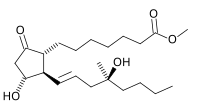
Misoprostol
 | |
| Clinical data | |
|---|---|
| Trade names | Cytotec, Misodel, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a689009 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, rectal, vaginal, under the tongue |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | extensively absorbed |
| Protein binding | 80–90% (active metabolite, misoprostol acid) |
| Metabolism | Liver (extensive to misoprostic acid) |
| Elimination half-life | 20–40 minutes |
| Excretion | Urine (80%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.190.521 |
| Chemical and physical data | |
| Formula | C22H38O5 |
| Molar mass | 382.541 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Misoprostol is a synthetic prostaglandin medication used to prevent and treat stomach and duodenal ulcers, induce labor, cause an abortion, and treat postpartum bleeding due to poor contraction of the uterus.[10][11] It is taken by mouth when used to prevent gastric ulcers in people taking nonsteroidal anti-inflammatory drugs (NSAID).[11] For abortions it is used by itself or in conjunction with mifepristone or methotrexate.[12] By itself, effectiveness for abortion is between 66% and 90%.[13][14] For labor induction or abortion, it is taken by mouth, dissolved in the mouth, or placed in the vagina.[12][15][16][17][18] For postpartum bleeding it may also be used rectally.[19]
Common side effects include diarrhea and abdominal pain.[11] It is in pregnancy category X, meaning that it is known to result in negative outcomes for the fetus if taken during pregnancy.[11] In rare cases, uterine rupture may occur.[11] It is a prostaglandin analogue—specifically, a synthetic prostaglandin E1 (PGE1).[11]
Misoprostol was developed in 1973.[20] It is on the World Health Organization's List of Essential Medicines.[21] It is available as a generic medication.[11]
Medical uses
[edit]Ulcer prevention
[edit]Misoprostol is used for the prevention of NSAID-induced gastric ulcers. It acts upon gastric parietal cells, inhibiting the secretion of gastric acid by G-protein coupled receptor-mediated inhibition of adenylate cyclase, which leads to decreased intracellular cyclic AMP levels and decreased proton pump activity at the apical surface of the parietal cell. Misoprostol is sometimes coprescribed with NSAIDs to prevent their common adverse effect of gastric ulceration (e.g., with diclofenac in Arthrotec).[citation needed]
However, even in the treatment of NSAID-induced ulcers, omeprazole proved to be at least as effective as misoprostol,[22] but was significantly better tolerated, so misoprostol should not be considered a first-line treatment. Misoprostol-induced diarrhea and the need for multiple daily doses (typically four) are the main issues impairing compliance with therapy.[medical citation needed]
Labor induction
[edit]Misoprostol is commonly used for labor induction. It causes uterine contractions and the ripening (effacement or thinning) of the cervix.[23] It can be less expensive than the other commonly used ripening agent, dinoprostone.[24]
Oxytocin has long been used as the standard agent for labor induction, but does not work well when the cervix is not yet ripe. Misoprostol also may be used in conjunction with oxytocin.[24]
Between 2002 and 2012, a misoprostol vaginal insert was studied, and was approved in the EU.[25][26][27] It was not approved for use in the United States, and the US FDA still considers cervical ripening and labor induction to be outside of the approved uses for misoprostol.[28][29]
Myomectomy
[edit]When administered prior to myomectomy in women with uterine fibroids, misoprostol reduces operative blood loss and requirement of blood transfusion.[30]
Abortion
[edit]Misoprostol is used either alone or in conjunction with another medication (mifepristone or methotrexate) for medical abortions as an alternative to surgical abortion.[31] Medical abortion has the advantage of being less invasive, and more autonomous, self-directed, and discreet. It is preferred by some women because it feels more natural, as the drugs induce a miscarriage.[32] It is also more easily accessible in places where abortion is illegal.[33] The World Health Organization (WHO) provides clear guidelines on the use, benefits and risks of misoprostol for abortions.[34]
Misoprostol is most effective when it is used in combination with methotrexate or mifepristone (RU-486).[35] Mifepristone blocks signaling by progesterone, causing the uterine lining to degrade, the blood vessels of the cervix and uterus to dilate and causing uterine contraction, similar to a menstrual period, which causes the embryo to detach from the uterine walls.[36] Misoprostol then dilates the cervix and induces muscle contractions which clear the uterus.[citation needed] Misoprostol alone is less effective (typically 88% up to eight-weeks gestation). It is not inherently unsafe if medically supervised, but 1% of women will have heavy bleeding requiring medical attention, some women may have ectopic pregnancy, and the 12% of pregnancies that continue after misoprostol failure are more likely to have birth defects and are usually followed up with a more effective method of abortion.[37]
Most large studies recommend a protocol for the use of misoprostol in combination with mifepristone.[38][39] Together they are effective in around 95% for early pregnancies.[40] Misoprostol alone may be more effective in earlier gestation.[41]
Misoprostol can also be used to dilate the cervix in preparation for a surgical abortion, particularly in the second trimester (either alone or in combination with osmotic dilators). Vaginal misoprostol can also be used to facilitate intrauterine device insertion after previous insertion failure.[42]
Misoprostol by mouth is the least effective treatment for producing complete abortion in a period of 24 hours due to the liver's first-pass effect which reduces the bioavailability of the misoprostol. Vaginal and sublingual routes result in greater efficacy and extended duration of action because these routes of administration allow the drug to be directly absorbed into circulation by bypassing the liver first-pass effect.[43][17][18]
Hematocrit or Hb tests and Rh testing are recommended before use for abortion confirmation of pregnancy.[44] Following use, it is recommended that people attend a follow-up visit 2 weeks after treatment. If used for treatment of complete abortion, a pregnancy test, physical examination of the uterus, and ultrasound should be performed to ensure success of treatment. Surgical management is possible in the case of failed treatment.[43]
Early pregnancy loss
[edit]Misoprostol may be used to complete a miscarriage or missed abortion when the body does not expel the embryo or fetus on its own. Compared to no medication or placebo, it could decrease the time to complete expulsion.[45] Use of a single dose of misoprostol vaginally or buccally is preferred, with additional doses as needed. It also can be used in combination with mifepristone, with a similar regimen to medical abortion.[17][18]
Misoprostol is regularly used in some Canadian hospitals for labour induction for fetal deaths early in pregnancy, and for termination of pregnancy for fetal anomalies. A low dose is used initially, then doubled for the remaining doses until delivery. In the case of a previous Caesarian section, however, lower doses are used.
Postpartum bleeding
[edit]Misoprostol is also used to prevent and treat post-partum bleeding. Orally administered misoprostol was marginally less effective than oxytocin.[46] The use of rectally administered misoprostol is optimal in cases of bleeding; it was shown to be associated with lower rates of side effects compared to other routes. Rectally administered misoprostol was reported in a variety of case reports and randomised controlled trials.[47][48] However, it is inexpensive and thermostable (thus does not require refrigeration like oxytocin), making it a cost-effective and valuable drug to use in the developing world.[49] A randomised control trial of misoprostol use found a 38% reduction in maternal deaths due to post partum haemorrhage in resource-poor communities.[50] Misoprostol is recommended due to its cost, effectiveness, stability, and low rate of side effects.[51] Oxytocin must also be given by injection, while misprostol can be given orally or rectally for this use, making it much more useful in areas where nurses and physicians are less available.[52]
Insertion of intrauterine contraceptive device
[edit]In women with prior caesarean section or prior failure of insertion of an intrauterine contraceptive device, pre-procedure administration of misoprostol reduces the rate of failure of insertion of intrauterine contraceptive device. However, due to a higher rate of adverse effects, routine use of misoprostol for this purpose in other women is not supported by the data.[53]
Other
[edit]For cervical ripening in advance of endometrial biopsy to reduce the need for use of a tenaculum or cervical dilator.[citation needed]
There is limited evidence supporting the use of misoprostol for the treatment of trigeminal neuralgia in patients with multiple sclerosis.[54][55]
Adverse effects
[edit]The most commonly reported adverse effect of taking misoprostol by mouth for the prevention of stomach ulcers is diarrhea. In clinical trials, an average 13% of people reported diarrhea, which was dose-related and usually developed early in the course of therapy (after 13 days) and was usually self-limiting (often resolving within 8 days), but sometimes (in 2% of people) required discontinuation of misoprostol.[56]
The next most commonly reported adverse effects of taking misoprostol by mouth for the prevention of gastric ulcers are: abdominal pain, nausea, flatulence, headache, dyspepsia, vomiting, and constipation, but none of these adverse effects occurred more often than when taking placebos.[56]
There are increased side effects with sublingual or oral misoprostol, compared to a low dose (400 μg) vaginal misoprostol. However, low dose vaginal misoprostol was linked with low complete abortion rate.[43] The study concluded that sublingually administered misoprostol dosed at 600 μg or 400 μg had greater instances of fever and diarrhea due to its quicker onset of action, higher peak concentration and bioavailability in comparison to vaginal or oral misoprostol.[43]
For the indication of medical abortion, bleeding and cramping is commonly experienced after administration of misoprostol. Bleeding and cramping is likely to be greater than that experienced with menses, however, emergency care is advised if bleeding is excessive.[44]
Misoprostol should not be taken by pregnant women with wanted pregnancies to reduce the risk of NSAID-induced gastric ulcers because it increases uterine tone and contractions in pregnancy, which may cause partial or complete abortions, and because its use in pregnancy has been associated with birth defects.[56][57]
All cervical ripening and induction agents can cause uterine hyperstimulation, which can negatively affect the blood supply to the fetus and increases the risk of complications such as uterine rupture.[58] Concern has been raised that uterine hyperstimulation that occurs during a misoprostol-induced labor is more difficult to treat than hyperstimulation during labors induced by other drugs.[59] Because the complications are rare, it is difficult to determine if misoprostol causes a higher risk than do other cervical ripening agents. One estimate is that it would require around 61,000 people enrolled in randomized controlled trials to detect a difference in serious fetal complications and about 155,000 people to detect a difference in serious maternal complications.[60]
Contraindications
[edit]It is recommended that medical treatment for missed abortion with misoprostol should only be considered in people without the following contraindications: suspected ectopic pregnancy, use of non-steroidal drugs, signs of pelvic infections or sepsis, unstable hemodynamics, known allergy to misoprostol, previous caesarean section, mitral stenosis, hypertension, glaucoma, bronchial asthma, and remote areas without a hospital nearby.[43]
Pharmacology
[edit]Mechanism of action
[edit]Misoprostol, a prostaglandin analogue, binds to myometrial cells to cause strong myometrial contractions leading to expulsion of tissue. This agent also causes cervical ripening with softening and dilation of the cervix. Misoprostol binds to and stimulates prostaglandin EP2 receptors, prostaglandin EP3 receptor and prostaglandin EP4 receptor but not prostaglandin EP1 receptor and therefore is expected to have a more restricted range of physiological and potentially toxic actions than prostaglandin E2 or other analogs which activate all four prostaglandin receptors.[61]
Society and culture
[edit]In August 2000, a letter from G.D. Searle, LLC, the inventor of the drug,[62][63] generated controversy by warning against its use by pregnant women because of its ability to induce abortion, citing reports of maternal and fetal deaths when it was used to induce labor.[64] The American College of Obstetricians and Gynecologists holds that substantial evidence supports the use of misoprostol for induction of labor, a position it reaffirmed in response to the Searle letter.[65] It is on the World Health Organization's List of Essential Medicines.[21]
A vaginal form of the medication is sold in the EU under the names Misodel[66] and Mysodelle[67] for use in labor induction.[medical citation needed]
Black market
[edit]Misoprostol is used for self-induced abortions in Brazil, where black market prices exceed US$100 per dose. Illegal medically unsupervised misoprostol abortions in Brazil are associated with a lower complication rate than other forms of illegal self-induced abortion, but are still associated with a higher complication rate than legal, medically supervised surgical and medical abortions. Failed misoprostol abortions are associated with birth defects in some cases.[68][69][70][71][72] Low-income and immigrant populations in New York City have also been observed to use self-administered misoprostol to induce abortions, as this method is much cheaper than a surgical abortion (about $2 per dose).[73] The drug is readily available in Mexico.[74] Use of misoprostol has also increased in Texas in response to increased regulation of abortion providers.[75] Following the United States Supreme Court decision of Dobbs v. Jackson Women's Health Organization, many states restricted access to legal abortion services, including medication abortion using misoprostol. As a result of these restrictions, it was reported that there was an increase in self-managed abortions by women in the United States. Many women purchased the pills from overseas online pharmacies or obtained misoprostol from Mexico.[76]
References
[edit]- ^ "TGA eBS - Product and Consumer Medicine Information Licence". Archived from the original on 14 April 2023. Retrieved 14 April 2023.
- ^ "TGA eBS - Product and Consumer Medicine Information Licence". Archived from the original on 14 April 2023. Retrieved 14 April 2023.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Angusta (Norgine Pty Ltd)". Therapeutic Goods Administration (TGA). 13 January 2023. Archived from the original on 18 March 2023. Retrieved 9 April 2023.
- ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 15 August 2023.
- ^ "Angusta 25 microgram tablets - Summary of Product Characteristics (SmPC)". (emc). 8 April 2022. Archived from the original on 10 April 2023. Retrieved 9 April 2023.
- ^ "Cytotec- misoprostol tablet". DailyMed. 9 July 2021. Archived from the original on 18 April 2023. Retrieved 13 April 2023.
- ^ "List of nationally authorised medicinal products. Misoprostol (gastrointestinal indication). Procedure no.: PSUSA/00010353/202005" (PDF). European Medicines Agency. 14 January 2021. Archived (PDF) from the original on 29 June 2022. Retrieved 8 August 2021.
- ^ "List of nationally authorised medicinal products. Misoprostol (gastrointestinal indication). Procedure no.: PSUSA/00010291/202006" (PDF). European Medicines Agency. 14 January 2021. Archived (PDF) from the original on 29 June 2022. Retrieved 8 August 2021.
- ^ Rostom A, Dube C, Wells G, Tugwell P, Welch V, Jolicoeur E, et al. (2002). "Prevention of NSAID-induced gastroduodenal ulcers". The Cochrane Database of Systematic Reviews. 2011 (4): CD002296. doi:10.1002/14651858.CD002296. PMC 8439413. PMID 12519573. S2CID 1052260.
- ^ a b c d e f g "Misoprostol". The American Society of Health-System Pharmacists. Archived from the original on 21 February 2015. Retrieved 20 February 2015.
- ^ a b Zhang J, Zhou K, Shan D, Luo X (May 2022). "Medical methods for first trimester abortion". The Cochrane Database of Systematic Reviews. 2022 (5): CD002855. doi:10.1002/14651858.CD002855.pub5. PMC 9128719. PMID 35608608.
- ^ Bryant AG, Regan E, Stuart G (January 2014). "An overview of medical abortion for clinical practice". Obstetrical & Gynecological Survey. 69 (1): 39–45. doi:10.1097/OGX.0000000000000017. PMID 25102250. S2CID 28486936.
- ^ Raymond EG, Harrison MS, Weaver MA (January 2019). "Efficacy of Misoprostol Alone for First-Trimester Medical Abortion: A Systematic Review". Obstetrics and Gynecology. 133 (1): 137–147. doi:10.1097/AOG.0000000000003017. PMC 6309472. PMID 30531568.
- ^ Marret H, Simon E, Beucher G, Dreyfus M, Gaudineau A, Vayssière C, et al. (April 2015). "Overview and expert assessment of off-label use of misoprostol in obstetrics and gynaecology: review and report by the Collège national des gynécologues obstétriciens français". European Journal of Obstetrics, Gynecology, and Reproductive Biology. 187: 80–4. doi:10.1016/j.ejogrb.2015.01.018. PMID 25701235.
- ^ Prager S. "Early Pregnancy Loss" (PDF). ACOG Practice Bulletin (200). ACOG. Archived (PDF) from the original on 2 June 2021. Retrieved 2 June 2021.
- ^ a b c American College of Obstetricians Gynecologists' Committee on Practice Bulletins—Gynecology (November 2018). "ACOG Practice Bulletin No. 200: Early Pregnancy Loss". Obstetrics and Gynecology. 132 (5): e197–e207. doi:10.1097/AOG.0000000000002899. PMID 30157093. S2CID 13149908.
- ^ a b c "Early Pregnancy Loss". ACOG. 20 January 2015. Archived from the original on 19 January 2023. Retrieved 25 June 2023.
- ^ Blum J, Alfirevic Z, Walraven G, Weeks A, Winikoff B (December 2007). "Treatment of postpartum hemorrhage with misoprostol". International Journal of Gynaecology and Obstetrics. 99 (Suppl 2): S202-5. doi:10.1016/j.ijgo.2007.09.013. PMID 17961565. S2CID 10997666.
- ^ Paul M (2011). "Misoprostol". Management of Unintended and Abnormal Pregnancy: Comprehensive Abortion Care. John Wiley & Sons. ISBN 9781444358476. Archived from the original on 29 June 2022. Retrieved 18 August 2020.
- ^ a b World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ Hawkey CJ, Karrasch JA, Szczepañski L, Walker DG, Barkun A, Swannell AJ, et al. (March 1998). "Omeprazole compared with misoprostol for ulcers associated with nonsteroidal antiinflammatory drugs. Omeprazole versus Misoprostol for NSAID-induced Ulcer Management (OMNIUM) Study Group". The New England Journal of Medicine. 338 (11): 727–34. doi:10.1056/NEJM199803123381105. hdl:2445/178575. PMID 9494149.
- ^ Goldberg AB, Greenberg MB, Darney PD (January 2001). "Misoprostol and pregnancy". The New England Journal of Medicine. 344 (1): 38–47. doi:10.1056/NEJM200101043440107. PMID 11136959.
- ^ a b Summers L (1997). "Methods of cervical ripening and labor induction". Journal of Nurse-Midwifery. 42 (2): 71–85. doi:10.1016/S0091-2182(96)00138-3. PMID 9107114.
- ^ "Misodel, Ferring's Removable Misoprostol Vaginal Delivery System, Approved for Labour Induction in European Decentralised Procedure" (Press release). Ferring Pharmaceuticals. 17 October 2013. Retrieved 25 June 2023 – via Business Wire.
- ^ Wing DA, Brown R, Plante LA, Miller H, Rugarn O, Powers BL (August 2013). "Misoprostol vaginal insert and time to vaginal delivery: a randomized controlled trial". Obstetrics and Gynecology. 122 (2 Pt 1): 201–9. doi:10.1097/AOG.0b013e31829a2dd6. PMID 23857539. S2CID 31090241.
- ^ Varlas VN, Bostan G, Nasui BA, Bacalbasa N, Pop AL (April 2021). "Is Misoprostol Vaginal Insert Safe for the Induction of Labor in High-Risk Pregnancy Obese Women?". Healthcare. 9 (4): 464. doi:10.3390/healthcare9040464. PMC 8070889. PMID 33919898.
- ^ "Safety Information, Cytotec (misoprostol) Tablets". U.S. Food and Drug Administration (FDA). 11 December 2012. Archived from the original on 20 January 2017. Retrieved 16 March 2017.
- ^ "Misoprostol (marketed as Cytotec) Information". U.S. Food and Drug Administration (FDA). 10 July 2015. Archived from the original on 27 May 2020. Retrieved 17 August 2020.
- ^ Wali S, Balfoussia D, Touqmatchi D, Quinn S (February 2021). "Misoprostol for open myomectomy: a systematic review and meta-analysis of randomised control trials". BJOG. 128 (3): 476–483. doi:10.1111/1471-0528.16389. PMID 32613769. S2CID 220307235.
- ^ "Medical methods for first trimester abortion". World Health Organization (WHO). Archived from the original on 14 February 2016. Retrieved 4 February 2016.
- ^ Harvey SM, Beckman LJ, Castle MA, Coeytaux F (1 October 1995). "Knowledge and perceptions of medical abortion among potential users". Family Planning Perspectives. 27 (5): 203–7. doi:10.2307/2136276. JSTOR 2136276. PMID 9104607. S2CID 30491792.
- ^ Bazelon E (28 August 2014). "The Dawn of the Post-Clinic Abortion". The New York Times. ISSN 0362-4331. Archived from the original on 28 April 2016. Retrieved 4 February 2016.
- ^ "Medical methods for first trimester abortion". The WHO Medical Reproductive Library. Archived from the original on 2 August 2014. Retrieved 22 June 2014.
- ^ Safe abortion : technical and policy guidance for health systems (Second ed.). Geneva: World Health Organization. 2012. ISBN 9789241548434. OCLC 812323067.
- ^ "Mifepristone". The American Society of Health System Pharmacists. 18 January 2023. Archived from the original on 22 December 2015. Retrieved 25 February 2023.
- ^ What is the "Mexican abortion pill" and how safe is it? Archived 30 July 2013 at the Wayback Machine Jen Gunter, 27 July 2013
- ^ "Annotated Bibliography on Misoprostol Alone for Early Abortion" (PDF). Gynuity Health Projects. Archived (PDF) from the original on 29 September 2007. Retrieved 22 August 2006.
- ^ "Annotated Bibliography - Misoprostol for Early Abortion". Gynuity Health Projects. 1 September 2004. Archived from the original on 18 May 2022. Retrieved 25 June 2023.
- ^ Providing medical abortion in low-resource settings (PDF) (2 ed.). Gynuity Health Projects. 2009. p. 4. Archived (PDF) from the original on 22 February 2016. Retrieved 31 August 2015.
- ^ "Instructions for Use: Abortion Induction with Misoprostol in Pregnancies up to 9 Weeks LMP" (PDF). Gynuity Health Projects. 2003. Archived (PDF) from the original on 29 September 2007. Retrieved 24 August 2006.
- ^ Rasheedy R, Tamara TF, Allam IS, Abbas AM, Essam El-Din Abd El Salam N, Ferhad Ahmed A (June 2019). "Vaginal misoprostol before copper IUD insertion after previous insertion failure: a double-blind, placebo-controlled, parallel-group, randomised clinical trial". The European Journal of Contraception & Reproductive Health Care. 24 (3): 222–226. doi:10.1080/13625187.2019.1610871. PMID 31112079. S2CID 160014019. (Retracted, see doi:10.1080/13625187.2023.2171653, PMID 36744398)
- ^ a b c d e Wu HL, Marwah S, Wang P, Wang QM, Chen XW (May 2017). "Misoprostol for medical treatment of missed abortion: a systematic review and network meta-analysis". Scientific Reports. 7 (1): 1664. Bibcode:2017NatSR...7.1664W. doi:10.1038/s41598-017-01892-0. PMC 5431938. PMID 28490770.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License Archived 16 October 2017 at the Wayback Machine.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License Archived 16 October 2017 at the Wayback Machine.
- ^ a b American College of Obstetricians Gynecologists (March 2014). "Practice bulletin no. 143: medical management of first-trimester abortion". Obstetrics and Gynecology. 123 (3): 676–92. doi:10.1097/01.AOG.0000444454.67279.7d. PMID 24553166. S2CID 23951273.
- ^ Lemmers M, Verschoor MA, Kim BV, Hickey M, Vazquez JC, Mol BW, et al. (June 2019). "Medical treatment for early fetal death (less than 24 weeks)". The Cochrane Database of Systematic Reviews. 2019 (6): CD002253. doi:10.1002/14651858.CD002253.pub4. PMC 6574399. PMID 31206170.
- ^ Villar J, Gülmezoglu AM, Hofmeyr GJ, Forna F (December 2002). "Systematic review of randomized controlled trials of misoprostol to prevent postpartum hemorrhage". Obstetrics and Gynecology. 100 (6): 1301–12. doi:10.1016/S0029-7844(02)02371-2. PMID 12468178. S2CID 24561459.
- ^ O'Brien P, El-Refaey H, Gordon A, Geary M, Rodeck CH (August 1998). "Rectally administered misoprostol for the treatment of postpartum hemorrhage unresponsive to oxytocin and ergometrine: a descriptive study". Obstetrics and Gynecology. 92 (2): 212–4. doi:10.1016/S0029-7844(98)00161-6. PMID 9699753.
- ^ Lokugamage AU, Sullivan KR, Niculescu I, Tigere P, Onyangunga F, El Refaey H, et al. (September 2001). "A randomized study comparing rectally administered misoprostol versus Syntometrine combined with an oxytocin infusion for the cessation of primary post partum hemorrhage". Acta Obstetricia et Gynecologica Scandinavica. 80 (9): 835–9. doi:10.1034/j.1600-0412.2001.080009835.x. PMID 11531635. S2CID 23335277.
- ^ Bradley SE, Prata N, Young-Lin N, Bishai DM (April 2007). "Cost-effectiveness of misoprostol to control postpartum hemorrhage in low-resource settings". International Journal of Gynaecology and Obstetrics. 97 (1): 52–6. doi:10.1016/j.ijgo.2006.12.005. PMID 17316646. S2CID 23593148.
- ^ Derman RJ, Kodkany BS, Goudar SS, Geller SE, Naik VA, Bellad MB, et al. (October 2006). "Oral misoprostol in preventing postpartum haemorrhage in resource-poor communities: a randomised controlled trial". Lancet. 368 (9543): 1248–53. doi:10.1016/S0140-6736(06)69522-6. PMID 17027730. S2CID 22275092.
- ^ Sanghvi H, Zulkarnain M, Chanpong G (2009). Blouse A, Lewison D (eds.). Prevention of Postpartum Hemorrhage at Home Birth: A Program Implementation Guide (PDF). United States Agency for International Development. Archived (PDF) from the original on 6 December 2013.[page needed]
- ^ Prata N, Passano P, Bell S, Rowen T, Potts M (July 2013). "New hope: community-based misoprostol use to prevent postpartum haemorrhage". Health Policy and Planning. 28 (4): 339–46. doi:10.1093/heapol/czs068. PMID 22879523.
- ^ Tassi A, Parisi N, Londero AP (February 2020). "Misoprostol administration prior to intrauterine contraceptive device insertion: a systematic review and meta-analysis of randomised controlled trials". Eur J Contracept Reprod Health Care. 25 (1): 76–86. doi:10.1080/13625187.2019.1706079. PMID 31914331. S2CID 210120219. Archived from the original on 29 June 2022. Retrieved 21 June 2022.
- ^ DMKG study group (May 2003). "Misoprostol in the treatment of trigeminal neuralgia associated with multiple sclerosis". Journal of Neurology. 250 (5): 542–545. doi:10.1007/s00415-003-1032-1. PMID 12736732. S2CID 36493785.
- ^ Di Stefano G, Truini A, Cruccu G (September 2018). "Current and Innovative Pharmacological Options to Treat Typical and Atypical Trigeminal Neuralgia". Drugs. 78 (14): 1433–1442. doi:10.1007/s40265-018-0964-9. PMC 6182468. PMID 30178160.
- ^ a b c "Cytotec- misoprostol tablet". DailyMed. 9 July 2021. Archived from the original on 18 April 2023. Retrieved 25 June 2023.
- ^ "Cytotec 200mcg Tablets - Summary of Product Characteristics (SmPC)". (emc). 26 April 2021. Archived from the original on 1 July 2022. Retrieved 25 June 2023.
- ^ Briggs GG, Wan SR (June 2006). "Drug therapy during labor and delivery, part 2". American Journal of Health-System Pharmacy. 63 (12): 1131–9. doi:10.2146/ajhp050265.p2. PMID 16754739.
- ^ Wagner M (2006). Born in the USA: how a broken maternity system must be fixed to put mothers and infants first. Berkeley: University of California Press. ISBN 0-520-24596-2., which cites:
- Wing DA, Paul RH (July 1996). "A comparison of differing dosing regimens of vaginally administered misoprostol for preinduction cervical ripening and labor induction". American Journal of Obstetrics and Gynecology. 175 (1): 158–64. doi:10.1016/s0002-9378(96)70267-3. PMID 8694043.
- Wing DA, Rahall A, Jones MM, Goodwin TM, Paul RH (June 1995). "Misoprostol: an effective agent for cervical ripening and labor induction". American Journal of Obstetrics and Gynecology. 172 (6): 1811–6. doi:10.1016/0002-9378(95)91416-1. PMID 7778637.
- ^ Goldberg & Wing 2003, which cites:
- ^ Moreno JJ (February 2017). "Eicosanoid receptors: Targets for the treatment of disrupted intestinal epithelial homeostasis". European Journal of Pharmacology. 796: 7–19. doi:10.1016/j.ejphar.2016.12.004. PMID 27940058. S2CID 1513449.
- ^ Tyman JH (January 1999). Lipids in Health and Nutrition. Elsevier. ISBN 9781845698386. Archived from the original on 29 June 2022. Retrieved 10 May 2022.
- ^ Featherstone L (June 2016). False Choices: The Faux Feminism of Hillary Rodham Clinton. Verso Books. ISBN 9781784784638. Archived from the original on 29 June 2022. Retrieved 10 May 2022.
- ^ Goldberg AB, Wing DA (2003). "Induction of labor: the misoprostol controversy". Journal of Midwifery & Women's Health. 48 (4): 244–8. doi:10.1016/S1526-9523(03)00087-4. PMID 12867908.
- ^ Goldberg & Wing 2003, which cites:
- American College of Obstetricians and Gynecologists (November 1999). "Induction of labor with misoprostol". ACOG Committee Opinion No. 228. Washington, DC.
- American College of Obstetricians and Gynecologists (November 1999). "Response to Searle's drug warning on misoprostol". ACOG Committee Opinion No. 248. Washington, DC.
- ^ "List of nationally authorized medicinal products" (PDF). Archived (PDF) from the original on 2 November 2021. Retrieved 29 June 2022.
- ^ "List of nationally authorised medicinal products" (PDF). Archived (PDF) from the original on 3 March 2022. Retrieved 26 June 2023.
- ^ Costa SH, Vessey MP (May 1993). "Misoprostol and illegal abortion in Rio de Janeiro, Brazil". Lancet. 341 (8855): 1258–61. doi:10.1016/0140-6736(93)91156-G. PMID 8098402. S2CID 30907894.
- ^ Coêlho HL, Teixeira AC, Cruz M, Gonzaga SL, Arrais PS, Luchini L, et al. (February 1994). "Misoprostol: the experience of women in Fortaleza, Brazil". Contraception. 49 (2): 101–10. doi:10.1016/0010-7824(94)90084-1. PMID 8143449.
- ^ Barbosa RM, Arilha M (1993). "The Brazilian experience with Cytotec". Studies in Family Planning. 24 (4): 236–40. doi:10.2307/2939191. JSTOR 2939191. PMID 8212093.
- ^ Rocha J (September 1994). "Brazil investigates drug's possible link with birth defects". BMJ. 309 (6957): 757–8. doi:10.1136/bmj.309.6957.757a. PMC 2540993. PMID 7950553.
- ^ Gonzalez CH, Vargas FR, Perez AB, Kim CA, Brunoni D, Marques-Dias MJ, et al. (August 1993). "Limb deficiency with or without Möbius sequence in seven Brazilian children associated with misoprostol use in the first trimester of pregnancy". American Journal of Medical Genetics. 47 (1): 59–64. doi:10.1002/ajmg.1320470113. PMID 8368254.
- ^ Leland J (2 October 2005). "Abortion Might Outgrow Its Need for Roe v. Wade". The New York Times. Archived from the original on 18 May 2013. Retrieved 6 March 2014.
- ^ Eckholm E (13 July 2013). "In Mexican Pill, a Texas Option for an Abortion". The New York Times. Archived from the original on 14 July 2013. Retrieved 14 July 2013.
- ^ Hellenstein E (27 June 2014). "The Rise of the DIY Abortion in Texas". The Atlantic. Archived from the original on 2 March 2017.
- ^ Sanger-Katz M, Miller CC (30 October 2022). "Legal Abortions Fell Around 6 Percent in Two Months After End of Roe". The New York Times. ISSN 0362-4331. Archived from the original on 8 February 2023. Retrieved 8 February 2023.
