gut and acting as a neurotransmitter.[2] Histamine triggers the inflammatory response. As part of an immune response to foreign pathogens, histamine is produced by basophils and by mast cells found in nearby connective tissues. Histamine increases the permeability of the capillaries to white blood cells and some proteins, to allow them to engage pathogens in the infected tissues.[3]
Properties
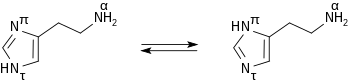
Histamine forms colorless hygroscopic crystals that melt at 84°C, and are easily dissolved in water or ethanol, but not in ether. In aqueous solution histamine exists in two tautomeric forms, Nπ-H-histamine and Nτ-H-histamine. The imidazole ring has two nitrogens. The nitrogen farthest away from the side chain is the 'tele' nitrogen and is denoted by a lowercase tau sign. The nitrogen closest to the side chain is the 'pros' nitrogen and is denoted by the pi sign. Whichever position the nitrogen, which has the hydrogen on it, is in, is how the tautomer is named. If the nitrogen with the hydrogen is in the tele position, then histamine is in the tele-tautomer form. The tele-tautomer is preferred in solution.
Histamine has two basic centres, namely the aliphatic amino group and whichever nitrogen atom of the imidazole ring does not already have a proton. Under physiological conditions, the aliphatic amino group (having a pKa around 9.4) will be protonated, whereas the second nitrogen of the imidazole ring (pKa ≈ 5.8) will not be protonated.[4] Thus, histamine is normally protonated to a singly charged cation.
Synthesis and metabolism
Histamine is derived from the decarboxylation of the amino acid histidine, a reaction catalyzed by the enzyme L-histidine decarboxylase. It is a hydrophilic vasoactive amine.

Once formed, histamine is either stored or rapidly inactivated by its primary degradative enzymes, histamine-N-methyltransferase or diamine oxidase. In the central nervous system, histamine released into the synapses is primarily broken down by histamine-N-methyltransferase, while in other tissues both enzymes may play a role. Several other enzymes, including MAO-B and ALDH2, further process the immediate metabolites of histamine for excretion or recycling.
Bacteria also are capable of producing histamine using histidine decarboxylase enzymes unrelated to those found in animals. A non-infectious form of foodborne disease, scombroid poisoning, is due to histamine production by bacteria in spoiled food, particularly fish. Fermented foods and beverages naturally contain small quantities of histamine due to a similar conversion performed by fermenting bacteria or yeasts. Sake contains histamine in the 20–40 mg/L range; wines contain it in the 2–10 mg/L range.[5]
Storage and release

Most histamine in the body is generated in granules in mast cells or in white blood cells called basophils. Mast cells are especially numerous at sites of potential injury - the nose, mouth, and feet, internal body surfaces, and blood vessels. Non-mast cell histamine is found in several tissues, including the brain, where it functions as a neurotransmitter. Another important site of histamine storage and release is the enterochromaffin-like (ECL) cell of the stomach.
The most important pathophysiologic mechanism of mast cell and basophil histamine release is immunologic. These cells, if sensitized by IgE antibodies attached to their membranes, degranulate when exposed to the appropriate antigen. Certain amines and alkaloids, including such drugs as morphine, and curare alkaloids, can displace histamine in granules and cause its release. Antibiotics like polymyxin are also found to stimulate histamine release.
Histamine release occurs when allergens bind to mast-cell-bound IgE antibodies. Reduction of IgE overproduction may lower the likelihood of allergens finding sufficient free IgE to trigger a mast-cell-release of histamine.
Mechanism of action
Histamine exerts its actions by combining with specific cellular histamine receptors. The four histamine receptors that have been discovered in humans and animals are designated H1 through H4, and are all G protein-coupled receptors (GPCR). Histamine biology is a series of weak interactions. In all of the known physiological reactions, the histamine backbone is unchanged. [6]
In the H2 receptor mechanism, histamine is protonated at the end-chain amine group. This amine group interacts with aspartic acid in the transmembrane domains of cells. The other nitrogens in the molecule interact with threonine and aspartic acid in different transmembrane domains. This is a three pronged interaction. It brings the transmembrane domains close to each other, causing a signal transduction cascade. [7]
Histamine receptors in insects, like Drosophila melanogaster, are histamine-gated chloride channels that function in inhibition of neurons.[8] Histamine-gated chloride channels are implicated in neurotransmission of peripheral sensory information in insects, especially in photoreception/vision. Two receptors subtypes have been identified in Drosophila, HClA and HClB.[9] There are no known GPCRs for histamine in insects.
| Type | Location | Function |
| H1 histamine receptor | Found on smooth muscle, endothelium, and central nervous system tissue | Causes, bronchoconstriction, bronchial smooth muscle contraction, vasodilation, separation of endothelial cells (responsible for hives), and pain and itching due to insect stings; the primary receptors involved in allergic rhinitis symptoms and motion sickness; sleep and appetite suppression. |
| H2 histamine receptor | Located on parietal cells and vascular smooth muscle cells | Primarily involved in vasodilation. Also stimulate gastric acid secretion |
| H3 histamine receptor | Found on central nervous system and to a lesser extent peripheral nervous system tissue | Decreased neurotransmitter release: histamine, acetylcholine, norepinephrine, serotonin |
| H4 histamine receptor | Found primarily in the basophils and in the bone marrow. It is also found on thymus, small intestine, spleen, and colon. | Plays a role in chemotaxis. |
Effects on nasal mucous membrane
Increased vascular permeability causes fluid to escape from capillaries into the tissues, which leads to the classic symptoms of an allergic reaction: a runny nose and watery eyes. Allergens can bind to IgE-loaded mast cells in the nasal cavity's mucous membranes. This can lead to three clinical responses:[10]
- sneezing due to histamine-associated sensory neural stimulation;
- hyper-secretion from glandular tissue; and
- nasal congestion due to vascular engorgement associated with vasodilation and increased capillary permeability.
Roles in the body
Although histamine is small compared to other biological molecules (containing only 17 atoms), it plays an important role in the body. It is known to be involved in 23 different physiological functions. Histamine is known to be involved in so many physiological functions because of its chemical properties that allow it to be so versatile in binding. It is Coulombic (able to carry a charge), conformational, and flexible. This allows it to interact and bind more easily. [11]
Sleep regulation
Histamine is released as a neurotransmitter. The cell bodies of histaminergics, the neurons which release histamine, are found in the posterior hypothalamus, in various tuberomammillary nuclei. From here, these neurons project throughout the brain, to the cortex through the medial forebrain bundle. Histaminergic action is known to modulate sleep. Classically, antihistamines (H1 histamine receptor antagonists) produce sleep. Likewise, destruction of histamine releasing neurons, or inhibition of histamine synthesis leads to an inability to maintain vigilance. Finally, H3 receptor antagonists increase wakefulness.
It has been shown that histaminergic cells have the most wakefulness-related firing pattern of any neuronal type thus far recorded. They fire rapidly during waking, fire more slowly during periods of relaxation/tiredness and completely stop firing during REM and NREM (non-REM) sleep. Histaminergic cells can be recorded firing just before an animal shows signs of waking.
Suppressive effects
While histamine has stimulatory effects upon neurons, it also has suppressive ones that protect against the susceptibility to convulsion, drug sensitization, denervation supersensitivity, ischemic lesions and stress.[12] It has also been suggested that histamine controls the mechanisms by which memories and learning are forgotten.[13]
Erection and sexual function
Libido loss and erectile failure can occur following histamine (H2) antagonists such as cimetidine and ranitidine.[14] The injection of histamine into the corpus cavernosum in men with psychogenic impotence produces full or partial erections in 74% of them.[15] It has been suggested that H2 antagonists may cause sexual difficulties by reducing the uptake[clarification needed] of testosterone.[14]
Schizophrenia
Metabolites of histamine are increased in the cerebrospinal fluid of people with schizophrenia, while the efficiency of H(1) receptor binding sites is decreased. Many atypical antipsychotic medications have the effect of increasing histamine turnover.[16]
Multiple Sclerosis
Histamine therapy for treatment of multiple sclerosis is currently being studied. The different H receptors have been known to have different effects on the treatment of this disease. The H1 and H4 receptors, in one study, have been shown to be counterproductive in the treatment of MS. The H1 and H4 receptors are thought to decrease permeability in the Blood Brain Barrier, thus increasing infiltration of unwanted cells in the Central Nervous System. This can cause inflammation, and MS symptom worsening. The H2 and H3 receptors are thought to be helpful when treating MS patients. Histamine has been shown to help with T-cell differentiation. This is important because in MS, the immune system attacks its own myelin sheaths on nerve cells (which causes loss of signaling function and eventual nerve degeneration). By helping T cells to differentiate, the T cells will be less likely to attack the body's own cells, and instead attack invaders. [17]
Disorders
As an integral part of the immune system, histamine may be involved in immune system disorders and allergies. Mastocytosis is a rare disease in which there is a proliferation of mast cells that produce excess histamine. [18] Histamine intolerance results from a disequilibrium of accumulated histamine and the capacity for histamine degradation. Histamine is a biogenic amine that occurs to various degrees in many foods. In healthy persons, dietary histamine can be rapidly detoxified by amine oxidases, whereas persons with low amine oxidase activity are at risk of histamine toxicity. Diamine oxidase (DAO) is the main enzyme for the metabolism of ingested histamine. It has been proposed that DAO, when functioning as a secretory protein, may be responsible for scavenging extracellular histamine after mediator release. Conversely, histamine N-methyltransferase, the other important enzyme inactivating histamine, is a cytosolic protein that can convert histamine only in the intracellular space of cells. An impaired histamine degradation based on reduced DAO activity and the resulting histamine excess may cause numerous symptoms mimicking an allergic reaction. [19]
History
The properties of histamine, then called β-iminazolylethylamine, were first described in 1910 by the British scientists Henry H. Dale and P.P. Laidlaw.[20]
"H substance" or "substance H" are occasionally used in medical literature for histamine or a hypothetical histamine-like diffusible substance released in allergic reactions of skin and in the responses of tissue to inflammation.[citation needed]
See also
References
- ^ a b http://www.sciencelab.com/msds.php?msdsId=9924264
- ^ Marieb, E. (2001). Human anatomy & physiology. San Francisco: Benjamin Cummings. p. 414. ISBN 0-8053-4989-8.
- ^ Di Giuseppe, M.; et al. (2003). Nelson Biology 12. Toronto: Thomson Canada Ltd. p. 473. ISBN 0-17-625987-2.
{{cite book}}: Explicit use of et al. in:|author=(help) - ^ Paiva, T. B.; Tominaga, M.; Paiva, A. C. M. (1970). "Ionization of histamine, N-acetylhistamine, and their iodinated derivatives". Journal of Medicinal Chemistry. 13 (4): 689–692. doi:10.1021/jm00298a025. PMID 5452432.
- ^ http://astrobiology.berkeley.edu/PDFs_articles/WineAnalysisAnalChem.pdf
- ^ Noszal, B.; Kraszni, M.; Racz, A. Histamine: fundamentals of biological chemistry. In Histamine: Biology and Medical Aspects; Falus, A., Grosman, N. and Darvas, Z., Eds.; SpringMed Publishing Ltd.: Budapest, 2004; pp 15-28.
- ^ Noszal, B.; Kraszni, M.; Racz, A. Histamine: fundamentals of biological chemistry. In Histamine: Biology and Medical Aspects; Falus, A., Grosman, N. and Darvas, Z., Eds.; SpringMed Publishing Ltd.: Budapest, 2004; pp 15-28.
- ^ Hardie RC (1989). "A histamine-activated chloride channel involved in neurotransmission at a photoreceptor synapse". Nature. 339 (6227): 704–6. doi:10.1038/339704a0. PMID 2472552.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Pantazis A, Segaran A, Liu CH; et al. (2008). "Distinct roles for two histamine receptors (hclA and hclB) at the Drosophila photoreceptor synapse". J. Neurosci. 28 (29): 7250–9. doi:10.1523/JNEUROSCI.1654-08.2008. PMID 18632929.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Monroe EW, Daly AF, Shalhoub RF (1997). "Appraisal of the validity of histamine-induced wheal and flare to predict the clinical efficacy of antihistamines". J. Allergy Clin. Immunol. 99 (2): S798–806. PMID 9042073.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Noszal, B.; Kraszni, M.; Racz, A. Histamine: fundamentals of biological chemistry. In Histamine: Biology and Medical Aspects; Falus, A., Grosman, N. and Darvas, Z., Eds.; SpringMed Publishing Ltd.: Budapest, 2004; pp 15-28.
- ^ Yanai, K; Tashiro, M (2007). "The physiological and pathophysiological roles of neuronal histamine: an insight from human positron emission tomography studies". Pharmacology & therapeutics. 113 (1): 1–15. doi:10.1016/j.pharmthera.2006.06.008. PMID 16890992.
- ^ Alvarez, EO (2009). "The role of histamine on cognition". Behavioural Brain Research. 199 (2): 183–9. doi:10.1016/j.bbr.2008.12.010. PMID 19126417.
- ^ a b White, JM; Rumbold, GR (1988). "Behavioural effects of histamine and its antagonists: a review". Psychopharmacology. 95 (1): 1–14. PMID 3133686.
- ^ Cará, AM; Lopes-Martins, RA; Antunes, E; Nahoum, CR; De Nucci, G (1995). "The role of histamine in human penile erection". British journal of urology. 75 (2): 220–4. doi:10.1111/j.1464-410X.1995.tb07315.x. PMID 7850330.
- ^ Ito, C (2004). "The role of the central histaminergic system on schizophrenia". Drug news & perspectives. 17 (6): 383–7. doi:10.1358/dnp.2004.17.6.829029. PMID 15334189.
- ^ Jadidi Niaragh, F.; Mirshafiey, A. Histamine and histamine receptors in pathogenesis and treatment of multiple sclerosis. Neuropharmacology 2010, 59, 180-189.
- ^ Valent P, Horny HP, Escribano L; et al. (2001). "Diagnostic criteria and classification of mastocytosis: a consensus proposal". Leuk. Res. 25 (7): 603–25. PMID 11377686.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Laura Maintz and Natalija Novak. "Histamine and histamine intolerance1,2,3". American Journal of Clinical Nutrition.
- ^ Dale HH, Laidlaw PP (1910). "The physiological action of β-iminazolylethylamine" (PDF). J. Physiol. (Lond.). 41 (5): 318–44. PMC 1512903. PMID 16993030.
{{cite journal}}: Unknown parameter|month=ignored (help)
External links
- Histamine bound to proteins in the PDB
