Ebola: Difference between revisions
m Reverted edits by 64.201.88.5 (talk) to last version by ClueBot |
|||
| Line 35: | Line 35: | ||
Ebola first emerged in 1976 in Zaire. It, however, remained largely obscure until 1989 in the outbreak in Reston, Virginia. |
Ebola first emerged in 1976 in Zaire. It, however, remained largely obscure until 1989 in the outbreak in Reston, Virginia. |
||
== Etymology == |
== Etymology ==& here. |
||
The virus is named after the [[Ebola River]] Valley in the [[Democratic Republic of the Congo]] (formerly [[Zaire|Zaïre]]), which is near the site of the first recognized outbreak in 1976, in a mission hospital run by [[Flemish people|Flemish]] [[nun]]s.<ref>{{cite journal |last=Bardi |first=Jason Socrates |authorlink= |coauthors= |year=2002 |month= |title=Death Called a River |journal=Scripps Research Institute |volume=2 |issue=1 |pages= |id= |url=http://www.scripps.edu/newsandviews/e_20020114/ebola1.html |accessdate=2006-12-08 |quote= }}</ref> |
The virus is named after the [[Ebola River]] Valley in the [[Democratic Republic of the Congo]] (formerly [[Zaire|Zaïre]]), which is near the site of the first recognized outbreak in 1976, in a mission hospital run by [[Flemish people|Flemish]] [[nun]]s.<ref>{{cite journal |last=Bardi |first=Jason Socrates |authorlink= |coauthors= |year=2002 |month= |title=Death Called a River |journal=Scripps Research Institute |volume=2 |issue=1 |pages= |id= |url=http://www.scripps.edu/newsandviews/e_20020114/ebola1.html |accessdate=2006-12-08 |quote= }}</ref> |
||
Revision as of 20:11, 11 February 2009
| Ebola | |
|---|---|

| |
| An electron micrograph of an Ebola virus | |
| Virus classification | |
| Group: | Group V ((−)ssRNA)
|
| Order: | |
| Family: | |
| Genus: | Ebolavirus
|
| Type species | |
| Zaïre virus | |
| Species | |
|
Ivory Coast ebolavirus | |
| Ebola | |
|---|---|
| Specialty | Infectious diseases |
Ebola is the common term for a group of viruses belonging to genus Ebolavirus (EBOV), family Filoviridae, and for the disease that they cause, Ebola hemorrhagic fever. The virus is named after the Ebola River, where the first recognized outbreak of Ebola hemorrhagic fever occurred. The viruses are characterized by long filaments, and have a shape similar to that of the Marburg virus, also in the family Filoviridae, and possessing similar disease symptoms.
There are a number of species within the ebolavirus genus, which in turn have a number of specific strains or serotypes. The Zaïre virus is the type species, which is also the first discovered and recorded to be the most lethal. Ebola is transmitted primarily through bodily fluids and to a limited extent through skin and mucous membrane contact. The virus interferes with the endothelial cells lining the interior surface of blood vessels and platelet cells. As the blood vessel walls become damaged and the platelets are unable to coagulate, patients succumb to shock.
Ebola first emerged in 1976 in Zaire. It, however, remained largely obscure until 1989 in the outbreak in Reston, Virginia.
== Etymology ==& here.
The virus is named after the Ebola River Valley in the Democratic Republic of the Congo (formerly Zaïre), which is near the site of the first recognized outbreak in 1976, in a mission hospital run by Flemish nuns.[1]
Classification
Ebola is thought to be a zoonotic virus, as it is currently devastating the populations of Western Lowland Gorillas in Central Africa.
Zaïre virus
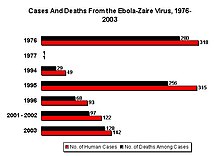
The Zaïre virus, formerly named Zaïre Ebola Virus, has the highest case-fatality rate, up to 90% in some epidemics, with an average case fatality rate of approximately 83% over 27 years. The case-fatality rates were 88% in 1976, 100% in 1977, 59% in 1994, 81% in 1995, 73% in 1996, 80% in 2001-2002, and 90% in 2003. There have been more outbreaks of Zaïre ebolavirus than any other strain.
The first outbreak took place on August 26, 1976, in Yambuku, a town in the north of Zaïre. The first recorded case was Mabalo Lokela, a 44-year-old schoolteacher returning from a trip around the north of the state. His high fever was diagnosed as possible malaria, and he was subsequently given a quinine shot. Lokela returned to the hospital every day. A week later, his symptoms included uncontrolled vomiting, bloody diarrhea, headache, dizziness, and trouble breathing. Later, he began bleeding from his nose, mouth, and anus. Lokela died on September 8, 1976, roughly 14 days after the onset of symptoms.
Soon after, more patients arrived with varying but similar symptoms including fever, headache, muscle and joint aches, fatigue, nausea, and dizziness. These often progressed to bloody diarrhea, severe vomiting, and bleeding from the nose, mouth, and anus. The initial transmission was believed to be due to reuse of the needle for Lokela's injection without sterilization. Subsequent transmission was also due to care of the sick patients without barrier nursing and the traditional burial preparation method, which involves washing and gastrointestinal tract cleansing.
Two nuns working in Yambuku as nurses also died in the same outbreak.[2]
Sudan ebolavirus

Sudan ebolavirus was the second species of Ebola emerging simultaneous with the Zaïre virus. It was believe to originated amongst cotton factory workers in Nzara, Sudan; with the first case reported as a worker exposed to a potential natural reservoir. Scientists tested all animals and insects in response to this, however none tested positive for the virus. The carrier is still unknown.
A second case involved a nightclub owner in Nzara, Sudan. The local hospital, Maridi, tested and attempted to treat the patient; however, nothing was successful, and he died. The hospital did not advocate safe and practical procedures in sterilizing and disinfecting the medical tools used on the nightclub owner, likely facilitating the spread of the virus in the hospital.
The most recent outbreak of Sudan ebolavirus occurred in May 2004. As of May 2004, 20 cases of Sudan ebolavirus were reported in Yambio County, Sudan, with five deaths resulting. The Centers for Disease Control and Prevention (CDC) confirmed the virus a few days later. The neighbouring countries of Uganda and the Democratic Republic of Congo have increased surveillance in bordering areas, and other similar measures have been taken to control the outbreak. The average fatality rates for Sudan ebolavirus were 54% in 1976, 68% in 1979, and 53% in 2000/2001.
Reston ebola virus
Reston ebolavirus is classified as species of Ebola, however it may be a new filovirus of Asian origin. It was discovered during an outbreak of Simian hemorrhagic fever virus (SHFV) in crab-eating macaques from Hazleton Laboratories (now Covance) in 1989. Since the initial outbreak in Reston, it has emerged in the Phillipines, Sienna Italy, Texas,[3] and recently among pigs in the Phillipines.[4] Despite its status as a Level-4 organism, it is non-pathogenic to humans however hazardous to monkeys.[5]
Ivory Coast ebolavirus
Ivory Coast ebolavirus was first discovered among chimpanzees of the Tai Forest in Côte d'Ivoire, Africa. On November 1, 1994, the corpses of two chimpanzees were found in the forest. Necropsies showed blood within the heart to be liquid and brown; no obvious marks were seen on the organs; and one necropsy displayed lungs filled with liquid blood. Studies of tissues taken from the chimps showed results similar to human cases during the 1976 Ebola outbreaks in Zaïre and Sudan. Later in 1994, more dead chimpanzees were discovered, with many testing positive to Ebola using molecular techniques. The source of contamination was believed to be the meat of infected Western Red Colobus monkeys, upon which the chimpanzees preyed.[6]
One of the scientists performing the necropsies on the infected chimpanzees contracted Ebola. She developed symptoms similar to those of dengue fever approximately a week after the necropsy, and was transported to Switzerland for treatment. After two weeks she was discharged from hospital, and was fully recovered six weeks after the infection.
Bundibugyo ebolavirus
On November 24, 2007, the Uganda Ministry of Health confirmed an outbreak of Ebola in the Bundibugyo District. After confirmation of samples tested by the United States National Reference Laboratories and the CDC, the World Health Organization confirmed the presence of the new species. On February 20, 2008, the Uganda Ministry officially announced the end of the epidemic in Bundibugyo with the last infected person discharged on January 8, 2008.[7] Ugandan officials confirmed a total of 149 cases of this new Ebola species, with 37 deaths attributed to the strain (24.83%).[8] It has yet to be classified.
Virology
Structure
Electron micrographs of members of genus Ebolavirus show them to have the characteristic thread-like structure of a filovirus.[9] EBOV VP30 is around 288 amino acids long.[9] The virions are tubular in general form but variable in overall shape and may appear as the classic shepherd's crook or eyebolt, as a U or a 6, or coiled, circular, or branched. However, laboratory purification techniques, such as centrifugation, may contribute to some of these.[9] Virions are generally 80 nm in diameter.[9] They are of variable length, typically around 1000 nm, but may be up to 1400 nm long. In the center of the virion is a structure called nucleocapsid, which is formed by the helically-wound viral genomic RNA complexed with the proteins NP, VP35, VP30, and L. It has a diameter of 40-50 nm and contains a central channel of 20–30 nm in diameter. Virally-encoded glycoprotein (GP) spikes 10 nm long and 10 nm apart are present on the outer viral envelope of the virion, which is derived from the host cell membrane. Between envelope and nucleocapsid, in the so-called matrix space, the viral proteins VP40 and VP24 are located.
Genome
Each virion contains one minor molecule of linear, single-stranded, negative-sense RNA, totaling 18,959 to 18,961 nucleotides in length. The 3′ terminus is not polyadenylated and the 5′ end is not capped. It was found that 472 nucleotides from the 3' end and 731 nucleotides from the 5' end are sufficient for replication.[9] It codes for seven structural proteins and one non-structural protein. The gene order is 3′ - leader - NP - VP35 - VP40 - GP/sGP - VP30 - VP24 - L - trailer - 5′; with the leader and trailer being non-transcribed regions, which carry important signals to control transcription, replication, and packaging of the viral genomes into new virions. The genomic material by itself is not infectious, because viral proteins, among them the RNA-dependent RNA polymerase, are necessary to transcribe the viral genome into mRNAs, as well as for replication of the viral genome.
Life cycle
- Virus attaches to host receptors through the GP (glycoprotein) surface peplomer and is endocytosed into vesicles in the host cell.
- Fusion of virus membrane with the vesicle membrane occurs; nucleocapsid is released into the cytoplasm.
- The encapsidated, negative-sense genomic ssRNA is used as a template for the synthesis ( 3' - 5') of polyadenylated, monocistronic mRNAs.
- Translation of the mRNA into viral proteins occurs using the host cell's machinery.
- Post-translational processing of viral proteins occurs. GP0 (glycoprotein precursor) is cleaved to GP1 and GP2, which are heavily glycosylated. These two molecules assemble, first into heterodimers, and then into trimers to give the surface peplomers. SGP (secreted glycoprotein) precursor is cleaved to SGP and delta peptide, both of which are released from the cell.
- As viral protein levels rise, a switch occurs from translation to replication. Using the negative-sense genomic RNA as a template, a complementary +ssRNA is synthesized; this is then used as a template for the synthesis of new genomic (-)ssRNA, which is rapidly encapsidated.
- The newly-formed nucleocapsides and envelope proteins associate at the host cell's plasma membrane; budding occurs, and the virions are released.
Natural reservoirs
Despite numerous studies, the wildlife reservoir of Ebolavirus has not been identified. Between 1976 and 1998, from 30,000 mammals, birds, reptiles, amphibians, and arthropods sampled from outbreak regions, no Ebolavirus was detected[10] apart from some genetic material found in six rodents (Mus setulosus and Praomys species) and a shrew (Sylvisorex ollula) collected from the Central African Republic in 1998.[11] Ebolavirus was detected in the carcasses of gorillas, chimpanzees, and duikers during outbreaks in 2001 and 2003 (the carcasses were the source of the initial human infections), but the high mortality from infection in these species precludes them from acting as reservoirs.[10] As of late 2005, three species of fruit bat have been identified as carrying the virus but not showing disease symptoms, and they are now believed to be the natural host species, or reservoir, of the virus.[12]
Plants, arthropods, and birds have also been considered as reservoirs; however, bats are considered the most likely candidate.[13] Bats were known to reside in the cotton factory in which the index cases for the 1976 and 1979 outbreaks were employed, and they have also been implicated in Marburg infections in 1975 and 1980.[10] Of 24 plant species and 19 vertebrate species experimentally inoculated with Ebolavirus, only bats became infected.[14] The absence of clinical signs in these bats is characteristic of a reservoir species. In 2002-03, a survey of 1,030 animals from Gabon and the Republic of the Congo including 679 bats found Ebolavirus RNA in 13 fruit bats (Hyspignathus monstrosus, Epomops franquetti and Myonycteris torquata).[15] Bats are also known to be the reservoirs for a number of related viruses including Nipah virus, Hendra virus and lyssaviruses.
Pathogenesis
Ebolavirus interferes mainly with the endothelial cells lining the interior surface of blood vessels and platelet cells. As the vessel walls become damaged from infection and the platelet cells are unable to coagulate, patients quickly lose blood and succumb to shock.[16][9] Filoviruses replicate well in a wide range of organs and cell types such as hepatocytes, epithelial cells, fibroblasts, fibroblastic reticular cells, and adrenal cortical cells.[9]
Epidemiology
Prevalence
This section needs expansion. You can help by adding to it. (December 2008) |
Outbreaks of Ebola have mainly been restricted to Africa, from which many of the outbreaks consume the population before it can effectively spread.[citation needed]
Reston ebolavirus (EBO-R), believed to potentially be either another subtype of Ebola or another filovirus, has spread to numerous areas via air transport of infected monkeys. It has emerged in: Reston, Virgina; Alice, Texas; the Philippines; and Italy.
On 11 December 2008, pigs from farms slightly north of Manila, Philippines tested positive for the virus. [17]
Transmission
This section needs additional citations for verification. (August 2007) |
Among humans, the virus is transmitted by direct contact with infected body fluids or, to a lesser extent, skin or mucous membrane contact. The incubation period can range from 2 to 21 days, but is, in general, 5–10 days.
Although airborne transmission between monkeys has been demonstrated by an accidental outbreak in a laboratory located in Virginia, USA, there is very limited evidence for human-to-human airborne transmission in any reported epidemics. Nurse Mayinga might represent the only possible case. The means by which she contracted the virus remains uncertain.
The infection of human cases with Ebolavirus has been documented through the handling of infected chimpanzees, gorillas, and forest antelopes — both dead and alive — as was documented in Côte d'Ivoire, the Republic of Congo, and Gabon. The transmission of the Ebola Reston strain through the handling of cynomolgus monkeys has also been reported.[18] Ebola Reston has been found in pigs in Luzon.
So far, all epidemics of Ebola have occurred in sub-optimal hospital conditions, where practices of basic hygiene and sanitation are often either luxuries or unknown to caretakers and where disposable needles and autoclaves are unavailable or too expensive. In modern hospitals with disposable needles and knowledge of basic hygiene and barrier nursing techniques, Ebola has never spread on such a large scale.
In the early stages, Ebola may not be highly contagious. Contact with someone in early stages may not even transmit the disease. As the illness progresses, bodily fluids from diarrhea, vomiting, and bleeding represent an extreme biohazard. Due to lack of proper equipment and hygienic practices, large-scale epidemics occur mostly in poor, isolated areas without modern hospitals or well-educated medical staff. Many areas where the infectious reservoir exists have just these characteristics. In such environments, all that can be done is to immediately cease all needle-sharing or use without adequate sterilization procedures, to isolate patients, and to observe strict barrier nursing procedures with the use of a medical rated disposable face mask, gloves, goggles, and a gown at all times. This should be strictly enforced for all medical personnel and visitors.
Ebola is unlikely to develop into a pandemic, or world-wide infection, due to its difficulty in spreading by airborne transmission and the period of time that the virus can use a living and contagious victim to spread compared to other infectious diseases. In isolated settings such as a quarantined hospital or a remote village, most victims are infected shortly after the first case of infection is present. In addition, the quick onset of symptoms from the time the disease becomes contagious in an individual makes it easy to identify sick individuals and limits an individual's ability to spread the disease by traveling. Because bodies of the deceased are still infectious, some doctors had to take measures to make sure that the disposal of dead bodies were conducted in a safe manner despite any local traditional burial rituals.[19]
Recent cases
As of August 30, 2007, 103 people (100 adults and three children) were infected by a suspected hemorrhagic fever outbreak in the village of Kampungu, Democratic Republic of the Congo. The outbreak started after the funerals of two village chiefs, and 217 people in four villages fell ill. The World Health Organization sent a team to take blood samples for analysis and confirmed that many of the cases are the result of Ebolavirus.[20] The Congo's last major Ebola epidemic killed 245 people in 1995 in Kikwit, about 200 miles from the source of the August 2007 outbreak.[21]
On November 30, 2007, the Uganda Ministry of Health confirmed an outbreak of Ebola in the Bundibugyo District. After confirmation of samples tested by the United States National Reference Laboratories and the Centers for Disease Control, the World Health Organization confirmed the presence of a new species of Ebolavirus.[22] The epidemic came to an official end on February 202008. While it lasted, 149 cases of this new strain were reported, and 37 of those led to deaths.
The virus appeared in southern Western Kasai province on November 27, and blood and stool samples were sent to laboratories in Gabon and South Africa for identification.
A mysterious disease that has killed eleven and infected twenty-one people in southern Democratic Republic of Congo has been identified as the Ebola virus.[23] Medecins Sans Frontieres reports 11 deaths as of Monday 29 December 2008 in the Western Kasai province of the Democratic Republic of Congo. A further 24 cases are said to being treated.
Medical aspects
Prevention
Vaccines have been produced for both Ebola[24] and Marburg[25] that were 99% effective in protecting a group of monkeys from the disease. These vaccines are based on either a recombinant Vesicular stomatitis virus or a recombinant Adenovirus[26] carrying the Ebola spike protein on its surface. A recent vaccine trial conducted by the Vaccine Research Center (VRC) of the National Institutes of Health (NIH) in Bethesda, MD succesfully demonstrated an immune response to the virus in humans.[27] The biggest problem with the vaccine is that, unless the patient is given it near the onset of the virus (1-4 days after the symptoms begin), there will be too much damage to the human body to repair, e.g., ruptured arteries and capillaries, vomiting, and other symptoms that may still cause enough harm to kill or seriously traumatize the patient. Nevertheless, such vaccines may play a crucial role by allowing health care workers and primary responders to safely help patients [28][29]
Symptoms
Symptoms are varied and often appear suddenly. Initial symptoms include high fever (at least 38.8°C; 101.8°F), severe headache, muscle, joint, or abdominal pain, severe weakness, and exhaustion, sore throat, nausea, and dizziness.[18] Before an outbreak is suspected, these early symptoms are easily mistaken for malaria, typhoid fever, dysentery, influenza, or various bacterial infections, which are all far more common and reliably less fatal.
Ebola may progress to cause more serious symptoms, such as diarrhea, dark or bloody feces, vomiting blood, red eyes due to distension and hemorrhage of sclerotic arterioles, petechia, maculopapular rash, and purpura. Other, secondary symptoms include hypotension (low blood pressure), hypovolemia, tachycardia, organ damage (especially the kidneys, spleen, and liver) as a result of disseminated systemic necrosis, and proteinuria. The interior bleeding is caused by a reaction between the virus and the platelets that produces a chemical that will cut cell-size holes into the capillary walls.
On occasion, internal and external hemorrhage from orifices, such as the nose and mouth, may also occur, as well as from incompletely-healed injuries such as needle-puncture sites. Ebola virus can affect the levels of white blood cells and platelets, disrupting clotting.[citation needed] More than 50% of patients will develop some degree of hemorrhaging.[citation needed]
Diagnosis
Methods of diagnosis of Ebola include testing saliva and urine samples. The span of time from onset of symptoms to death is usually between 2 and 21 days. By the second week of infection, patients will either defervesce (the fever will lessen) or undergo systemic multi-organ failure. Mortality rates are generally high, ranging from 50 to 90%.[18] The cause of death is usually due to hypovolemic shock or organ failure.[30]
Ebola is diagnosed with Enzyme-Linked ImmunoSorbent Assay (ELISA) test. This diagnosis method has produced potentially ambiguous results during non-outbreak situations.
Following Reston, and in an effort to evaluate the original test, Dr. Karl Johnson of the CDC tested San Blas Indians from Central America: which have no history of Ebola infection. It produced a two percent positive. Other researchers later tested sera from Native Americans in Alaska and found a similar percentage of positive. To combat the false positives a more complex test based on the ELISA system was developed by Tom Kzaisek at USAMRIID which was later improved with Immunofluorescent antibody analysis (IFA). It was however not used during the serosurvey following Reston.[31]
Prognosis
Ebola hemorrhagic fever is potentially lethal and encompasses a range of symptoms including fever, vomiting, diarrhea, generalized pain or malaise, and sometimes internal and external bleeding. Mortality rates are extremely high, with the human case-fatality rate ranging from 50–89%, depending on virus.[32] The cause of death is usually due to hypovolemic shock or organ failure.
Treatment

There is no standard treatment for Ebola HF. Treatment is primarily supportive and includes minimizing invasive procedures, balancing electrolytes, and, since patients are frequently dehydrated, replacing lost coagulation factors to help stop bleeding, maintaining oxygen and blood levels, and treating any complicating infections. Convalescent plasma (factors from those that have survived Ebola infection) shows promise as a treatment for the disease [citation needed]. Ribavirin is ineffective. Interferon is also thought to be ineffective. In monkeys, administration of an inhibitor of coagulation (rNAPc2) has shown some benefit, protecting 33% of infected animals from a usually 100% (for monkeys) lethal infection (however, this inoculation does not work on humans). In early 2006, scientists at USAMRIID announced a 75% recovery rate after infecting four rhesus monkeys with Ebolavirus and administering antisense drugs.[33]
History
Emergence
Ebolavirus first emerged in 1976 in outbreaks of Ebola hemorrhagic fever in Zaire and Sudan.[34] The strain of Ebola that broke out in Zaire has one of the highest case fatality rates of any human pathogenic virus, roughly 90%.[35] The strain that broke out later in Sudan has a case fatality rate of around 50%.[35] The virus is believed to be transmitted to humans via contact with an infected animal host. The virus is then transmitted to other people that come into contact with blood and bodily fluids of the infected person, and by human contact with contaminated medical equipment such as needles. Both of these infectious mechanisms will occur in clinical (nosocomial) and non-clinical situations. Due to the high fatality rate, the rapidity of demise, and the often remote areas where infections occur, the potential for widespread epidemic outbreaks is considered low.
Public attention
While investigating on an outbreak of Simian hemorrhagic fever (SHFV) in the November of 1989, an electron microscopist from USAMRIID discovered filoviruses similar in appearance to Ebola in tissue samples taken from Crab-eating Macaque imported from the Philippines to Hazleton Laboratories Reston, Virginia.[36] Due to the lethality of the suspected and previously obscure virus, the investigation quickly attracted attention.[citation needed]
Blood samples were taken from 178 animal handlers during the incident.[37] Of them, six animal handlers eventually became seroconverted. When the handler failed to become ill, the Centers for Disease Control and Prevention (CDC) concluded that the virus had a very low pathogenicity to humans.[38]
Both the Phillipines and the United States had no previous cases of infection, and upon further isolation it was concluded to be another species of Ebola or a new filovirus of Asian origin, and named Reston ebolavirus (REBOV) after the location of the incident.
Post-Reston
In 1992, Ebola has been considered by members of Japan's Aum Shinrikyo cult, whose leader, Shoko Asahara, led about 40 members to Zaire under the guise of offering medical aid to Ebola victims in what was, it is presumed, an attempt to acquire a sample of the virus.[39]
Given the lethal nature of Ebola, and, since no approved vaccine or treatment is available, it is classified as a biosafety level 4 agent, as well as a Category A bioterrorism agent by the Centers for Disease Control and Prevention. It has the potential to be weaponized for use in biological warfare.[40] The effectiveness as a biological weapon is compromised by its rapid lethality as patients quickly die off before they are capable of effectively spreading the contagion.[citation needed]
The attention gather from the outbreak in Reston prompted in increase in public interest, leading the publication of numerous fictional works.
- Ebola Syndrome (1996) a movie following a resistant carrier spreading the virus to Hong Kong.
- Executive Orders and Rainbow Six, two of Tom Clancy's novels, describe scenarios in which Ebola is used as a biological weapon
- Outbreak (1995), a movie following the outbreak of an Ebola-like virus called Motaba.
- Resident Evil movie and video game series, Ebola serves as the basis for the T-virus.
- The Hot Zone a non-fiction book by Richard Preston.
- The Transall Saga, a novel by Gary Paulsen, describes strain of Ebola wipes out humanity.
References
Notes
- ^ Bardi, Jason Socrates (2002). "Death Called a River". Scripps Research Institute. 2 (1). Retrieved 2006-12-08.
{{cite journal}}: Cite has empty unknown parameters:|month=and|coauthors=(help) - ^ Isaacson, Margaretha. "Two Belgian nurses died of Ebola".
{{cite journal}}: Cite journal requires|journal=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Special Pathogens Branch CDC (2008-01-14). "Known Cases and Outbreaks of Ebola Hemorrhagic Fever". Center for Disease Control and Prevention. Retrieved 2008-08-02.
{{cite web}}: Cite has empty unknown parameters:|month=and|coauthors=(help) - ^ "Pig-to-Human Ebola Case Suspected in Philippines". New York Times. 2009-1-24. Retrieved 2009-1-26.
{{cite web}}: Check date values in:|accessdate=and|date=(help); Cite has empty unknown parameters:|month=and|coauthors=(help) - ^ Level 4: Virus Hunters of the CDC (1999), p.300.
- ^ Ebola Cote d'Ivoire Outbreaks
- ^ "End of Ebola outbreak in Uganda" (Press release). World Health Organization. 2008-02-20.
- ^ Cocks, Tim (2007-12-11). "Uganda confirms 113 suspected Ebola cases". Reuters. Retrieved 2008-02-25.
{{cite news}}: Cite has empty unknown parameter:|coauthors=(help) - ^ a b c d e f g Klenk, Hans-Dieter (2004). Ebola and Marburg Viruses, Molecular and Cellular Biology. Wymondham, Norfolk: Horizon Bioscience. ISBN 0954523237.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c Pourrut X, Kumulungui B, Wittmann T; et al. (2005). "The natural history of Ebola virus in Africa". Microbes Infect. 7 (7–8): 1005–14. doi:10.1016/j.micinf.2005.04.006. PMID 16002313.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Morvan JM, Deubel V, Gounon P; et al. (1999). "Identification of Ebola virus sequences present as RNA or DNA in organs of terrestrial small mammals of the Central African Republic". Microbes Infect. 1 (14): 1193–201. doi:10.1016/S1286-4579(99)00242-7. PMID 10580275.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ X. Pourrut, A. Délicat, PE Rollin, TG Ksiazek, J-P Gonzalez, EM Leroy (2007) "Spatial and temporal patterns of Zaire ebolavirus antibody prevalence in the possible reservoir bat species" Journal of Infectious Diseases 196: 176–183.
- ^ "Fruit bats may carry Ebola virus". BBC News. 2005-12-11. Retrieved 2008-02-25.
{{cite news}}: Cite has empty unknown parameter:|coauthors=(help) - ^ Swanepoel R, Leman PA, Burt FJ; et al. (1996). "Experimental inoculation of plants and animals with Ebola virus". Emerging Infect. Dis. 2 (4): 321–5. PMID 8969248.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Leroy EM, Kumulungui B, Pourrut X; et al. (2005). "Fruit bats as reservoirs of Ebola virus". Nature. 438 (7068): 575–6. doi:10.1038/438575a. PMID 16319873.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ [1]
- ^ Gale, Jason (2008-12-11). "Pig Ebola May Lead Scientists to 'Elusive Reservoir' of Virus". New York City: Bloomberg L.P. Retrieved 2008-12-22.
{{cite web}}: Cite has empty unknown parameters:|month=and|coauthors=(help) - ^ a b c WHO Fact Sheet Ebola haemorrhagic fever
- ^ Harden, Blaine (2001-02-18). "Dr. Matthew's Passion". New York Times Magazine. Retrieved 2008-02-25.
{{cite news}}: Cite has empty unknown parameter:|coauthors=(help) - ^ "Ebola Outbreak Confirmed in Congo". NewScientist.com. 2007-09-11. Retrieved 2008-02-25.
{{cite news}}: Cite has empty unknown parameter:|coauthors=(help) - ^ "Mystery DR Congo fever kills 100". BBC News. 2007-08-31. Retrieved 2008-02-25.
{{cite news}}: Cite has empty unknown parameter:|coauthors=(help) - ^ "Uganda: Deadly Ebola Outbreak Confirmed - UN". UN News Service. 2007-11-30. Retrieved 2008-02-25.
{{cite news}}: Cite has empty unknown parameter:|coauthors=(help) - ^ [2]
- ^ Jones SM, Feldmann H, Ströher U; et al. (2005). "Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses". Nat. Med. 11 (7): 786–90. doi:10.1038/nm1258. PMID 15937495.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Hevey M, Negley D, Pushko P, Smith J, Schmaljohn A (1998). "Marburg virus vaccines based upon alphavirus replicons protect guinea pigs and nonhuman primates". Virology. 251 (1): 28–37. doi:10.1006/viro.1998.9367. PMID 9813200.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sullivan NJ, Geisbert TW, Geisbert JB; et al. (2003). "Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates". Nature. 424 (6949): 681–4. doi:10.1038/nature01876. PMID 12904795.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ "Ebola/Marburg Vaccine Development" (Press release). National Institute of Allergy and Infectious Diseases. 2008-09-15.
- ^ , 2003-11-18 http://news.bio-medicine.org/medicine-news-2/NIAID-Ebola-vaccine-enters-human-trial-4881-3/
{{citation}}: Missing or empty|title=(help) - ^ , 2008-03-30 http://www.forbes.com/feeds/hscout/2008/03/31/hscout614041.html
{{citation}}: Missing or empty|title=(help) - ^ Bray M, Geisbert TW (2005). "Ebola virus: the role of macrophages and dendritic cells in the pathogenesis of Ebola hemorrhagic fever". Int. J. Biochem. Cell Biol. 37 (8): 1560–6. doi:10.1016/j.biocel.2005.02.018. PMID 15896665.
- ^ Level 4: Virus Hunters of the CDC (1999), pp.302-303.
- ^ Rouquet P, Froment JM, Bermejo M; et al. (2005). "Wild animal mortality monitoring and human Ebola outbreaks, Gabon and Republic of Congo, 2001-2003". Emerging Infect. Dis. 11 (2): 283–90. PMID 15752448.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ "USAMRIID press release" (PDF) (Press release).
- ^ Bennett D, Brown D (1995). "Ebola virus". BMJ. 310 (6991): 1344–5. PMID 7787519.
- ^ a b King, John W (April 2, 2008). "Ebola Virus". eMedicine. WebMd. Retrieved 2008-10-06.
- ^ Level 4: Virus Hunters of the CDC (1999), pp.277-279.
- ^ Waterman, Tara (1999). "Ebola Reston Outbreak Standford Honors Thesis". Stanford University. Retrieved 2008-08-02.
{{cite web}}: Cite has empty unknown parameters:|month=and|coauthors=(help) - ^ Level 4: Virus Hunters of the CDC (1999), ppgs.298-299.
- ^ "Chronology of Aum Shinrikyo's CBW Activities" (PDF). Monterey Institute for International Studies.
{{cite journal}}: Cite has empty unknown parameter:|coauthors=(help); Cite journal requires|journal=(help) - ^ Borio L, Inglesby T, Peters CJ; et al. (2002). "Hemorrhagic fever viruses as biological weapons: medical and public health management". JAMA. 287 (18): 2391–405. PMID 11988060.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link)
Bibliography
- McCormick M.D., Joseph B. (1999) [1996]. Level 4: Virus Hunters of the CDC. Horvitz, Leslie Alan ("Updated edition" 3rd ed.). Barnes & Noble. ISBN 9780760712085.
{{cite book}}:|access-date=requires|url=(help); Cite has empty unknown parameters:|accessyear=,|accessmonth=,|month=, and|origdate=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|origmonth=ignored (help) - Preston, Richard (1995-07-20) [1994]. The Hot Zone, A Terrifying True Story. Anchor Books (Random House), Sagebrush Education Resources, Tandem Library Books. ISBN 0-385-47956-5.
{{cite book}}:|access-date=requires|url=(help); Cite has empty unknown parameters:|origmonth=and|origdate=(help) - Waterman, Tara (1999). "Ebola Reston Outbreak Standford Honors Thesis". Stanford University. Retrieved 2008-08-02.
{{cite web}}: Cite has empty unknown parameters:|month=and|coauthors=(help)
External links
Overviews
- ViralZone: Ebolavirus
- Database entry on genus Ebolavirus - ICTVdB
- Ebola Virus Haemorrhagic Fever - Proceedings of an International Colloquium on Ebola Virus Infection and Other Haemorrhagic Fevers held in Antwerp, Belgium, 6-8 December, 1977
- Questions and Answers about Ebola Hemorrhagic Fever - Center for Disease Control (CDC), retrieved 10 July 2006
- WHO Factsheet - retrieved 10 July 2006
- Ebola and Marburg haemorrhagic fever (10 July 2008) factsheet from European Centre for Disease Prevention and Control
- Vaccine Research Center (VRC) - Information concerning Ebola vaccine research studies
Outbreaks
- Ebola Alert Shuts Angolan Border retrieved 2009-01-06.
- "Ebola outbreak in Congo". CBC News. 2007-09-12.
- "Ebola 'kills over 5,000 gorillas'". BBC News. 2006-12-08.
- History of Ebola Outbreaks - Centers for Disease Control Special Pathogens Branch, retrieved 2006-07-10.
- Infection Control for Viral Hemorrhagic Fevers in the African Health Care Setting - Center for Disease Control and Prevention, Atlanta, December 1998.
- Filovirus Global Symposium - Filomeeting 2008
Life Cycle
- Biomarker Database - information on Ebola
Infectivity
- U.S. Army Medical Research Institute of Infectious Diseases: Gene-Specific Ebola Therapies Protect Nonhuman Primates from Lethal Disease
- Jaax NK, Davis KJ, Geisbert TJ; et al. (1996). "Lethal experimental infection of rhesus monkeys with Ebola-Zaire (Mayinga) virus by the oral and conjunctival route of exposure". Arch. Pathol. Lab. Med. 120 (2): 140–55. PMID 8712894.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Johnson E, Jaax N, White J, Jahrling P (1995). "Lethal experimental infections of rhesus monkeys by aerosolized Ebola virus". Int J Exp Pathol. 76 (4): 227–36. PMID 7547435.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Leffel EK, Reed DS (2004). "Marburg and Ebola viruses as aerosol threats". Biosecur Bioterror. 2 (3): 186–91. PMID 15588056.
- Salvaggio MR, Baddley JW (2004). "Other viral bioweapons: Ebola and Marburg hemorrhagic fever". Dermatol Clin. 22 (3): 291–302, vi. doi:10.1016/j.det.2004.03.003. PMID 15207310.
- Jaax N, Jahrling P, Geisbert T; et al. (1995). "Transmission of Ebola virus (Zaire strain) to uninfected control monkeys in a biocontainment laboratory". Lancet. 346 (8991–8992): 1669–71. PMID 8551825.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - What is the probability of a dangerous strain of Ebola mutating and becoming airborne? Brett Russel, retrieved 2006-07-10.
