Tetryzoline
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.001.384 |
| Chemical and physical data | |
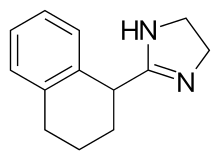
| Formula | C13H16N2 |
| Molar mass | 200.285 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 117–119 °C (243–246 °F) [1] 256-257°C for HCl-salt[2] |
| Solubility in water | Very soluble in water and ethanol, slightly soluble in chloroform and insoluble in diethylether[2] mg/mL (20 °C) |
| |
| |
| | |
Tetryzoline (also known as tetrahydrozoline) is a drug used in some over-the-counter eye drops and nasal sprays.
It was patented in 1954 and came into medical use in 1959.[3]
Mechanism of action
Tetryzoline is an alpha agonist for alpha-2 receptor and imidazoline receptor I-1 agonist. Mainly due to its alpha-2 agonism it can constrict conjuctival blood vessels of the eye when taken in the form of eye drops.[4] This relieves the redness of the eye caused by minor ocular irritants. To treat allergic conjunctivitis, tetryzoline can be combined in a solution with antazoline.[5]
In an overdose, slow heart rate and low blood pressure are mainly due to imidazoline-1 receptor agonism. Dry mouth and sedating effects are due to alpha-2 agonism.[4]
Side effects
Tetryzoline eye drops may cause blurred vision, eye irritation and dilated pupils.[6] Tetryzoline is not suitable for prolonged use as its vasoconstrictive effects within the eye eventually decrease or stop. If tolerance to the drug has developed, ceasing its use may cause a rebound effect and increase redness of the eyes — a vasodilatory effect.[7]
Intranasal use of tetryzoline may cause transient burning, stinging, or dryness of the mucosa and sneezing. Prolonged intranasal use often causes opposite effects in the form of rebound congestion with effects such as chronic redness, swelling and rhinitis. Prolonged use thus may result in overuse of the drug.[6]
Overdose most often causes slow heart rate. Respiratory depression, low blood pressure, constricted pupils, hypothermia, brief episodes of high blood pressure,[8] drowsiness, headaches and vomiting may also occur.[9] In serious cases some of these effects may result in circulatory shock.[6] Most often overdoses occur in children who have ingested the drug.[8]
There is no antidote for tetryzoline or other similar imidazoline analogue poisoning, but the symptoms can be alleviated and with treatment, death is rare.[10]
Pharmacokinetics
Half-life of tetryzoline in healthy people is about 6 hours and it is excreted unchanged in urine, at least in part. In one study 10 people were given 2 drops of 0.5 mg/ml tetryzoline eye drops (0.025–0.05 mg) at 0, 4, 8 and 12 h. Within 24 h time window since the last dose, tetryzoline blood serum concentration of the subjects was 13.0–210.0 ng/ml and urine concentration was 11–400 ng/ml. Both reached their maximum about 9 h post last dose. These levels correspond to normal ocular use of tetryzoline. Higher concentrations may indicate misuse of the drug or poisoning.[10]
Chemistry
Chemically, tetryzoline is a derivative of imidazoline. It has two enantiomers.
Society and culture
Names
Urban legend
An urban legend suggests that tetryzoline can cause violent diarrhea if given orally, such as by putting a few drops of Visine in an unsuspecting person's beverage. However, the actual results of the prank may be worse, varying from severe nausea and vomiting to seizures or a coma. Diarrhea is not a side effect.[12]
Criminal use
In late August 2018, a South Carolina woman was charged with murdering her husband by putting eye drops containing tetryzoline in his drinking water. An autopsy found high concentrations of tetryzoline in his body.[13][14][15]
Tetryzoline has been used as a date rape drug in a number of cases due to its ability to cause dizziness and unconsciousness.[9]
References
- ^ "US 2731471 A - IP.com". ip.com. Retrieved 2018-09-05.
- ^ a b The Merck index. Chapman & Hall Electronic Publishing Division. (12th ed.). Whitehouse Station, NJ, United States: Chapman & Hall Electronic Pub. Division. 2000. p. 1453. ISBN 1584881291. OCLC 46987702.
{{cite book}}: CS1 maint: others (link) - ^ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 552. ISBN 9783527607495.
- ^ a b Lowry JA, Brown JT (June 2014). "Significance of the imidazoline receptors in toxicology". Clinical Toxicology. 52 (5): 454–69. doi:10.3109/15563650.2014.898770. PMID 24666288.
- ^ Castillo M, Scott NW, Mustafa MZ, Mustafa MS, Azuara-Blanco A (June 2015). "Topical antihistamines and mast cell stabilisers for treating seasonal and perennial allergic conjunctivitis" (PDF). The Cochrane Database of Systematic Reviews. 6 (6): CD009566. doi:10.1002/14651858.CD009566.pub2. PMID 26028608.
- ^ a b c "Tetrahydrozoline". toxnet.nlm.nih.gov. Archived from the original on 2017-01-03. Retrieved 2018-09-05.
- ^ McLaurin E, Cavet ME, Gomes PJ, Ciolino JB (March 2018). "Brimonidine Ophthalmic Solution 0.025% for Reduction of Ocular Redness: A Randomized Clinical Trial". Optometry and Vision Science. 95 (3): 264–271. doi:10.1097/OPX.0000000000001182. PMC 5839712. PMID 29461408.
- ^ a b Al-Abri SA, Yang HS, Olson KR (December 2014). "Unintentional pediatric ophthalmic tetrahydrozoline ingestion: case files of the medical toxicology fellowship at the University of California, San Francisco". Journal of Medical Toxicology. 10 (4): 388–91. doi:10.1007/s13181-014-0400-9. PMC 4252297. PMID 24760708.
- ^ a b Stillwell ME, Saady JJ (September 2012). "Use of tetrahydrozoline for chemical submission". Forensic Science International. 221 (1–3): e12-6. doi:10.1016/j.forsciint.2012.04.004. PMID 22554870.
- ^ a b Carr ME, Engebretsen KM, Ho B, Anderson CP (November 2011). "Tetrahydrozoline (Visine®) concentrations in serum and urine during therapeutic ocular dosing: a necessary first step in determining an overdose". Clinical Toxicology. 49 (9): 810–4. doi:10.3109/15563650.2011.615064. PMID 21972870.
- ^ "International Non-Proprietary Names for Pharmaceutical Preparations. Recommended International Non-Proprietary Names: List 3" (PDF). World Health Organization. p. 474. Archived (PDF) from the original on 2016-09-11. Retrieved 30 August 2016.
- ^ "Visine Prank: Mickey Red Eyes". Snopes. 29 June 2009. Retrieved 28 July 2010.
- ^ "US wife accused of 'fatally poisoning husband with eyedrops'". BBC. 4 September 2018. Retrieved 4 September 2018.
- ^ Police: Woman kills husband by putting eye drops in water, Associated Press, Aug 31, 2018
- ^ Connelly, Eileen (1 September 2018). "Wife admits fatally poisoning 'unfaithful' hubby with eye drops: cops" (Newspaper). New York Post. Archived from the original on 2018-09-02. Retrieved 3 September 2018.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help)
