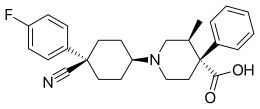
Levocabastine
 | |
| Clinical data | |
|---|---|
| Trade names | Livostin |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| Routes of administration | Ophthalmic, intranasal[1] |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C26H29FN2O2 |
| Molar mass | 420.528 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Levocabastine (trade name Livostin or Livocab, depending on the region) is a selective second-generation H1 receptor antagonist which was discovered at Janssen Pharmaceutica in 1979. It is used for allergic conjunctivitis.[3]
As well as acting as an antihistamine, levocabastine has also subsequently been found to act as a potent and selective antagonist for the neurotensin receptor NTS2, and was the first drug used to characterise the different neurotensin subtypes.[4][5] This has made it a useful tool for the study of this receptor.[6][7][8][9][10]
The pharmaceutical drug Bilina is a combination of Levocabastine, benzalkonium chloride, and other components and is typically used in a 0.5 mg/ml suspension as eye-drops, dispensed in 4ml bottles for the treatment of allergic conjunctivitis or similar allergic ocular conditions. Another formulation is available as a nasal spray for the management of allergic rhinitis.[11][12]
References
[edit]- ^ "Livostin Nasal Spray". RxMed: Pharmaceutical Information. Retrieved 13 November 2005.
- ^ "Livostin - levocabastine hydrochloride suspension". DailyMed. U.S. National Library of Medicine. Retrieved 4 January 2016.
- ^ Pipkorn U, Bende M, Hedner J, Hedner T (October 1985). "A double-blind evaluation of topical levocabastine, a new specific H1 antagonist in patients with allergic conjunctivitis". Allergy. 40 (7): 491–496. doi:10.1111/j.1398-9995.1985.tb00255.x. PMID 2866725. S2CID 8681108.
- ^ Schotte A, Leysen JE, Laduron PM (August 1986). "Evidence for a displaceable non-specific [3H]neurotensin binding site in rat brain". Naunyn-Schmiedeberg's Archives of Pharmacology. 333 (4): 400–405. doi:10.1007/BF00500016. PMID 3022160. S2CID 23692347.
- ^ Kitabgi P, Rostène W, Dussaillant M, Schotte A, Laduron PM, Vincent JP (August 1987). "Two populations of neurotensin binding sites in murine brain: discrimination by the antihistamine levocabastine reveals markedly different radioautographic distribution". European Journal of Pharmacology. 140 (3): 285–293. doi:10.1016/0014-2999(87)90285-8. PMID 2888670.
- ^ Chalon P, Vita N, Kaghad M, Guillemot M, Bonnin J, Delpech B, et al. (May 1996). "Molecular cloning of a levocabastine-sensitive neurotensin binding site". FEBS Letters. 386 (2–3): 91–94. doi:10.1016/0014-5793(96)00397-3. PMID 8647296. S2CID 5802578.
- ^ Mazella J, Botto JM, Guillemare E, Coppola T, Sarret P, Vincent JP (September 1996). "Structure, functional expression, and cerebral localization of the levocabastine-sensitive neurotensin/neuromedin N receptor from mouse brain". The Journal of Neuroscience. 16 (18): 5613–5620. doi:10.1523/JNEUROSCI.16-18-05613.1996. PMC 6578974. PMID 8795617.
- ^ Sarret P, Esdaile MJ, Perron A, Martinez J, Stroh T, Beaudet A (September 2005). "Potent spinal analgesia elicited through stimulation of NTS2 neurotensin receptors". The Journal of Neuroscience. 25 (36): 8188–8196. doi:10.1523/JNEUROSCI.0810-05.2005. PMC 6725526. PMID 16148226.
- ^ Bredeloux P, Costentin J, Dubuc I (December 2006). "Interactions between NTS2 neurotensin and opioid receptors on two nociceptive responses assessed on the hot plate test in mice". Behavioural Brain Research. 175 (2): 399–407. doi:10.1016/j.bbr.2006.09.016. PMID 17074405. S2CID 24790151.
- ^ Yamauchi R, Wada E, Kamichi S, Yamada D, Maeno H, Delawary M, et al. (September 2007). "Neurotensin type 2 receptor is involved in fear memory in mice". Journal of Neurochemistry. 102 (5): 1669–1676. doi:10.1111/j.1471-4159.2007.04805.x. PMID 17697051. S2CID 19774998.
- ^ "Levocabastine ophthalmic". vademecum.es. Retrieved 11 September 2014.
- ^ "★ Levocabastina 🥇". www.vademecum.es. Retrieved 2024-09-16.
External links
[edit]- "Levocabastine". Drug Information Portal. U.S. National Library of Medicine.
- "Levocabastine hydrochloride". Drug Information Portal. U.S. National Library of Medicine.
