Wikipedia:Reference desk/Science: Difference between revisions
→Name that beetle: new section |
Undid revision 615633142 by Baseball Bugs (talk)rvv |
||
| Line 264: | Line 264: | ||
::Wouldn't the survival rate depend mainly on the configuration of the drainage system immediately after the U-bend? A gentle but free-draining gradient with some irregularities to hang on to should ensure that most mammals and insects would survive a brief immersion in (not too polluted) water. For fish, the quality of the water lower down would be critical. If flood-water drainage is mixing with the sewage then there would be a chance of survival for a time. [[User:Dbfirs|''<font face="verdana"><font color="blue">D</font><font color="#00ccff">b</font><font color="#44ffcc">f</font><font color="66ff66">i</font><font color="44ee44">r</font><font color="44aa44">s</font></font>'']] 07:07, 4 July 2014 (UTC) |
::Wouldn't the survival rate depend mainly on the configuration of the drainage system immediately after the U-bend? A gentle but free-draining gradient with some irregularities to hang on to should ensure that most mammals and insects would survive a brief immersion in (not too polluted) water. For fish, the quality of the water lower down would be critical. If flood-water drainage is mixing with the sewage then there would be a chance of survival for a time. [[User:Dbfirs|''<font face="verdana"><font color="blue">D</font><font color="#00ccff">b</font><font color="#44ffcc">f</font><font color="66ff66">i</font><font color="44ee44">r</font><font color="44aa44">s</font></font>'']] 07:07, 4 July 2014 (UTC) |
||
:<small>"Personal freedom infringed? Ring Slater-Nazi, CITy 0478 or if closed, the Department of Trade and Industry." [[User:Tevildo|Tevildo]] ([[User talk:Tevildo|talk]]) 16:50, 4 July 2014 (UTC)</small> |
|||
== Lost city of Ubar == |
== Lost city of Ubar == |
||
Revision as of 08:23, 5 July 2014
of the Wikipedia reference desk.
Main page: Help searching Wikipedia
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Wikipedia:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
July 1
Superconductor critical temperature graph
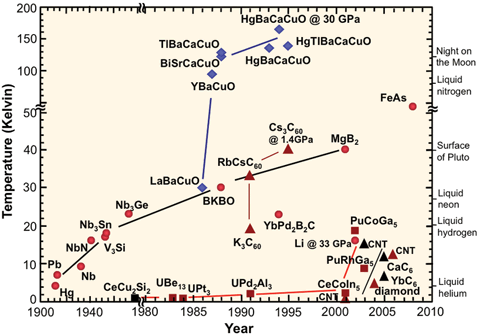
I would like to redraw File:Sc_history.gif to address the misleading axes (the year axis before 1980, and temperature axis above 50 K are compressed).
Could anyone familiar with the subject please tell me what the colours and shapes of the markers for each substance denote? I tried searching for a legend of the graphic online but couldn't find anything. It's no longer available at the original US government site.
Secondly, FeAs appears under 50 K in the top part of the graph, yet doesn't appear in the bottom part (which is under 50 K), so what is its critical temperature? Iron-based_superconductor lists many FeAs compounds but no plain FeAs.
Thanks, cmɢʟee⎆τaʟκ 19:17, 1 July 2014 (UTC)
- Not an answer, but rather a suggestion: If you redo that chart, I'd take the chemical formulas off the graph and place them in a legend, instead, and just put a number in place of each on the graph. This will make the graph less busy and easier to read. Also, ideally the legend would have links to the articles on each chemical. StuRat (talk) 23:30, 1 July 2014 (UTC)
- ..."easier to read" is often in the eye of the beholder. Replacing all the formulas with numbers means that at a single glance it is impossible to identify important compounds or chemical families. Someone wanting that sort of basic information would have to constantly shift their focus from the graph to the legend and back again. (Note, as well, that the "formulas" already present are, in many cases, abbreviated; for example, the XBaCuO and XCaCuO compounds are most assuredly not actually present in anything like 1:1:1:1 quantity.) Stripping out too much information can cause as much confusion as putting too much in.
- Arguably, one might be able to label the families of compounds in some cases (the blue diamonds are all YBCO-related structures, for instance) and add smaller notations for the individual family members, but there's limited gains to be made there. Some of the 'families' are pretty tenuously related. TenOfAllTrades(talk) 07:58, 2 July 2014 (UTC)
- I'd love to see the original legend for the categories. As I note above, the blue diamonds are YBCO/LBCO-related compounds. The dark red triangles are "3D"-carbon compounds (conjugates to carbon nanotubes or buckyballs, or diamond), the black triangles are "2D"-carbon compounds (intercalated graphite structures). The red circles seem to be mostly metals and simple binary compounds...except for the ones that aren't(?) The red squares are..."weird-ass actinide compounds"? Note that FeAs doesn't likely refer to a straight-up "iron arsenide" binary compound; presumably it is a catch-all for the ferropnictide [FeAs] layered structure, of which the best have (as you've seen in the linked article) critical temps just above 50 K.
- As for adjusting the vertical and horizontal scales, do be careful. You can linearize the vertical scale without too much harm if you're willing to accept a much taller figure; it works well if the point you want to convey is that the YBCO compounds were an astonishing leap forward in the field. If you try to keep the figure the same height, though, you squish together all the lower-Tc points. Similar crowding problems arise if you try to linearize the date axis. There are only eight points in the eighty years (one point per decade) between 1900 and 1980, whereas there are nearly thirty points in the subsequent thirty (almost one point per year). If the point you want to make is that the field was relatively quiet until the mid-1980s, that's fine; if you want the reader to be able to read approximate date and Tc information for particular compounds or classes...then completely linear date and Tc scales may not be as effective. TenOfAllTrades(talk) 07:58, 2 July 2014 (UTC)
- Looks like FeAs is ~54K, according to the introduction of [1].
- I would suggest trying out a semilog plot format for the Tc, since temperature practically wants a log before it anyway. Also a cool aspect of that plot is that you could have room temperature taunt the reader from the top of the graph. Wnt (talk) 14:45, 2 July 2014 (UTC)
- I second that suggestion for a semilog plot. This is a far more natural way to squeeze this information into a graph, compared to the current cut axes. The jumps in the axes essentially hide what the graph is trying to communicate, which is the progression in time. Crowding can be dealt with via fine lines from data points to the individual labels. —Quondum 16:13, 2 July 2014 (UTC)
Plastic in the Sun
I live in the Mojave Desert. I regularly find old plastic soda and water bottles out in the sand. Often, the bottles are so thin and brittle that the side facing up (in the sunlight) turns to dust when you touch it. This is, to me, degraded plastic. However, I am strongly into recycling and one common claim that I've read (and I use in my writings) is that it takes about 500 years for plastic to degrade. I know that these soda bottles are not anywhere near 500 years old. So, is this dust state of plastic not considered degraded plastic? If it is degraded, what is the justification for the standard "500 year" time for plastic degradation? I want to sound as though I have some knowledge of the matter because I'm sure others around here have seen plastic turned to dust as well. — Preceding unsigned comment added by 209.149.113.71 (talk) 19:54, 1 July 2014 (UTC)
- See the articles Biodegradeable and Biodegradable plastic for information. 84.209.89.214 (talk) 22:57, 1 July 2014 (UTC)
- The UV light causes this degradation in polymers such as plastics and rubber, unless they take precautions, such as adding dye to absorb the UV. This is why, incidentally, they quickly stopped making white rubber tires, as they rapidly degraded in sunlight, too. So, the 500 year time frame is for buried plastics, kept safe from UV light. StuRat (talk) 23:26, 1 July 2014 (UTC)
- Plastics degrade at differing rates depending on the composition of the material and on the environment it is in, such as exposure to UV. Some contain stabilizers to resist UV, others do not. The book "Green plastics" says (p53) that some polyolefins basically do not degrade, while more recently biodegradable plastics have been introduced which break down after use via the action of moisture, daylight, heat or biological activity. But I have seen tables which imply that it is bad to use plastic because it takes 500 uers to break down, without considering the composition and the environment it is exposed to. Edison (talk) 02:32, 2 July 2014 (UTC)
- I was too lazy to look it up but certainly "turning to dust" is not the same as degradation; see microbeads. :( Some of the "biodegradable" plastics of the past simply broke up into fibers. Of course, plastic will still degrade faster in the sun, while even newspapers might remain readable in landfills on a paleontological time scale. Wnt (talk) 14:37, 2 July 2014 (UTC)
- YES. I could take a new plastic bottle and run it through a shredder a few times, but it would not be decomposed, just broken into little pieces. As with microbeads, these small particles can still be potentially harmful. Though some depolymerization may occur in the desert sun over a few years, those small particles are still plastic, and in some ways even more harmful, since they can be easily dispersed and cannot be picked up and recycled the way an intact bottle can. I'd encourage the OP to tell friends and neighbors that picking up bottles before they disintegrate is the best option for avoiding environmental contamination. See also Plastic_particle_water_pollution and Microplastics. Most of the documented damage is in marine environments, but remember everything eventually gets washed to the sea. SemanticMantis (talk) 14:57, 2 July 2014 (UTC)
- Most Plastic is actually brittle in its raw form. That was a major problem with early plastic like Bakelite (1907). Today Plasticizers are used widely to put it in the state we are so familiar with that we take it for its oiginal state. Unfortunately these additives evapurate or degenetate rather fast over 20 - 30 years and much faster under exposure of Light, especially ultraviolet wavelength. So actually plastic "degenerates" much faster in 20-30 years. The 500 years are needed to decompose plastic completely. --Kharon (talk) 20:56, 2 July 2014 (UTC)
- The brittle plastics are thermoset plastics. Thermoplastics, on the other hand, despite the similar name, are soft and flexible at a certain temperature (typically room temperature), how we normally think of plastics. StuRat (talk) 21:19, 2 July 2014 (UTC)
- Good distinction. The problem that apparently none of us want to invest time in researching is: what exactly constitutes decomposition of a plastic? Small pieces of e.g. HDPE (commonly used for bottles) are still HDPE, and that can cause problems in both natural ecosystems, and in human health. When is HDPE no longer HDPE? It sounds like a koan, but that is the type of question we need to research to fully answer this question. I'm no good a this kind of detailed chemistry, but I suspect the long-chain molecules must be cut/broken until the constituent ingredients no longer have the physical and chemical properties of the parent plastic. UV light can apparently break such bonds, but that is similar to dilution -- how many drops of water must I add to my coca cola before it no longer coca cola? I think in the OP's description of bottles turning to dust some chains are broken, but many more remain. SemanticMantis (talk) 21:41, 2 July 2014 (UTC)
- The brittle plastics are thermoset plastics. Thermoplastics, on the other hand, despite the similar name, are soft and flexible at a certain temperature (typically room temperature), how we normally think of plastics. StuRat (talk) 21:19, 2 July 2014 (UTC)
- Thanks for all the comments. This sent me off to do more research. Water bottles are normally HDPE, which degrades quickly in UV light, and still degrades quickly without UV light. The 500 year statement is the average time for LDPE plastic to degrade. I found a nice chart that relates the recycle number to breakdown estimates. I don't feel that this negates my work in trying to limit plastic use. It helps focus on bad plastics and worse plastics. The site with the chart is http://www.brighthub.com/environment/green-living/articles/107380.aspx 209.149.113.71 (talk) 20:09, 3 July 2014 (UTC)
- Re. HDPE vs. LDPE: Note that HDPE molecules are practically linear, whereas LDPE molecules are highly branched. This has a TREMENDOUS impact on the rate of bacterial degradation, with linear molecules being degraded MUCH more readily than branched ones -- as we've already seen with synthetic detergents, which break down over time in water if the molecule is linear, but not if it's branched. 24.5.122.13 (talk) 22:21, 3 July 2014 (UTC)
July 2
Enthalpy of formation
Is it possible to calculate a vapoural heat of formation of when IR spectroscopic data is the only experimental data available? Plasmic Physics (talk) 00:23, 2 July 2014 (UTC)
- Maybe, but I don't see how that would be possible. For measuring heat of formation, calorimetry is the normal technique. 24.5.122.13 (talk) 01:11, 2 July 2014 (UTC)
- You mean if the ONLY thing you know is a single ir spectrum? No. But IR does capture TEMPERATURE information - I hope that is clear to you. So, theoretically, there are probably some gas phase reactions which have sufficiently strong and sufficiently sensitive peaks in the IR so that you could determine the initial and final composition and temperatures. Generally, IR is only semi-quantitative, meaning that its good for composition to 5% (optimistically) and maybe ½% in very good circumstances. This means that IR would be a poor method if you wanted accuracy to 1% or better. (And this ignores the issue, which I am not competent to deal with of the accuracy of the temperature measurement using IR. I know that IR guns are commonly used to measure temp., and I know they can be calibrated to within a couple of degrees, but I don't know how much more accurate a calibrated lab IR spectrometer can be (since a couple of degrees is probably not good enough).) Could you get "an answer" ? Sure. How accurate would it be? Depends, but without a lot of calibrating, I doubt it would be more than plus or minus a factor of 10 of the right Hf...maybe a factor of +/- 5X ... Your question basically doesn't give sufficient context for an answer. A lab IR uses IR to excite various modes, while temperature is all about the existing modes (or black-body radiation emission). Meaning if you want to see if something in a room is giving off light, you don't turn on more lights, you turn all the lights off... — Preceding unsigned comment added by 173.189.75.163 (talk) 15:54, 2 July 2014 (UTC)
- What a shame, thanks though. Plasmic Physics (talk) 04:23, 3 July 2014 (UTC)
Will Ebola become a global pandemic?
There are about 600 Ebola cases in West Africa and it seems that the number of cases is doubling every month. At this rate, in about a year there could be a million cases and in two years time there could be more than a billion cases. With an incubation period of up to 21 days, what is going to stop this virus from getting to Asia, the US or Europe? Count Iblis (talk) 18:11, 2 July 2014 (UTC)
- Our Ebola article states that "The potential for widespread EVD epidemics is considered low due to the high case-fatality rate, the rapidity of demise of patients, and the often remote areas where infections occur". It also says that "Due to lack of proper equipment and hygienic practices, large-scale epidemics occur mostly in poor, isolated areas without modern hospitals or well-educated medical staff". Your 'doubling every month' prediction (which isn't actually borne out by the latest data - see 2014 West Africa Ebola outbreak#Temporal evolution) is predicated on the same lack of appropriate medical care occurring elsewhere. AndyTheGrump (talk) 18:25, 2 July 2014 (UTC)
- As Andy says, Ebola epidemics are usually more or less self-contained because of the rapidity of the death of patients, and because the epidemics tend to occur in isolated areas, where the patients are unlikely to go to the US, Europe, or Asia. If the patients come in contact with visitors from an industrial country who then return home and develop Ebola, they will be treated in isolation units to control the spread. Influenza is far more of a pandemic threat than Ebola or similar hemorrhagic fevers. Get your flu shot this coming fall. (If you are in the Southern Hemisphere and haven't gotten your flu shot yet, it isn't too late.) Robert McClenon (talk) 18:54, 2 July 2014 (UTC)
- Yes. Ebola kills hundreds per year and may expand to thousands. Flu kills hundreds of thousands and has sometimes expanded to millions. Our OP is relying on news to guide action. A disease makes news when it is interesting, thus odd, thus small. Newspeople aren't in the business of guding action. Jim.henderson (talk) 19:06, 2 July 2014 (UTC)
- Also note that increasing at a rate, were it to continue, which would rapidly infect the entire human population, is a feature of the early stages of many diseases or disease strains. That rate of increase inevitable slows down, as it moves out of the most vulnerable populations, survivors develop immunity, and people start taking precautions, like breathing masks, in the case of SARS. Of course, we should avoid getting lazy and just assuming it will slow down, but rather study it and actively find ways to slow it down. StuRat (talk) 19:11, 2 July 2014 (UTC)
- See Richard II Act II Scene 1:
- John of Gaunt:
- ...His rash fierce blaze of riot cannot last,
- For violent fires soon burn out themselves;
- Small showers last long, but sudden storms are short;
- He tires betimes that spurs too fast betimes;
- With eager feeding food doth choke the feeder:
- Light vanity, insatiate cormorant,
- Consuming means, soon preys upon itself.
- (Reminding all of WP:NOTCRYSTAL) -- the question in the header cannot be answered with references. I will not answer this question, but instead provide references relevant to the topic.
- If OP would like reliable information on the current Ebola situation, he should look at WP:RS such as the USA's CDC page on the topic [2], or the UK's HPA [3]. If you prefer an NGO perspective, check out info from Doctors without borders here [4], [5]
- Since this is "breaking news" in the field of epidemiology, there are very few peer-reviewed papers published on the current outbreak, and reports from those agencies are probably the best sources. If you want to see some of the science of Ebola-specific control and mitigation, here's a paper on an Ebola vaccine and vaccination strategies [6]. For the general topic of control strategies for multiple outbreaks, see this nice paper on epidemic control in a more disease-agnostic sense [7]. SemanticMantis (talk) 19:53, 2 July 2014 (UTC)
- Hygiene, communication and medicine are advanced enough today to contain any epidemic virus that spreads tru physical contact and/or exchange of body fluids. Only airborne contagious Vira have a high potential to cause a Global pandemic. Thus not Ebola. --Kharon (talk) 21:24, 2 July 2014 (UTC)
- "Viruses" if you're writing in English, "virus" (plural) if you're writing in Latin. "Vira" is entirely beyond the pale, as there is no Latin declension that has "-us" in the singular and "-a" in the plural. "Virii" (the most common error) has at least the excuse of being plausible. Tevildo (talk) 21:59, 2 July 2014 (UTC)
- Virus isn't originally Latin. It is originally Greek. I don't know Greek plurals. However, Tevildo is right about the Latin. If virus is fully naturalized in Latin, it is fifth declension, and its plural is virus. So if it is fully naturalized in English, and it is, its plural is viruses. Any other plural is just a misguided attempt to use a classical language without understanding the classical language. Fortunately, this has not been litigated, so that no one needs to be warned of discretionary sanctions. But do not edit war over the plural. It is viruses, unless the rest of the article is in Latin. Robert McClenon (talk) 03:22, 3 July 2014 (UTC)
- No and no. Vīrus is not from Greek (how could it be? - classical Greek had lost its w sound before the Roman period). It's a native Latin word. The equivalent Greek word is īos, both the Latin and Greek forms representing regular development from something like like *wīsos. And it's not fifth declension. The genitive singular is documented as vīrī, making it second declension. In fact it's an almost unique example of a second declension neuter noun in -us: nominative and accusative both vīrus, dative and ablative apparently not found, and more relevant to the current question, it never occurs in the plural. --rossb (talk) 16:14, 3 July 2014 (UTC)
- Robert may have been led astray by the modern barbarism "virion", which is no better and no worse than "television", when it comes down to it. Did we not at one time have a very long section on the etymology of "virus" in our article, or did I read it elsewhere? Tevildo (talk) 21:56, 3 July 2014 (UTC)
- No and no. Vīrus is not from Greek (how could it be? - classical Greek had lost its w sound before the Roman period). It's a native Latin word. The equivalent Greek word is īos, both the Latin and Greek forms representing regular development from something like like *wīsos. And it's not fifth declension. The genitive singular is documented as vīrī, making it second declension. In fact it's an almost unique example of a second declension neuter noun in -us: nominative and accusative both vīrus, dative and ablative apparently not found, and more relevant to the current question, it never occurs in the plural. --rossb (talk) 16:14, 3 July 2014 (UTC)
- Virus isn't originally Latin. It is originally Greek. I don't know Greek plurals. However, Tevildo is right about the Latin. If virus is fully naturalized in Latin, it is fifth declension, and its plural is virus. So if it is fully naturalized in English, and it is, its plural is viruses. Any other plural is just a misguided attempt to use a classical language without understanding the classical language. Fortunately, this has not been litigated, so that no one needs to be warned of discretionary sanctions. But do not edit war over the plural. It is viruses, unless the rest of the article is in Latin. Robert McClenon (talk) 03:22, 3 July 2014 (UTC)
- To answer the original question, it's not as if the locals are doing nothing; in Liberia, the legislature appropriated a huge portion of the national budget to fight it (if I remember rightly), and although the news outlets currently aren't talking as much about Ebola as they were a few months ago, it still sometimes makes the headlines; this story, for example, is on the main page of the Heritage website, while the Liberian Observer hasn't been running any above-the-fold stories about it in the last few days. A big contributor to the spread of such a disease is ignorance — people who are aware of it will be more careful, and that alone will reduce transmission rates, especially with a disease like this that requires some sort of contact with a patient. Nyttend (talk) 02:06, 3 July 2014 (UTC)
- "Viruses" if you're writing in English, "virus" (plural) if you're writing in Latin. "Vira" is entirely beyond the pale, as there is no Latin declension that has "-us" in the singular and "-a" in the plural. "Virii" (the most common error) has at least the excuse of being plausible. Tevildo (talk) 21:59, 2 July 2014 (UTC)
- Hygiene, communication and medicine are advanced enough today to contain any epidemic virus that spreads tru physical contact and/or exchange of body fluids. Only airborne contagious Vira have a high potential to cause a Global pandemic. Thus not Ebola. --Kharon (talk) 21:24, 2 July 2014 (UTC)
Air conditioner settings
I have a window air conditioner that has only two dials. One is a dial that can be turned from max cooling (7) to min cooling (1). The other dial has four settings, labeled "low cool", "high cool", "low fan", and "high fan". What is the difference between "low cool" and "high cool" (the manual is slim and gives no useful information on this)?
- One of my friends argues that the words "low" and "high" in "low cool" and "high cool" refers to the fan, so that compressor usage is the same in both settings, with only fan speed differing. In other words, he argues that the settings mean:
- "low cool" - compressor on; low fan speed
- "high cool" - compressor on; high fan speed
- "low fan" - compressor off; low fan speed
- "high fan" - compressor off; high fan speed
- Another of my friends argues that the words "low" and "high" in "low cool" and "high cool" refers to compressor usage, and that "high cool" uses the compressor more than "low cool" does. In other words, he argues that the settings mean:
- "low cool" - compressor on less; fan off
- "high cool" - compressor on more; fan off
- "low fan" - compressor off; low fan speed
- "high fan" - compressor off; high fan speed
Who is right?
—SeekingAnswers (reply) 21:54, 2 July 2014 (UTC)
- We can't predict the response of an unknown model of air conditioner. You could specify the model, but I don't understand why you can't just listen to what the fan sounds like (surely it is more than loud enough, and even a deaf person would feel it blowing and also feel the vibration of the compressor) Wnt (talk) 22:14, 2 July 2014 (UTC)
- Re: "We can't predict the response of an unknown model of air conditioner." Googling suggests these setting labels are fairly standard for many air conditioners. There would be a standard meaning to them, then, no? And no matter what the setting, there is sound, there is air, and there is vibration, so I can't tell the difference between them that way. —SeekingAnswers (reply) 22:20, 2 July 2014 (UTC)
- (e/c) Top option. The compressor is only 'On' or 'Off'; the fan has 2 speeds in this case. (In my case, it set so that when the compressor is on, the fan is high, and when the compressor is off, the fan is low; for circulation, air filtering and even distribution of temp/humidity). This link provides some general info: [8] (A web search can easily find plenty of other links). BTW, the fan doesn't use much electricity, especially when compared to the compressor. —71.20.250.51 (talk) 22:26, 2 July 2014 (UTC)
Okay, after changing around the settings and listening carefully as User:Wnt suggested, I agree with User:71.20.250.51 and User:StuRat that top option is correct: when set to "high cool", I can hear the compressor periodically automatically turning off, presumably due to the thermostat detecting that it has reached the desired temperature set by the other dial, at which point it sounds exactly like "high fan". But now I have a new question. When initially turning the air conditioner on, the manual recommends "high cool" to cool down the room to the desired temperature as quickly as possible. My question, however, is about what to do once the room cool downs to the desired temperature: given that the compressor automatically turns off when it detects that the desired temperature has been reached, at that point, would it be more energy-efficient / cheaper for my electricity bill to leave the air conditioner on "high cool" or to switch to "low cool"? First of all, everyone agrees that the compressor comes on or off automatically based on the thermostat, and that the compressor costs far more electricity than the fan. However, that still leads my friends to different conclusions:
- Friend #1 argues that once you only want to maintain a temperature rather than cool further, "high cool" is more energy-efficient and cheaper than "low cool", because the fan uses so little electricity in comparison to the compressor that all that matters is how often the compressor comes on. He argues that "high cool", with the fan running at high speed, will do more to circulate the cool air, therefore doing more to maintain the cool temperature, and therefore causing the compressor to automatically turn on less often and thereby saving more electricity.
- Friend #2 argues that once you only want to maintain a temperature rather than cool further, "low cool" is more energy-efficient and cheaper than "high cool", because the fan is running at a lower speed, which obviously consumes less electricity. He rejects friend #1's argument about the fan running at high speed doing anything to maintain a cool temperature to cause the compressor to turn on less often. In other words, friend #2 believes how often the compressor automatically turns on/off is entirely unaffected by fan speed.
- Friend #3 agrees with friend #2 that once you only want to maintain a temperature rather than cool further, "low cool" is more energy-efficient and cheaper than "high cool". However, he differs from friend #2 in also accepting friend #1's argument that the fan running at high speed will cause the compressor to turn on less often -- but he ultimately still agrees with friend #2 that "low cool" is more energy-efficient and cheaper than "high cool" because he believes the effect of greater circulation is very small and he does not believe that the compressor will turn on less often enough to compensate for the higher cost from running the fan at high speed.
Now who is right among these 3 viewpoints?
—SeekingAnswers (reply) 04:07, 3 July 2014 (UTC)
- It depends on the size and layout of your room. In a small square room, with the A/C in the center of one wall, and nothing in the way, the low fan is probably adequate. But in a large, irregular shaped room with obstacles, like a big-screen TV in the center, more fan speed might be required to distribute the "coolth". I've even supplemented the anemic fan in my window A/C unit with a box fan blowing across the vents.
- My experience based on a lot of motels with that sort of air conditioner is that "high" is usually too damn noisy, and that's enough reason to prefer "low" if possible. --50.100.189.160 (talk) 19:47, 3 July 2014 (UTC)
- I'm not asking "which gets the job done" -- both "low cool" and "high cool" successfully maintain the cool temperature of the room -- I'm asking which of them does it with less electricity, as several arguments have been given as to why either might use less electricity. —SeekingAnswers (reply) 02:08, 4 July 2014 (UTC)
- I think this depends on where the thermostat actually is (the part taking the measurement) and if it is drawing in outside air, the temperature of the air. If the air outside is hotter and being drawn in, the more you have the fan on the more you're drawing in the heat from outside. If the air is from inside, the metal is probably still transmitting more heat in from outside when more air is blowing through it but I doubt it is much. Now when the air passes over the compressor, it warms it up, and a warmer compressor should be able to cool by 1 degree using less energy than a cold compressor, per Carnot cycle. OTOH who knows what really is going on inside it? But if the thermostat measures the exact temperature of the compressor gas then the compressor will be the same temperature when on no matter how much air is passing over it; it just will be warmer in the room when the setting is to a lower fan. (I doubt that's the case, but again, who really knows?) That said, the choice of fan setting for many might have more to do with the amount of noise it produces (some of us don't even use those things because the heat is less annoying), or what it feels like to stand directly in front of it, or whatever. Wnt (talk) 04:38, 3 July 2014 (UTC)
- While the compressor is running, its efficiency depends (in part) on how many air molecules contact the heat exchanger (coils w/fins, like a car's radiator) –which, of course, depends on the fan speed. So, while the compressor is running, the fan should be 'high'. I'd suggest finding the manual for your specific thermostat model, which is probably online if you don't have a copy. There might be an automatic mode where the fan continuously runs at low speed and switches to high speed when the compressor comes on. Something like 'Auto low'; ('Auto high' would have the fan always on high). The "best" setting depends on a lot of things. For example, if you live in a very hot location (e.g. Phoenix) and you have an older home with non-insulated ducts in a very hot attic, then having the fan run continuously would not be recommended -at least not during mid-day. However, when the compressor turns off, the heat exchanger is still cold (and probably has some ice on it) -and if the fan shuts off at the same time, then this "thermal mass" is wasted, and literally goes down the drain as the ice melts. This effect only lasts for a few (5~10?) minutes with the fan on, so efficiency also depends on how often the compressor turns on/off. —I hope this helps, ~E:71.20.250.51 (talk) 05:35, 3 July 2014 (UTC)
Iron as essential nutrient, rust, and water pipes
Two parts to this question:
- If iron is an essential nutrient, does that mean people can just eat things like iron bolts? Yet no one eats iron bolts, and eating pieces of iron instinctively seems to me to be a very bad and unhealthy idea. Similarly, rust is just iron and oxygen, the former of which is an essential nutrient and the latter which we need to breathe. So, again, wouldn't that mean it would be a good idea to eat rust? But again, no one eats rust, and eating rust instinctively seems to me to be a very bad and unhealthy idea.
- If water pipes are made of iron, doesn't that mean they would rust? And wouldn't that mean our drinking water is full of rust? Wouldn't that bad for people's health, if indeed the answer to the previous question is that eating rust is a bad/unhealthy idea?
—SeekingAnswers (reply) 22:09, 2 July 2014 (UTC)
- One could get dietary iron by eating iron or rust in bulk. But it will likely damage the person. Consult your doctor if you need more iron or not. Some people need to consume less iron. But you don't need that much that you need a bolt's worth. If you get a breakfast cereal wheat biscuit and run a magnet through it you should be able to extract the iron added by the manufacturer. Too much iron degrades the water quality, leading to bad taste and staining. Graeme Bartlett (talk) 22:59, 2 July 2014 (UTC)
- (1) Generally, eating pieces of metallic iron is a bad idea because of the physical damage they can cause to interior organs. However, some prepared foods contain "reduced iron", which is essentially just very finely powdered iron that dissolves rapidly in stomach acid.
- (2) Yes, steel water pipes DO rust (sometimes pretty rapidly, if used for very hot water), so your drinking water MAY be full of rust. This, however, does NOT impact people's health, because the rust particles are very small and dissolve in the stomach -- but it DOES impact your home repair bill when the pipe eventually rusts right through and floods your whole house with scalding hot water (as happened to me on one occasion). 24.5.122.13 (talk) 23:05, 2 July 2014 (UTC)
- I don't know how common the practice actually was at the time, but at least in the "Law of the Land" episode of Dr. Quinn, Medicine Woman, Dr. Quinn tells someone suffering from iron-deficiency anemia to boil rusty nails in water and then drink the rusty water.[9] Red Act (talk) 00:36, 3 July 2014 (UTC)
- Note that an Iron overload can be quite dangerous, so eating an entire iron bolt could be very bad for that reason, too. Also, an iron bolt probably contains additives that are toxic. And if you've every tasted rust, you probably spit it right back out. It tastes horrid.
- You might also be interested in pica (disorder)#Causes, a condition in which people eat strange things, like bolts, at times due to a deficiency in some mineral like iron.
- As for pipes rusting, they often accumulate scale (minerals that come out of solution from the water and stick to the pipes), so that can protect the iron from the water and slow down rusting. But, if your water is shut off, when it comes back on you may very well notice a rusty color for a bit. StuRat (talk) 01:09, 3 July 2014 (UTC)
- The Iron poisoning article talks about how much iron you can eat before it has toxic effects. From the numbers that article gives, it sounds like even a rather small iron bolt, finely ground up to aid in digestion and to avoid poking a hole in your throat or something, would be too much to safely eat at once. Monsieur Mangetout ate a lot of bolts during his lifetime, but he was a rather special case who shouldn't be emulated. Please don't eat bolts, kids.
- See also Human iron metabolism. Red Act (talk) 02:24, 3 July 2014 (UTC)
- As much of iron goes to blood, blood is a good nutritional source, or for the more Mosaic palette, liver (food) provides a more solid relative. I dare say even in the wild west they must have had liver now and then when they shot something. Wnt (talk) 05:46, 3 July 2014 (UTC)
As strange as it may seem, placing a piece of iron in cooking vessels does actually help fight iron deficiency. See this article about an iron fish used in Cambodia for that purpose [10]. --Xuxl (talk) 08:22, 3 July 2014 (UTC)
- Yeah, cast-iron cookware has been known for some time to contribute nutritionally significant quantities of iron to food, which is why people thought to create the lucky iron fish to provide the same benefit. Red Act (talk) 08:52, 3 July 2014 (UTC)
Why the hell haven't bacteria evolved to eat plastic by now anyway?
The question above about plastic degradation and plastic particle water pollution by microplastics reminds me of an old mystery: why haven't the bacteria evolved to eat this stuff? I mean, polyethylene is basically fatty acid, minus the ends. And living organisms routinely chow down fatty acids two carbons at a time (beta oxidation) for variable distances; there's no special mechanism for just a certain length (well, OK, I'm sure there is if you look hard enough but it's not the main metabolic pathway). Discarded in every possible environment, all the necessary nutrients are close at hand to at least some of the pieces of plastic. I've been expecting to see news reports of bacteria dissolving plastic in landfills for decades now, and it dawns on me suddenly that I'm still waiting. Why? Wnt (talk) 22:40, 2 July 2014 (UTC)
- You may be interested in Nylon-eating bacteria, although it's actually byproducts of nylon manufacture that that bacterium eats. Red Act (talk) 22:50, 2 July 2014 (UTC)
- You are probably more interested in [11]. EllenCT (talk) 22:54, 2 July 2014 (UTC)
- There are plenty of search examples for "plastic eating bacteria", many referencing: Nature —71.20.250.51 (talk) 23:02, 2 July 2014 (UTC)
- That "minus the ends" bit is pretty critical. Polyethylene in the form of plastic isn't water-soluble, and beta oxidation, like almost every chemical reaction, takes place in solution. In order to digest it, a bacterium would first need to evolve a way to dissolve it. --Carnildo (talk) 00:11, 3 July 2014 (UTC)
- Hmmmm... there's some merit to this, although I'd think that in some sense the plastic is already dissolved in plasticizer, I'm getting the impression that truly dissolving polyethylene requires elevated temperatures. [12] Though I know that polyethylene terephthalate (bottle cap) doesn't need to be all that warm to dissolve into olive oil (it's amazing what I've had to resort to during power outages). Bacteria clearly can form biofilm on fat globules and somehow use them for energy; but the devil may indeed be in the details. To begin with I should admit that upon RTFA I note that beta oxidation of lipids over 22 carbons actually has to take place in peroxisomes for some reason (which may be a clue?). But also, there's the question of whether a fatty acid has to get into the cell before beta-oxidation in order to be used for energy. It doesn't seem like that ought to be an inflexible requirement, but it is hard to picture how to separate the cycle from the external environment otherwise. And for all the marvels of bacterial design I have to admit I've never read about one with teeth to bite off and chew little bits of plastic. Wnt (talk) 05:24, 3 July 2014 (UTC)
- Maybe they already have, and we just don't know it yet? There are a lot of landfills, and prospecting is slow and tedious. Granted, this is "just" a science fair project, and I haven't looked yet for follow up since 2008, but this kid thinks he's found a bacteria (from a landfill) that can speed plastic degradation [13] [14]. Also, recall that it took fungi a long time to figure out how to eat dead wood, that's why we had the carboniferous era. So like you, I've expected to hear more about this by now, but I also think such a discovery and serious scientific investigation will take place long after bacteria have started munching our plastic bags. SemanticMantis (talk) 16:37, 3 July 2014 (UTC)
- That is indeed heartening to read, and I think that it's great that a teenager saw this and saw fit to carry out the work. I don't think that reducing the weight of the plastic by 43% in three months is the limit, though. Apparently there are Pseudomonas and Sphingomonas involved, but now some pros need to get involved, repeating this in many landfills, tracking down the genes involved, and assembling them all in a few organisms. Or maybe some other kids around the world will just repeat the experiment and swap samples on the internet relying on old-fashioned bacterial conjugation. :) In any case, progress! Wnt (talk) 02:36, 4 July 2014 (UTC)
July 3
Mach's Principle
I am wondering if there has been an experimental confirmation of Mach principle? I am wondering also it such an experiment makes sense? Well, one of the formulations of Mach principle is as follows: "Events in local inertial frame are dependent on mass distribution of distant stars."
A few questions: how distant is "distant?" Can it be defined in terms of the parsecs? Ten parsecs? One hundred? A kilo parsec or what? Where does the influence end?
Our Sun is located on the periphery of the Galaxy, off center, thus it can be surmised that the gravitational pull of the "distant stars" that determine local inertial frames should be one sided to an extent. Can it be verified?
Imagine a plank rotating around its center, sort of a carrying pole. The two shoulders of the plank must be absolutely symmetrical of course. While rotating they will generate centrifugal force. Provided the angular velocity is stable, can it be measured if the force is absolutely the same over the 360 degrees of circumference? Or the pull toward the center of our Galaxy is stronger than in other directions? Thanks, --AboutFace 22 (talk) 01:24, 3 July 2014 (UTC)
- That plank length (pardon the pun) would have to be many light years long to have any measurable effect of being pulled more towards the galactic center when on the side closer to it. However, closer stars might have an effect on somewhat smaller scales, enough to noticeably perturb Oort Cloud objects, for example. StuRat (talk) 02:29, 3 July 2014 (UTC)
StuRat, I am at a loss. I cannot understand what you are talking about. What does Planck Length have to do with my question? --AboutFace 22 (talk) 02:39, 3 July 2014 (UTC)
- Mach's principle by itself is really too vague to be testable. From the article, "... because the principle is so vague, many distinct statements can be (and have been) made which would qualify as a Mach principle, and some of these are false." On the other hand, general relativity was to a certain extent inspired by Mach's principle, although it isn't 100% compatible with it, and general relativity certainly is precise enough to be testable; see Tests of general relativity. Red Act (talk) 02:56, 3 July 2014 (UTC)
StuRat, thanks. I must have been tired last night, I did not notice the pun when I typed my question. I guess Planck and plank are separated by many convolutions in my brain. Anyhow, RedAct says Mach's Principle is too vague to be tested, so what? What about the centrifugal force? Is it caused by the whole mass of the Universe or not? --AboutFace 22 (talk) 13:39, 3 July 2014 (UTC)
- The only way you could answer that question scientifically is by extracting a numerical prediction from Mach's principle that's different from a prediction of general relativity, but Mach's principle is too vague for that. At best you might "predict" something totally different from GR, in which case it's probably ruled out by existing data.
- You can alternately ask "how Machian" general relativity or some rival theory is, philosophically. People have argued about this for the last century. On the one hand, in GR the gravitational field is spacetime and does completely determine inertial behavior, and massive objects are sources of the field, at least in the conventional terminology. On the other hand in the Kerr solution for a rotating massive object, the object rotates relative to the vacuum despite being the only object in the universe, which seems quite non-Machian.
- Brans–Dicke gravitation may still be consistent with experiment, and I seem to recall that it was promoted as more Machian than GR by its creators. I'm not sure what they meant by that or whether anyone else agreed with them. -- BenRG (talk) 15:43, 3 July 2014 (UTC)
- As others have pointed out, Mach's principle is more a suggestive idea than a definite predictive theory. This makes it hard to pin down. One reading is that, according to Mach's principle, it's impossible for the matter in the universe to have an overall net rotation. In the bucket experiment, this means that the surface of the water has to be flat when the bucket isn't rotating relative to distant galaxies. This is hard to test, because we find that the matter in the universe doesn't have any overall net rotation, and our LCDM cosmology doesn't predict any such rotation. So the question becomes whether such a net rotation can't exist in principle, or could exist but doesn't. Since this is a question of hypotheticals, there's no way to test it empirically. Of course, there are other readings of Mach's principles and other Machian ideas, which have been suggested above. --Amble (talk) 20:17, 3 July 2014 (UTC)
Thank you everyone who contributed but the answers left me disappointed. I posed a specific question but the answers drifted into much criticized vagueness of Mach's Principle. Phrased differently my question is about a possibility of detecting the contribution the gravitational field 26,000 light years away makes to the curvature tensor here on earth if you like. With that rotating plank one can imagine one-directional dynamometers built into respective ends of the plank and while it is rotating the readings could be made. If the readings (and I am talking about the centrifugal force F here) are uniform and do not change at different angles then either the contribution is below threshold or nonexistent. I also assume that the experimenter took care of finding the most advantageous initial orientation of the plank which should roughly coincide with the Galactic Plane. What kind of numerical predictions are needed here? The idea of the net rotation of the Universe I don't even want to touch. I consider it absurd. Net rotation in respect to what? Is there a reference point "outside the known Universe" to effect such a measurement? Thanks, --AboutFace 22 (talk) 16:40, 4 July 2014 (UTC)
- I think the answers fit the question you asked about Mach's principle. Your rephrased question seems to be about general relativity instead (since you mention the curvature tensor).
- To get a very rough estimate of the locally detectable effect of the mass of the Milky Way, I'll treat it as a point mass of 1042 kg at a distance of 26,000 ly. Plugging that into the tidal acceleration formula 2GM/R³ gives ~10−30 gee/meter. I think it's safe to say that this is undetectable by experiments in the solar system since the tidal effect of the sun even at 100 AU is ~1010 times larger.
- In GR it is possible for the universe as a whole to rotate in a detectable way. The rotation would have no center, but would have an angular velocity (magnitude and direction), which we could detect as an anisotropic Doppler shift of the cosmic microwave background. There is in fact a large-angle anisotropy in the CMBR which selects a preferred axis, but it's small enough that it can be blamed on cosmic variance. In fact cosmic variance implies that the visible universe must (with probability 1) have a nonzero net angular momentum. -- BenRG (talk) 17:55, 4 July 2014 (UTC)
BenRG, thank you for the calculations which I certainly could have never done myself. Using your numbers I am tempted to say that centrifugal forces on Earth do NOT have any contribution from the mass of the distant stars. It seems to indicate that the Mach's principle is false? Then what is the physical explanation of the centrifugal force? Thanks, --AboutFace 22 (talk) 18:45, 4 July 2014 (UTC)
That net angular momentum of the Universe is another fascinating topic I've tried to understand. I even posted about it before. Since you mentioned it here I want to ask you (1) if the net angular momentum is the sum of ALL angular momenta of rotating galaxies and other mass? And (2) if the individual angular momenta of visible large scale structures of the universe originated because of the net Universe angular momentum which must have appeared in the first super small fraction of the second after the Big Bang. When I posted on this topic before the meaning of the answers that came seemed to indicate that all local rotations are derived from unequal mass distribution or sound waves in the early Universe. --AboutFace 22 (talk) 19:10, 4 July 2014 (UTC)
Flush survivors
In general, which of the following will survive a flush down the toilet if healthy before the flush?
(Before you animal lovers get up in arms, the question is hypothetical.)
—SeekingAnswers (reply) 05:23, 3 July 2014 (UTC)
- Rats will drown, and goldfish would suffocate from toxic methane gas. Some insects might survive, though, but I'm not sure about that. 24.5.122.13 (talk) 05:31, 3 July 2014 (UTC)
- Flushing is a brief, transient event. What would really matter is where the results of a flush end up.Sewerage systems vary a lot from place to place. Some would be not much different from open flowing water. Some would be far worse. HiLo48 (talk) 05:40, 3 July 2014 (UTC)
- My thought was that a rat ought not to go gently into that good night. Fortunately, through the use of YouTube one can obtain objective data, with a certain risk of fraud. [17] seemed persuasive enough that the second flush is enough to put a mousy-looking "rat" down the hole - that I never expected. On the other hand, sometimes the rat makes it the other way. [18] According to the Daily Mirror even a puppy can survive the trip, though I don't think it would do well in the sewers. (With alligators YMMV) Wnt (talk) 05:58, 3 July 2014 (UTC)
- I've seen videos of and heard reports of rats climbing out from toilet drain holes, and I've personally seen, on multiple occasions, insects doing the same, both of which lead me to believe that rats and insects will sometimes (on a semi-frequent basis, but not always) survive flushes. I'm looking for more insight into how they do so and under what conditions they will survive. —SeekingAnswers (reply) 02:17, 4 July 2014 (UTC)
- Wouldn't the survival rate depend mainly on the configuration of the drainage system immediately after the U-bend? A gentle but free-draining gradient with some irregularities to hang on to should ensure that most mammals and insects would survive a brief immersion in (not too polluted) water. For fish, the quality of the water lower down would be critical. If flood-water drainage is mixing with the sewage then there would be a chance of survival for a time. Dbfirs 07:07, 4 July 2014 (UTC)
Lost city of Ubar
I need some help finding the Lost city of Ubar, or more specifically some clarification on what SIR-C/X-SAR found and how Iram of the Pillars relates (or not). Please follow up here. Thanks! -- ke4roh (talk) 21:04, 3 July 2014 (UTC)
- This paper says the shuttle data suggested a number of sites and that "these sites were targeted for a subsequent ground based expedition ... Most useful were the Landsat image data which revealed a network of tracks that converge at the modern day village of Shisr. Archaeological investigations of the ruins at Shisr indicate that it is most likely the site which inspired the Uhar legends." Shisr (also transliterated as Shis'r or Shasar) is in the Dhofar Governorate of Oman, here - note that Google Maps labels it "Ubar". This archeology, and a discussion of whether it really is Ubar or anything like it, is discussed in at Atlantis of the Sands#The Shisr discoveries and following sections. A few photos of Shisr, again with things claimed to be Ubar, are at commons:Category:Shisr, Dhofar province, Oman. -- Finlay McWalterᚠTalk 21:51, 3 July 2014 (UTC)
- Note, incidentally, that the co-author of that paper is NASA scientist Robert E. Crippen and not NASA astronaut Robert L. Crippen. -- Finlay McWalterᚠTalk 21:58, 3 July 2014 (UTC)
July 4
Drone bees
Can someone help me understand the role of drone bees please? I thought that the only egg-laying bee is the Queen bee and her fertilised eggs become Worker bees and the unfertilised eggs become Drone bees. Since Drone bees fertilise the Queen bee's eggs, why aren't all resulting bees genetically identical since both sets of genes come from the queen bee (as the drone bee is from the QB's unfertliised egg)? I read that there are sometimes laying worker bees but this is only in the absence of a queen bee. Thank you — Preceding unsigned comment added by 81.91.232.122 (talk) 01:57, 4 July 2014 (UTC)
- There's a good discussion here: [19]. Look for the "4.1. Genetic explanations" section. My reading is that the drones have half the queen's genes, and the queen also contributes half of her genes when mating, but these halves can include overlapping genes, and miss other genes. So, the workers each get a different set of duplicates and missing genes, making them slightly different genetically.
- What I'm unclear on is how the next queen gets her genes. Is she a clone of the old queen ? If so, how is this accomplished ? StuRat (talk) 02:40, 4 July 2014 (UTC)
- "My reading is that the drones have half the queen's genes"
- So each of the unfertilised eggs if the queen bee are genetically distinct as they divide in half differently? And some genes are missing entirely in worker bees? Didn't realise that was possible. Do you have any more sources I can use? — Preceding unsigned comment added by 81.91.232.122 (talk) 02:55, 4 July 2014 (UTC)
- Yea, you'd expect that the absence of some genes would be fatal. Perhaps they do have a high defect rate and just lay more eggs to compensate, killing off any worker that manages to hatch, but is obviously defective. They might also have many copies of critical genes on different chromosomes, to increase the likelihood that at least one will be passed on to each worker. If there's only one copy of a gene, there should be a 25% chance it will be missing, while, if there were 10 copies, each on a different chromosome, that chance drops to less than one in a million, ignoring mutations, etc. StuRat (talk) 03:16, 4 July 2014 (UTC)
- I don't know what the heck you're talking about here, but there is no random gene loss. You could think of the drone as being a sperm, in the sense that it is already haploid, but it doesn't cut that down further. See haplodiploidy. Wnt (talk) 03:21, 4 July 2014 (UTC)
- I think Stu is trying to get at recombination. In honey bees, when the queen lays a fertilized egg, the results are not clones, but because of haplodiploidy, they share 75% of alleles on average. When she lays an unfertilized egg, they become males, but these share only 50% of their alleles on average. In honey bees, queen determination is solely a developmental pathway, genetics have nothing to do with it. Proto-queens are just fertilized eggs layed in a special larger cell (queen cell), and fed with more royal jelly. In a laboratory setting, we can make any fertilized bee egg into a queen. Note that this is not a general rule though, in e.g. the Melipona and some other bees, there is genetic caste determination (redlink, I mean genetic control of caste_(biology), see a paper on how this work in some ants here [20]), though that is a more recent finding and less well understood. SemanticMantis (talk) 15:41, 4 July 2014 (UTC)
- Lovers' lanes. Secluded, south-facing lovers' lanes, it would appear, at least in Puerto Rico, with bees commuting 500 m to 5 km to get there from hundreds of hives. The magic word is "drone congregation areas". See [21] and especially [22] for more about them. Thanks for asking this!!! Wnt (talk) 02:56, 4 July 2014 (UTC)
- (Ha, this reminds me in grad school, there was a secluded road by the ag research farms called Bee Biology road. It was often used by humans as a "lovers' lane." Something about birds and bees I guess...) SemanticMantis (talk) 15:03, 4 July 2014 (UTC)
- I think you've got your answers already; it's all about haplodiploidy. Also note that while technically possible, it is not the norm for a queen to mate with her own drone. See nuptial flight. Also, in honeybees, virgin queens can mate with many drones in one flight. 10-15 separate inseminations is not uncommon if I recall correctly. I also want to point out that among the bees there are very different colonial systems. In Apis mellifera, male drones are basically flying sperm that don't do much of anything other than mate and die. In some sweat bees and some solitary bees however, males life roughly as long as the female and in some cases help with provisioning offspring. Incidentally, haplodiploidy is seen as a key feature for allowing Evolution_of_eusociality in the worker castes of the eusocial hymenoptera. The idea is, sister workers share 75% of their alleles with each other, while their hypothetical offspring would only share 50% on average. Kin selection is widely accepted as an explanation for this process, but the analogous group selection is somewhat more contentious. SemanticMantis (talk) 14:59, 4 July 2014 (UTC)
Species
What's the definition of a species? 24.5.122.13 (talk) 05:28, 4 July 2014 (UTC)
- So would the spinner dolphin and the striped dolphin be considered two separate species, or two subspecies of one species, along with their hybrid the Clymene dolphin??? 24.5.122.13 (talk) 08:02, 4 July 2014 (UTC)
- See Species problem. The more we study these things the more we realise that "species" is a human cultural concept, with limited application to reality. That's why cladistics is becoming the preferred method of biological classification. Rojomoke (talk) 12:42, 4 July 2014 (UTC)
- It's the borderlines that cause the classification problem. This is one example. Some things called hybrids aren't really hybrids. However, you can't easily mate a dolphin with a horse, for example. Those are clearly separate species. ←Baseball Bugs What's up, Doc? carrots→ 14:41, 4 July 2014 (UTC)
- See Species problem. The more we study these things the more we realise that "species" is a human cultural concept, with limited application to reality. That's why cladistics is becoming the preferred method of biological classification. Rojomoke (talk) 12:42, 4 July 2014 (UTC)
- See biological species definition. There are instances as in Europe where two neighboring species such as the Hooded Crow and Carrion Crow overlap in a narrow band, and hybridization occurs. The hybrids, while fertile, do not seem to fare as well as the pure species and backbreeding into the general populations is not significant enough to cause a wide transitional area of confusion among the main types. This is also common with oaks, where many species are specialized for habitat. A species specialized for humid valleys and on for dry hilltops can interbreed with fertile offspring, but the hybrids do not do as well in valleys or on mountainsides as the parent species, so the difference is maintained. The fact that there are transitional forms in no way invalidates the usefulness of the concepts as tools, when used properly. One can even clean one's earwax with a phillip's head. μηδείς (talk) 20:48, 4 July 2014 (UTC)
Job prospects
If it took 2 years longer to get a degree due to repeat years as a result of failed exams, your grades were only average and you filled the gaps with voluntary work or work in industry, how likely is that to affect your future career? — Preceding unsigned comment added by 94.10.247.171 (talk) 09:34, 4 July 2014 (UTC)
- There are pro's and con's. Failed exams and repeat years isn't good - but time spent in industry and in voluntary work are a plus. I can't speak for every field - but in mine (Computer science) we probably wouldn't even ask about the reason for the extra time - probably we'd be more interested in what you did when you were "working in industry". When you get a fresh graduate with zero work experience, you always wonder how they'll adapt from easy-going college hours to a 9 to 5 job, how they'll work in a team, and how they'll cope when they are handed a project that they must complete by some deadline. Having worked in a "real job" (ideally in your field of future employment) is a huge win because it is concrete confirmation that you know what it's like to be in a 9-5 job.
- You don't have to say why you took longer to get your degree on your resume and I doubt most employers would ask. But you might want to be ready with some well-thought out answers to that question. "I felt the need to dive more deeply into the subject matter" or something like that.
- You also mention "your future career" - presumably beyond your first job until retirement. Again, I can only speak for my industry - but generally, your degree is what gets you your first job. Your first job is what gets your your second job and so on. The things you worked on most recently are by far more interesting than what you did 5, 10 or 20 years ago. This is especially noticeable for people with advanced degrees - masters and PhD's - for those people, it's a massive benefit for getting their first job. It matters less for their second - and after they've been out working for 20 years, recruiters may not even read that far back down their resume!
- So I don't think this should affect you too badly. Certainly it mustn't affect your confidence in going into those first interviews. Make much of what you learned in your industry jobs - explain the responsibility that came with your volunteer work - how you enjoyed working in a team environment - your sense of achievement at pulling off something "real"...that kind of thing.
- SteveBaker (talk) 12:10, 4 July 2014 (UTC)
- Normally you'd just put your year of graduation on your resume, along with what degree or diploma you ended up with. You're not likely to be asked how long it took you to reach it. 173.228.123.145 (talk) 23:04, 4 July 2014 (UTC)
orang - chimp - human image origin
What is the origin of this much-parodied image on human evolution? Image:Human-evolution-man.png ---- CS Miller (talk) 12:32, 4 July 2014 (UTC)
- See March of Progress. The original was drawn by Rudolph Zallinger, and first appeared in a book of the Life Nature Library series in 1965. Tevildo (talk) 13:15, 4 July 2014 (UTC)
DIS1
Dear Sirs,
Do you know what "DIS1" means? I know it is a microtubule-associated protein, but I do not know what it means, from what words it is composed. It is similar as TOG (tumor overexpressing gene), but I do not know from what words it is composed. I think that it is a TOG of the S. pombe.
Yours sincerely,
Đekić Miloš
Serbia — Preceding unsigned comment added by 109.93.106.27 (talk) 14:48, 4 July 2014 (UTC)
- Possibly "defect in sister chromatid disjoining", as per this, which I found by googling "dis1". ←Baseball Bugs What's up, Doc? carrots→ 15:06, 4 July 2014 (UTC)
- Yup, I got http://www.pombase.org/spombe/result/SPCC736.14 , but don't know anything of the area to make anything of it. CS Miller (talk) 15:38, 4 July 2014 (UTC)
- Sister chromatid separation is defined here. הסרפד (call me Hasirpad) 15:57, 4 July 2014 (UTC)
- Yup, I got http://www.pombase.org/spombe/result/SPCC736.14 , but don't know anything of the area to make anything of it. CS Miller (talk) 15:38, 4 July 2014 (UTC)
Is there such a thing as leaf toner?
There is a plant hormone marketed as root toner which induces the growth of roots on plant cuttings.
Is there a parallel hormone which will induce the growth of leaves on (presumably wounded) living hardwood branches that lack leaves?
I have a holly which had to have some branch ends removed. One sprouted new leaves, the other didn't.
Searches of google pretty much return only skin toner for women based on plant leaf hormone extracts.
Thanks. μηδείς (talk) 20:06, 4 July 2014 (UTC)
- You'll want to consult an actual expert in person who can answer your questions more directly, but this site recommends a high nitrogen fertilizer. --Jayron32 20:42, 4 July 2014 (UTC)
- I don't think there's a widely available commercial product like that.
- You may be able to get your hands on some
auxin orGibberellic_acid, pasting some of that on the tips might help. (Oops, you want to not use auxin, that's what suppresses buds). - If you have access to any willow cuttings, (Salix spp.), you can make up a nice little batch of plant hormones in the following way: take several pencil-diameter cuttings, about 12" long (smaller or bigger is ok). Put in a small-mouth bottle to prevent evaporation, fill with water, and place in location with fairly bright indirect light. After a few weeks, you should see vigorous root growth and some bud formation. Grind up the buds and bark in the water, soak for another day, and use that to make a paste with some peat moss or leaf mulch. This paste will contain decent amounts of jasmonic acid, salycylic acid, and a few others. It can help other plants root and bud (I have not personally tested the latter).
- You can also work with the plant's natural hormonal system. Apical meristems suppress the formation of other meristems, and each of those ultimately comes from stem cells, sometimes in a structure like an epicormic bud. Think of how, for many plants, a single pruning will be followed by two or more buds emerging near that tip. The the tip sends "no bud" signals to the rest of the plant, and when that signal is removed, buds form. So, you can induce bud growth on one branch by removing many bud tips/meristems from the other branch. You don't even need to remove many leaves if you don't want to, as long as the tips are taken off. This can process take some time, and may require subsequent pruning of the productive branch. If it's only been a few weeks since pruning, I'd just wait a bit more before I did much of anything.
- Good luck! (post EC with Jayron, a little fertilizer couldn't hurt, but I'd personally go for a milder N level than a stronger one.) SemanticMantis (talk) 20:46, 4 July 2014 (UTC)
- Thanks, guys, I actually did growth experiments in high school biochemistry and one of my undergrad majors was plant ecology.
- What has happened is a holly in my father's back yard was losing half its leaves to yellow blight every year, to the point where it would soon be dead. The google solution was to remove the lower branches to improve air circulation and to remove any fallen yellow leaves in the fall. I did this, which freaked out my father, who has no idea how plants work, but who liked the ground-dragging branches. I had cut off most branches to the trunk, and had left a few at about 4 ft high as stumps one foot out from the trunk, as I would need a saw rather than the clippers I had been using.
- Once he saw what I had done I was forbidden to touch the tree. I told him it was possible leaves might return on the not-fully-removed branches, just as the oak he had topped off had sprouted new branches lower down. This year the tree has three times as many leaves and no blight and one of the three stub-branches has indeed leafed. So I want to lightly scar the bark of the other two, appl something waxy, and see what happens. I might be able to get my hands on auxin, but I was hoping there was something commercially formulated and easy to use not intended as a pesticide. I am not around all the time to maintain a will compress etc. (The other problem is the oly place I'd know to get willow cuttings would be on private land.) But at least I am encouraged by the above suggestions. μηδείς (talk) 21:08, 4 July 2014 (UTC)
July 5
Name that beetle


Can anybody ID this beetle. I found it in Cambridge Bay on Victoria Island (Canada) so it is obviously an Arctic dweller. It does not appear to be either Upis ceramboides or Cucujidae. While I forgot to measure it I would estimate that it was about 1 cm (0.39 in) long. Thanks. CBWeather, Talk, Seal meat for supper? 03:50, 5 July 2014 (UTC)
