Forodesine
Appearance
| |
| Names | |
|---|---|
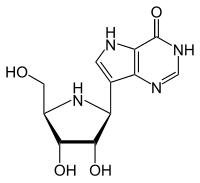
| IUPAC name
7-[(2S,3S,4R,5R)-3,4-dihydroxy-5-(hydroxymethyl)-2-pyrrolidinyl]-1,5-dihydropyrrolo[2,3-e]pyrimidin-4-one
| |
| Other names
Immucillin H
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C11H14N4O4 | |
| Molar mass | 266.25 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Forodesine (INN; also known as Immucillin H) is a transition-state analog inhibitor of purine nucleoside phosphorylase[1] under development for the treatment of relapsed B-cell chronic lymphocytic leukemia
Immucillin H was originally discovered by Vern Schramm's laboratory at the Albert Einstein College of Medicine in New York and Industrial Research Limited in New Zealand.
Forodesine is being developed by BioCryst Pharmaceuticals as Fodosine. As of 2008[update], it is currently in phase II clinical trials.[2][needs update]
References
- ^ Kicska GA, Long L, Hörig H; et al. (April 2001). "Immucillin H, a powerful transition-state analog inhibitor of purine nucleoside phosphorylase, selectively inhibits human T lymphocytes". Proc. Natl. Acad. Sci. U.S.A. 98 (8): 4593–8. doi:10.1073/pnas.071050798. PMC 31879. PMID 11287638.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Complete list of clinical trials for forodesine (BCX-1777) (ClinicalTrials.gov)". Retrieved 2008-07-22.
External links
- "From cell biology to therapy: forodesine". Hematology Meeting Reports. 2 (5): 106–111. 2008.
