Lithium oxide

| |

| |
 | |

| |
| Names | |
|---|---|
| IUPAC name
Lithium oxide
| |
| Other names
Lithia, Kickerite
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.031.823 |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Li 2O | |
| Molar mass | 29.88 g/mol |
| Appearance | white solid |
| Density | 2.013 g/cm3 |
| Melting point | 1,438 °C (2,620 °F; 1,711 K) |
| Boiling point | 2,600 °C (4,710 °F; 2,870 K) |
| Reacts to form LiOH | |
| log P | 9.23 |
Refractive index (nD)
|
1.644 [1] |
| Structure | |
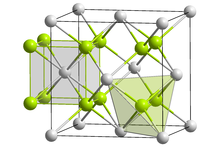
| Antifluorite (cubic), cF12 | |
| Fm3m, No. 225 | |
| Tetrahedral (Li+); cubic (O2−) | |
| Thermochemistry | |
Heat capacity (C)
|
1.8105 J/g K or 54.1 J/mol K |
Std molar
entropy (S⦵298) |
37.89 J/mol K |
Std enthalpy of
formation (ΔfH⦵298) |
-20.01 kJ/g or -595.8 kJ/mol |
Gibbs free energy (ΔfG⦵)
|
-562.1 kJ/mol |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Corrosive, reacts violently with water |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
Lithium sulfide Lithium selenide Lithium telluride Lithium polonide |
Other cations
|
Sodium oxide Potassium oxide Rubidium oxide Caesium oxide |
| Lithium peroxide Lithium superoxide | |
Related compounds
|
Lithium hydroxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Lithium oxide (Li
2O) or lithia is an inorganic chemical compound. It is a white solid. Although not specifically important, many materials are assessed on the basis of their Li2O content. For example, the Li2O content of the principal lithium mineral spodumene (LiAlSi2O6) is 8.03%.[2]
Production

Lithium oxide is produced by thermal decomposition of lithium peroxide at 300–400 °C.[2]
Lithium oxide forms along with small amounts of lithium peroxide when lithium metal is burned in the air at and combines with oxygen at temperatures above 100 °C:[3]
- 4Li + O
2 → 2Li
2O.
Pure Li
2O can be produced by the thermal decomposition of lithium peroxide, Li
2O
2, at 450 °C[3]
- 2Li
2O
2 → 2Li
2O + O
2
Structure
Solid lithium oxide adopts an antifluorite structure with four-coordinated Li+ centers and eight-coordinated oxides.[4]
The ground state gas phase Li
2O molecule is linear with a bond length consistent with strong ionic bonding.[5][6] VSEPR theory would predict a bent shape similar to H
2O.
Uses
Lithium oxide is used as a flux in ceramic glazes; and creates blues with copper and pinks with cobalt. Lithium oxide reacts with water and steam, forming lithium hydroxide and should be isolated from them.
Its usage is also being investigated for non-destructive emission spectroscopy evaluation and degradation monitoring within thermal barrier coating systems. It can be added as a co-dopant with yttria in the zirconia ceramic top coat, without a large decrease in expected service life of the coating. At high heat, lithium oxide emits a very detectable spectral pattern, which increases in intensity along with degradation of the coating. Implementation would allow in situ monitoring of such systems, enabling an efficient means to predict lifetime until failure or necessary maintenance.
Lithium metal might be obtained from lithium oxide by electrolysis, releasing oxygen as by-product.
Reactions
Lithium oxide absorbs carbon dioxide forming lithium carbonate:
- Li
2O + CO
2 → Li
2CO
3
The oxide reacts slowly with water, forming lithium hydroxide
- Li
2O + H
2O → 2LiOH
See also
References
- ^ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0-07-049439-8
- ^ a b Wietelmann, Ulrich and Bauer, Richard J. (2005) "Lithium and Lithium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH: Weinheim. doi:10.1002/14356007.a15_393.
- ^ a b Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. pp. 97–99. ISBN 978-0-08-022057-4.
- ^ Zintl, Eduard; Harder, A.; Dauth, B. (1934). "Gitterstruktur der Oxyde, Sulfide, Selenide und Telluride des Lithiums, Natriums und Kaliums". Zeitschrift für Elektrochemie und Angewandte Physikalische Chemie (in German). 40 (8): 588–593. doi:10.1002/bbpc.19340400811. S2CID 94213844.
- ^ Wells A. F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
- ^ A spectroscopic determination of the bond length of the LiOLi molecule: Strong ionic bonding, D. Bellert, W. H. Breckenridge, J. Chem. Phys. 114, 2871 (2001); doi:10.1063/1.1349424

