Lupus: Difference between revisions
Adding HMGB1 with citation, also a recent finding, was pub in 2009 Feb 27. |
adding Renal transplantation with possible recurrence, with a citation to a recent research, pub in 2009 Feb 27 |
||
| Line 199: | Line 199: | ||
Fasting and massive nutrition changes, towards a low fat, mostly strict vegetarian, wholesome diet, also have been reported as a possibility to lessen the symptoms or even induce a remission.<ref>[http://www.drmcdougall.com/stars/mayra.html Mayra. Almost Lost to Lupus. As Told by Her Father, Victor Dengler]</ref> |
Fasting and massive nutrition changes, towards a low fat, mostly strict vegetarian, wholesome diet, also have been reported as a possibility to lessen the symptoms or even induce a remission.<ref>[http://www.drmcdougall.com/stars/mayra.html Mayra. Almost Lost to Lupus. As Told by Her Father, Victor Dengler]</ref> |
||
===Renal transplantation=== |
|||
is the treatment of choice for end-stage renal disease, which is one of the complications of [[lupus nephritis]], but the recurrence of the full disease is common in up to 30% of patients.<ref name="pmid19247694">{{cite journal |author=Cochat P, Fargue S, Mestrallet G, ''et al'' |title=Disease recurrence in paediatric renal transplantation |journal=[[Pediatr. Nephrol.]] |volume= |issue= |pages= |year=2009 |month=February |pmid=19247694 |doi=10.1007/s00467-009-1137-6 |url=http://dx.doi.org/10.1007/s00467-009-1137-6 |issn=}}</ref> |
|||
==Prevention== |
==Prevention== |
||
Revision as of 15:28, 7 March 2009
| Lupus | |
|---|---|
| Specialty | Immunology, rheumatology, dermatology |
Systemic lupus erythematosus (SLE or lupus, pronounced ) is a chronic autoimmune connective tissue disease that can affect any part of the body. As occurs in other autoimmune diseases, the immune system attacks the body’s cells and tissue, resulting in inflammation and tissue damage.[1]
SLE most often harms the heart, joints, skin, lungs, blood vessels, liver, kidneys, and nervous system. The course of the disease is unpredictable, with periods of illness (called flares) alternating with remissions. The disease occurs nine times more often in women than in men, especially between the ages of 15 and 50, and is more common in those of non-European descent.[2][3][4]
SLE is treatable through addressing its symptoms, mainly with corticosteroids and immunosuppressants; there is currently no cure. SLE can be fatal, although with recent medical advances, fatalities are becoming increasingly rare. Survival for people with SLE in the United States, Canada, and Europe is approximately 95% at five years, 90% at 10 years, and 78% at 20 years.[4]
Classification

There are several types of lupus; in general, when the word lupus alone is used, reference is to systemic lupus erythematosus, as discussed in this article. Other types include:[1]
- Chronic cutaneous lupus erythematosus
- Discoid lupus erythematosus, a skin disorder that causes a red, raised rash on the face and scalp. Discoid lupus occasionally (1–5%) develops into SLE.[5]
- Verrucous lupus erythematosus (Hypertrophic lupus erythematosus)
- Lupus erythematosus-lichen planus overlap syndrome
- Chilblain lupus erythematosus (Hutchinson)
- Tumid lupus erythematosus
- Lupus erythematosus panniculitis (Lupus erythematosus profundus)
- Subacute cutaneous lupus erythematosus, which causes nonscarring skin lesions on patches of skin exposed to sunlight.[6]
- Neonatal lupus erythematosus, a rare disease affecting babies born to women with SLE, Sjögren's syndrome, or sometimes no autoimmune disorder. It is theorized that maternal antibodies attack the fetus, causing skin rash; liver problems; low blood counts, which gradually fade; and heart block, leading to bradycardia.[6]
- Childhood systemic lupus erythematosus, the pediatric variant of systemic lupus erythematosus.
- Drug-induced lupus erythematosus, a drug-induced form of SLE; this type of lupus can occur equally in either sex.
- Lupus nephritis, an inflammation of the kidneys caused by SLE.
- Complement deficiency syndromes
Signs and symptoms
SLE is one of several diseases known as "the great imitators" because it often mimics or is mistaken for other illnesses.[7] SLE is a classical item in differential diagnosis,[2] because SLE symptoms vary widely and come and go unpredictably. Diagnosis can thus be elusive, with some people suffering unexplained symptoms of untreated SLE for years.
Common initial and chronic complaints are fever, malaise, joint pains, myalgias, fatigue, and temporary loss of cognitive abilities. Because they are so often seen with other diseases, these signs and symptoms are not part of the diagnostic criteria for SLE. When occurring in conjunction with other signs and symptoms (see below), however, they are considered suggestive.[8]
- Dermatological manifestations

As many as 30% of sufferers have some dermatological symptoms (and 65% suffer such symptoms at some point), with 30% to 50% suffering from the classic malar rash (or butterfly rash) associated with the disease. Some may exhibit thick, red scaly patches on the skin (referred to as discoid lupus). Alopecia; mouth, nasal, and vaginal ulcers; and lesions on the skin are also possible manifestations.
- Musculoskeletal manifestations
The most commonly sought medical attention is for joint pain, with the small joints of the hand and wrist usually affected, although all joints are at risk. The Lupus Foundation of America estimates that more than 90 percent will experience joint and/or muscle pain at some time during the course of their illness.[9] Unlike rheumatoid arthritis, lupus arthritis is less disabling and usually does not cause severe destruction of the joints. Fewer than ten percent of people with lupus arthritis will develop deformities of the hands and feet.[9]
it is suggested that there might be an assiciation between rheumatoid arthritis and SLE.[10]
- Hematological manifestations
Anemia and iron deficiency may develop in up to 50% of case. Low platelet and white blood cell counts may be due to the disease or a side-effect of pharmacological treatment. People with SLE may have an association with antiphospholipid antibody syndrome (a thrombotic disorder), wherein autoantibodies to phospholipids are present in their serum. Abnormalities associated with antiphospholipid antibody syndrome include a paradoxical prolonged PTT (which usually occurs in hemorrhagic disorders) and a positive test for antiphospholipid antibodies; the combination of such findings have earned the term lupus anticoagulant-positive. Another autoantibody finding in SLE is the anticardiolipin antibody, which can cause a false positive test for syphilis.
- Cardiac manifestations
A person with SLE may have inflammation of various parts of the heart, such as pericarditis, myocarditis, and endocarditis. The endocarditis of SLE is characteristically noninfective (Libman-Sacks endocarditis) and involves either the mitral valve or the tricuspid valve. Atherosclerosis also tends to occur more often and advances more rapidly than in the general population.[11][12][13]
- Pulmonary manifestations
Lung and pleura inflammation can cause pleuritis, pleural effusion, lupus pneumonitis, chronic diffuse interstitial lung disease, pulmonary hypertension, pulmonary emboli, pulmonary hemorrhage, and shrinking lung syndrome.
- Renal involvement
Painless hematuria or proteinuria may often be the only presenting renal symptom. Acute or chronic renal impairment may develop with lupus nephritis, leading to acute or end-stage renal failure. Because of early recognition and management of SLE, end-stage renal failure occurs in less than 5% of cases.
A histological hallmark of SLE is membranous glomerulonephritis with "wire loop" abnormalities.[14] This finding is due to immune complex deposition along the glomerular basement membrane, leading to a typical granular appearance in immunofluorescence testing.
- Neuropsychiatric manifestations
Neuropsychiatric syndromes can result when SLE affects the central or peripheral nervous system. The American College of Rheumatology defines 19 neuropsychiatric syndromes in systemic lupus erythematosus.[15] The most common neuropsychiatric disorder people with SLE have is headache, although the existence of a specific lupus headache and the optimal approach to headache in SLE cases remains controversial.[16] Other common neuropsychiatric manifestation of SLE include cognitive dysfunction, mood disorder, cerebrovascular disease, seizures, polyneuropathy, anxiety disorder and psychosis.
More rare manifestations are acute confusional state, Guillain-Barré syndrome, aseptic meningitis, autonomic disorder, demyelinating syndrome, mononeuropathy (which might manifest as mononeuritis multiplex), movement disorder (more specifically, chorea), myasthenia gravis, myelopathy, cranial neuropathy and plexopathy.
- Systemic manifestations
Fatigue in SLE is probably multifactorial and has been related not only to disease activity or complications such as anemia or hypothyroidism but also to pain, depression, poor sleep quality, poor physical fitness and perceived lack of social support.[17][18]
- Other rarer manifestations
Lupus gastroenteritis, lupus pancreatitis[19], lupus cystitis, autoimmune inner ear disease, parasympathetic dysfunction, retinal vasculitis[citation needed], hemophagocytic syndrome[20], systemic vasculitis[21], and like many autoimmune disease can be complicated by myeloid malignancies.[22]
Causes
There is no one specific cause of SLE. There are however a number of environmental triggers and a number of genetic susceptibilities.[23][24]
Genetics
The first mechanism may arise genetically. Research indicates that SLE may have a genetic link. SLE does run in families, but no single, causal, gene has been identified. Instead, multiple genes appear to influence a person's chance of developing lupus when triggered by environmental factors. The most important genes are located in the HLA region on chromosome 6, where mutations may occur randomly (de novo) or may be inherited. HLA class I, class II, and class III are associated with SLE, but only class I and class II contribute independently to increased risk of SLE.[25] Other genes which contain risk variants for SLE are IRF5, PTPN22, STAT4, CDKN1A,[26] ITGAM, BLK, TNFSF4 and BANK1.[27]
Environmental triggers
The second mechanism may be due to environmental factors. These factors may not only exacerbate existing SLE conditions but also trigger the initial onset. They include certain medications (such as some antidepressants and antibiotics), extreme stress, exposure to sunlight, hormones, and infections. UV radiation has been shown to trigger the photosensitive lupus rash and some evidence suggests that UV light might be capable of altering the structure of the DNA, leading to the creation of autoantibodies. Sex hormones such as estrogen play an important role in the occurrence of SLE and it is observed that during reproductive years, the frequency of SLE is 10 times greater in females than in males. Researchers have sought to find a connection between certain infectious agents (viruses and bacteria), but no pathogen can be consistently linked to the disease. Some researchers have found that women with silicone gel-filled breast implants have produced antibodies to their own collagen, but it is not known how often these antibodies occur in the general population, and there is no data that show that these antibodies cause connective tissue diseases such as SLE. There is also a small but growing body of evidence linking SLE to lipstick usage,[28] [29] although lipstick manufacturers do not appear to be overly concerned about it.[30]
Drug reactions
Drug-induced lupus erythematosus is a reversible condition that usually occurs in people being treated for a long-term illness. Drug-induced lupus mimics SLE. However, symptoms of drug-induced lupus generally disappear once the medication that triggered the episode is stopped. There are about 400 medications that can cause this condition, the most common of which are procainamide, hydralazine, quinidine, and phenytoin.[2]
Non-SLE forms of lupus
Other forms of lupus can predispose to SLE. About 1–5% of cases of discoid lupus eventually develop into SLE.[citation needed] Discoid (cutaneous) lupus is limited to skin symptoms and is diagnosed by biopsy of skin rash on the face, neck, or scalp. Often an antinuclear antibody (ANA) test for discoid lupus is negative or a low-titer positive.[citation needed]
Pathophysiology
One manifestation of SLE is abnormalities in apoptosis, a type of programmed cell death in which aging or damaged cells are neatly disposed of as a part of normal growth or functioning.
Transmission
In SLE, the body's immune system produces antibodies against itself, particularly against proteins in the cell nucleus. SLE is triggered by environmental factors that are unknown.
"All the key components of the immune system are involved in the underlying mechanisms" of SLE, according to Rahman, and SLE is the prototypical autoimmune disease. The immune system must have a balance (homeostasis) between being sensitive enough to protect against infection, and being too sensitive and attacking the body's own proteins (autoimmunity). From an evolutionary perspective, according to Crow, the population must have enough genetic diversity to protect itself against a wide range of possible infection; some genetic combination's result in autoimmunity. The likely environmental triggers include ultraviolet light, drugs, and viruses. These stimuli cause the destruction of cells and expose their DNA, histones, and other proteins, particularly parts of the cell nucleus. Because of genetic variations in different components of the immune system, in some people the immune system attacks these nuclear-related proteins and produces antibodies against them. In the end, these antibody complexes damage blood vessels in critical areas of the body, such as the glomeruli of the kidney; these antibody attacks are the cause of SLE. Researchers are now identifying the individual genes, the proteins they produce, and their role in the immune system. Each protein is a link on the autoimmune chain, and researchers are trying to find drugs to break each of those links. [2][31][32]
SLE is a chronic inflammatory disease believed to be a type III hypersensitivity response with potential type II involvement.[33]
Abnormalities in apoptosis
- Apoptosis is increased in monocytes and keratinocytes
- Expression of Fas by B cells and T cells is increased
- There are correlations between the apoptotic rates of lymphocytes and disease activity.
Tingible body macrophages (TBMs) are large phagocytic cells in the germinal centers of secondary lymph nodes; they express CD68 protein. These cells normally engulf B cells that have undergone apoptosis after somatic hypermutation. In some people with SLE, significantly fewer TBMs can be found, and these cells rarely contain material from apoptotic B cells. Also, uningested apoptotic nuclei can be found outside of TBMs. This material may present a threat to the tolerization of B cells and T cells. Dendritic cells in the germinal center may endocytose such antigenic material and present it to T cells, activating them. Also, apoptotic chromatin and nuclei may attach to the surfaces of follicular dendritic cells and make this material available for activating other B cells that may have randomly acquired self-specificity through somatic hypermutation.[34]
Clearance deficiency

The exact mechanisms for the development of SLE are still unclear, since the pathogenesis is a multifactorial event. Beside discussed causation's, impaired clearance of dying cells is a potential pathway for the development of this systemic autoimmune disease. This includes deficient phagocytic activity and scant serum components in addition to increased apoptosis.
Monocytes isolated from whole blood of SLE sufferers show reduced expression of CD44 surface molecules involved in the uptake of apoptotic cells. Most of the monocytes and tingible body macrophages (TBM), which are found in the germinal centres of lymph nodes, even show a definitely different morphology; they are smaller or scarce and die earlier. Serum components like complement factors, CRP, and some glycoproteins are, furthermore, decisively important for an efficiently operating phagocytosis. With SLE, these components are often missing, diminished, or inefficient.
The clearance of early apoptotic cells is an important function in multicellular organisms. It leads to a progression of the apoptosis process and finally to secondary necrosis of the cells if this ability is disturbed. Necrotic cells release nuclear fragments as potential autoantigens as well as internal danger signals, inducing maturation of dendritic cells (DC), since they have lost their membranes' integrity. Increased appearance of apoptotic cells also simulates inefficient clearance. That leads to maturation of DC and also to the presentation of intracellular antigens of late apoptotic or secondary necrotic cells, via MHC molecules. Autoimmunity possibly results by the extended exposure to nuclear and intracellular autoantigens derived from late apoptotic and secondary necrotic cells. B and T cell tolerance for apoptotic cells is abrogated, and the lymphocytes get activated by these autoantigens; inflammation and the production of autoantibodies by plasma cells is initiated. A clearance deficiency in the skin for apoptotic cells has also been observed in people with cutaneous lupus erythematosus (CLE).[35]
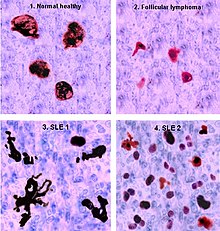
Accumulation in germinal centres (GC)
In healthy conditions, apoptotic lymphocytes are removed in germinal centres by specialised phagocytes, the tingible body macrophages (TBM), which is why no free apoptotic and potential autoantigenic material can be seen. In some people with SLE, accumulation of apoptotic debris can be observed in GC because of an ineffective clearance of apoptotic cells. In close proximity to TBM, follicular dendritic cells (FDC) are localised in GC, which attach antigen material to their surface and, in contrast to bone marrow-derived DC, neither take it up nor present it via MHC molecules. Autoreactive B cells can accidentally emerge during somatic hypermutation and migrate into the GC light zone. Autoreactive B cells, maturated coincidentally, normally do not receive survival signals by antigen planted on follicular dendritic cells, and perish by apoptosis. In the case of clearance deficiency, apoptotic nuclear debris accumulates in the light zone of GC and gets attached to FDC. This serves as a germinal centre survival signal for autoreactive B-cells. After migration into the mantle zone, autoreactive B cells require further survival signals from autoreactive helper T cells, which promote the maturation of autoantibody-producing plasma cells and B memory cells. In the presence of autoreactive T cells, a chronic autoimmune disease may be the consequence.
Anti-nRNP autoimmunity
Autoantibodies to nRNP A and nRNP C initially targeted restricted, proline-rich motifs. Antibody binding subsequently spread to other epitopes. The similarity and cross-reactivity between the initial targets of nRNP and Sm autoantibodies identifies a likely commonality in cause and a focal point for intermolecular epitope spreading.[36]
Others
Elevated expression of HMGB1 was found in the sera of patients and mice with systemic lupus erythematosus, High Mobility Group Box 1 (HMGB1) is a nuclear protein participating in chromatin architecture and transcriptional regulation. Recently, there is increasing evidence that HMGB1 contributes to the pathogenesis of chronic inflammatory and autoimmune diseases due to its pro-inflammatory and immunostimulatory properties.[37]
Diagnosis

Laboratory tests
Antinuclear antibody (ANA) testing and anti-extractable nuclear antigen (anti-ENA) form the mainstay of serologic testing for SLE.Several techniques are used to detect ANAs.Clinically the most widely used method is indirect immunofluorescence.The pattern of fluorescence suggests the type of antibody present in the patient's serum.
ANA screening yields positive results in many connective tissue disorders and other autoimmune diseases, and may occur in normal individuals. Subtypes of antinuclear antibodies include anti-Smith and anti-double stranded DNA (dsDNA) antibodies (which are linked to SLE) and anti-histone antibodies (which are linked to drug-induced lupus). Anti-dsDNA antibodies are highly specific for SLE; they are present in 70% of cases, whereas they appear in only 0.5% of people without SLE.[2] The anti-dsDNA antibody titers also tend to reflect disease activity, although not in all cases.[2] Other ANA that may occur in SLE sufferers are anti-U1 RNP (which also appears in systemic sclerosis), SS-A (or anti-Ro) and SS-B (or anti-La; both of which are more common in Sjögren's syndrome). SS-A and SS-B confer a specific risk for heart conduction block in neonatal lupus.[38]
Other tests routinely performed in suspected SLE are complement system levels (low levels suggest consumption by the immune system), electrolytes and renal function (disturbed if the kidney is involved), liver enzymes, complete blood count and recently By proteomics, we can directly detect proteins as gene products as well as their alterations by post-translational modification and internal abscission which are characteristically observed in proteins.[39]
Previously, the lupus erythematosus (LE) cell test was not commonly used for diagnosis because those LE cells are only found in 50–75% of SLE cases, and are also found in some people with rheumatoid arthritis, scleroderma, and drug sensitivities. Because of this, the LE cell test is now performed only rarely and is mostly of historical significance.[40]
Diagnostic criteria
Some physicians make a diagnosis on the basis of the American College of Rheumatology (ACR) classification criteria. The criteria, however, were established mainly for use in scientific research including use in randomized controlled trials which require higher confidence levels, so some people with SLE may not pass the full criteria.
The American College of Rheumatology established eleven criteria in 1982,[41] which were revised in 1997[42] as a classificatory instrument to operationalise the definition of SLE in clinical trials. They were not intended to be used to diagnose individuals and do not do well in that capacity. For the purpose of identifying patients for clinical studies, a person has SLE if any 4 out of 11 symptoms are present simultaneously or serially on two separate occasions.
- Serositis: Pleuritis (inflammation of the membrane around the lungs) or pericarditis (inflammation of the membrane around the heart); sensitivity = 56%; specificity = 86% (pleural is more sensitive; cardiac is more specific).[43]
- Oral ulcers (includes oral or nasopharyngeal ulcers).
- Arthritis: nonerosive arthritis of two or more peripheral joints, with tenderness, swelling, or effusion; sensitivity = 86%; specificity = 37%.[43]
- Photosensitivity (exposure to ultraviolet light causes skin rash, or other symptoms of SLE flareups); sensitivity = 43%; specificity = 96%.[43]
- Blood—hematologic disorder—hemolytic anemia (low red blood cell count) or leukopenia (white blood cell count<4000/µl), lymphopenia (<1500/µl) or thrombocytopenia (<100000/µl) in the absence of offending drug; sensitivity = 59%; specificity = 89%.[43] Hypocomplementemia is also seen, due to either consumption of C3 and C4 by immune complex-induced inflammation or to congenitally complement deficiency, which may predispose to SLE.
- Renal disorder: More than 0.5g per day protein in urine or cellular casts seen in urine under a microscope; sensitivity = 51%; specificity = 94%.[43]
- Antinuclear antibody test positive; sensitivity = 99%; specificity = 49%.[43]
- Immunologic disorder: Positive anti-Smith, anti-ds DNA, antiphospholipid antibody, and/or false positive serological test for syphilis; sensitivity = 85%; specificity = 93%.[43] Presence of anti-ss DNA in 70% of cases (though also positive with rheumatic disease and healthy persons)[44]).
- Neurologic disorder: Seizures or psychosis; sensitivity = 20%; specificity = 98%.[43]
- Malar rash (rash on cheeks); sensitivity = 57%; specificity = 96%.[43]
- Discoid rash (red, scaly patches on skin that cause scarring); sensitivity = 18%; specificity = 99%.[43]
The mnemonic to remember the 11 symptoms is 'SOAP BRAIN MD'.[45]
Some people, especially those with antiphospholipid syndrome, may have SLE without four criteria, and also SLE may present with features other than those listed in the criteria.[46][47][48]
Recursive partitioning has been used to identify more parsimonious criteria.[43] This analysis presented two diagnostic classification trees:
1. Simplest classification tree: SLE is diagnosed if a person has an immunologic disorder (anti-DNA antibody, anti-Smith antibody, false positive syphilis test, or LE cells) or malar rash.
- sensitivity = 92%
- specificity = 92%
2. Full classification tree: Uses 6 criteria.
- sensitivity = 97%
- specificity = 95%
Other alternative criteria have been suggested.[49]
Treatment
Being a chronic disease with no known cure, the treatment of SLE is symptomatic. In essence, this involves preventing flares and reducing their severity and duration when they occur. Currently, medication is the main form of treatment.
Medications
Due to the variety of symptoms and organ system involvement with SLE, its severity in an individual must be assessed in order to successfully treat SLE. Mild or remittent disease can sometimes be safely left untreated. If required, nonsteroidal anti-inflammatory drugs and antimalarials may be used.
Disease-modifying antirheumatic drugs
Disease-modifying antirheumatic drugs (DMARDs) are used preventively to reduce the incidence of flares, the process of the disease, and lower the need for steroid use; when flares occur, they are treated with corticosteroids. DMARDs commonly in use are antimalarials and immunosuppressants (e.g. methotrexate and azathioprine). Hydroxychloroquine is an FDA-approved antimalarial used for constitutional, cutaneous, and articular manifestations, whereas cyclophosphamide is used for severe glomerulonephritis or other organ-damaging complications. In 2005, mycophenolic acid became accepted for treatment of lupus nephritis.
Immunosuppressive drugs
In more severe cases, medications that modulate the immune system (primarily corticosteroids and immunosuppressants) are used to control the disease and prevent recurrence of symptoms (known as flares). Depending on the dosage, people that require steroids may develop side-effects such as central obesity, puffy round face, diabetes mellitus, large appetite, difficulty sleeping and osteoporosis. Those side-effects can subside if and when the large initial dosage is reduced, but long-term use of even low doses can cause elevated blood pressure and cataracts.
Analgesia
Since a large percentage of people with SLE suffer from varying amounts of chronic pain, stronger prescription analgesics (pain killers) may be used if over-the-counter drugs (mainly nonsteroidal anti-inflammatory drugs) do not provide effective relief. Moderate pain is typically treated with mild prescription opiates such as dextropropoxyphene and co-codamol. Moderate to severe chronic pain is treated with stronger opioids, such as hydrocodone or longer-acting continuous-release opioids, such as oxycodone, MS Contin, or Methadone. The Fentanyl duragesic transdermal patch is also a widely-used treatment option for the chronic pain caused by complications because of its long-acting timed release and ease of use. When opioids are used for prolonged periods, drug tolerance, chemical dependency, and addiction may occur. Opiate addiction is not typically a concern, since the condition is not likely to ever completely disappear. Thus, lifelong treatment with opioids is fairly common for chronic pain symptoms, accompanied by periodic titration that is typical of any long-term opioid regimen.
Lifestyle changes
Avoiding sunlight is the primary change to the lifestyle of SLE sufferers, as sunlight is known to exacerbate the disease. Drugs unrelated to SLE should be prescribed only when known not to exacerbate the disease. Occupational exposure to silica, pesticides and mercury can also make the disease worsen.[23]
Fasting and massive nutrition changes, towards a low fat, mostly strict vegetarian, wholesome diet, also have been reported as a possibility to lessen the symptoms or even induce a remission.[50]
Renal transplantation
is the treatment of choice for end-stage renal disease, which is one of the complications of lupus nephritis, but the recurrence of the full disease is common in up to 30% of patients.[51]
Prevention
SLE is not understood well enough to be prevented, but, when the disease develops, quality of life can be improved through flare prevention. The warning signs of an impending flare include increased fatigue, pain, rash, fever, abdominal discomfort, headache, and dizziness. Early recognition of warning signs and good communication with a doctor can help individuals remain active, experience less pain, and reduce medical visits.[6]
Complications during pregnancy
While most infants born to mothers who have SLE are healthy, pregnant mothers with SLE should remain under a doctor's care until delivery. Neonatal lupus is rare, but identification of mothers at highest risk for complications allows for prompt treatment before or after birth. In addition, SLE can flare during pregnancy, and proper treatment can maintain the health of the mother longer. Women pregnant and known to have the antibodies for anti-Ro (SSA) or anti-La (SSB) should have echocardiograms during the 16th and 30th weeks of pregnancy to monitor the health of the heart and surrounding vasculature.[6]
Even contraception was routinely advised in treating SLE patients, getting pregnant during active disease was eventually found. Lupus nephritis was the most common manifestation. Overall live-birth was 72.7%. Pregnancy lost was due to abortion and dead fetus in utero. Pregnancy outcome was worse in SLE patients who had disease flares up or emerging during pregnancy.[52]
Prognosis
In the 1950s, most people diagnosed with SLE lived fewer than five years. Advances in diagnosis and treatment have improved survival to the point where over 90% now survive for more than ten years, and many can live relatively asymptomatically. Prognosis is normally worse for men and children than for women; however, if symptoms are present after age 60, the disease tends to run a more benign course. Early mortality, within 5 years, is due to organ failure or overwhelming infections, both of which can be modified by early diagnosis and treatment. The mortality risk is five-fold when compared to the normal population in the late stages, which can be attributed to cardiovascular diseases acquired from corticosteroid therapy. To reduce potential for cardiovascular issues, steroids should be used at the lowest dose for the shortest possible period. High serum creatinine, hypertension, nephrotic syndrome, anemia and hypoalbuminemia are poor prognostic factors.[53] The ANA is the most sensitive screening test for evaluation, whereas anti-Sm (anti-Smith) is the most specific. The dsDNA (double-stranded DNA) antibody is also fairly specific and often fluctuates with disease activity; as such, the dsDNA titer is sometimes useful to monitor disease flares or response to treatment.[54]
Epidemiology
The rate of SLE varies considerable between countries, ethnicity, by gender, and has changed over time.[55] In the United States the prevalence of SLE is estimated to be about 53 per 100,000, translating to about 3% or to 1 million people in the US being affected.[56] [55] In Northern Europe the rate is about 40 per 100,000 people.[57] SLE occurs more frequently and with greater severity among those of non-European descent. [56] That rate has been found to be as high as 159 per 100,000 among those of Afro-Caribbean decent.[55]
SLE, like many autoimmune diseases, affects females more frequently, at a rate of almost 9 to 1. [55]
The incidence of SLE in the United States increased from 1.0 in 1955 to 7.6 in 1974. Whether the increase is due to better diagnosis or to increasing frequency of the disease is unknown.[55]
History and culture
Etymology
There are several explanations ventured for the term lupus erythematosus. Lupus is Latin for wolf, and "erythro" is derived from ερυθρός[] Error: {{Lang}}: no text (help), Greek for "red." All explanations originate with the reddish, butterfly-shaped malar rash that the disease classically exhibits across the nose and cheeks.
- In various accounts, some doctors thought the rash resembled the pattern of fur on a wolf's face.
- In other accounts, doctors thought that the rash, which was often more severe in earlier centuries, created lesions that resembled wolf bites or scratches.
- Another account claims that the term "lupus" did not come from Latin directly, but from the term for a French style of mask that women reportedly wore to conceal the rash on their faces. The mask is called a "loup," French for "wolf."
- Another common explanation for the term is that the disease's course involves repeated attacks like those of a voracious predator, leaving behind the red blotches.
History
The history of SLE can be divided into three periods: classical, neoclassical, and modern. The classical period began when the disease was first recognized in the Middle Ages and saw the description of the dermatological manifestation of the disorder. The term lupus is attributed to 12th-century physician Rogerius, who used it to describe the classic malar rash. The neoclassical period was heralded by Móric Kaposi's recognition in 1872 of the systemic manifestations of the disease. The modern period began in 1948 with the discovery of the LE cell (the lupus erythematosus cell—a misnomer, as it occurs with other diseases as well) and is characterised by advances in our knowledge of the pathophysiology and clinical-laboratory features of the disease, as well as advances in treatment.[58]
Medical historians have theorized that people with porphyria (a disease that shares many symptoms with SLE) generated folklore stories of vampires and werewolves, due to the photosensitivity, scarring, hair growth, and porphyrin brownish-red stained teeth in severe recessive forms of porphyria (or combinations of the disorder, known as dual, homozygous, or compound heterozygous porphyrias).[58]
Useful medication for the disease was first found in 1894, when quinine was first reported as an effective therapy. Four years later, the use of salicylates in conjunction with quinine was noted to be of still greater benefit. This was the best available treatment until the middle of the twentieth century, when Hench discovered the efficacy of corticosteroids in the treatment of SLE.[58]
Notable cases
- Stephanie Smith, artist who died of SLE complications in 1969 at the age of 22. The anti-Smith (or anti-Sm) antigen was discovered in her and is the basis of a SLE diagnostic test.
- Michael Jackson has been reported to suffer from both SLE and vitiligo.[59] Diagnosed in 1986, and comfirmed by his dermatologist, Dr. Arnold Klein, who presented legal documments during court despositions.
- Seal, singer, was reportedly diagnosed with discoid lupus of the face as a child, leading to scarring[60]
- Caroline Dorough-Cochran, sister of Howie D. of the Backstreet Boys who died of SLE complications and founded the Dorough Lupus Foundation in her memory.
- Inday Ba (also known as N'Deaye Ba), a Swedish-born actress who died from SLE complications at age 32.[61]
- Donald Byrne, American chess player who died from SLE complications in 1976.[62]
- J Dilla (also known as Jay Dee), a hip-hop producer and beatmaker who died of SLE complications in 2006.[63]
- Hugh Gaitskell, British politician who died of SLE complications in 1963 aged 56.[64]
- Teddi King, American singer, died of SLE complications in 1977.[65]
- Charles Kuralt, former anchor of CBS Sunday Morning, died of SLE complications in 1997.[66]
- Ferdinand Marcos, former Philippine president, who died of SLE complications in 1989.[67]
- Flannery O'Connor, American fiction writer who died of SLE complications in 1964.[68]
- Michael Wayne, Hollywood director, and producer, part owner of Batjac Productions, son of legendary actor John Wayne, died of heart failure resulting from SLE complications in 2003.[69]
- Ray Walston, character actor who died of SLE complications in 2001 after a 6-year battle with the disease.[70]
- Tim Raines, former major league baseball player, primarily with the Montreal Expos and Chicago White Sox who was diagnosed with SLE in 1999 and spent the rest of the year undergoing treatment and recovery.[71]
- Mary Elizabeth McDonough, American actress; blames her SLE on leaky silicone breast implants.[72]
- Elaine Paige, British actress and singer.[73]
- Mercedes Scelba-Shorte, America's Next Top Model Season Two runner-up and model.[74]
- Louisa May Alcott, American author best known for her novel Little Women, has been suggested to have SLE.[75]
- Sophie Howard, British glamour model.[76]
- Lauren Shuler Donner, American movie director.[77]
- Fred Williamson[citation needed]
See also
- Abzyme
- Antinuclear antibody
- Canine discoid lupus erythematosus in dogs
- Lupus erythematosus
- Lupus band test
References
- ^ a b James, William; Berger, Timothy; Elston, Dirk (2005). Andrews' Diseases of the Skin: Clinical Dermatology. (10th ed.). Saunders. ISBN 0721629210. Cite error: The named reference "Andrews" was defined multiple times with different content (see the help page).
- ^ a b c d e f Anisur Rahman and David A. Isenberg (February 28, 2008). "Review Article: Systemic Lupus Erythematosus". N Engl J Med. 358 (9): 929–939. doi:10.1056/NEJMra071297. PMID 18305268.
{{cite journal}}: CS1 maint: date and year (link) - ^ "LUPUS FOUNDATION OF AMERICA". Retrieved 2007-07-04.
- ^ a b Harrison's Internal Medicine, 17th ed. Chapter 313. Systemic Lupus Erythematosus.
- ^ Discoid Lupus Erythematosus
- ^ a b c d "Handout on Health: Systemic Lupus Erythematosus". The National Institute of Arthritis and Musculoskeletal and Skin Diseases. National Institutes of Health. 2003. Retrieved 2007-11-23.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ "Lupus, "The Great Imitator"". University Health Care. Retrieved 2009-02-03.
- ^ "Lupus: Symptoms - MayoClinic.com". Retrieved 2008-07-14.
- ^ a b Joint and Muscle Pain Lupus Foundation of America
- ^ Hemminki K, Li X, Sundquist J, Sundquist K (2009). "Familial associations of rheumatoid arthritis with autoimmune diseases and related conditions". Arthritis Rheum. 60 (3): 661–668. doi:10.1002/art.24328. PMID 19248111.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Yu Asanuma, M.D., Ph.D., Annette Oeser, B.S., Ayumi K. Shintani, Ph.D., M.P.H., Elizabeth Turner, M.D., Nancy Olsen, M.D., Sergio Fazio, M.D., Ph.D., MacRae F. Linton, M.D., Paolo Raggi, M.D., and C. Michael Stein, M.D. (2003). "Premature coronary-artery atherosclerosis in systemic lupus erythematosus". New England Journal of Medicine. 349 (Dec. 18): 2407–2414. doi:10.1056/NEJMoa035611. PMID [http://content.nejm.org/cgi/content/abstract/349/25/2407 Abstract (full text requires registration) 14681506 [http://content.nejm.org/cgi/content/abstract/349/25/2407 Abstract] (full text requires registration)].
{{cite journal}}: Check|pmid=value (help); External link in|pmid= - ^ Bevra Hannahs Hahn, M.D. (2003). "Systemic lupus erythematosus and accelerated atherosclerosis". New England Journal of Medicine. 349 (Dec. 18): 2379–2380. doi:10.1056/NEJMp038168. PMID [http://content.nejm.org/cgi/content/extract/349/25/2379 Extract (full text requires registration) 14681501 [http://content.nejm.org/cgi/content/extract/349/25/2379 Extract] (full text requires registration)].
{{cite journal}}: Check|pmid=value (help); External link in|pmid= - ^ Mary J. Roman, M.D., Beth-Ann Shanker, A.B., Adrienne Davis, A.B., Michael D. Lockshin, M.D., Lisa Sammaritano, M.D., Ronit Simantov, M.D., Mary K. Crow, M.D., Joseph E. Schwartz, Ph.D., Stephen A. Paget, M.D., Richard B. Devereux, M.D., and Jane E. Salmon, M.D. (2003). "Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus". New England Journal of Medicine. 349 (Dec. 18): 2399–2406. doi:10.1056/NEJMoa035471. PMID [http://content.nejm.org/cgi/content/abstract/349/25/2399 Abstract (full text requires registration) 14681505 [http://content.nejm.org/cgi/content/abstract/349/25/2399 Abstract] (full text requires registration)].
{{cite journal}}: Check|pmid=value (help); External link in|pmid= - ^ "General Pathology Images for Immunopathology". Retrieved 2007-07-24.
- ^ "The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes". Arthritis Rheum. 42 (4): 599–608. 1999. doi:10.1002/1529-0131(199904)42:4<599::AID-ANR2>3.0.CO;2-F. PMID 10211873.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Omdal R (2002). "Some controversies of neuropsychiatric systemic lupus erythematosus". Scand. J. Rheumatol. 31 (4): 192–7. PMID 12369649.
- ^ D'Cruz DP (2006). "Systemic lupus erythematosus". BMJ. 332 (7546): 890–4. doi:10.1136/bmj.332.7546.890. PMC 1440614. PMID 16613963.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Jump RL, Robinson ME, Armstrong AE, Barnes EV, Kilbourn KM, Richards HB (2005). "Fatigue in systemic lupus erythematosus: contributions of disease activity, pain, depression, and perceived social support". J. Rheumatol. 32 (9): 1699–705. PMID 16142863.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Abdallah M, B'Chir Hamzaoui S, Bouslama K; et al. (2005). "[Acute pancreatitis associated with hemophagocytic syndrome in systemic lupus erythematous: a case report]". Gastroenterol. Clin. Biol. (in French). 29 (10): 1054–6. PMID 16435516.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Yoshida S, Takeuchi T, Itami Y; et al. (2009). "[Hemophagocytic syndrome as primary manifestation in a patient with systemic lupus erythematosus after parturition]". Nihon Rinsho Meneki Gakkai Kaishi (in Japanese). 32 (1): 66–70. PMID 19252381.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Carr EJ, Clatworthy MR, Lowe CE; et al. (2009). "Contrasting genetic association of IL2RA with SLE and ANCA - associated vasculitis". BMC Med. Genet. 10 (1): 22. doi:10.1186/1471-2350-10-22. PMID 19265545.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Anderson LA, Pfeiffer RM, Landgren O, Gadalla S, Berndt SI, Engels EA (2009). "Risks of myeloid malignancies in patients with autoimmune conditions". Br. J. Cancer. 100 (5): 822–8. doi:10.1038/sj.bjc.6604935. PMID 19259097.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b D'Cruz DP, Khamashta MA, Hughes GR (2007). "Systemic lupus erythematosus". Lancet. 369 (9561): 587–96. doi:10.1016/S0140-6736(07)60279-7. PMID 17307106. Retrieved 2009-02-01.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Kanta H, Mohan C (2009). "Three checkpoints in lupus development: central tolerance in adaptive immunity, peripheral amplification by innate immunity and end-organ inflammation". Genes Immun. doi:10.1038/gene.2009.6. PMID 19262576.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Martens HA, Nolte IM, van der Steege G; et al. (2009). "An extensive screen of the HLA region reveals an independent association of HLA class I and class II with susceptibility for systemic lupus erythematosus". Scand. J. Rheumatol.: 1–7. doi:10.1080/03009740802552469. PMID 19255932.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Kim K, Sung YK, Kang CP, Choi CB, Kang C, Bae SC (2009). "A regulatory SNP at position -899 in CDKN1A is associated with systemic lupus erythematosus and lupus nephritis". Genes Immun. doi:10.1038/gene.2009.5. PMID 19262578.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Rhodes B, Vyse TJ (2008). "The genetics of SLE: an update in the light of genome-wide association studies". Rheumatology (Oxford). 47 (11): 1603–11. doi:10.1093/rheumatology/ken247. PMID 18611920.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Burry, J. (1969). "Lipstick and lupus erythematosus". New England Journal of Medicine. 281: 620–621.
- ^ Wang J, Kay AB, Fletcher J, Formica MK & McAlindon TE (2008). "Is lipstick associated with the development of systemic lupus erythematosus (SLE)?". Clinical Rheumatology. 29 (9): 1183–1197.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Masters, K. (2009). "Lupus And Lipstick: The Industry Responds". The Internet Journal of Dermatology. 7 (1).
- ^ Mary K. Crow (February 28, 2008). "Collaboration, Genetic Associations, and Lupus Erythematosus". N Engl J Med. 358 (9): 956–961. doi:10.1056/NEJMe0800096. PMID 18204099.
{{cite journal}}: CS1 maint: date and year (link) - ^ Geoffrey Hom, Robert R. Graham, Barmak Modrek; et al. (February 28, 2008). "Association of Systemic Lupus Erythematosus with C8orf13–BLK and ITGAM–ITGAX". N Engl J Med. 358 (9): 900–909. doi:10.1056/NEJMoa0707865. PMID 18204098.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ University of South Carolina School of Medicine lecture notes, Immunology, Hypersensitivity reactions. General discussion of hypersensitivity, not specific to SLE.
- ^ Gaipl, U S; Kuhn, A; Sheriff, A; Munoz, L E; Franz, S; Voll, R E; Kalden, J R; Herrmann, M (2006). "Clearance of apoptotic cells in human SLE". Current directions in autoimmunity. 9: 173–87. PMID 1639466 [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16394661&dopt=Abstract Abstract (full text requires registration) : 1639466 [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16394661&dopt=Abstract Abstract] (full text requires registration)].
{{cite journal}}: Check|pmid=value (help); External link in|pmid= - ^ Gaipl US, Munoz LE, Grossmayer G; et al. (2007). "Clearance deficiency and systemic lupus erythematosus (SLE)". J. Autoimmun. 28 (2–3): 114–21. doi:10.1016/j.jaut.2007.02.005. PMID 17368845.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Poole BD, Schneider RI, Guthridge JM; et al. (2009). "Early targets of nuclear RNP humoral autoimmunity in human systemic lupus erythematosus". Arthritis Rheum. 60 (3): 848–859. doi:10.1002/art.24306. PMID 19248110.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Pan HF, Wu GC, Li WP, Li XP, Ye DQ (2009). "High Mobility Group Box 1: a potential therapeutic target for systemic lupus erythematosus". Mol. Biol. Rep. doi:10.1007/s11033-009-9485-7. PMID 19247800.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Buyon JP, Clancy RM (2003). "Maternal autoantibodies and congenital heart block: mediators, markers, and therapeutic approach". Semin. Arthritis Rheum. 33 (3): 140–54. PMID 14671725.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Iizuka N, Okamoto K, Hirohata S, Kato T (2009). "[Analysis of autoantigens in patients with systemic lupus erythematosus by using proteomic approach]". Nihon Rinsho Meneki Gakkai Kaishi (in Japanese). 32 (1): 43–7. PMID 19252377.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ NIM encyclopedic article on the LE cell test
- ^ Rheumatology.org article on the classification of rheumatic diseases
- ^ Revision of Rheumatology.org's diagnostic criteria
- ^ a b c d e f g h i j k Edworthy SM, Zatarain E, McShane DJ, Bloch DA (1988). "Analysis of the 1982 ARA lupus criteria data set by recursive partitioning methodology: new insights into the relative merit of individual criteria". J. Rheumatol. 15 (10): 1493–8. PMID 3060613.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ UpToDate Patient information article on DNA antibodies
- ^ Mnemonic for 11 criteria of American College of Rheumatology
- ^ Asherson RA, Cervera R, de Groot PG; et al. (2003). "Catastrophic antiphospholipid syndrome: international consensus statement on classification criteria and treatment guidelines". Lupus. 12 (7): 530–4. doi:10.1191/0961203303lu394oa. PMID 12892393.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Sangle S, D'Cruz DP, Hughes GR (2005). "Livedo reticularis and pregnancy morbidity in patients negative for antiphospholipid antibodies". Ann. Rheum. Dis. 64 (1): 147–8. doi:10.1136/ard.2004.020743. PMID 15608315.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hughes GR, Khamashta MA (2003). "Seronegative antiphospholipid syndrome". Ann. Rheum. Dis. 62 (12): 1127. doi:10.1136/ard.2003.006163. PMID 14644846.
- ^ Hughes GR (1998). "Is it lupus? The St. Thomas' Hospital "alternative" criteria". Clin. Exp. Rheumatol. 16 (3): 250–2. PMID 9631744.
- ^ Mayra. Almost Lost to Lupus. As Told by Her Father, Victor Dengler
- ^ Cochat P, Fargue S, Mestrallet G; et al. (2009). "Disease recurrence in paediatric renal transplantation". Pediatr. Nephrol. doi:10.1007/s00467-009-1137-6. PMID 19247694.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Foocharoen C, Nanagara R, Salang L, Suwannaroj S, Mahakkanukrauh A (2009). "Pregnancy and disease outcome in patients with systemic lupus erythematosus (SLE): a study at Srinagarind Hospital". J Med Assoc Thai. 92 (2): 167–74. PMID 19253790.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Poor prognostic factors,Sudheer, SLE document
- ^ [EARLY STEROIDS MAY PREVENT RELAPSES IN LUPUS, P Jarman (Published in Journal Watch (General) July 18, 1995)
- ^ a b c d e Danchenko N, Satia JA, Anthony MS (2006). "Epidemiology of systemic lupus erythematosus: a comparison of worldwide disease burden". Lupus. 15 (5): 308–18. PMID 16761508.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b "OMHD|AMH|Factsheets|Lupus".
- ^ Rahman A, Isenberg DA (2008). "Systemic lupus erythematosus". N. Engl. J. Med. 358 (9): 929–39. doi:10.1056/NEJMra071297. PMID 18305268.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c Hochberg MC (1991). "The history of lupus erythematosus". Md Med J. 40 (10): 871–3. PMID 1943516.
{{cite journal}}:|access-date=requires|url=(help); Unknown parameter|month=ignored (help) - ^ Jeri Jewett-Tennant. Celebrities with Lupus: Michael Jackson. Updated: August 11, 2008.
- ^ Jeri Jewett-Tennant. Celebrities with Lupus: Seal. Updated: August 11, 2008.
- ^ A battle with the wolf. Anthony Gardner, Mail on Sunday, October 10, 2008.
- ^ Former chess coach named to Hall of Fame. Gary Cramer. Penn State Intercom, September 26, 2002.
- ^ J Dilla/Jay Dee. Retrieved February 2, 2009.
- ^ 1963: Labour leader Hugh Gaitskell dies. BBC News, On This Day, January 18, 1963.
- ^ In the Beginning, 1949~1954 - Teddi King. Allaboutjazz.com. Retrieved February 2, 2009.
- ^ Celebrities with Lupus: Charles Kuralt. Updated: August 11, 2008.
- ^ Famous Lupus Patient: Ferdinand Marcos. Updated: August 11, 2008.
- ^ Flannery O'Connor (1925-1964). New Georgia Encyclopedia. Sarah Gordon, Georgia College and State University. Updated 3/21/2008
- ^ Dennis Mclellan. Michael Wayne, 68; Producer, Guardian of His Father’s Legacy. Los Angeles Times, April 4, 2003.
- ^ Jeri Jewett-Tennant. Celebrities with Lupus: Ray Walston. Updated: August 11, 2008.
- ^ Jeri Jewett-Tennant. Celebrities with Lupus: Tim Raines. Updated: August 28, 2008
- ^ Celebrities with Lupus: Mary Elizabeth McDonough. Updated: October 28, 2008.
- ^ Biography. Retrieved February 2, 2009.
- ^ Jeri Jewett-Tennant. Celebrities with Lupus: Mercedes Scelba-Shorte. Updated: August 28, 2008.
- ^ Hirschhorn N, Greaves IA. Louisa May Alcott, her mysterious illness. Perspectives in Biology and Medicine 2007;50(2):243-259.
- ^ Angie Davidson interviews top glamour model Sophie Howard Lupus.org.uk, accessed 21 November 2008
- ^ Jeri Jewett-Tennant. Celebrities with Lupus: Lauren Schuler Donner. Updated: August 28, 2008
