Rutecarpine: Difference between revisions
expand |
|||
| Line 37: | Line 37: | ||
}} |
}} |
||
'''Rutecarpine''' or '''rutaecarpine''' is a [[COX-2 inhibitor]] isolated from ''[[Tetradium ruticarpum]]''.<ref>{{cite journal |last1=Moon |first1=T. C. |last2=Murakami |first2=M. |last3=Kudo |first3=I. |last4=Son |first4=K. H. |last5=Kim |first5=H. P. |last6=Kang |first6=S. S. |last7=Chang |first7=H. W. |date=1999 |title=A new class of COX-2 inhibitor, rutaecarpine from ''Evodia rutaecarpa'' |journal=Inflammation Research |volume=48 |issue=12 |pages=621–625 |doi=10.1007/s000110050512 |pmid=10669112 |s2cid=19555209}}</ref> It is claimed as one of the non-basic alkaloids.<ref>{{cite book |last=Manske |first=R. H. F. |date=1950 |chapter=Sources of alkaloids and their isolation |editor1-last=Manske |editor1-first=R. H. F. |editor2-last=Holmes |editor2-first=H. L. |title=The Alkaloids: Chemistry and Physiology |volume=1 |publisher=Academic Press |pages=1–14 |doi=10.1016/S1876-0813(08)60184-0 |isbn=978-0-12-469501-6 |s2cid=82529003}}</ref> |
'''Rutecarpine''' or '''rutaecarpine''' is a [[COX-2 inhibitor]] isolated from ''[[Evodia rutaecarpa]]'' and ''[[Tetradium ruticarpum]]''.<ref>{{cite journal |last1=Moon |first1=T. C. |last2=Murakami |first2=M. |last3=Kudo |first3=I. |last4=Son |first4=K. H. |last5=Kim |first5=H. P. |last6=Kang |first6=S. S. |last7=Chang |first7=H. W. |date=1999 |title=A new class of COX-2 inhibitor, rutaecarpine from ''Evodia rutaecarpa'' |journal=Inflammation Research |volume=48 |issue=12 |pages=621–625 |doi=10.1007/s000110050512 |pmid=10669112 |s2cid=19555209}}</ref> It is claimed as one of the non-basic alkaloids.<ref>{{cite book |last=Manske |first=R. H. F. |date=1950 |chapter=Sources of alkaloids and their isolation |editor1-last=Manske |editor1-first=R. H. F. |editor2-last=Holmes |editor2-first=H. L. |title=The Alkaloids: Chemistry and Physiology |volume=1 |publisher=Academic Press |pages=1–14 |doi=10.1016/S1876-0813(08)60184-0 |isbn=978-0-12-469501-6 |s2cid=82529003}}</ref> |
||
In contrast to synthetic COX-2 inhibitors like [[Etoricoxib]] and [[Celecoxib]] Rutecarpine does not show their negative effects on the cardiovascular system; in fact it even does compensate for the adverse effects usually caused by COX-2 inhibition.<ref>{{cite journal |last1=Jia |first1=Sujie |last2=Hu |first2=Changping |date=2010 |title=Pharmacological effects of rutaecarpine as a cardiovascular protective agent |title-link=doi |journal=Molecules |volume=15 |issue=3 |pages=1873–1881 |doi=10.3390/molecules15031873 |doi-access=free |pmc=6257227 |pmid=20336017 |s2cid=21968872 |s2cid-access=free}}</ref> |
In contrast to synthetic COX-2 inhibitors like [[Etoricoxib]] and [[Celecoxib]] Rutecarpine does not show their negative effects on the cardiovascular system; in fact it even does compensate for the adverse effects usually caused by COX-2 inhibition.<ref>{{cite journal |last1=Jia |first1=Sujie |last2=Hu |first2=Changping |date=2010 |title=Pharmacological effects of rutaecarpine as a cardiovascular protective agent |title-link=doi |journal=Molecules |volume=15 |issue=3 |pages=1873–1881 |doi=10.3390/molecules15031873 |doi-access=free |pmc=6257227 |pmid=20336017 |s2cid=21968872 |s2cid-access=free}}</ref> |
||
Microsome studies suggest that rutecarpine may be at least a weak inhibitor of [[CYP1A2]], [[CYP2C9]], [[CYP2C19]], [[CYP2E1]], and [[CYP3A4]] enzymes.<ref>{{cite journal |last1=Zhang |first1=Fang-Liang |last2=He |first2=Xin |last3=Zhai |first3=Yi-Ran |last4=He |first4=Li-Na |last5=Zhang |first5=Si-Chao |last6=Wang |first6=Li-Li |last7=Yang |first7=Ai-Hong |last8=An |first8=Li-Jun |title=Mechanism-based inhibition of CYPs and RMs-induced hepatoxicity by rutaecarpine |journal=Xenobiotica |date=2 November 2015 |volume=45 |issue=11 |pages=978–989 |doi=10.3109/00498254.2015.1038742}}</ref><ref>{{cite journal |last1=Ueng |first1=Yune-Fang |last2=Jan |first2=Woan-Ching |last3=Lin |first3=Lie-Chwen |last4=Chen |first4=Ta-Liang |last5=Guengerich |first5=F. Peter |last6=Chen |first6=Chieh-Fu |title=The Alkaloid Rutaecarpine Is a Selective Inhibitor of Cytochrome P450 1A in Mouse and Human Liver Microsomes |journal=Drug Metabolism and Disposition |date=1 March 2002 |volume=30 |issue=3 |pages=349–353 |doi=10.1124/dmd.30.3.349}}</ref> At the same time, it is believed to be a rather strong inducer of the [[CYP1A2]] and [[CYP1A1]] enzymes.<ref>{{cite journal |last1=Ueng |first1=Yune-Fang |last2=Wang |first2=Jong-Jing |last3=Lin |first3=Lie-Chwen |last4=Park |first4=Sang Shin |last5=Chen |first5=Chieh-Fu |title=Induction of cytochrome P450-dependent monooxygenase in mouse liver and kidney by rutaecarpine, an alkaloid of the herbal drug Evodia rutaecarpa |journal=Life Sciences |date=November 2001 |volume=70 |issue=2 |pages=207–217 |doi=10.1016/S0024-3205(01)01390-X}}</ref> Rutecarpine has been shown to decrease the overall bioavailability of caffeine in rats by to 80%.<ref>{{cite journal |last1=Estari |first1=Rohit Kumar |last2=Dong |first2=Jin |last3=Chan |first3=William K. |last4=Park |first4=Miki Susanto |last5=Zhou |first5=Zhu |title=Time effect of rutaecarpine on caffeine pharmacokinetics in rats |journal=Biochemistry and Biophysics Reports |date=1 December 2021 |volume=28 |pages=101121 |doi=10.1016/j.bbrep.2021.101121}}</ref> Caffeine metabolism in rats given rutecarpine is modified greatly, likely through the induction of both the [[CYP1A2]] and [[CYP2E1]] enzymes.<ref>{{cite journal |last1=Noh |first1=Keumhan |last2=Seo |first2=Young Min |last3=Lee |first3=Sang Kyu |last4=Bista |first4=Sudeep R. |last5=Kang |first5=Mi Jeong |last6=Jahng |first6=Yurngdong |last7=Kim |first7=Eunyoung |last8=Kang |first8=Wonku |last9=Jeong |first9=Tae Cheon |title=Effects of rutaecarpine on the metabolism and urinary excretion of caffeine in rats |journal=Archives of Pharmacal Research |date=January 2011 |volume=34 |issue=1 |pages=119–125 |doi=10.1007/s12272-011-0114-3}}</ref> A natural product, rutecarpine can be purchased legally online, where it is sold as a "decafeineator". The long-term safety of rutecarpine supplementation has not been established. |
|||
Rutecarpine metabolism is complex and proceeds along several routes, primarily involving the addition of a single hydroxyl group by [[CYP3A4]]. Six monohydroxylated and four dihydroxylated metabolites have been identified. To a much lesser extent rutecarpine may also be metabolized by [[CYP2C9]] and [[CYP1A2]], according to liver microsome studies.<ref>{{cite journal |last1=Lee |first1=Seung |last2=Son |first2=Jong-Keun |last3=Jeong |first3=Byeong |last4=Jeong |first4=Tae-Cheon |last5=Chang |first5=Hyeon |last6=Lee |first6=Eung-Seok |last7=Jahng |first7=Yurngdong |title=Progress in the Studies on Rutaecarpine |journal=Molecules |date=6 February 2008 |volume=13 |issue=2 |pages=272–300 |doi=10.3390/molecules13020272}}</ref> |
|||
==References== |
==References== |
||
Revision as of 16:32, 2 October 2022
This article needs additional citations for verification. (September 2013) |
| |
| Names | |
|---|---|
| Preferred IUPAC name
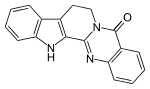
8,13-Hydroindolo[2′,3′:3,4]pyrido[2,1-b]quinazolin-5(7H)-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.163.752 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C18H13N3O | |
| Molar mass | 287.322 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Rutecarpine or rutaecarpine is a COX-2 inhibitor isolated from Evodia rutaecarpa and Tetradium ruticarpum.[1] It is claimed as one of the non-basic alkaloids.[2]
In contrast to synthetic COX-2 inhibitors like Etoricoxib and Celecoxib Rutecarpine does not show their negative effects on the cardiovascular system; in fact it even does compensate for the adverse effects usually caused by COX-2 inhibition.[3]
Microsome studies suggest that rutecarpine may be at least a weak inhibitor of CYP1A2, CYP2C9, CYP2C19, CYP2E1, and CYP3A4 enzymes.[4][5] At the same time, it is believed to be a rather strong inducer of the CYP1A2 and CYP1A1 enzymes.[6] Rutecarpine has been shown to decrease the overall bioavailability of caffeine in rats by to 80%.[7] Caffeine metabolism in rats given rutecarpine is modified greatly, likely through the induction of both the CYP1A2 and CYP2E1 enzymes.[8] A natural product, rutecarpine can be purchased legally online, where it is sold as a "decafeineator". The long-term safety of rutecarpine supplementation has not been established.
Rutecarpine metabolism is complex and proceeds along several routes, primarily involving the addition of a single hydroxyl group by CYP3A4. Six monohydroxylated and four dihydroxylated metabolites have been identified. To a much lesser extent rutecarpine may also be metabolized by CYP2C9 and CYP1A2, according to liver microsome studies.[9]
References
- ^ Moon, T. C.; Murakami, M.; Kudo, I.; Son, K. H.; Kim, H. P.; Kang, S. S.; Chang, H. W. (1999). "A new class of COX-2 inhibitor, rutaecarpine from Evodia rutaecarpa". Inflammation Research. 48 (12): 621–625. doi:10.1007/s000110050512. PMID 10669112. S2CID 19555209.
- ^ Manske, R. H. F. (1950). "Sources of alkaloids and their isolation". In Manske, R. H. F.; Holmes, H. L. (eds.). The Alkaloids: Chemistry and Physiology. Vol. 1. Academic Press. pp. 1–14. doi:10.1016/S1876-0813(08)60184-0. ISBN 978-0-12-469501-6. S2CID 82529003.
- ^ Jia, Sujie; Hu, Changping (2010). "Pharmacological effects of rutaecarpine as a cardiovascular protective agent". Molecules. 15 (3): 1873–1881. doi:10.3390/molecules15031873. PMC 6257227. PMID 20336017. S2CID 21968872.
- ^ Zhang, Fang-Liang; He, Xin; Zhai, Yi-Ran; He, Li-Na; Zhang, Si-Chao; Wang, Li-Li; Yang, Ai-Hong; An, Li-Jun (2 November 2015). "Mechanism-based inhibition of CYPs and RMs-induced hepatoxicity by rutaecarpine". Xenobiotica. 45 (11): 978–989. doi:10.3109/00498254.2015.1038742.
- ^ Ueng, Yune-Fang; Jan, Woan-Ching; Lin, Lie-Chwen; Chen, Ta-Liang; Guengerich, F. Peter; Chen, Chieh-Fu (1 March 2002). "The Alkaloid Rutaecarpine Is a Selective Inhibitor of Cytochrome P450 1A in Mouse and Human Liver Microsomes". Drug Metabolism and Disposition. 30 (3): 349–353. doi:10.1124/dmd.30.3.349.
- ^ Ueng, Yune-Fang; Wang, Jong-Jing; Lin, Lie-Chwen; Park, Sang Shin; Chen, Chieh-Fu (November 2001). "Induction of cytochrome P450-dependent monooxygenase in mouse liver and kidney by rutaecarpine, an alkaloid of the herbal drug Evodia rutaecarpa". Life Sciences. 70 (2): 207–217. doi:10.1016/S0024-3205(01)01390-X.
- ^ Estari, Rohit Kumar; Dong, Jin; Chan, William K.; Park, Miki Susanto; Zhou, Zhu (1 December 2021). "Time effect of rutaecarpine on caffeine pharmacokinetics in rats". Biochemistry and Biophysics Reports. 28: 101121. doi:10.1016/j.bbrep.2021.101121.
- ^ Noh, Keumhan; Seo, Young Min; Lee, Sang Kyu; Bista, Sudeep R.; Kang, Mi Jeong; Jahng, Yurngdong; Kim, Eunyoung; Kang, Wonku; Jeong, Tae Cheon (January 2011). "Effects of rutaecarpine on the metabolism and urinary excretion of caffeine in rats". Archives of Pharmacal Research. 34 (1): 119–125. doi:10.1007/s12272-011-0114-3.
- ^ Lee, Seung; Son, Jong-Keun; Jeong, Byeong; Jeong, Tae-Cheon; Chang, Hyeon; Lee, Eung-Seok; Jahng, Yurngdong (6 February 2008). "Progress in the Studies on Rutaecarpine". Molecules. 13 (2): 272–300. doi:10.3390/molecules13020272.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
